Summary
Bone marrow mesenchymal stem/stromal cells (BM-MSCs) are key components of the hematopoietic niche thought to have a direct role in leukemia pathogenesis. BM-MSCs from patients with acute myeloid leukemia (AML) have been poorly characterized due to disease heterogeneity. We report a functional, genetic, and immunological characterization of BM-MSC cultures from 46 AML patients, stratified by molecular/cytogenetics into low-risk (LR), intermediate-risk (IR), and high-risk (HR) subgroups. Stable MSC cultures were successfully established and characterized from 40 of 46 AML patients irrespective of the risk subgroup. AML-derived BM-MSCs never harbored tumor-specific cytogenetic/molecular alterations present in blasts, but displayed higher clonogenic potential than healthy donor (HD)-derived BM-MSCs. Although HD- and AML-derived BM-MSCs equally provided chemoprotection to AML cells in vitro, AML-derived BM-MSCs were more immunosuppressive/anti-inflammatory, enhanced suppression of lymphocyte proliferation, and diminished secretion of pro-inflammatory cytokines. Multivariate analysis revealed that the level of interleukin-10 produced by AML-derived BM-MSCs as an independent prognostic factor negatively affected overall survival. Collectively our data show that AML-derived BM-MSCs are not tumor related, but display functional differences contributing to therapy resistance and disease evolution.
Keywords: BM-MSC, AML, risk-stratification, immunosuppression, characterization, chemoprotection, IL-10
Highlights
-
•
Functional, genetic, and immunological characterization of BM-MSCs from 46 AML patients
-
•
BM-MSCs never harbored tumor-specific cytogenetic/molecular alterations as in blasts
-
•
HD- and AML-BM-MSCs equally provided chemoprotection to AML cells in vitro
-
•
IL-10 produced by AML-BM-MSCs is a prognostic factor with negative impact on OS
In this article, Díaz de la Guardia and colleagues report a functional, genetic, and immunological characterization of BM-MSC cultures from 46 AML patients, stratified by molecular/cytogenetics into low-risk (LR), intermediate-risk (IR), and high-risk (HR) subgroups. BM-MSCs never harbored tumor-specific cytogenetic/molecular alterations present in blasts, and IL-10 produced by AML-derived BM-MSCs is an independent prognostic factor negatively impacting on overall survival.
Introduction
Acute myeloid leukemia (AML) comprises a biologically and genetically heterogeneous group of disorders characterized by the rapid expansion of immature myeloid blasts in bone marrow (BM) (Bene et al., 2015, Grimwade et al., 2016). Disease heterogeneity is well documented and patients are stratified based on cytogenetic, molecular, and immunophenotypic data. A significant proportion of patients fail to respond to standard first-line chemotherapy regimens and current salvage therapy rarely yields durable remissions, with relapse being common (Hills et al., 2016, Medinger et al., 2016). Failure of current therapies to eradicate leukemia-initiating cells and chemotherapy refractoriness are major mechanisms underlying AML progression/relapse. The high rate of mortality and morbidity in AML guides the search for new compounds with higher efficiency and lower toxicity.
Mesenchymal stem/stromal cells (MSCs) are an essential component of the BM hematopoietic microenvironment as well as a potential source of progenitors for mesodermal tissues (Dominici et al., 2006, Garcia-Castro et al., 2008, Horwitz et al., 2005, Pittenger et al., 1999). MSCs have emerged as excellent candidates for clinical applications due to their immunomodulatory properties and their ability to support normal hematopoiesis (Garcia-Castro et al., 2008, Garcia-Gomez et al., 2010, Gonzalo-Gil et al., 2016, Rodriguez et al., 2012, Sanchez et al., 2011). BM-MSCs have been shown to modulate hematopoiesis by regulating the balance between self-renewal and differentiation of hematopoietic stem/progenitor cells (HSPCs) through cell-cell interactions and paracrine secretion of cytokines and extracellular matrix molecules (Konopleva et al., 2009). Moreover, a role for BM-MSCs has been implied in the pathogenesis of a variety of hematologic malignances including acute lymphoblastic leukemia (ALL), AML, multiple myeloma (MM), lymphomas, chronic myeloid leukemia (CML), and myelodysplastic syndromes (MDS) (Blau et al., 2007, Blau et al., 2011, Corre et al., 2007, Lopez-Villar et al., 2009, Medyouf et al., 2014, Menendez et al., 2009, Shalapour et al., 2010, Streubel et al., 2004, Walkley et al., 2007).
The interaction of leukemic cells with the BM microenvironment in functional niches is hypothesized to be a major mechanism underlying leukemia maintenance (Medyouf et al., 2014, Schepers et al., 2015, Sison and Brown, 2011, Tabe and Konopleva, 2014). BM stroma has also been suggested to contribute to therapy resistance and promote residual disease and relapse by favoring leukemic cell growth and clonal evolution of malignant cells (Iwamoto et al., 2007, Konopleva et al., 2002).
To date, BM-MSCs from AML patients have been poorly characterized, and conflicting results have made it unclear whether or not these cells play a role in the disease and/or treatment outcomes (Chandran et al., 2015, Geyh et al., 2016, Klopp et al., 2011, Le et al., 2016, von der Heide et al., 2016). To address these contradictions, we undertook a functional, genetic, and immunological characterization of BM-MSC cultures from a cohort of 46 patients with AML stratified into three risk groups according to molecular/cytogenetic features: low-risk (LR), intermediate-risk (IR), and high-risk (HR) AML. Stable MSC cultures were successfully established and characterized from the BM of the majority AML patients irrespective of the molecular/cytogenetic subgroup. AML-derived BM-MSCs from all molecular AML subgroups exhibited higher clonogenic and in vitro immunosuppressive/anti-inflammatory potential than BM-MSCs from healthy donors (HDs), whereas only BM-MSCs derived from HR-AML patients possessed a significantly reduced adipogenic/osteogenic differentiation potential. Importantly, regardless of the molecular subgroup, all AML-derived BM-MSC cultures were devoid of leukemia cell-specific cytogenetic/molecular alterations, verifying that HSPCs, rather than pre-hematopoietic precursors, represent the cell of origin in AML. Furthermore, multivariate analysis revealed that the levels of the anti-inflammatory cytokine interleukin-10 (IL-10) produced by AML-derived BM-MSCs negatively affects overall survival (OS). Collectively, we demonstrate that AML-derived BM-MSCs are not tumor related but are functionally distinct from HD-derived BM-MSCs. Importantly, our results provide a link between in vitro properties of MSC and AML treatment outcomes, providing clinical evidence that BM-MSCs play a role in therapy responsiveness regardless of molecular/cytogenetic classification.
