Summary
The Nucleosome Remodeling and Deacetylase (NuRD) complex is a chromatin regulatory complex that functions as a transcriptional co-repressor in metazoans. The NuRD subunit MBD3 is essential for targeting and assembly of a functional NuRD complex as well as embryonic stem cell (ESC) pluripotency. Three MBD3 isoforms (MBD3A, MBD3B, and MBD3C) are expressed in mouse. Here, we find that the MBD3C isoform contains a unique 50-amino-acid N-terminal region that is necessary for MBD3C to specifically interact with the histone H3 binding protein WDR5. Domain analyses of WDR5 reveal that the H3 binding pocket is required for interaction with MBD3C. We find that while Mbd3c knockout ESCs differentiate normally, MBD3C is redundant with the MBD3A and MBD3B isoforms in regulation of gene expression, with the unique MBD3C N terminus required for this redundancy. Together, our data characterize a unique NuRD complex variant that functions specifically in ESCs.
Keywords: NuRD, Mbd3, Wdr5, chromatin, differentiation
Graphical Abstract

Highlights
-
•
Mbd3c binds Wdr5 through its unique N-terminal domain
-
•
Wdr5 interaction is critical for Mbd3c function
-
•
Mbd3c/NuRD can substitute for canonical NuRD complex in ESC gene regulation
Ee and colleagues characterize an embryonic stem cell (ESC)-specific isoform of the Mbd3 subunit of the NuRD chromatin remodeling complex. This variant, called Mbd3c, forms a variant NuRD complex that incorporates the histone binding protein Wdr5, and this interaction is critical for its functions in gene regulation. The presence of either Mbd3c/NuRD or canonical NuRD complex (containing either the Mbd3a or Mbd3b isoform) is necessary for normal gene regulation and ESC pluripotency.
Introduction
To maintain their cellular identity, embryonic stem cells (ESCs) utilize a network of core transcription factors and chromatin remodeling enzymes that bind and regulate pluripotency genes and differentiation genes in response to developmental signaling (Kim et al., 2008). The Nucleosome Remodeling and Deacetylase (NuRD) complex is unique among chromatin regulators because it couples ATP-dependent nucleosome remodeling activity with histone modification (deacetylase) activity (Tong et al., 1998, Wade et al., 1998, Xue et al., 1998, Zhang et al., 1998). NuRD alters nucleosome occupancy to block the binding of transcriptional machinery at gene promoters, thus functioning primarily as a co-repressor (Denslow and Wade, 2007, Yildirim et al., 2011). In addition, mice deleted for the Mbd3 gene, which encodes a NuRD subunit important for NuRD targeting and assembly, are nonviable (Hendrich et al., 2001). ESCs derived from Mbd3-null mouse embryos do not differentiate and are capable of self-renewal in culture in the absence of leukemia inhibitory factor (LIF) (Kaji et al., 2006). Mbd3 was subsequently shown to be important for differentiation and development through silencing of pluripotency genes (Reynolds et al., 2012), functioning in part by deacetylation of H3K27 (Reynolds et al., 2011).
MBD3 was originally identified as a member of the methyl-CpG binding domain (MBD) family of proteins (Hendrich and Bird, 1998). However, unlike MBD members MECP2 and MBD1, 2, and 4, MBD3 does not bind methylated DNA (Hendrich and Bird, 1998, Zhang et al., 1999). Three MBD3 isoforms (MBD3A, B, and C) are expressed in mouse ESCs, and only MBD3A has a full-length MBD (Kaji et al., 2006). Thus, the possibility exists for formation of multiple NuRD complexes of varying subunit combinations and functional specificities.
Here, we have characterized a unique variant of the NuRD chromatin remodeling complex that harbors MBD3C, an ESC-specific isoform of MBD3, as well as the histone H3 binding protein WDR5. MBD3C is expressed almost exclusively in ESCs via an alternative CpG island (CGI)-containing promoter located in the second intron of the Mbd3 gene. We further show that MBD3C contains a unique 50-amino-acid N terminus that is necessary for WDR5 interaction. MBD3C interacts with the WDR5 H3 binding pocket through an arginine-containing motif also utilized by MLL1 for WDR5 binding. RNA sequencing (RNA-seq) analysis revealed that the three MBD3 isoforms are largely redundant for gene regulation, since knockout (KO) of all three isoforms had a more severe effect on gene expression than individual KO of Mbd3c or simultaneous KO of Mbd3a and Mbd3b. Importantly, the WDR5-interaction domain of Mbd3c is critical for its gene regulatory function, suggesting that WDR5 plays critical roles in MBD3C/NuRD complex.
