Abstract
Mannan-binding lectin (MBL), L-ficolin, and H-ficolin are pattern recognition molecules of the innate immune system. We investigated their ability to bind to different serotypes and noncapsulated variants of two gram-positive bacterial species, Streptococcus pneumoniae and Staphylococcus aureus. MBL did not bind to capsulated S. aureus or capsulated S. pneumoniae but did bind to a noncapsulated S. aureus variant (Wood). L-ficolin bound to some capsulated S. aureus serotypes (serotypes 1, 8, 9, 11, and 12) and capsulated S. pneumoniae serotypes (11A, 11D, and 11F) but not to noncapsulated strains. H-ficolin did not bind to any of the S. pneumoniae and S. aureus serotypes included in this study but did bind to one strain of Aerococcus viridans. The concentrations of the three proteins in 97 plasma samples were estimated. The median concentrations were 0.8 μg per ml for MBL, 3.3 μg per ml for L-ficolin, and 18.4 μg per ml for H-ficolin.
The immune system responds to infections in two different but highly integrated ways, i.e., through the innate and the adaptive systems. The innate immune system comprises a number of pattern recognition and effector mechanisms enabling it to target a large array of microorganisms, thereby preventing or limiting the spread and the severity of infections. One component of the innate immune system is the complement system, encompassing more than 30 different recognition, effector, and control proteins. The activation of the complement system is initiated through three different pathways: the classical, the alternative, and the mannan-binding lectin (MBL) pathways. The activation pathways converge in the formation of C3 convertase complexes, which lead to the activation of further complement factors.
Upon binding to carbohydrate-based pathogen-associated molecular patterns (PAMPs) on microorganisms, MBL activates complement via the MBL-associated serine proteases (MASPs) (6, 22, 37). The three MASPs that have been characterized circulate in complexes with MBL as inactive single-chain proenzymes. When the complexes bind to a target, the MASPs are cleaved to become active enzymes. Recently, L-ficolin and H-ficolin also were found to form complexes with the MASPs and to be capable of activating complement (19, 23).
MBL, L-ficolin, and H-ficolin are plasma proteins synthesized mainly in the liver, although H-ficolin is also found in secretions, i.e., in bile, bronchiolar, and alveolar fluids (1, 15). In plasma, L-ficolin, H-ficolin, and MBL are found as higher-order oligomers comprising several homotrimeric subunits. A collagen-like region in the N-terminal part enables three polypeptides to form the collagen-like triple helix and thus the trimeric subunit. The three pattern recognition domains are located in the C-terminal part of this subunit. MBL has a C-type carbohydrate recognition domain, while the ficolins have fibrinogen-like recognition domains (21). The oligomerization of MBL and of the ficolins enables multiple interactions, i.e., pattern recognition, thereby increasing avidity and stabilizing interactions in encounters with suitably spaced ligands (39). The carbohydrate specificity of MBL has been thoroughly investigated and determined to be directed against carbohydrates having equatorial 3′ and 4′ hydroxyl groups (43). The specificity of the ficolins has not been fully elucidated, but L-ficolin has been shown to bind to N-acetylglucosamine (GlcNAc) (15).
MBL is able to bind to various microorganisms, and clinical studies have shown MBL to be involved in the first line of defense against invading pathogens (10, 41). The two ficolins have also been shown to bind to bacteria. The structural and functional properties of MBL, L-ficolin, and H-ficolin appear very similar, suggesting that the three proteins may serve similar biological functions through activation of the complement system upon binding to microorganisms. Assuming that the three recognition molecules bind to different microorganisms, we compared the reactivities of MBL and the ficolins with the important human pathogens Staphylococcus aureus and Streptococcus pneumoniae as well as with strains of Escherichia coli and Aerococcus viridans. We included both capsulated and noncapsulated strains. Most pneumococcal and staphylococcal strains possess two kinds of cell wall-associated polysaccharide antigens, the capsule and teichoic acids. It is generally assumed that the capsule covers the bacterial surface during infections, although the levels of expression of the capsule may vary during different phases (33). Therefore, in studies of the mutual interactions between bacterial surface structures and components of the innate immune system, it is important to include noncapsulated homologous strains in the experiments. Bacterial cultures in growth media often contain a mixture of capsulated and noncapsulated cells (34).
The results of the present study show that the three proteins target different PAMPs and that the three proteins selectively bind to a limited number of bacterial polysaccharide antigens. Binding to the capsule may occur or, indeed, the capsule may mask the binding sites, depending on the bacteria.