Results
Functional Characterization of BM-MSCs from Cytogenetically Distinct AML Subgroups
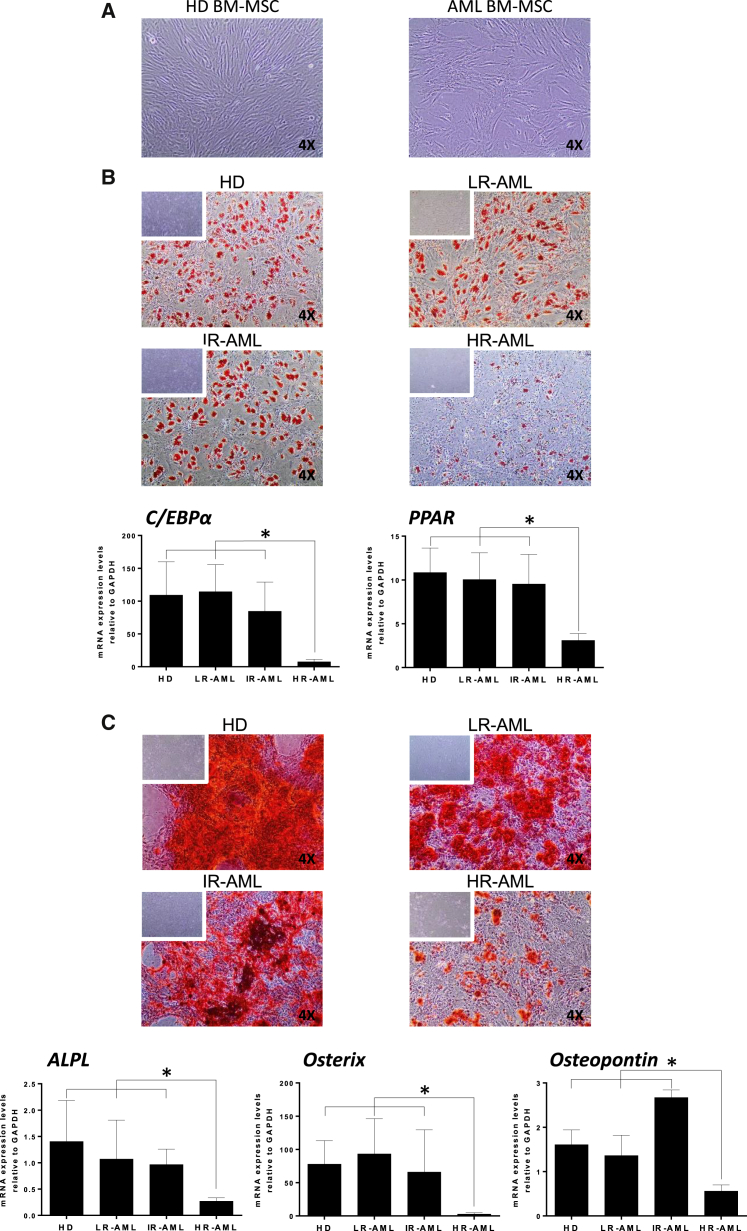
To characterize the MSC component of the BM stroma of AML at presentation, we sought to generate stable BM-MSC cultures from 46 patients with AML and ten age-matched HDs. Table 1 shows the main biological and molecular/cytogenetic features, allowing patient stratification into LR-AML (n = 16, displaying favorable cytogenetics/molecular features), IR-AML (n = 11, normal karyotype and lacking mutations in NPM, FLT3, or cEBPa), and HR-AML (n = 19, displaying unfavorable cytogenetics/molecular features). Cultures of adherent fibroblast-like cells were successfully established from the BM of AML patients using standard MSC culture conditions regardless of cytogenetic/molecular subgroup or patient age (Figure 1A), with cultures being generated at a high frequency from all risk groups (81%, 91%, and 90% for LR-AML, IR-AML, and HR-AML patients, respectively) (Table 2). To determine whether these cells were bona fide MSCs, we compared them against MSCs derived from the BM of HDs using standardized criteria outlined by the International Society for Cellular Therapy (ISCT) (Dominici et al., 2006). Similarly to HD-derived BM-MSC, AML-derived cultures were consistently devoid of contaminating hematopoietic and endothelial cells, being negative for CD45, CD34, CD31, and HLA-DR but expressing common MSC markers including CD73, CD105, CD90, and CD13, as defined by ISCT guidelines on MSC characterization (Figure S1). Early passage (p1–p3) BM-MSC cultures were further evaluated to measure their adipogenic and osteoblastic differentiation potential. While all AML patient-derived BM-MSC cultures demonstrated multipotent differentiating capacity, regardless of donor age, BM-MSC cultures from HR-AML patients (complex karyotypes, TP53 mutated, FLT3-ITD+ or MLL-rearranged) possessed a very poor adipogenic and osteogenic differentiation potential compared with HD-derived BM-MSCs (Figures 1B and 1C). Differences were evident by both qualitatively, limited staining with oil red O and alizarin red, and quantitatively by a significant decrease in the expression of early and late master regulator genes of adipogenic (cEBPa, PPAR) and osteogenic (ALPL, Osterix, and Ostepontin) differentiation (Figures 1B and 1C). Overall, these data demonstrate that bona fide BM-MSC cultures can be successfully derived from LR-, IR- and HR-AML patients; however, HR-AML BM-MSC cultures show intrinsic impairments in their ability to form adipocytes and osteocytes in vitro.
Table 1.
Biological and Cytogenetic-Molecular Characteristics of Blasts and BM-MSCs from Diagnostic AML Patients
| Patient ID | Diagnostic | Cytogenetics | Molecular | Age (years) | Gender | Blasts (%) | Risk | Cytogenetic/Molecular Alteration in BM-MSCs |
|---|---|---|---|---|---|---|---|---|
| AML01 | AML-M4 | 46, XY, inv(16) | Cbfb-MYH11 | 20 | M | 56 | low | negative |
| AML02 | AML-M5 | 46, XY, inv(16) | Cbfb-MYH11 | 1 | M | 50 | low | negative |
| AML03 | AML-M2 | 46, XY, t(8;21) | AML1-ETO | 30 | M | 39 | low | ND |
| AML04 | AML | 46, XX | NPM1MUT, IDH1MUT | 20 | F | 92 | low | negative |
| AML05 | AML | 46, XY | NPM1MUT | 18 | M | 32 | low | negative |
| AML06 | AML | 46, XY, t(5;15) | cEBPaMUT | 66 | M | 15 | low | negative |
| AML07 | AML-M4 | 46, XX | NPM1MUT | 37 | F | 80 | low | negative |
| AML08 | AML-M3 | 46, XX t(15;17) | PML-RARa | 59 | F | 90 | low | negative |
| AML09 | AML-5b | 46, XX | NPM1MUT | 70 | F | 34 | low | ND |
| AML10 | AML-M3 | 46, XY, t(15;17) | PML-RARa | 27 | M | 92 | low | negative |
| AML11 | AML | 46, XY | NPM1MUT | 81 | M | 45 | low | ND |
| AML12 | AML | 46, XY | NPM1MUT | 80 | M | 49 | low | negative |
| AML13 | AML | 46, XX | NPM1MUT | 71 | F | 45 | low | ND |
| AML14 | AML | 46, XX | NPM1MUT | 78 | F | 67 | low | negative |
| AML15 | AML-M3 | 46, XY, t(15;17) | PML-RARa | 25 | M | 90 | low | negative |
| AML16 | AML-M3 | 46, XY, inv(16) | Cbfb-MYH11 | 27 | M | 40 | low | negative |
| AML17 | AML | 46, XY | – | 10 | M | 92 | int | ND |
| AML18 | AML | 46, XY | – | 81 | M | 35 | int | ND |
| AML19 | AML | 46, XY | – | 77 | M | 45 | int | ND |
| AML20 | AML | 46, XX | – | 61 | F | 71 | int | ND |
| AML21 | AML-M4 | 46, XY | – | 86 | M | 50 | int | ND |
| AML22 | AML | 46, XX | – | 52 | F | 79 | int | ND |
| AML23 | AML | 46, XY | – | 78 | M | 60 | int | ND |
| AML24 | AML-M4 | 46, XX | – | 44 | F | 65 | int | ND |
| AML25 | AML | 46, XY | – | 61 | M | 1 | int | ND |
| AML26 | AML-M7 | 46, XY | – | 60 | M | 60 | int | ND |
| AML27 | AML | 46, XY | – | 56 | M | 40 | int | ND |
| AML28 | AML-M5 | 46, XX, t(11;19) | MLL-ENL | 48 | F | 77 | high | negative |
| AML29 | AML | 45, XY, −7 | – | 80 | M | 85 | high | negative |
| AML30 | AML-M2 | 46, XX, t(8;21) | AML1-ETO, FLT3-ITD | 4 | F | 40 | high | negative/negative |
| AML31 | AML-M4 | 46, XX, t(9;11) | MLL-AF9 | 63 | F | 90 | high | negative |
| AML32 | AML | 45, XY, −7 | – | 73 | M | 15 | high | negative |
| AML33 | AML-M5 | 46, XY | NPM1MUT, FLT3del | 77 | M | 80 | high | negative/negative |
| AML34 | AML-M5 | 46, XX | NPM1MUT, FLT3-ITD | 65 | F | 90 | high | negative/negative |
| AML35 | AML-M1 | 46, XX | NPM1MUT, FLT3-ITD | 73 | F | 90 | high | negative |
| AML36 | AML | 46, XX, 11q23 | MLL-MLL (PTD) | 74 | F | 70 | high | ND |
| AML37 | AML-M1 | 46, XX, t(9;11) | MLL-AF9 | 77 | F | 80 | high | negative |
| AML38 | AML | 45, XY, −7 | – | 69 | M | 22 | high | negative |
| AML39 | AML-M4 | 46, XY | NPM1MUT, FLT3MUT, WT1MUT | 42 | M | 99 | high | negative |
| AML40 | AML | CK | – | 35 | M | 80 | high | negative |
| AML41 | AML | 47, XY (+mar) | FLT3 D835MUT | 81 | M | 47 | high | negative |
| AML42 | AML-M2 | 46, XX | MLL-PTD+ | 76 | F | 40 | high | negative |
| AML43 | AML | 46, XY, −7q | – | 53 | M | 56 | high | negative |
| AML44 | AML-M4 | 47, XY, +8 | WT1+, TP53 exon 6+ | 69 | M | 35 | high | ND |
| AML45 | AML-M4 | 46, XX | MLL-PTD+ | 68 | F | 65 | high | negative |
| AML46 | AML | 46, XX | FLT3-ITD | 8 | F | 95 | high | negative |
| HD01 | normal | 46, XX | – | 37 | F | 0 | HD | ND |
| HD02 | normal | 46, XY | – | 34 | M | 0 | HD | ND |
| HD03 | normal | 46, XY | – | 41 | M | 0 | HD | ND |
| HD04 | normal | 46, XY | – | 42 | M | 0 | HD | ND |
| HD05 | normal | 46, XY | – | 42 | M | 0 | HD | ND |
| HD06 | normal | 46, XX | – | 34 | F | 0 | HD | ND |
| HD07 | normal | 46, XY | – | 28 | M | 0 | HD | ND |
| HD08 | normal | 46, XX | – | 49 | F | 0 | HD | ND |
| HD09 | normal | 46, XY | – | 56 | M | 0 | HD | ND |
| HD10 | normal | 46, XX | – | 34 | F | 0 | HD | ND |
HD, healthy donor; CK, complex karyotype; int, intermediate risk; M, male; F, female; –, no mutations found for FLT3, NPM1, cEBPa, WT, and IDH1; ND, not determined.