Results and Discussion
MBD3C/NuRD Co-purifies with WDR5
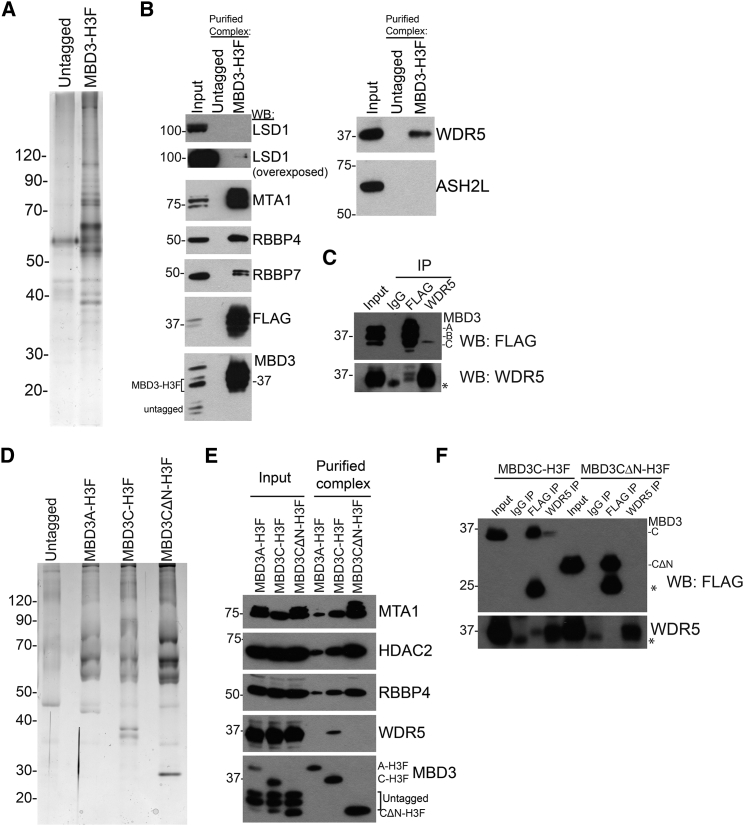
To identify proteins co-purifying with MBD3 in ESCs, we used a cell line in which one copy of endogenous MBD3 is fused to a C-terminal 6xHis-3xFLAG tag (Mbd3-H3F; Yildirim et al., 2011), allowing for affinity purification of MBD3A, B, and C simultaneously (Figures 1A and S1A). Liquid chromatography-tandem mass spectrometry (LC-MS/MS) of purified MBD3 complexes identified all canonical NuRD subunits, several of which were subsequently confirmed by western blot (Figure 1B and Table S1).
Figure 1.
The NuRD Subunit MBD3C Co-purifies with WDR5
(A) Silver stain of MBD3-H3F complex.
(B) Western blot (WB) of purified complex from (A) showing interaction of MBD3 with NuRD subunits (left) and with WDR5 or MLL subunit ASH2L (right).
(C) Western blots for MBD3-H3F or WDR5 upon immunoprecipitation (IP) of each. Asterisk indicates immunoglobulin G (IgG).
(D) Silver stain of MBD3 complex expressing individually H3F-tagged MBD3 isoforms.
(E) Western blot of purified complexes from (D) showing interaction with WDR5 and NuRD subunits.
(F) WDR5 or FLAG immunoprecipitation from individually tagged MBD3C-H3F and MBD3CΔN-H3F ESCs. Asterisks indicate IgG.
Consistent with recent MS analyses of NuRD components (Bode et al., 2016), we detected an interaction between MBD3 and the SET/MLL complex component WDR5 (Figures 1B and S1B; Table S1). The MLL complex is a histone methyltransferase that catalyzes methylation of H3K4, a mark found at transcriptionally active genes (Bernstein et al., 2002, Santos-Rosa et al., 2002). WDR5 binds the histone H3 tail in vitro and is essential for H3K4 trimethylation and MLL complex formation (Couture et al., 2006, Dou et al., 2006, Ruthenburg et al., 2006, Schuetz et al., 2006, Wysocka et al., 2005). We did not observe any other MLL subunits co-purifying with MBD3 (Figure 1B and Table S1), suggesting that WDR5 interacts with MBD3/NuRD independently of MLL complex. To validate these data, we performed co-immunoprecipitation (coIP) assays. Interestingly, WDR5 immunoprecipitation pulled down MBD3C, but not the more abundant isoforms, MBD3A and B (Figure 1C). These data suggest that WDR5 interacts specifically with this smallest and least characterized isoform of MBD3.
To further investigate the composition of the MBD3C/NuRD complex, we generated an ESC line expressing Mbd3c-H3F from a viral vector, such that only the MBD3C isoform is epitope-tagged. To this end, we first performed 5′ rapid amplification of cDNA ends (5′-RACE) to obtain the Mbd3c coding sequence. We found that MBD3C is translated from a start codon within intron 2 of the Mbd3 gene, consistent with a recent report (dos Santos et al., 2014). Thus, MBD3C lacks the entire MBD and contains a unique 50-amino-acid N terminus (Figure S1C). MBD3C-H3F complexes were affinity purified (Figure 1D) and analyzed by LC-MS/MS. As expected, WDR5 co-purifies with MBD3C-H3F but not MBD3A-H3F (Figures 1E and S1D; Table S2). Importantly, we found that WDR5 interaction was disrupted by deletion of the unique MBD3C N terminus (MBD3CΔN; Figures 1E and S1D), demonstrating that this domain is necessary for WDR5 binding. CoIP experiments confirmed these results (Figure 1F). Furthermore, we observed that MBD3C-H3F, MBD3A-H3F, and MBD3CΔN-H3F all co-purify with the canonical NuRD subunits (Figures 1E and S1D; Table S2). Together with data showing that WDR5 also co-purifies with NuRD subunits (Figure S1B; Bode et al., 2016) and that MBD3C co-fractionates exclusively with NuRD subunit MTA1 (Figure S1E), these data demonstrate that MBD3C assembles into a canonical NuRD complex that also includes WDR5. Although the MBD3 MBD was previously shown to directly interact with NuRD subunits HDAC1 and MTA2 in vitro (Saito and Ishikawa, 2002), our findings suggest that HDAC1 and MTA2 can also associate with the NuRD complex by MBD-independent mechanisms in vivo. In addition, while the unique MBD3C N terminus is required for interaction with WDR5, it is dispensable for interaction with the other known NuRD subunits (Figure S1D and Table S2).