MATERIALS AND METHODS
Bacteriological procedures.
The following bacterial strains were examined in the binding assays: S. pneumoniae serotypes 1, 4, 14, 6A, 6B, 7A, 7F, 9L, 9V, 11A, 11B, 11C, 11D, 11F, 19C, 19F, 23F, 27, 32F, and 45 and noncapsulated variant strain SCR2 (Statens Serum Institut, Copenhagen, Denmark). The strains were grown in Todd-Hewitt broth medium (Oxoid, Basingstoke, England) overnight at 37°C in 5% CO2. S. aureus serotypes 1 to 13 (T-1 to T-13) and noncapsulated variant strain Wood (National Institutes of Health, Bethesda, Md.) were cultured on Columbia agar plates (Difco, Kansas City, Kans.) supplemented with 1% (wt/vol) yeast extract and 0.1% (wt/vol) glucose at 37°C overnight to ensure maximum production of capsules (4, 9, 16, 29). A. viridans strains 86965 and Ring 44 were isolated from mice kept in the local animal facility and kindly provided by Frederik Dagnæs-Hansen (5). E. coli strains 74924 and 74285, with known potential to bind to MBL (25), were also included as controls. A. viridans and E. coli were grown in L broth (Q-Biogene, Carlsbad, Calif.) overnight at 37°C. Following incubation, formaldehyde (Sigma-Aldrich, St. Louis, Mo.) was added to the broth cultures to a final concentration of 1% (wt/vol), and the cultures were kept at room temperature until the next day. This treatment stabilizes the cells but does not alter the polysaccharide antigens. S. aureus organisms were washed off the agar plates, resuspended in 5 ml of 137 mM NaCl-2.7 mM KCl-1.5 mM KH2PO4-8.1 mM Na2HPO4 (pH 7.4) (PBS), and fixed with formaldehyde as described above. Residual reactive aldehyde groups were blocked by incubation with a 1/10 volume of 1 M ethanolamine (pH 9.0). After 1 h of incubation with ethanolamine, the bacterial cells were washed three times with 20 mM Tris-HCl-140 mM NaCl-1.5 mM NaN3 (pH 7.4) (TBS) and stored at 4°C. The concentrations of bacteria in the suspensions were estimated by reading the optical density at 600 nm. As determined by microscopy, an optical density of 1.0 corresponds to approximately 1.8 × 109 bacteria/ml.
Bacterial binding assays.
Bacteria (4.5 × 108) were incubated with 6 μl of normal human serum (NHS) and TBS containing 5 mM CaCl2 and 0.05% (vol/vol) Tween 20 (TBS/Tw/Ca) in a total volume of 300 μl. Samples were incubated for 2 h at room temperature. After centrifugation (9,000 × g, 5 min), MBL, L-ficolin, or H-ficolin in the supernatants was quantified as described below. In some experiments, a five-step twofold dilution series starting with 3.6 × 109 bacteria was used instead of a fixed amount of bacterial cells. The percentage of unbound protein was estimated by dividing the signal obtained from the supernatant sample by the signal obtained from a sample free of bacteria and multiplying the results by 100. Each test was repeated three times. Significant differences (P < 0.05) were determined with Student's t test by use of the statistical utilities included in the Microsoft Excel program (Microsoft, Seattle, Wash.).
MBL, L-ficolin, and H-ficolin quantification assays.