Figure 1.
Differentiation Capacity of BM-MSCs from HDs and AML Patients
(A) Phase-contrast morphology of BM-MSCs from HD and AML patients.
(B) Top: oil red O staining indicative of adipogenic differentiation potential of BM-MSCs from HD, LR-, IR-, and HR-AML subjects. Bottom: quantitative expression by qRT-PCR for the pan-adipogenesis transcription factors cEBPa and PPAR.
(C) Top: alizarin red staining revealing the osteogenic differentiation capacity of BM-MSCs from HD, LR-, IR-, and HR-AML subjects. Bottom: quantitative expression by qRT-PCR for the pan-osteogenesis transcription factors ALPL, OSTERIX, and OSTEOPONTIN. Original magnification is indicated in microscopy images. n = 30 patients (10 LR-, 10 IR-, and 10 HR-AML) and n = 10 healthy donor controls.
Error bars indicate the SEM values of the biological replicates. ∗p < 0.05.
Table 2.
Establishment Rate of MSC Cultures from BM-AML According to Cytogenetic/Molecular Risk
| Prognostic Groups | Cytogenetics/Molecular | Efficiency of BM-MSC Culture Establishment | Mean Age (years) ± SD | Gender Ratio M/F | Mean Blasts (%) |
|---|---|---|---|---|---|
| LR-AML | inv(16)/CBFB-MYH11 | 3/3 (100%) | 44.4 ± 26.8 | 10/6 | 57.3 ± 24.8 |
| t(8;21)/AML1-ETO | 1/1 (100%) | ||||
| t(15;17)/PML-RARa | 2/3 (67%) | ||||
| NK (NPMmut,FLT3wt) | 6/8 (75%) | ||||
| t(5;15)/TP53BP1-PDGFRB | 1/1 (100%) | ||||
| IR-AML | NK (NPMwt, FLT3wt, cEBPawt) | 10/11 (91%) | 55.2 ± 27.9 | 8/3 | 54.4 ± 24.5 |
| HR-AML | CK | 7/7 (100%) | 59.7 ± 22.9 | 9/10 | 66.1 ± 25.9 |
| NK (NPMwt,FLT3mut) | 4/5 (83%) | ||||
| MLL-r (11q23) | 5/6 (83%) | ||||
| NK/NPM1mut, IDH1mut | 1/1 (100%) | ||||
| HD | 10/10 (100%) | 40 ± 8 | 6/4 | 0 |
NK, normal karyotype; CK, complex karyotype; MLL-r, MLL rearranged; MUT, mutated; HR, high risk; IR, intermediate risk; LR, low risk; HD, healthy donor.
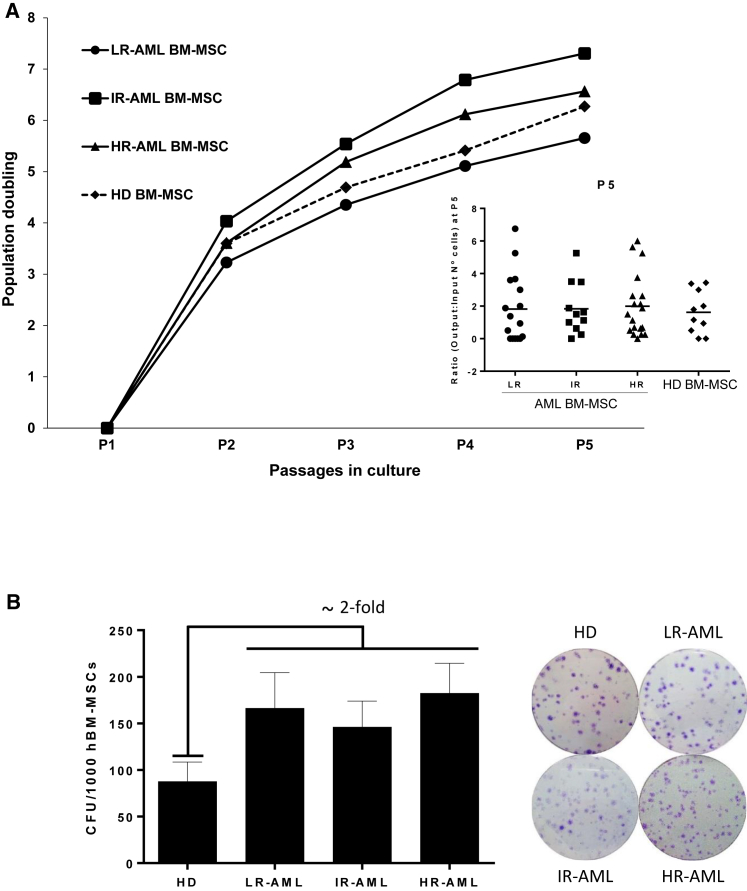
We next assessed whether BM-MSCs from LR-, IR- and HR-AML patients had a similar proliferative potential to HD-derived BM-MSCs by assessing both their growth rate in vitro and their clonogenic potential. BM-MSCs from both HD and the three molecularly different subgroups of AML grew similarly, achieving sic to seven population doublings over five passages (∼42–45 days, Figure 2A). Interestingly, colony forming unit-fibroblast (CFU-F) activity in BM derived from LR-, IR- and HR-AML patients was 2-fold greater (p = 0.04) than that detected from HDs (Figure 2B). These data indicate that BM-MSCs can be detected at a greater frequency from all molecular AML subgroups compared with HDs but that this is not related to a greater proliferative capacity of BM-MSCs ex vivo.
Figure 2.
Functional Characterization of BM-MSCs from HDs and AML Patients
(A) Proliferation measured as population doublings of BM-MSC cultures from LR-, IR-, and HR-BM-MSC compared with BM-MSCs from HD. Inset represents patient variability. n = 46 patients (16 LR-, 11 IR-, and 19 HR-AML) and n = 10 healthy donor controls.
(B) Clonogenic capacity of BM-MSCs (number of CFU per 1,000 cells seeded) from HD and LR-, IR- and HR-AML subjects. p < 0.05. Right: representative crystal violet staining of BM-MSC-CFU/colonies from HD- and AML-derived BM-MSCs. n = 40 patients (13 LR-, 10 IR-, and 17 HR-AML) and n = 10 healthy donor controls. Error bars indicate the SEM values of the biological replicates.