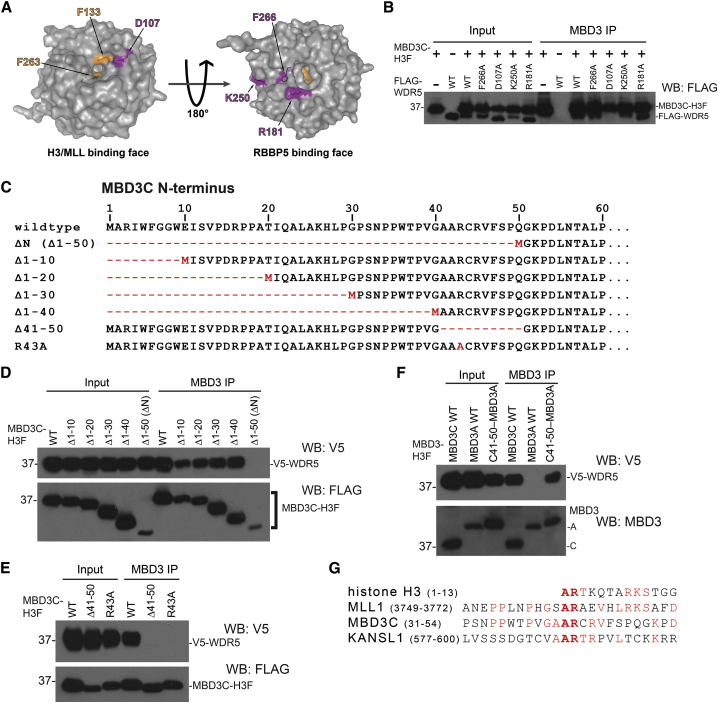
The WDR5 Histone H3 Binding Pocket Is Required for Interaction with MBD3C
To gain insight into the functions of the MBD3C-WDR5 interaction, we dissected the domains within MBD3C and WDR5 important for their interaction. WDR5 contains two binding surfaces on opposite sides of the protein, one that binds the histone H3 N-terminal tail or the SET/MLL complex subunit MLL1, and another that binds both the SET/MLL subunit RBBP5 (Figure 2A; Odho et al., 2010, Patel et al., 2008, Song and Kingston, 2008) and long noncoding RNAs (Yang et al., 2014). To test whether MBD3C interacts with either WDR5 binding surface, we performed coIPs in 293T cells co-transfected with vectors expressing MBD3C-H3F and FLAG-tagged point mutants from both binding surfaces of WDR5: D107A on the H3K4/MLL1 binding surface and F266A, K250A, and R181A on the RBBP5/RNA binding surface (Yang et al., 2014). We found that the F266A, K250A, and R181A mutants of WDR5 co-immunoprecipitate with antibodies recognizing endogenous MBD3 (Figure 2B). In contrast, the D107A mutant was absent from MBD3 immunoprecipitates, suggesting that MBD3C binds near or within the WDR5 H3K4/MLL1 binding pocket.
Figure 2.
The WDR5 Histone H3 Binding Pocket Is Required to Bind MBD3C
(A) PyMol depiction of WDR5 crystal structure (PDB: 2GNQ) showing the H3K4/MLL1 (left) and RBBP5 (right) binding pockets. Residues individually mutated to alanine (Yang et al., 2014) are shown in magenta. Residues necessary for MLL1 R3761 or histone H3 R2 binding are shown in orange.
(B) CoIPs with MBD3 antibody from 293T cells co-transfected with expression vectors carrying MBD3C-H3F and indicated FLAG-tagged WDR5 mutants.
(C) Schematic of Mbd3c N-terminal mutant constructs used in (D) and (E).
(D–F) CoIPs with MBD3 antibody in 293T cells performed as in (B), using V5-tagged WDR5 constructs and H3F-tagged MBD3 constructs. For “C41-50−MBD3A,” MBD3C amino acids 41–50 were fused to the N terminus of the MBD3A isoform.
(G) Alignment of the MBD3C N terminus with WDR5-binding regions of mouse MLL1, KANSL1, and histone H3.
To extend these findings, we generated a series of truncation mutants of the 50-amino-acid MBD3C N terminus to pinpoint the residues necessary for binding (Figure 2C). Deletion of the first 40 amino acids of H3F-tagged MBD3C did not disrupt the interaction with V5-tagged WDR5 (Figure 2D). However, upon deletion of amino acids 41–50 of MBD3C, interaction with WDR5 was completely lost (Figure 2E), as we observed for MBD3C mutants lacking amino acids 1–50 (Figures 1E, 1F, and 2D). Furthermore, an N-terminal fusion of amino acids 41–50 to the MBD3A isoform was sufficient to allow MBD3A to bind WDR5 (Figure 2F). These data demonstrate that amino acids 41–50 of MBD3C mediate WDR5 binding (see also Figure S1B).