The wells of FlouroNunc microtiter plates (Nunc, Kamstrup, Denmark) were coated with 100 ng of the following monoclonal antibodies in 100 μl of PBS: anti-MBL antibody (36) (Hyb 131-1; Immunolex, Copenhagen, Denmark), anti-L-ficolin antibody (35) (GN5; HyCult Biotechnology, Uden, The Netherlands), and anti-H-ficolin antibody (34) (4H5; HyCult Biotechnology). All incubations were carried out overnight at 4°C in a humid chamber. The wells were blocked by the addition of 200 μg of human serum albumin (HSA; Statens Serum Institut) in 200 μl of TBS for 1 h at room temperature; this step was followed by three washes with TBS/Tw/Ca. Samples (100 μl) were added to the wells, and the plates were incubated overnight at 4°C, washed as described above, and incubated with 100 μl of TBS/Tw/Ca containing 100 ng of biotinylated anti-MBL antibody (Hyb 131-1), 100 ng of biotinylated anti-L-ficolin antibody (2F5) (35), or 25 ng of biotinylated anti-H-ficolin antibody (4H5). The anti-MBL and L-ficolin antibodies were biotinylated with 167 μg of biotinyl-N-hydroxy-succinimide (Sigma-Aldrich) per mg of protein. The anti-H-ficolin antibody was biotinylated with 33 μg of biotinyl-N-hydroxy-succinimide per mg of protein, since a loss of activity was observed at a higher degree of biotinylation. The plates were incubated for 1 h at room temperature and washed three times. Next, 10 ng of Eu-labeled streptavidin (Wallac, Turku, Finland) in 100 μl of TBS containing 0.05% (vol/vol) Tween 20 and 25 μM EDTA was added per well; this step was followed by incubation for 1 h. The plates were finally washed three times as described above. After the addition of 200 μl of enhancement solution (Wallac) per well, the bound europium was quantified by time-resolved fluorometry with a 1232 Delfia fluorometer (Wallac). NHS (standard serum) with known contents of MBL (1.5 μg/ml), L-ficolin (5.0 μg/ml), and H-ficolin (20.0 μg/ml) was used as a standard in the assays by including a twofold dilution series starting at 1/5 on every plate. The concentrations of MBL, L-ficolin, and H-ficolin in the standard serum were determined by using highly purified preparations of the three proteins as primary standards (3, 23). The concentrations of MBL and the ficolins in plasma samples from 97 apparently healthy individuals were estimated. Plasma samples were diluted 1/100, 1/50, and 1/150 in the assays for MBL, L-ficolin, and H-ficolin, respectively. Dilutions were made in 20 mM Tris-HCl-1 M NaCl-0.05% Triton X-100 (Sigma-Aldrich)-10 mM CaCl2-1 mg of HSA/ml (pH 7.4) (1 M salt buffer).
Size permeation chromatography.
To analyze the sizes and thus the selectivity of the serum proteins producing signals in the assays, i.e., MBL, L-ficolin, and H-ficolin, 50 μl of serum was fractionated on a Superose 6 column (10 mm, 30 cm; Amersham Biosciences, Uppsala, Sweden) with TBS containing 0.01% (vol/vol) Tween 20 and 5 mM calcium as the elution buffer, and fractions of 250 μl were tested. A standard curve for estimation of the apparent molecular weights was made from the elution profiles for thyroglobin, ferritin, catalase, aldolase, immunoglobulin G, HSA, and ovalbumin.
Flow cytometry.
Recombinant MBL (rMBL) (42) (a kind gift from NatImmune A/S, Copenhagen, Denmark) was biotinylated as described above for Hyb 131-1. Stabilized bacterial cells (2.3 × 108) of each S. aureus strain were incubated with 1.5 μg of biotinylated rMBL in a total volume of 300 μl of TBS/Tw/Ca for 2 h at room temperature with end-over-end rotation. In the negative controls, either 100 mM GlcNAc (Sigma-Aldrich) was included or 1.5 μg of nonbiotinylated rMBL was used instead of biotinylated rMBL. Samples were centrifuged, and the pellets were washed twice with 1 ml of TBS/Tw/Ca, resuspended in TBS-Tween 20-Ca, and incubated at room temperature for 1 h with 3 μg of fluorescein isothiocyanate (FITC)-labeled streptavidin (Dako, Glostrup, Denmark) in 300 μl of TBS/Tw/Ca. Bacterial cells were washed three times, resuspended in 1.5 ml of TBS/Tw/Ca, and examined with a FACSCalibur flow cytometer (BD Biosciences, San Jose, Calif.). The data were analyzed by use of the Cellquest program (BD Bioscience).
C4 consumption assay.