BM-MSCs from Cytogenetically Distinct AML Subgroups Are Highly Immunosuppressive
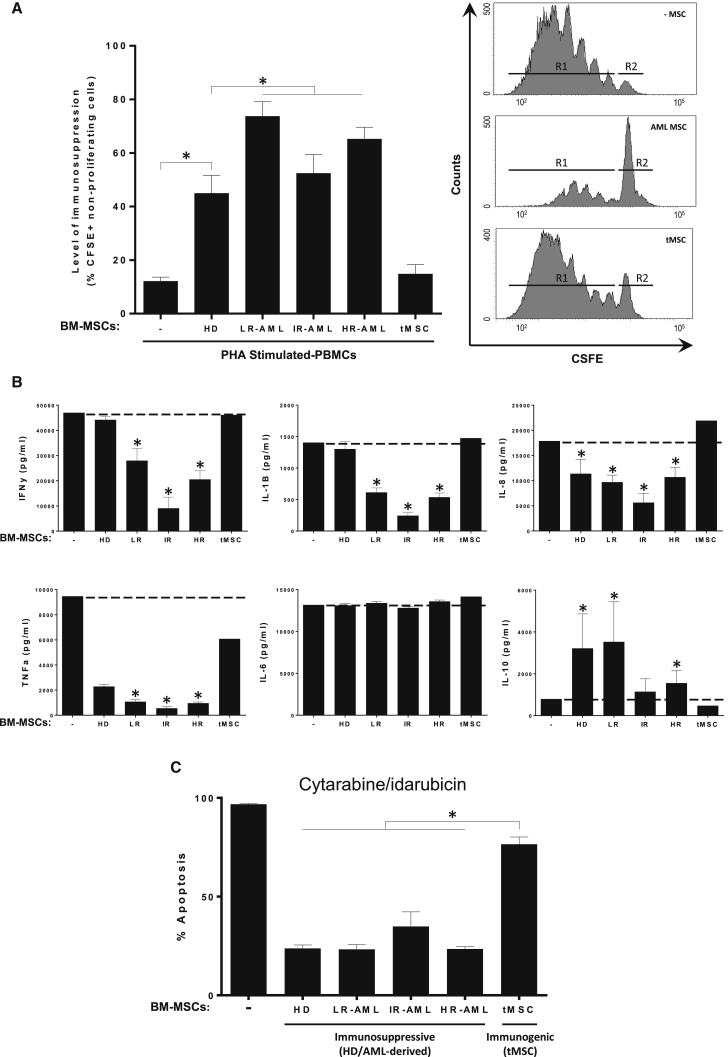
Increasing evidence suggests a potential contribution of the stroma milieu and immune escape to therapy resistance in AML (Klopp et al., 2011). Whether or not this contribution is in part mediated by BM-MSCs remains unclear, partially due to the fact that the immunosuppressive and anti-inflammatory properties of AML-derived BM-MSCs have yet to be tested. To address this, we compared the immunosuppressive properties of the different AML subgroups with HD-derived BM-MSCs. As a negative control in these experiments, T cells were co-cultured with transformed BM-MSCs (tMSCs), an oncogenic cell line derived from primary BM-MSCs that has been previously demonstrated to lack immunosuppressive properties (Funes et al., 2007, Rodriguez et al., 2014). Using carboxyfluorescein succinimidyl ester (CFSE) dilution assays to monitor cell division, we found that AML-derived BM-MSCs suppressed the proliferation of phytohemagglutinin (PHA)-stimulated human peripheral blood mononuclear cells (PBMCs) irrespective of the molecular subgroup (Figure 3A). In line with previous studies, the level of immunosuppression (measured as non-proliferating PHA-stimulated lymphocytes) was 44% ± 20% and 14% ± 8% for HD-derived BM-MSCs and tMSCs, respectively (Figure 3A; Funes et al., 2007, Rodriguez et al., 2014, Sanchez et al., 2011). Intriguingly, AML-derived BM-MSCs had a more robust immunosuppressive potential (74% ± 18% for LR-AML, 55% ± 20% for IR-AML and 66% ± 16% for HR-AML; p < 0.05) than HD-derived BM-MSCs.
Figure 3.
In Vitro Immunosuppressive and Anti-Inflammatory Properties of BM-MSCs from HDs and AML Patients
(A) Left: level of immunosuppression measured as percentage of CFSE+ non-proliferating cells. CSFE-labeled PBMCs were stimulated with PHA and then co-cultured with BM-MSCs from LR-, IR-, and HR-AML subjects for 5 days. BM-MSCs from HD and transformed BM-MSCs (tMSCs) were used as positive and negative controls, respectively. Right: the number of cycling (CSFEmild/low) cells was determined by flow cytometry. Representative flow-cytometry histograms of CSFE-labeled PBMCs: R1, proliferating cells; R2 non-proliferating cells. n = 35 patients (11 LR-, 10 IR-, and 14 HR-AML) and n = 10 healthy donor controls. ∗p < 0.05. Error bars indicate the SEM values of the biological replicates.
(B) Concentration of the indicated cytokines in cell-culture supernatants determined by Luminex Multiplex assays. PBMCs from HD were co-cultured with BM-MSCs from 10 HD, 2 tMSCs, and 35 BM-MSCs from LR-, IR-, and HR-AML subjects. Error bars indicate the SEM values of the biological replicates. ∗p < 0.05.
(C) Similar protective effect of immunosuppressive BM-MSCs from HD and AML (LR-, IR- and HR-) individuals, but not tMSCs, to Ara-C/idarubicin cytotoxic treatment of HL60 AML cells. Error bars indicate the SEM values of the biological replicates. ∗p < 0.05.
To further confirm this result, we analyzed the cytokine profile in PHA/lipopolysaccharide (LPS)-activated human PBMCs co-cultured or not with the different BM-MSC subgroups (Lopez-Millan et al., 2017). The production of master pro-inflammatory cytokines, such as interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), IL-1β, and IL-8, was significantly inhibited by LR-, IR- and HR-AML BM-MSCs, whereas the levels of the non-inflammatory cytokine IL-6 were unchanged (Figure 3B). Consistent with the results of the CSFE/proliferation assay of stimulated PBMCs, AML-derived BM-MSCs were superior to HD-derived BM-MSCs in inhibiting the secretion of these pro-inflammatory cytokines (Figure 3B). Conversely, LR- and HR-AML BM-MSCs significantly increased the levels of the anti-inflammatory cytokine IL-10 compared with HD-derived BM-MSCs (Figure 3B). These in vitro data show that AML-derived BM-MSCs are immunosuppressive/anti-inflammatory by both inhibiting lymphocyte proliferation and modulating cytokine secretion.
BM-MSCs have been shown to confer in vitro therapy resistance to leukemic blasts (Iwamoto et al., 2007, Klopp et al., 2011, Konopleva et al., 2002), suggesting that MSCs in BM niches have anti-apoptotic/protective properties that could provide a safe haven for leukemic cells. To determine whether this property is retained in AML-derived BM-MSCs, we treated AML HL60 cells with cytarabine/idarubicin for 72 hr in the presence or absence of immunosuppressive BM-MSCs (HD- and AML-derived). To determine whether BM-MSCs which have lost immunosuppressive potential had the capacity to confer resistance, we also measured the chemoprotective capacity of tMSCs. BM-MSCs from HD and AML (irrespective of the cytogenetic risk-group) were equally chemoprotective and conferred a significantly higher level of resistance to AML cells compared with tMSCs, as shown by a significant reduction of apoptosis (Figure 3C).
IL-10 Secretion Levels from AML-Derived BM-MSCs Correlate Directly with Patient Overall Survival
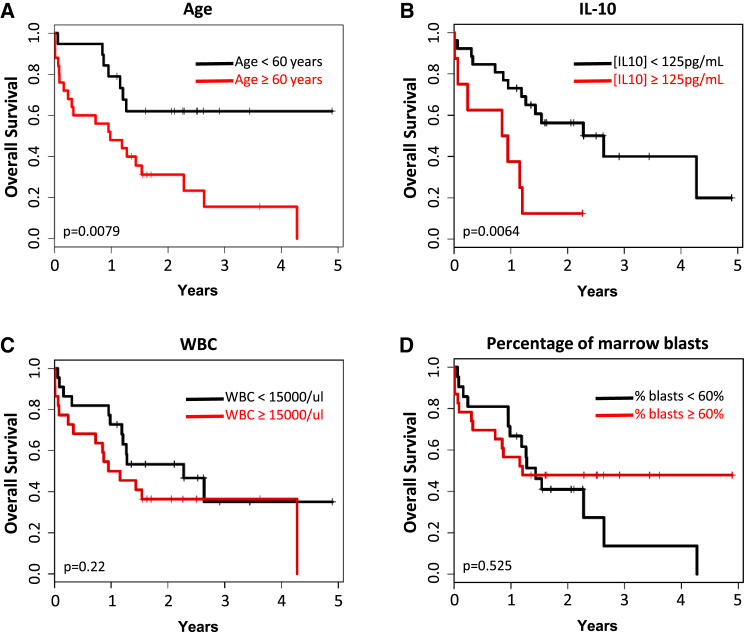
Since a primary method of action for MSCs is the secretion of growth factors and cytokines, we next used Luminex assays to evaluate the concentration of various factors secreted to the media of cultured AML- and HD-derived BM-MSCs in the presence of unstimulated PBMCs. Secreted levels of TNF-α, IFN-γ, IL-1β, IL-8, IL-6, and IL-10 were variable from donor to donor such that no significant differences were detected between HD- and AML-derived BM-MSCs. More interestingly, however, when the cytokine concentrations produced by AML-derived BM-MSCs were included in a multivariate analysis, IL-10 secretion levels by AML-derived BM-MSCs and patient age were the only variables that had a significant impact on patient outcomes among all the clinical-biological variables analyzed, which include eight cytokines, molecular/cytogenetic risk stratification, white blood cell (WCB) counts, and percentage of BM blasts, among others. Specifically, a patient age of <60 versus ≥60 years (Figure 4A) and levels of IL-10 (<125 versus ≥125 pg/mL) released by AML-derived BM-MSCs (Figure 4B) had a significant effect on patient OS (p = 0.03) while other variables such as WBC count (Figure 4C, p = 0.22) and percentage of BM blasts (Figure 4D, p = 0.525) did not. As such, the median survival of patients with [IL-10] ≥ 125 pg/mL was 11 months, compared with 32 months for those patients with [IL-10] < 125 pg/mL. The multivariate analysis confirmed that the secretion of high levels of IL-10 shortened the survival of our patients independent of other variables. The hazard ratios for age and IL-10 concentration were 4.0 (95% confidence interval [CI] = 1.5–10.4, p = 0.026) and 5.1 (95% CI = 1.8–14.8, p = 0.013), respectively. Collectively these data represent evidence linking a property of BM-MSCs with clinical disease outcomes in treated AML patients.