MLL1, KANSL1, and histone H3 all bind the same domain on WDR5 via a 2-amino-acid alanine-arginine (AR) motif present on each protein (Couture et al., 2006, Han et al., 2006, Patel et al., 2008, Ruthenburg et al., 2006, Schuetz et al., 2006, Song and Kingston, 2008, Dias et al., 2014; Figures 2A and 2G). MBD3C contains an AR dipeptide within its N-terminal WDR5 binding domain (A42-R43; Figure 2G), which we hypothesized to be necessary for WDR5 binding. Confirming our hypothesis, an R43A mutant of MBD3C failed to pull down WDR5 (Figure 2E). Together, these data indicate that MBD3C, histone H3, and MLL1 use a common motif to bind the same surface of WDR5. We observed slightly reduced MBD3C protein levels in cells expressing mutants of MBD3C or WDR5 that disrupt MBD3C-WDR5 binding (Figures 2D and 2E), raising the possibility that interaction with WDR5 plays a role in stabilizing MBD3C.
Mbd3c Expression Is Largely Restricted to Pluripotent Stem Cells
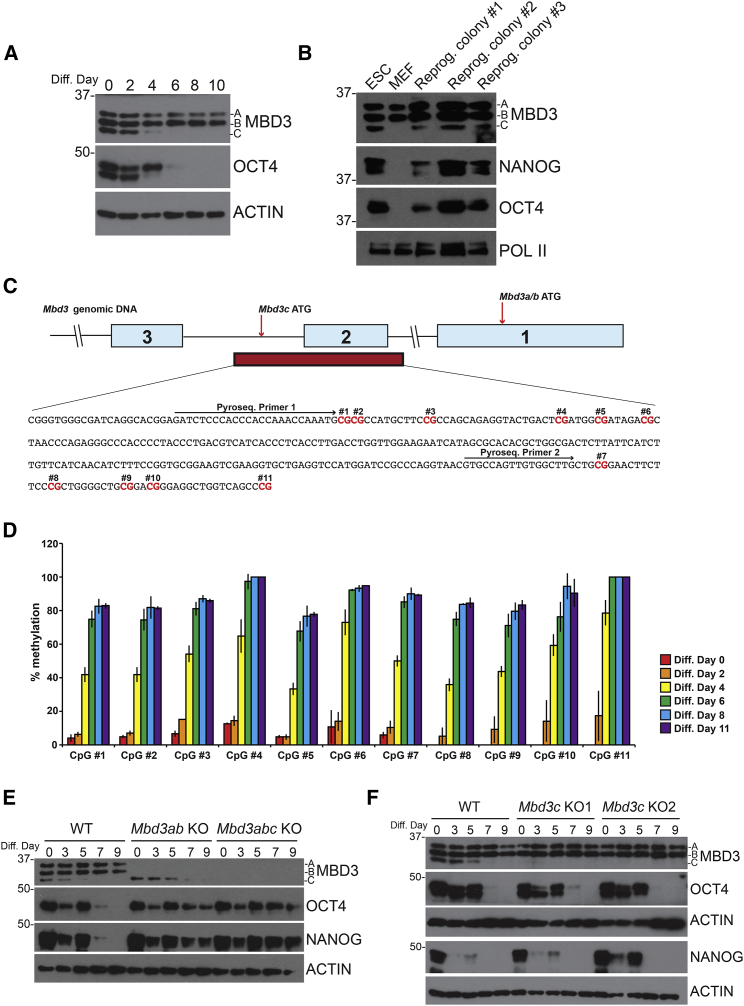
The Mbd3c isoform appears to be highly expressed only in ESCs, as it is absent or weakly expressed in mouse embryonic fibroblasts (MEFs) and all adult tissues tested (Figures S1A and S2A). To determine when Mbd3c expression is lost during differentiation, we subjected ESCs to a 10-day embryoid body (EB) differentiation time course. We observed that MBD3C protein was lost between days 4 and 6 of the time course with kinetics similar to loss of OCT4 protein during differentiation (Figure 3A).
Figure 3.
Mbd3c Is Dispensable for ESC Differentiation
(A) Western blots from ESCs differentiated over 10 days. Actin serves as a loading control.
(B) Western blots from primary MEFs reprogrammed to iPSCs.
(C) Schematic of the Mbd3 gene showing the sequence and location of the Mbd3c promoter CpG island (red bar). Light-blue boxes indicate exons. CpGs tested for methylation are highlighted in red.
(D) Pyrosequencing of bisulfite-converted DNA from cells collected at the indicated differentiation time points. Error bars represent the SD of three biological replicates.
(E and F) Western blots of differentiating Mbd3ab and Mbd3abc KO cells (E) and two clonal Mbd3c KO lines (F). WT, wild-type.