Bacteria (1.05 × 109) were incubated for 2 h at 4°C with 15 μl of NHS in a final volume of 700 μl of 10 mM Tris-HCl-0.5 M NaCl-0.05% (vol/vol) Tween 20-5 mM CaCl2 (0.5 M salt buffer) with end-over-end rotation. In the negative controls for MBL and L-ficolin, 100 mM GlcNAc was added to inhibit binding to the bacteria. After centrifugation (9,000 × g, 5 min), the concentrations of MBL, L-ficolin, and H-ficolin in the supernatants were determined as described above. The pellets were washed twice with 1 ml of TBS/Tw/Ca. Bacterial cells were resuspended in 300 μl of 4 mM barbital-145 mM NaCl-2 mM CaCl2-1 mM MgCl2-0.05% Tween 20-1.5 mM NaN3 (pH 7.4) (activation buffer) containing 360 ng of complement factor C4 (7). Samples were incubated with end-over-end rotation for 2 h at 37°C. After centrifugation, the supernatants were tested for residual active C4 on FluoroNunc microtiter plates that had been precoated with MBL-MASP complexes as follows: the wells were coated by overnight incubation at 4°C with 1 μg of mannan in 100 μl of 15 mM Na2CO3-35 mM NaHCO3-3 mM NaN3 (pH 9.6), blocked with HSA as described above, washed with TBS/Tw/Ca, and incubated with 100 μl of standard serum diluted 10-fold in 20 mM Tris-1 M NaCl-0.05% Triton X-100-1 mg of HSA/ml-10 mM CaCl2 for 2 h at room temperature. This procedure allows the attachment of MBL-MASP complexes, whereas the high salt concentration prevents the activation of C4 at this stage, and the MASPs are still retained on the attached MBL. After washing was done with TBS/Tw/Ca, 100-μl samples were taken from the C4 consumption step described above and incubated in wells for 1.5 h at 37°C. The wells were washed with TBS/Tw/Ca, and C4 deposited on the surface of the wells was measured by the addition of a mixture of two biotinylated anti-C4 monoclonal antibodies (Hyb 161-1 and Hyb 161-2; Immunolex), each at a concentration of 0.25 μg per ml of TBS/Tw/Ca. Biotinylation was carried out as described above for Hyb 131-1. After incubation overnight at 4°C, the plates were washed, developed with Eu-labeled streptavidin, and quantified as described above. A standard curve was made from a twofold dilution series of purified C4 in activation buffer (56 ng to 2.9 μg of C4 per 100 μl) in wells prepared with MBL-MASP. Residual C4 in the samples was quantified.
RESULTS
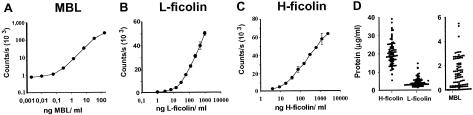
Time-resolved immunofluorometric assays for the quantification of MBL, L-ficolin, and H-ficolin were developed for determination of the concentrations of the proteins in human serum and in the sample supernatants from the bacterial binding assays. The specificity of the assays was verified by testing purified proteins. Only the relevant protein gave a signal, i.e., no cross-reactivity between the assays was revealed (data not shown). When serum was fractionated by size permeation chromatography, the molecular masses of MBL, H-ficolin, and L-ficolin were estimated at 700, 650, and 625 kDa, respectively. The dynamic ranges of the assays are shown in Fig. 1A to C. The median concentrations of MBL, L-ficolin, and H-ficolin in plasma samples from 97 healthy individuals were determined to be 0.76 μg/ml (5th and 95th percentiles: 8 ng/ml and 3.1 μg/ml), 3.3 μg/ml (1.8 and 9.0 μg/ml), and 18.4 μg/ml (11.2 and 33.8 μg/ml) (Fig. 1D), respectively. The concentrations in serum and plasma were identical for the three proteins. We selected a serum sample containing 1.5 μg of MBL, 5 μg of L-ficolin, and 20 μg of H-ficolin per ml for use in the binding experiments.
FIG. 1.
Assays for the determination of MBL, L-ficolin, and H-ficolin concentrations. Dilutions of standard sera with known concentrations of the respective molecules were applied to wells coated with monoclonal antibodies to MBL (A), L-ficolin (B), and H-ficolin (C). Bound proteins were detected by use of relevant biotinylated monoclonal antibodies. The signals are given as counts per second. Error bars indicate standard deviations. (D) Concentrations of H-ficolin, L-ficolin, and MBL in samples from 97 healthy individuals. Note the different y axis for MBL. Each filled circle represents an individual.