Figure 4.
Five-Year OS Kaplan-Meier Curve Reveals that Higher Production of IL-10 by BM-MSCs Is Independently Associated with Shorter OS in AML
Impact of patient's age (A), IL-10-secreted levels by BM-MSCs (B), white blood cell counts (WBC) (C), and percentage of blasts in the BM (D) on OS. n = 35 patients (11 LR-, 10 IR-, and 14 HR-AML) and n = 10 healthy donor controls.
BM-MSCs from AML Patients Do Not Harbor Tumor-Specific Cytogenetic/Molecular Alterations
BM-MSCs play a role in the pathogenesis of a variety of hematologic malignances (Garcia-Castro et al., 2008). The cellular origin of leukemia-specific fusion genes and recurrent genetic abnormalities is difficult to ascertain; however, the finding of tumor-specific genetic alterations in either the MSC or the endothelial BM compartment in CML, MDS, ALL, and MM suggest that stromal cells may be in part tumor related, and that such oncogenic insults may arise in a population of pre-hematopoietic precursors (Blau et al., 2007, Corre et al., 2007, Lopez-Villar et al., 2009, Menendez et al., 2009, Shalapour et al., 2010, Streubel et al., 2004, Walkley et al., 2007). We screened BM-MSC cultures from the majority of AML patients in a search for the molecular abnormalities found in the leukemia population at diagnosis. Using fluorescence in situ hybridization (FISH), G-banding, and PCR-based analysis, no diagnostic molecular/cytogenetic hallmarks, including CBFB-MYH11, AML1-ETO, PML-RARa, MLL rearrangements, complex karyotypes, trisomies, and monosomy 7, MLL-PTD, FLT3-ITD, FLT3 point mutations, NPM, wild-type, cEBPa, and TP53 mutations, were detected in BM-MSCs from a heterogeneous cohort of AML patients (Table 1). These data suggest that AML-derived BM-MSCs are not tumor related and that common AML-specific oncogenic drivers do not arise in pre-hematopoietic precursors.
Discussion
The BM microenvironment plays a role in the pathogenesis of a variety of hematologic malignances, including acute leukemia, MM, lymphomas, and MDS (Blau et al., 2007, Corre et al., 2007, Lopez-Villar et al., 2009, Menendez et al., 2009, Shalapour et al., 2010, Streubel et al., 2004, Walkley et al., 2007). MSCs are key components of the BM milieu, and many efforts are being undertaken to assess their role in several hematopoietic disorders (Garcia-Castro et al., 2008). BM-MSCs regulate normal hematopoiesis through cell-cell interaction with HSPCs or via released paracrine factors within functional BM niches. BM-MSCs contribute to the maintenance of leukemogenesis/hematopoiesis by regulating self-renewal, differentiation, survival, and apoptosis. In hematopoietic malignances, BM stroma is likely to be an accomplice to tumor development, promoting an environment that supports the growth and clonal evolution of malignant cells through as yet undefined mechanisms. Conversely, it may be the leukemic cells themselves which drive the reprogramming of BM-MSCs to provide a niche that is more permissive to the disease (Garcia-Gomez et al., 2014, Klopp et al., 2011, Medyouf et al., 2014). Regardless, a clearer understanding of the role of BM-MSCs in leukemias, and particularly in AML, could lead to improved treatments that reduce the occurrence of residual disease and relapse in patients (Iwamoto et al., 2007, Konopleva et al., 2002).
To date, our capacity to determine the role of BM-MSCs in AML has been hampered by a poor level of characterization and contradictory results (Chandran et al., 2015, Geyh et al., 2016, Klopp et al., 2011, Le et al., 2016, von der Heide et al., 2016). Here we report a detailed functional, genetic, and immunological characterization of BM-MSC cultures from a cohort of 46 diagnostic AMLs and ten age-matched HDs. Bona fide MSC cultures were successfully established from the BM of ∼90% of AML patients irrespective of the molecular/cytogenetic subgroup. BM-MSCs from all molecular AML subgroups showed intrinsic differences compared with those from HDs, including a higher clonogenic potential and greater immune suppressive capacity, while HR-AML-derived BM-MSCs exhibited further functional impairments in their adipogenic/osteogenic differentiation potential. Most importantly, we demonstrate that AML-derived BM-MSCs do not harbor the tumor-specific cytogenetic/molecular alterations and provide evidence of a clear clinical link between BM-MSCs and treatment outcomes for AML patients.
Compared with previous research, our prospective characterization of MSCs from BM of AML patients is unique in several aspects. First, the large number of patients analyzed allowed us to address the high level of heterogeneity associated with the disease. Accordingly, we were able to gain insights into the functional, genetic, and immunosuppressive properties of BM-MSCs derived from distinct LR, IR, and HR groups (Arber et al., 2016). One clear distinction in our study of 46 AML patients was that BM-MSCs from the HR-AML subgroup showed a severe impairment in both adipogenic and osteogenic differentiation potential. This finding supports a previous study from Geyh et al. (2016) showing impaired osteogenic differentiation of AML-derived BM-MSCs. However, our data contrast with a study published by Le et al. (2016), who reported that AML-derived BM-MSCs have markedly increased adipogenic differentiation. This may be explained by the limited cohort of patients (n = 5 LR) used in their study and underpins the importance of stratifying AML patients because of the high clinico-biological heterogeneity of the disease. Overall, we consider that the large number of samples analyzed in this study allows for increased clarity on potential differences in the function of AML BM-MSCs and suggests that defects in osteogenic and adipogenic differentiation may play a role in disease progression from HR subgroups.
Leukemia-specific translocations and recurrent genetic abnormalities have been found in both MSCs and endothelial cells in B-cell ALL, MM, and lymphoma, suggesting that stromal cells may be in part tumor related and that such oncogenic insults may arise in a population of pre-hematopoietic precursors such as early mesodermal precursors or hemangioblast-like progenitors (Blau et al., 2007, Corre et al., 2007, Lopez-Villar et al., 2009, Menendez et al., 2009, Prieto et al., 2016, Shalapour et al., 2010, Streubel et al., 2004, Walkley et al., 2007). The presence of AML-specific molecular hallmarks in BM-MSCs from AML patients has not been analyzed to date. We show that AML-derived BM-MSCs do not harbor tumor-specific cytogenetic/molecular alterations found at diagnosis, such as CBFB-MYH11, AML1-ETO, PML-RARa, MLL rearrangements, complex karyotypes, trisomies, and monosomy 7, MLL-PTD, FLT3-ITD, FLT3 point mutations, NPM, wild-type, cEBPa, and TP53 mutations. These data confirm that BM-MSCs are not tumor related and that the cell of origin for AML may be an early hematopoietic stem cell or a more committed myeloid progenitor. Three out of the 46 AMLs were pediatric cases, yet still their BM-MSCs were devoid of tumor-specific cytogenetic/molecular alterations, further emphasizing that even in those pediatric cases in which genetic drivers may have a prenatal origin during in utero hematopoietic development, the target cell for transformation seems to be an ontogenically early HSPC (Greaves and Wiemels, 2003, Menendez et al., 2009, Sanjuan-Pla et al., 2015).