Next, we tested whether Mbd3c expression was restored upon reprogramming of differentiated cells to induced pluripotent stem cells. Primary MEFs were infected with doxycycline (dox)-inducible lentiviruses expressing reprogramming factors OCT4, SOX2, and KLF4 marked with an mCherry reporter (OSK-mCherry), L-MYC, and a lentiviral EOS-EGFP reporter specifically activated in pluripotent cells (Hotta et al., 2009). Infected cells were cultured with dox for 20 days. After an additional 10 days in the absence of dox, cells were imaged and stained for alkaline phosphatase to verify silencing of OSK-mCherry and presence of ESC-like colonies (Figures S3A and S3B). Lysates for western blots were prepared from expanded induced pluripotent stem cell (iPSC) colonies picked at 30 days. We observed that reprogrammed iPSC colonies express Mbd3c (Figure 3B). These data demonstrate that Mbd3c expression is restored when somatic cells are reprogrammed to iPSCs.
Finally, to investigate how Mbd3c expression might be silenced during differentiation, we performed bisulfite pyrosequencing analysis on the Mbd3c promoter during an EB differentiation time course. The Mbd3 gene contains a ∼350-bp CGI which spans exon 2 and part of intron 2, and overlaps with the sequence encoding the MBD3C N-terminal domain (Figure 3C). We measured methylation of 11 individual CpGs within the Mbd3c promoter and observed a large increase in methylation at all sites over the differentiation time course (Figure 3D). Methylation increased most dramatically around day 4, which corresponds to the timing of MBD3C loss during differentiation (Figure 3A). Therefore, silencing of Mbd3c expression during differentiation is likely due to increased methylation of the Mbd3c promoter CGI.
Mbd3c Is Dispensable for ESC Differentiation
Mbd3-null ESCs exhibit pluripotency defects and are capable of self-renewal in the absence of LIF (Kaji et al., 2006). We therefore wanted to test whether the MBD3C isoform was specifically required for early stages of differentiation. We generated homozygous Mbd3c, Mbd3ab, and Mbd3abc KO ESCs (Figure S2B) using CRISPR/Cas9 cleavage and error-prone DNA repair (Cong et al., 2013). We found that Mbd3c KO ESCs proliferate similarly to wild-type (WT) cells. In contrast, Mbd3ab KO and especially Mbd3abc KO ESCs grow more slowly than WT (Figures S2C and S2D), consistent with previous observations of Mbd3-null ESCs (Kaji et al., 2006).
Next, we tested the differentiation capacity of each Mbd3 mutant. Consistent with previous studies (Kaji et al., 2006), we found that ESCs lacking all MBD3 isoforms (Mbd3abc KO) maintained expression of both OCT4 and NANOG over 9 days in medium without LIF (Figure 3E). Unlike Mbd3abc KOs, Mbd3c KOs did not show a noticeable differentiation defect (Figure 3F). ESCs expressing only Mbd3c (Mbd3ab KO) were largely defective in differentiation (although OCT4 and NANOG levels appeared slightly reduced relative to Mbd3abc KO lines). Since Mbd3c expression is lost early during differentiation (Figures 3A and 3E), Mbd3ab mutants are functionally equivalent to Mbd3abc mutants at mid- to late-differentiation time points, potentially accounting for this phenotype. We next asked whether constitutive overexpression of Mbd3c in the absence of MBD3A and MBD3B could allow for normal differentiation. To test this possibility, we replaced the entire Mbd3 gene with an H3F-tagged Mbd3c transgene overexpressed from the CAG promoter, which is not silenced during differentiation. Differentiation proceeds normally in these cells (Figure S2E, top panel), revealing that MBD3C can compensate for MBD3A and MBD3B when it is overexpressed. Unexpectedly, ESCs overexpressing Mbd3cΔN were also able to differentiate (Figure S2E, bottom panel), in marked contrast to cells expressing Mbd3cΔN at endogenous levels (Figure S2F). We conclude that Mbd3c is not required for differentiation but can substitute for Mbd3a and Mbd3b when overexpressed.
MBD3 Isoforms Function Redundantly in Gene Regulation
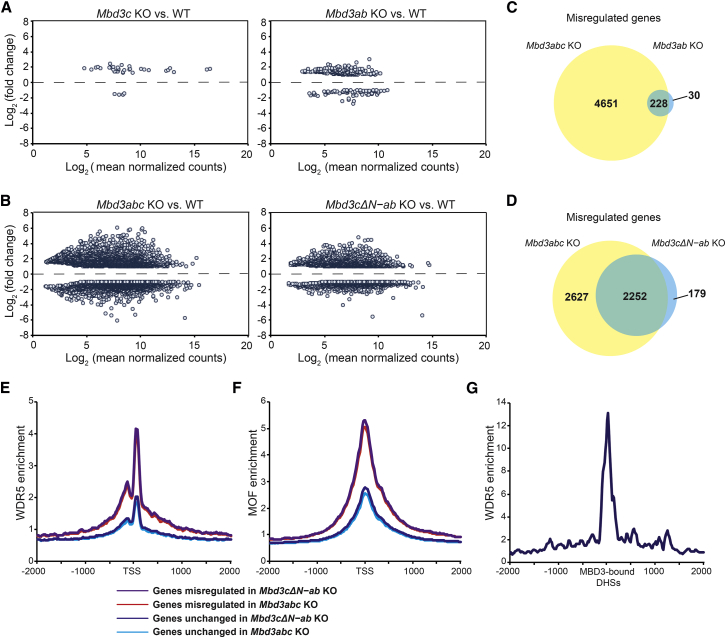
Although it is dispensable for differentiation, MBD3C binds chromatin (Figure S4) and could still be important for regulation of a subset of MBD3 target genes independently of the other MBD3 isoforms. To test this possibility, we analyzed the transcriptomes of Mbd3 isoform-specific mutant ESCs by RNA-seq. Gene expression in Mbd3c KO ESCs was largely normal (Figure 4A, left panel), with expression of only 38 genes changed more than 2-fold compared with WT. Similarly, we observed relatively few genes (258) misregulated in Mbd3ab KO ESCs that express only Mbd3c (Figure 4A, right panel) compared with a much larger number (4,879) misregulated in ESCs where all Mbd3 isoforms are deleted (Figures 4B [left panel] and 4C). These data suggest that Mbd3c can largely compensate for the loss of Mbd3a and Mbd3b at shared target genes.