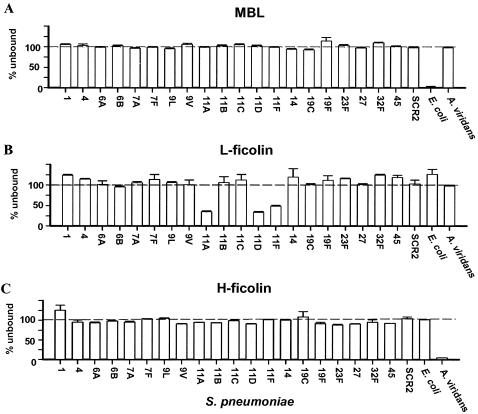
The abilities of MBL, L-ficolin, and H-ficolin to adhere to various strains of capsulated and noncapsulated bacteria were examined in binding assays. Binding was tested by incubating diluted serum and whole bacterial cells, followed by centrifugation and analysis for proteins left in the supernatants. The results are given as percentages of added proteins that were left unbound in the supernatants. Thus, a value of 100 indicates no binding, and a value of 0 means that all of the protein was bound. Neither MBL (Fig. 2A) nor H-ficolin (Fig. 2C) bound to strains of any of the S. pneumoniae serotypes, whereas each bound significantly to one of the control strains, E. coli strain 74924 or A. viridans strain 86965, respectively. L-ficolin, on the other hand, bound significantly to strains of three of the examined serotypes of S. pneumoniae (11A, 11D, and 11F) (Fig. 2B). When the cell concentrations of strains of these particular S. pneumoniae serotypes were increased, the depletion of L-ficolin was observed (data not shown). Another A. viridans strain (Ring 44) and another E. coli strain (74285) were examined. L-ficolin and H-ficolin did not bind to either of them, while MBL bound to the E. coli strain to some extent (data not shown).
FIG. 2.
Binding of MBL, L-ficolin, and H-ficolin to strains of S. pneumoniae and to control strains. MBL, L-ficolin, and H-ficolin in supernatants were measured after incubation of sera and bacteria; binding is seen as a decreased signal. Error bars indicate standard deviations for the individual strains. The results shown are typical of three experiments.
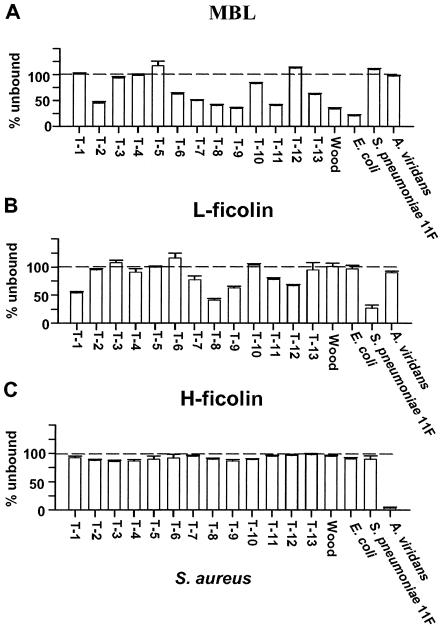
Figure 3 shows the results of the binding experiments with S. aureus. Again, H-ficolin (Fig. 3C) did not bind to any of the examined bacterial strains, except for the control A. viridans. In contrast, both MBL (Fig. 3A) and L-ficolin (Fig. 3B) bound to some S. aureus strains. However, MBL also bound efficiently to the noncapsulated variant (Wood). Thus, it is plausible that the binding of MBL to various staphylococcal strains was due to the presence of common teichoic acids and was not caused by capsular polysaccharides (see Discussion). Figure 3 also shows that some staphylococcal strains (serotypes T-1, T-3, T-4, T-5, and T-12) did not bind to MBL (Fig. 3A), indicating that the capsule in these strains masks the common polysaccharide antigens. L-ficolin bound significantly to strains of serotypes T-1, T-8, T-9, T-11, and T-12 but not to the noncapsulated variant (Wood). These data indicate that noncapsulated staphylococci do not present ligands for L-ficolin, and the observed binding therefore seems to be directed toward the capsule.
FIG. 3.
Binding of MBL, L-ficolin, and H-ficolin to S. aureus. The various serotypes are listed numerically from left to right, with the noncapsulated variant at the right. MBL, L-ficolin, and H-ficolin in supernatants were measured after incubation of sera and bacteria; binding is seen as a decreased signal. Error bars indicate standard deviations for the individual bacteria. The results shown are typical of three experiments.