BM-MSCs secrete cytokines that can suppress immune cell activation as well as enhance proliferation, vasculogenesis, migration, and chemoresistance, all key processes that may permit tumor progression and immune escape (Bernardo and Fibbe, 2013, Klopp et al., 2011). From the current level of understanding it is hypothesized that BM-MSCs play a role in maintaining hematopoiesis and supporting leukemogenesis in some hematopoietic malignancies (Iwamoto et al., 2007, Klopp et al., 2011, Konopleva et al., 2002). This may be mediated through one or more of the multiple functions attributed to MSCs, of which immune modulation is arguably the most clinically relevant (Knaan-Shanzer, 2014, Najar et al., 2016). In the case of solid tumors, there is concrete evidence that immune suppression mediated by the stromal environment enables cancer cell growth (Turley et al., 2015). A similar role for MSCs in AML, however, is less clear. This is partially due to the fact that no study has addressed the immunosuppressive and anti-inflammatory nature of BM-MSCs derived from AML patients. Our in vitro studies demonstrate that regardless of the molecular subgroup, AML-derived BM-MSCs are more highly immunosuppressive/anti-inflammatory than those derived from HDs. Immunosuppressive properties of MSCs can be considered a double-edged sword based on evidence suggesting that MSCs could be responsible for the enhanced tumor growth and chemoresistance in solid tumors and leukemia (Djouad et al., 2003, Low et al., 2015). It is interesting to note that T cell dysfunction has been reported in AML patients. The increased immune-suppressive function of BM-MSCs noted in our study implies that a potential role for BM-MSCs in the loss of T cell function in these patients warrants further investigation.
BM-MSCs have also been shown in vitro to protect ALL/AML cells from cytotoxic therapy (Iwamoto et al., 2007, Klopp et al., 2011, Konopleva et al., 2002). Here, co-culturing BM-MSCs with AML cells reduced apoptosis in AML cells in response to chemotherapy. Our data also reveal that while AML-derived BM-MSCs show functional differences from HD-derived MSCs, they maintain a similar capacity to protect leukemic cells from chemotherapy. Interestingly, BM-MSC-derived adipocytes have been implicated in leukemic cell apoptosis in response to doxorubicin through a cell contact-dependent mechanism (Klopp et al., 2011). Thus, our finding that HR-AML-derived BM-MSCs are highly immunosuppressive and chemoprotective, coupled with a marked impairment in adipogenic potential, may be associated with the ultimate poorer prognosis of HR-AML patients who frequently fail to respond to therapy or relapse.
Two of the most intriguing findings of our study are that BM-MSCs do not harbor the molecular alterations associated with the disease and that the concentration of IL-10 secreted by BM-MSCs in culture is directly linked to patient outcome. The nature of the molecular and (epi)genetic alterations of BM-MSCs for the pathogenesis of AML has just begun to be elucidated in a very recent single-center study using genetic, transcriptional, and epigenetic genome-wide omics approaches (von der Heide et al., 2016). While studies such as these may shed further light on the genetic and epigenetic changes involved in AML pathogenesis and disease relapse, our study goes further to demonstrate that any potential functional changes in MSCs that may be contributing to the disease are not related to the presence of the key molecular alterations associated with the different AML subgroups. More strikingly, however, our data provide comprehensive clinical evidence of a link between BM-MSC behaviors in vitro and response to treatment in AML patients. Our finding that the secretion of higher concentrations of IL-10 by BM-MSCs independently shortened the survival of our patients implies a direct role for BM-MSCs in AML and relapse from treatment. The contribution of potential molecular and cellular alterations of BM-MSCs to the pathogenesis of AML demands further integrative-functional studies, which may ultimately facilitate the design of niche-directed therapies in AML.
Experimental Procedures
Patients
Fresh BM aspirates were obtained at time of disease presentation from 46 patients (27 males and 19 females; median age of 53 ± 25 years) with cytogenetically different AML. Patients were diagnosed in the following hospitals: Hospital Clínico San Carlos (Madrid, Spain), Hospital Virgen de Arrixaca (Murcia, Spain), Hospital Universitario de Gran Canaria (Las Palmas, Spain), ICO-Hospital Germans Trias i Pujol (Badalona, Spain), and Hospital Santa Creu i San Pau (Barcelona, Spain). In addition, BM from ten age-matched HD (6 males and 4 females; median age of 40 ± 8 years) were used as controls. The diagnosis of AML was based on French-American-British (FAB) (Bennett et al., 1985) and World Health Organization (WHO) classifications (Dohner et al., 2010, Grimwade, 2002, Swerdlow et al., 2008). AML was classified as low-risk AML (LR-AML, displaying favorable cytogenetics/molecular features), intermediate-risk AML (IR-AML, with normal karyotype and lacking mutations in NPM, FLT3, or cEBPa), and high-risk AML (HR-AML, displaying unfavorable cytogenetics/molecular features) disease. Table 1 shows the main cytogenetic/molecular diagnostics and other clinical-biological features of each patient. Table 2 shows the success of establishing BM-MSC cultures from the three distinct AML subtypes. The study was IRB-approved by the Clinic Hospital of Barcelona and samples were accessed upon signed informed consent.
Isolation and Expansion of BM Mesenchymal Stem/Stromal Cells
Mononuclear cells (MNCs) from BM were isolated by centrifugation using Ficoll-Paque Plus (GE Healthcare) density gradients. MNCs were seeded at 2 × 105 cells/cm2 and maintained in a humidified atmosphere with 5% CO2 at 37°C for 48 hr, after which non-adherent cells were washed off and fresh medium was added. Adherent cells at >85% confluence were trypsinized and replated at 1.6 × 103 cells/cm2 (Bueno et al., 2014, Menendez et al., 2009, Rodriguez et al., 2012, Rodriguez et al., 2013). The resulting BM-MSC cultures were maintained in Advanced DMEM (Gibco) supplemented with 10% fetal calf serum (Invitrogen), L-glutamine, and penicillin-streptomycin-amphotericin B (Gibco). BM-MSC cultures were assessed daily for changes in growth rates and morphology. Established BM-MSC cultures were extensively characterized at p1–p3 (Bueno et al., 2014, Menendez et al., 2009, Rodriguez et al., 2012, Rodriguez et al., 2013).
Cytotoxic Assay
Irradiated BM-MSCs (2 × 104 cells/well) were co-cultured with the AML cell line HL60 (2 × 104 cells/well) in a 96-well plate. Co-cultures were treated or not with cytarabine (77 nM) and idarubicin (7 nM) for 72 hr. Floating cells were collected, washed in PBS, and stained with anti-CD33-APC (Becton Dickinson catalog no. 551378) for 15 min. After washing, cells were stained with Annexin-V-PE and 7-ADD (PE Annexin V Apoptosis Detection Kit I [RUO], BD cat. no. 559763) as described by Romero-Moya et al. (2013). Apoptotic cells (Annexin V+) were analyzed within the CD33+ population using a FACSCanto-II flow cytometer.
Molecular and Cytogenetic Analysis of BM-MSCs Derived from AML Patients
To evaluate whether the molecular-cytogenetic hallmarks found in diagnostic AML blasts were present in corresponding BM-MSCs, we analyzed cells at p2–p3 as described previously (Bueno et al., 2009, Catalina et al., 2008, Menendez et al., 2009). In brief, structural/numerical chromosome alterations were assessed by conventional G-banding (Bueno et al., 2009, Catalina et al., 2008, Menendez et al., 2009). Chromosomes were visualized using modified Wright staining. At least 20 metaphases were analyzed using a conventional microscope and IKAROS software (Metasystems). Metaphases of cells were GTG-banded and karyotyped in accordance with the International System for Human Cytogenetic Nomenclature recommendations (Standing Committee on Human Cytogenetic Nomenclature et al., 1985). Balanced translocations were analyzed by FISH using commercially available probes (Vysis). CBFB-MYH11, AML1-ETO, and PML-RARa fusions were detected using locus-specific LSI Dual Color probes, whereas MLL fusions were analyzed using LSI MLL Dual Color probes. At least 500 nuclei were analyzed. Slides were analyzed under a fluorescence microscope equipped with appropriate filters using ISIS software (Metasystems). Molecular analyses of the AML-associated mutations in NPM1, FLT3, cEBPa, MLL, TP53, and WT1 genes were performed using established protocols (Lasa et al., 2009, Nomdedeu et al., 2011, Nomdedeu et al., 2013).