Figure 4.
MBD3C Is Redundant with MBD3A and MBD3B in Regulation of Gene Expression
(A and B) MA plots showing log2 (fold change) in gene expression in Mbd3c KO (A, left), Mbd3ab KO (A, right), Mbd3abc KO (B, left), and Mbd3cΔN-ab KO (B, right) ESCs relative to wild-type (WT). Genes shown are misregulated ≥2-fold compared with WT.
(C and D) Venn diagrams showing overlap between misregulated genes in ESCs of indicated genotypes.
(E and F) WDR5 binding (Ang et al., 2011) (E) and MOF binding (Li et al., 2012) (F) averaged over transcription start sites (TSS) of misregulated or unchanged genes in Mbd3abc KO (red) and Mbd3cΔN-ab KO ESCs (purple).
(G) Average WDR5 binding over MBD3-bound (Yildirim et al., 2011), TSS-distal DNase I hypersensitive sites (DHSs) (GSM1014514).
To test whether the unique 50-amino-acid MBD3C N terminus (and thus the interaction with WDR5) is important for this compensatory effect, we also performed RNA-seq on ESCs lacking MBD3A and MBD3B and the N terminus of MBD3C (Mbd3cΔN-ab KO). In contrast to the relatively few genes misregulated in Mbd3ab KO and Mbd3c KO cells, we observed 2,431 genes misregulated in Mbd3cΔN-ab KO cells, with nearly twice as many genes upregulated as downregulated (1,577 versus 854, respectively; Figure 4B, right panel). The vast majority (∼93%) of misregulated genes overlapped with genes misregulated in Mbd3abc KO cells (Figure 4D), indicating that the MBD3C N terminus is largely required for MBD3C to compensate for loss of MBD3A and MBD3B. However, as Mbd3abc KO has a stronger phenotype than Mbd3cΔN-ab KO, MBD3C/NuRD may also regulate some genes independently of its N-terminal domain. Closer examination of the 2,627 genes misregulated only in Mbd3abc KO ESCs (Figure 4D) revealed similar, but weaker misregulation in Mbd3cΔN-ab KO cells in most cases that fell below our 2-fold cutoff. These data suggest that the Mbd3cΔN mutation is not a complete null and are consistent with our finding that MBD3CΔN can compensate for loss of MBD3A and MBD3B during ESC differentiation, but only when overexpressed (Figures S2E and S2F).
WDR5 is a component of multiple complexes with key regulatory functions in ESCs (Ang et al., 2011, Li et al., 2012). Wdr5 knockdown results in loss of ESC self-renewal (Ang et al., 2011), precluding the use of Wdr5 KO ESCs to compare the functions of MBD3C and WDR5 in gene regulation. However, to test whether the genes misregulated in Mbd3c mutant cells are targets of WDR5 and/or WDR5-associated complexes, we examined published ESC chromatin immunoprecipitation sequencing data for WDR5 and the MSL/NSL subunit MOF (Ang et al., 2011, Li et al., 2012). We observed considerably higher WDR5 binding at the promoter-proximal regions of genes that were misregulated in Mbd3abc KO and Mbd3cΔN-ab KO cells compared with genes that were unaffected by Mbd3 mutations (Figure 4E), consistent with a regulatory role for WDR5 at MBD3 target genes. However, it is likely that WDR5 regulates some of these genes through mechanisms independent of MBD3C/NuRD, as we also observed higher MOF binding at genes misregulated in Mbd3abc KO and Mbd3cΔN-ab KO cells (Figure 4F). Similarly, we found that WDR5 binding is enriched at promoter-distal DNase I hypersensitive sites that are co-bound by MBD3 (Figure 4G), suggesting that WDR5 and MBD3 co-regulate target gene expression at both promoter-distal enhancers and promoters.
We have identified a variant ESC-specific NuRD complex that includes the histone H3 binding protein WDR5. While WDR5 contributes to H3K4 trimethlyation by the SET/MLL complex (Ang et al., 2011, Yang et al., 2014) our data suggest that a WDR5-binding MBD3C/NuRD complex functions separately from SET/MLL. Consistent with these findings, we showed that MBD3C interacts with WDR5 at the same binding surface as MLL1 and histone H3, using a conserved arginine-containing motif (Couture et al., 2006, Patel et al., 2008). As MBD3/NuRD has previously been shown to repress pluripotency genes during differentiation (Reynolds et al., 2012), it is possible that MBD3C/NuRD functions to oppose SET/MLL activity.