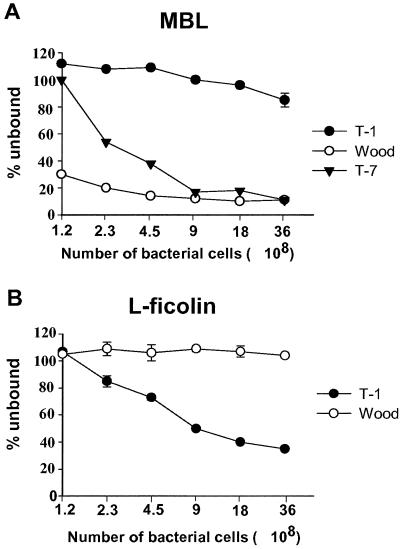
In order to compare the potentials of MBL and L-ficolin to bind to various S. aureus serotypes, dilution series of bacterial cells were made and then incubated with a fixed volume of serum. MBL binding to dilutions of serotypes T-1 and T-7 and strain Wood is shown in Fig. 4A, and L -ficolin binding to dilutions of T-1 and Wood is shown in Fig. 4B. These strains were chosen to illustrate the various binding potentials for MBL and L-ficolin. For strain Wood, a bacterial concentration of 4.5 × 108 was enough to deplete the added 6 μl of NHS for MBL, while hardly any MBL was removed by T-1, even at the highest concentration of bacteria. T-7 bound to MBL well, but more than four times as many bacteria were needed to bind to the same degree as strain Wood. This difference might be explained in two ways: most of the common antigens might have been masked by capsule material in T-7, or about 25% of the cells could possess microcapsules or might have lost their capsules, thus exposing teichoic acids and other cell wall antigens. Common to all of the MBL-binding strains was that dose-dependent binding was observed. L-ficolin (Fig. 4B) showed no ability to bind to strain Wood, regardless of the amount of bacteria added. In contrast, L-ficolin binding to T-1 was dose dependent. L-ficolin therefore seems to bind specifically to type 1 capsules and not to any antigen on noncapsulated bacteria. The number of bacteria needed to bind 50% of the added MBL or L-ficolin was estimated from the dilution series of all of the S. aureus strains studied (Table 1), making it possible to compare the binding potentials of the various bacterial strains.
FIG. 4.
Binding of MBL and L-ficolin to dilutions of S. aureus. (A) Amounts of MBL bound to various numbers of serotype T-1, serotype T-7, and strain Wood cells. (B) Amounts of L-ficolin bound to T-1 and Wood. Proteins in supernatants were measured after incubation of sera and bacteria; binding is seen as a decreased signal. Error bars indicate standard deviations.
TABLE 1.
Binding of MBL and L-ficolin to S. aureus
| S. aureus serotype or strain | B50a (108) for:
|
|
|---|---|---|
| MBL | L-Ficolin | |
| T-1 | NB | 9 |
| T-2 | 4.5 | NB |
| T-3 | NB | NB |
| T-4 | NB | NB |
| T-5 | NB | NB |
| T-6 | 2.3 | NB |
| T-7 | 1.2 | 36 |
| T-8 | 9 | 9 |
| T-9 | <1.1 | 18 |
| T-10 | 9 | NB |
| T-11 | <1.1 | 9 |
| T-12 | 9 | 9 |
| T-13 | 36 | NB |
| Wood | <1.1 | NB |
B50, number of bacteria needed to remove 50% of MBL or L-ficolin in 6 μl of serum; NB, no binding, i.e., 50% binding was not achieved, even at the highest concentration of bacteria (3.6 × 109) (see text for details).