Statistical Analysis
Median and mean values and their SDs and ranges were calculated for each variable under study using SPSS software. All key data were supported by statistical tests performed on at least three independent replicates and are expressed as mean ± SEM. Statistical comparisons between experimental groups were performed with either paired or unpaired Student's t test, as appropriate. Kaplan-Meier curves for OS were plotted and the effect of the different clinico-biological variables (molecular/cytogenetic classification, WBC, % of BM blasts, age, cytogenetics, MSC differentiation potential, CFU, and levels of TNF-α, IFN-γ, IL-1β, IL-8, IL-10, IL-6, IL-2, and IL-4) assessed using the log rank test. The Cox proportional hazards regression model was used to explore the independent effect of variables that showed a significant influence on OS by univariate analysis. The statistical significance cutoff was corrected according to the Bonferroni method. Statistical significance was defined as a p value of <0.05.
Author Contributions
R.D.d.l.G. conceived the study, designed and performed experiments, analyzed data, and wrote the manuscript. P.M. and M.R.-M. conceived the study, designed experiments, analyzed data, and wrote the manuscript. B.L.-M., C.B., J.C., M.J., L.B., L.P., J.R.L., and J.D. performed experiments and analyzed data. M.G.-C., S.V., A.C., J.N., J.L.F., and E.A. provided leukemic samples and clinical data.
Acknowledgments
This work was supported by Health Canada's Genomics Research and Development Initiative Phase VI (H4080-144541-2014-2019) to P.M., M.R.-M., and J.R.L.; the European Research Council (CoG-2014-646903), the RTC-2016-4603-1, the Obra Social La Caixa-Fundaciò Josep Carreras and the Generalitat de Catalunya (SGR330) to P.M.; the Asociación Española Contra el Cáncer (AECC-CI-2015) and the ISCIII (PI14-01191) to C.B. and the “Fundación Hay Esperanza” to E.A. P.M. and J.L.F. are investigators of the Spanish Cell Therapy cooperative network (TERCEL). We are indebted to Dominique Patry from Health Canada for his assistance with the Luminex experiments. We would like to thank Dr. Kenneth McCreath for editorial support.
Published: May 18, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and one figure and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.04.019.
Contributor Information
Rafael Diaz de la Guardia, Email: rdiaz@carrerasresearch.org.
Michael Rosu-Myles, Email: michael.rosu-myles@hc-sc.gc.ca.
Pablo Menéndez, Email: pmenendez@carrerasresearch.org.
Supplemental Information
References
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- Bene M.C., Grimwade D., Haferlach C., Haferlach T., Zini G., European L. Leukemia diagnosis: today and tomorrow. Eur. J. Haematol. 2015;95:365–373. doi: 10.1111/ejh.12603. [DOI] [PubMed] [Google Scholar]
- Bennett J.M., Catovsky D., Daniel M.T., Flandrin G., Galton D.A., Gralnick H.R., Sultan C. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann. Intern. Med. 1985;103:620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- Bernardo M.E., Fibbe W.E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Blau O., Hofmann W.K., Baldus C.D., Thiel G., Serbent V., Schumann E., Thiel E., Blau I.W. Chromosomal aberrations in bone marrow mesenchymal stroma cells from patients with myelodysplastic syndrome and acute myeloblastic leukemia. Exp. Hematol. 2007;35:221–229. doi: 10.1016/j.exphem.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Blau O., Baldus C.D., Hofmann W.K., Thiel G., Nolte F., Burmeister T., Turkmen S., Benlasfer O., Schumann E., Sindram A. Mesenchymal stromal cells of myelodysplastic syndrome and acute myeloid leukemia patients have distinct genetic abnormalities compared with leukemic blasts. Blood. 2011;118:5583–5592. doi: 10.1182/blood-2011-03-343467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno C., Catalina P., Melen G.J., Montes R., Sanchez L., Ligero G., Garcia-Perez J.L., Menendez P. Etoposide induces MLL rearrangements and other chromosomal abnormalities in human embryonic stem cells. Carcinogenesis. 2009;30:1628–1637. doi: 10.1093/carcin/bgp169. [DOI] [PubMed] [Google Scholar]
- Bueno C., Roldan M., Anguita E., Romero-Moya D., Martin-Antonio B., Rosu-Myles M., del Canizo C., Campos F., Garcia R., Gomez-Casares M. Bone marrow mesenchymal stem cells from patients with aplastic anemia maintain functional and immune properties and do not contribute to the pathogenesis of the disease. Haematologica. 2014;99:1168–1175. doi: 10.3324/haematol.2014.103580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalina P., Montes R., Ligero G., Sanchez L., de la Cueva T., Bueno C., Leone P.E., Menendez P. Human ESCs predisposition to karyotypic instability: is a matter of culture adaptation or differential vulnerability among hESC lines due to inherent properties? Mol. Cancer. 2008;7:76. doi: 10.1186/1476-4598-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J., Mahtouk K., Attal M., Gadelorge M., Huynh A., Fleury-Cappellesso S., Danho C., Laharrague P., Klein B., Reme T. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21:1079–1088. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran P., Le Y., Li Y., Sabloff M., Mehic J., Rosu-Myles M., Allan D.S. Mesenchymal stromal cells from patients with acute myeloid leukemia have altered capacity to expand differentiated hematopoietic progenitors. Leuk. Res. 2015;39:486–493. doi: 10.1016/j.leukres.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Djouad F., Plence P., Bony C., Tropel P., Apparailly F., Sany J., Noel D., Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- Dohner H., Estey E.H., Amadori S., Appelbaum F.R., Buchner T., Burnett A.K., Dombret H., Fenaux P., Grimwade D., Larson R.A. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Funes J.M., Quintero M., Henderson S., Martinez D., Qureshi U., Westwood C., Clements M.O., Bourboulia D., Pedley R.B., Moncada S. Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc. Natl. Acad. Sci. USA. 2007;104:6223–6228. doi: 10.1073/pnas.0700690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castro J., Trigueros C., Madrenas J., Perez-Simon J.A., Rodriguez R., Menendez P. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J. Cell. Mol. Med. 2008;12:2552–2565. doi: 10.1111/j.1582-4934.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gomez I., Elvira G., Zapata A.G., Lamana M.L., Ramirez M., Castro J.G., Arranz M.G., Vicente A., Bueren J., Garcia-Olmo D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin. Biol. Ther. 2010;10:1453–1468. doi: 10.1517/14712598.2010.519333. [DOI] [PubMed] [Google Scholar]
- Garcia-Gomez A., De Las Rivas J., Ocio E.M., Diaz-Rodriguez E., Montero J.C., Martin M., Blanco J.F., Sanchez-Guijo F.M., Pandiella A., San Miguel J.F. Transcriptomic profile induced in bone marrow mesenchymal stromal cells after interaction with multiple myeloma cells: implications in myeloma progression and myeloma bone disease. Oncotarget. 2014;5:8284–8305. doi: 10.18632/oncotarget.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyh S., Rodriguez-Paredes M., Jager P., Khandanpour C., Cadeddu R.P., Gutekunst J., Wilk C.M., Fenk R., Zilkens C., Hermsen D. Functional inhibition of mesenchymal stromal cells in acute myeloid leukemia. Leukemia. 2016;30:683–691. doi: 10.1038/leu.2015.325. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Gil E., Perez-Lorenzo M.J., Galindo M., Diaz de la Guardia R., Lopez-Millan B., Bueno C., Menendez P., Pablos J.L., Criado G. Human embryonic stem cell-derived mesenchymal stromal cells ameliorate collagen-induced arthritis by inducing host-derived indoleamine 2,3 dioxygenase. Arthritis Res. Ther. 2016;18:77. doi: 10.1186/s13075-016-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M.F., Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat. Rev. Cancer. 2003;3:639–649. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- Grimwade D. Screening for core binding factor gene rearrangements in acute myeloid leukemia. Leukemia. 2002;16:964–969. doi: 10.1038/sj.leu.2402421. [DOI] [PubMed] [Google Scholar]
- Grimwade D., Ivey A., Huntly B.J. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood. 2016;127:29–41. doi: 10.1182/blood-2015-07-604496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills R.K., Ivey A., Grimwade D., UK National Cancer Research Institute (NCRI) AML Working Group Assessment of minimal residual disease in standard-risk AML. N. Engl. J. Med. 2016;375:e9. doi: 10.1056/NEJMc1603847. [DOI] [PubMed] [Google Scholar]
- Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C., Deans R.J., Krause D.S., Keating A., International Society for Cellular Therapy Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- Iwamoto S., Mihara K., Downing J.R., Pui C.H., Campana D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J. Clin. Invest. 2007;117:1049–1057. doi: 10.1172/JCI30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopp A.H., Gupta A., Spaeth E., Andreeff M., Marini F., 3rd Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaan-Shanzer S. Concise review: the immune status of mesenchymal stem cells and its relevance for therapeutic application. Stem Cells. 2014;32:603–608. doi: 10.1002/stem.1568. [DOI] [PubMed] [Google Scholar]
- Konopleva M., Konoplev S., Hu W., Zaritskey A.Y., Afanasiev B.V., Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- Konopleva M., Tabe Y., Zeng Z., Andreeff M. Therapeutic targeting of microenvironmental interactions in leukemia: mechanisms and approaches. Drug Resist. Updat. 2009;12:103–113. doi: 10.1016/j.drup.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa A., Carricondo M., Estivill C., Bussaglia E., Gich I., Brunet S., Aventin A., Sierra J., Nomdedeu J.F. WT1 monitoring in core binding factor AML: comparison with specific chimeric products. Leuk. Res. 2009;33:1643–1649. doi: 10.1016/j.leukres.2009.03.046. [DOI] [PubMed] [Google Scholar]
- Le Y., Fraineau S., Chandran P., Sabloff M., Brand M., Lavoie J.R., Gagne R., Rosu-Myles M., Yauk C.L., Richardson R.B. Adipogenic mesenchymal stromal cells from bone marrow and their hematopoietic supportive role: towards understanding the permissive marrow microenvironment in acute myeloid leukemia. Stem Cell Rev. 2016;12:235–244. doi: 10.1007/s12015-015-9639-z. [DOI] [PubMed] [Google Scholar]
- Lopez-Millan B., Diaz de la Guardia R., Roca-Ho H., Garcia Herrero C.M., Lavoie J.R., Rosu-Myles M., Gonzalez-Rey E., O’Valle F., Criado G., Delgado M. Therapeutic effect of the immunomodulatory drug lenalidomide, but not pomalidomide, in experimental models of rheumatoid arthritis and inflammatory bowel disease. Exp. Mol. Med. 2017;49:e290. doi: 10.1038/emm.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Villar O., Garcia J.L., Sanchez-Guijo F.M., Robledo C., Villaron E.M., Hernandez-Campo P., Lopez-Holgado N., Diez-Campelo M., Barbado M.V., Perez-Simon J.A. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009;23:664–672. doi: 10.1038/leu.2008.361. [DOI] [PubMed] [Google Scholar]
- Low J.H., Ramdas P., Radhakrishnan A.K. Modulatory effects of mesenchymal stem cells on leucocytes and leukemic cells: a double-edged sword? Blood Cells Mol. Dis. 2015;55:351–357. doi: 10.1016/j.bcmd.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Medinger M., Lengerke C., Passweg J. Novel therapeutic options in acute myeloid leukemia. Leuk. Res. Rep. 2016;6:39–49. doi: 10.1016/j.lrr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H., Mossner M., Jann J.C., Nolte F., Raffel S., Herrmann C., Lier A., Eisen C., Nowak V., Zens B. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14:824–837. doi: 10.1016/j.stem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Menendez P., Catalina P., Rodriguez R., Melen G.J., Bueno C., Arriero M., Garcia-Sanchez F., Lassaletta A., Garcia-Sanz R., Garcia-Castro J. Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene. J. Exp. Med. 2009;206:3131–3141. doi: 10.1084/jem.20091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najar M., Raicevic G., Fayyad-Kazan H., Bron D., Toungouz M., Lagneaux L. Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy. 2016;18:160–171. doi: 10.1016/j.jcyt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Nomdedeu J., Bussaglia E., Villamor N., Martinez C., Esteve J., Tormo M., Estivill C., Queipo M.P., Guardia R., Carricondo M. Immunophenotype of acute myeloid leukemia with NPM mutations: prognostic impact of the leukemic compartment size. Leuk. Res. 2011;35:163–168. doi: 10.1016/j.leukres.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Nomdedeu J.F., Hoyos M., Carricondo M., Bussaglia E., Estivill C., Esteve J., Tormo M., Duarte R., Salamero O., de Llano M.P. Bone marrow WT1 levels at diagnosis, post-induction and post-intensification in adult de novo AML. Leukemia. 2013;27:2157–2164. doi: 10.1038/leu.2013.111. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prieto C., Stam R.W., Agraz-Doblas A., Ballerini P., Camos M., Castano J., Marschalek R., Bursen A., Varela I., Bueno C. Activated KRAS cooperates with MLL-AF4 to promote extramedullary engraftment and migration of cord blood CD34+ HSPC but is insufficient to initiate leukemia. Cancer Res. 2016;76:2478–2489. doi: 10.1158/0008-5472.CAN-15-2769. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Rubio R., Menendez P. Modeling sarcomagenesis using multipotent mesenchymal stem cells. Cell Res. 2012;22:62–77. doi: 10.1038/cr.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R., Tornin J., Suarez C., Astudillo A., Rubio R., Yauk C., Williams A., Rosu-Myles M., Funes J.M., Boshoff C. Expression of FUS-CHOP fusion protein in immortalized/transformed human mesenchymal stem cells drives mixoid liposarcoma formation. Stem Cells. 2013;31:2061–2072. doi: 10.1002/stem.1472. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Rosu-Myles M., Arauzo-Bravo M., Horrillo A., Pan Q., Gonzalez-Rey E., Delgado M., Menendez P. Human bone marrow stromal cells lose immunosuppressive and anti-inflammatory properties upon oncogenic transformation. Stem Cell Rep. 2014;3:606–619. doi: 10.1016/j.stemcr.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Moya D., Bueno C., Montes R., Navarro-Montero O., Iborra F.J., Lopez L.C., Martin M., Menendez P. Cord blood-derived CD34+ hematopoietic cells with low mitochondrial mass are enriched in hematopoietic repopulating stem cell function. Haematologica. 2013;98:1022–1029. doi: 10.3324/haematol.2012.079244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez L., Gutierrez-Aranda I., Ligero G., Rubio R., Munoz-Lopez M., Garcia-Perez J.L., Ramos V., Real P.J., Bueno C., Rodriguez R. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells. 2011;29:251–262. doi: 10.1002/stem.569. [DOI] [PubMed] [Google Scholar]
- Sanjuan-Pla A., Bueno C., Prieto C., Acha P., Stam R.W., Marschalek R., Menendez P. Revisiting the biology of infant t(4;11)/MLL-AF4+ B-cell acute lymphoblastic leukemia. Blood. 2015;126:2676–2685. doi: 10.1182/blood-2015-09-667378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers K., Campbell T.B., Passegue E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16:254–267. doi: 10.1016/j.stem.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S., Eckert C., Seeger K., Pfau M., Prada J., Henze G., Blankenstein T., Kammertoens T. Leukemia-associated genetic aberrations in mesenchymal stem cells of children with acute lymphoblastic leukemia. J. Mol. Med. 2010;88:249–265. doi: 10.1007/s00109-009-0583-8. [DOI] [PubMed] [Google Scholar]
- Sison E.A., Brown P. The bone marrow microenvironment and leukemia: biology and therapeutic targeting. Expert Rev. Hematol. 2011;4:271–283. doi: 10.1586/ehm.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standing Committee on Human Cytogenetic Nomenclature, Harnden D.G., Klinger H.P., March of Dimes Birth Defects Foundation . Karger; 1985. ISCN 1985: An International System for Human Cytogenetic Nomenclature (1985): Report of the Standing Committee on Human Cytogenetic Nomenclature. [PubMed] [Google Scholar]
- Streubel B., Chott A., Huber D., Exner M., Jager U., Wagner O., Schwarzinger I. Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N. Engl. J. Med. 2004;351:250–259. doi: 10.1056/NEJMoa033153. [DOI] [PubMed] [Google Scholar]
- Swerdlow S.H., International Agency for Research on Cancer, World Health Organization . Fourth Edition. International Agency for Research on Cancer; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- Tabe Y., Konopleva M. Advances in understanding the leukaemia microenvironment. Br. J. Haematol. 2014;164:767–778. doi: 10.1111/bjh.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nature reviews. Immunology. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- von der Heide E.K., Neumann M., Vosberg S., James A.R., Schroeder M.P., Tanchez J.O., Isaakidis K., Schlee C., Luther M., Johrens K. Molecular alterations in bone marrow mesenchymal stromal cells derived from acute myeloid leukemia patients. Leukemia. 2016 doi: 10.1038/leu.2016.324. [DOI] [PubMed] [Google Scholar]
- Walkley C.R., Olsen G.H., Dworkin S., Fabb S.A., Swann J., McArthur G.A., Westmoreland S.V., Chambon P., Scadden D.T., Purton L.E. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.