Our data reveal an additional layer of complexity to the composition and function of chromatin remodelers in ESCs and uncover a previously unidentified function for the WDR5 protein. The WDR5 binding domain appears to be essential for MBD3C/NuRD function in ESCs, while other MBD3/NuRD complexes are recruited to the same target genes via the MBD or other binding domains. However, since the differentiation defect of Mbd3ab KO cells can be overcome by constitutive overexpression of Mbd3cΔN, WDR5 may simply enhance the chromatin binding or remodeling activities of MBD3C/NuRD. Multiple independent mechanisms likely target different NuRD complexes to overlapping targets on chromatin, where the complexes function redundantly.
Experimental Procedures
MBD3 Purification and LC-MS/MS
MBD3/NuRD complex was purified from indicated H3F-tagged ESCs as described by Yildirim et al. (2011). Purified samples were separated by SDS-PAGE, and LC-MS/MS was performed as described by Chen et al. (2013).
Embryoid Body Differentiation
ESCs were differentiated to EBs in suspension culture. Cells (2.5 × 106) were plated in ESC medium without LIF in bacteriological plates. Cells were replated to nongelatinized cell culture plates after 3 days and harvested for western blots at the indicated time points.
RNA-Seq
Total RNA was isolated from WT and mutant ESCs using TRIzol (Life Technologies), and purified with the Zymo RNA Clean and Concentrator kit. Strand-specific libraries were prepared and sequenced by Applied Biological Materials, and analyzed as described in Supplemental Experimental Procedures.
Author Contributions
L.E., K.N.M., and T.G.F. designed and performed most experiments. F.C. designed MS experiments and analyzed data. Y.T. and N.F. performed mass spec experiments. L.E. analyzed RNA-seq data. W.R.H. and M.R.G. designed reprogramming experiments. W.R.H. cloned reprogramming vectors. L.E. and T.G.F. wrote the paper.
Acknowledgments
We thank Howard Chang for WDR5 binding domain mutant plasmids, Feng Wang and Yong Du for assistance in mouse tissue lysate preparation, and members of the T.G.F. laboratory for critical reading of the manuscript. This research was supported by NIH Grant HD072122 to T.G.F. and NSF Grant CLF1307367 to F.C. T.G.F. is a scholar of the Leukemia and Lymphoma Society. M.R.G. is an Investigator of the Howard Hughes Medical Institute.
Published: May 18, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.04.020.
Accession Numbers
RNA-seq data are available at GEO: GSE80708.
Supplemental Information
References
- Ang Y.S., Tsai S.Y., Lee D.F., Monk J., Su J., Ratnakumar K., Ding J., Ge Y., Darr H., Chang B. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B.E., Humphrey E.L., Erlich R.L., Schneider R., Bouman P., Liu J.S., Kouzarides T., Schreiber S.L. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc. Natl. Acad. Sci. USA. 2002;99:8695–8700. doi: 10.1073/pnas.082249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode D., Yu L., Tate P., Pardo M., Choudhary J. Characterization of two distinct nucleosome remodeling and deacetylase (NuRD) complex assemblies in embryonic stem cells. Mol. Cell. Proteomics. 2016;15:878–891. doi: 10.1074/mcp.M115.053207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.B., Hung J.H., Hickman T.L., Coles A.H., Carey J.F., Weng Z., Chu F., Fazzio T.G. Hdac6 regulates Tip60-p400 function in stem cells. Elife. 2013;2:e01557. doi: 10.7554/eLife.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture J.F., Collazo E., Trievel R.C. Molecular recognition of histone H3 by the WD40 protein WDR5. Nat. Struct. Mol. Biol. 2006;13:698–703. doi: 10.1038/nsmb1116. [DOI] [PubMed] [Google Scholar]
- Denslow S.A., Wade P.A. The human Mi-2/NuRD complex and gene regulation. Oncogene. 2007;26:5433–5438. doi: 10.1038/sj.onc.1210611. [DOI] [PubMed] [Google Scholar]
- Dias J., Van Nguyen N., Georgiev P., Gaub A., Brettschneider J., Cusack S., Kadlec J., Akhtar A. Structural analysis of the KANSL1/WDR5/KANSL2 complex reveals that WDR5 is required for efficient assembly and chromatin targeting of the NSL complex. Genes Dev. 2014;28:929–942. doi: 10.1101/gad.240200.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos R.L., Tosti L., Radzisheuskaya A., Caballero I.M., Kaji K., Hendrich B., Silva J.C.R. MBD3/NuRD facilitates induction of pluripotency in a context-dependent manner. Cell Stem Cell. 2014;15:102–110. doi: 10.1016/j.stem.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y., Milne T.A., Ruthenburg A.J., Lee S., Lee J.W., Verdine G.L., Allis C.D., Roeder R.G. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat. Struct. Mol. Biol. 2006;13:713–719. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- Han Z., Guo L., Wang H., Shen Y., Deng X.W., Chai J. Structural basis for the specific recognition of methylated histone H3 lysine 4 by the WD-40 protein WDR5. Mol. Cell. 2006;22:137–144. doi: 10.1016/j.molcel.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Hendrich B., Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol. 1998;18:6538–6547. doi: 10.1128/mcb.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich B., Guy J., Ramsahoye B., Wilson V.A., Bird A. Closely related proteins MBD2 and MBD3 play distinctive but interacting roles in mouse development. Genes Dev. 2001;15:710–723. doi: 10.1101/gad.194101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta A., Cheung A.Y.L., Farra N., Vijayaragavan K., Séguin C.A., Draper J.S., Pasceri P., Maksakova I.A., Mager D.L., Rossant J. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat. Methods. 2009;6:370–376. doi: 10.1038/nmeth.1325. [DOI] [PubMed] [Google Scholar]
- Kaji K., Caballero I.M., MacLeod R., Nichols J., Wilson V.A., Hendrich B. The NuRD component Mbd3 is required for pluripotency of embryonic stem cells. Nat. Cell Biol. 2006;8:285–292. doi: 10.1038/ncb1372. [DOI] [PubMed] [Google Scholar]
- Kim J., Chu J., Shen X., Wang J., Orkin S.H. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li L., Pandey R., Byun J.S., Gardner K., Qin Z., Dou Y. The histone acetyltransferase MOF is a key regulator of the embryonic stem cell core transcriptional network. Cell Stem Cell. 2012;11:163–178. doi: 10.1016/j.stem.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odho Z., Southall S.M., Wilson J.R. Characterization of a novel WDR5-binding site that recruits RbBP5 through a conserved motif to enhance methylation of histone H3 lysine 4 by mixed lineage leukemia protein-1. J. Biol. Chem. 2010;285:32967–32976. doi: 10.1074/jbc.M110.159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Vought V.E., Dharmarajan V., Cosgrove M.S. A conserved arginine-containing motif crucial for the assembly and enzymatic activity of the mixed lineage leukemia protein-1 core complex. J. Biol. Chem. 2008;283:32162–32175. doi: 10.1074/jbc.M806317200. [DOI] [PubMed] [Google Scholar]
- Reynolds N., Salmon-Divon M., Dvinge H., Hynes-Allen A., Balasooriya G., Leaford D., Behrens A., Bertone P., Hendrich B. NuRD-mediated deacetylation of H3K27 facilitates recruitment of Polycomb Repressive Complex 2 to direct gene repression. EMBO J. 2011;31:593–605. doi: 10.1038/emboj.2011.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds N., Latos P., Hynes-Allen A., Loos R., Leaford D., O'Shaughnessy A., Mosaku O., Signolet J., Brennecke P., Kalkan T. NuRD suppresses pluripotency gene expression to promote transcriptional heterogeneity and lineage commitment. Cell Stem Cell. 2012;10:583–594. doi: 10.1016/j.stem.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthenburg A.J., Wang W., Graybosch D.M., Li H., Allis C.D., Patel D.J., Verdine G.L. Histone H3 recognition and presentation by the WDR5 module of the MLL1 complex. Nat. Struct. Mol. Biol. 2006;13:704–712. doi: 10.1038/nsmb1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Ishikawa F. The mCpG-binding domain of human MBD3 does not bind to mCpG but interacts with NuRD/Mi2 components HDAC1 and MTA2. J. Biol. Chem. 2002;277:35434–35439. doi: 10.1074/jbc.M203455200. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H., Schneider R., Bannister A.J., Sherriff J., Bernstein B.E., Emre N.C.T., Schreiber S.L., Mellor J., Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- Schuetz A., Allali-Hassani A., Martín F., Loppnau P., Vedadi M., Bochkarev A., Plotnikov A.N., Arrowsmith C.H., Min J. Structural basis for molecular recognition and presentation of histone H3 by WDR5. EMBO J. 2006;25:4245–4252. doi: 10.1038/sj.emboj.7601316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.J., Kingston R.E. WDR5 interacts with mixed lineage leukemia (MLL) protein via the histone H3-binding pocket. J. Biol. Chem. 2008;283:35258–35264. doi: 10.1074/jbc.M806900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J.K., Hassig C.A., Schnitzler G.R., Kingston R.E., Schreiber S.L. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature. 1998;395:917–921. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- Wade P.A., Jones P.L., Vermaak D., Wolffe A.P. A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr. Biol. 1998;8:843–846. doi: 10.1016/s0960-9822(98)70328-8. [DOI] [PubMed] [Google Scholar]
- Wysocka J., Swigut T., Milne T.A., Dou Y., Zhang X., Burlingame A.L., Roeder R.G., Brivanlou A.H., Allis C.D. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Xue Y., Wong J., Moreno G.T., Young M.K., Côté J., Wang W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol. Cell. 1998;2:851–861. doi: 10.1016/s1097-2765(00)80299-3. [DOI] [PubMed] [Google Scholar]
- Yang Y.W., Flynn R.A., Chen Y., Qu K., Wan B., Wang K.C., Lei M., Chang H.Y. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. Elife. 2014;3:e02046. doi: 10.7554/eLife.02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim O., Li R., Hung J.H., Chen P.B., Dong X., Ee L.S., Weng Z., Rando O.J., Fazzio T.G. Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells. Cell. 2011;147:1498–1510. doi: 10.1016/j.cell.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., LeRoy G., Seelig H.P., Lane W.S., Reinberg D. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998;95:279–289. doi: 10.1016/s0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ng H.H., Erdjument-Bromage H., Tempst P., Bird A., Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.