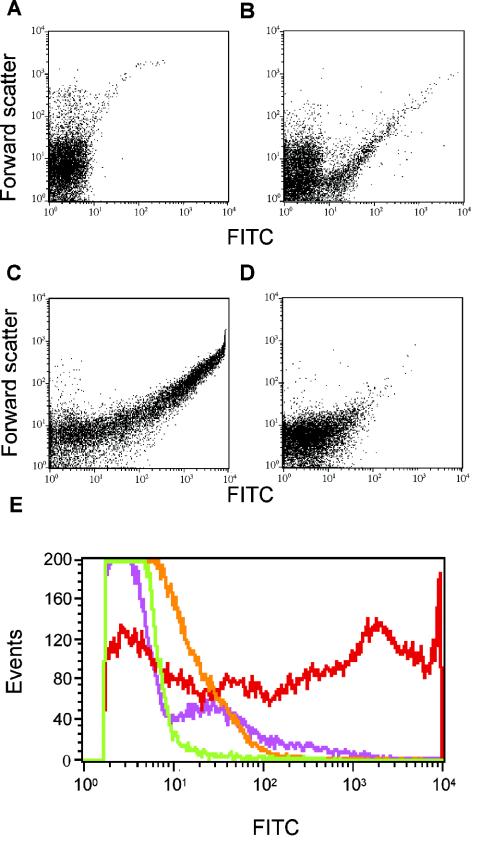
The results presented above indicate that several different structures might be involved in the binding of MBL to bacteria. Flow cytometry was therefore used to examine MBL binding to individual bacterial cells. The various S. aureus serotypes were incubated with biotinylated rMBL and then with FITC-labeled streptavidin. The biotin-streptavidin method was used because anti-MBL antibody may give nonspecific signals due to immunoglobulin G-binding protein A present on staphylococcal cells. This approach enabled us to evaluate whether the observed binding was uniform on all bacterial cells or whether subpopulations exhibited different binding capacities. Figure 5 shows the scatter plots of S. aureus Wood or T-1 and a compilation of the frequency histograms of the fluorescence distributions in the various experiments. Figure 5A shows the data obtained when strain Wood was incubated with nonbiotinylated rMBL. Figure 5B and C show the fluorescence data obtained when serotype T-1 and strain Wood, respectively, were examined after being labeled with biotinylated rMBL. Very few serotype T-1 cells were labeled compared to the number of labeled strain Wood cells. However, the signal seen from the few labeled T-1 cells was almost as high as that seen from the labeled Wood cells. The low level of binding to T-1 cells observed in the binding assays was thus likely due to significant binding to a minority of the cells only. The data from the flow experiments are compiled in Fig. 5E, where the MBL-binding T-1 subpopulation was seen as a shoulder on the histogram. These results are compatible with the interpretation that MBL binds to common antigens exposed on a few incompletely capsulated cells. The binding of MBL to strain Wood was significantly blocked by the relevant carbohydrate GlcNAc at a concentration of 100 mM (Fig. 5D).
FIG. 5.
Binding of MBL to selected S. aureus serotypes, as analyzed by flow cytometry. Bacteria were incubated with biotinylated rMBL (biotin-rMBL) followed by FITC-labeled streptavidin. (A) Control with bacteria (Wood) and nonbiotinylated rMBL. (B and C) Binding to S. aureus T-1 (B) and S. aureus Wood (C). (D) S. aureus Wood incubated with biotin-rMBL in the presence of 100 mM GlcNAc. The x axis depicts the fluorescence intensity, and the y axis depicts the forward scatter. (E) Compilation of the four flow cytometric histograms, with fluorescence on the x axis and events on the y axis: A, green line; B, purple line; C, red line; D, orange line.
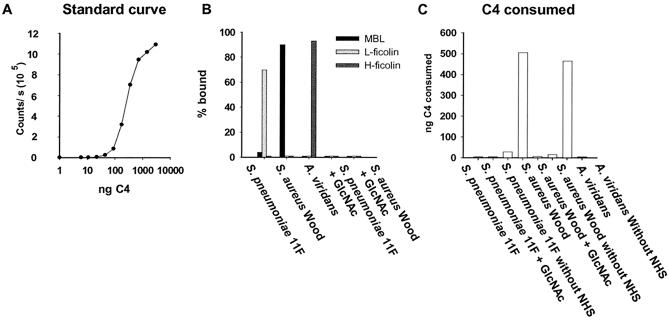
The results described above demonstrate that the binding of MBL, L-ficolin, and H-ficolin is directed toward specific and different PAMPs. Based on those results, we selected three bacteria exhibiting high levels and distinct patterns of binding to the three proteins. We estimated the relative complement activation potentials of the proteins by using these bacteria. MBL, L-ficolin, and H-ficolin were bound to the selected bacteria by incubation with the standard serum. Purified C4 was added, the remaining active C4 after incubation was quantified, and the C4 consumption was estimated. Figure 6A shows a standard curve used for the estimation of C4 consumption. Figure 6B shows the percentages of MBL, L-ficolin, and H-ficolin that bound to the selected bacteria. Incubation with noncapsulated S. aureus strain Wood removed more than 90% of the MBL, whereas neither L-ficolin nor H-ficolin was removed. Incubation with an S. pneumoniae 11F strain removed 70 to 80% of the L-ficolin, while neither of the other proteins was affected. The binding of MBL and L-ficolin to the bacteria was totally inhibited when GlcNAc was added to the sample. Finally, more than 90% of the H-ficolin was removed from the sample when it was incubated with A. viridans strain 86965, whereas no MBL or L-ficolin was bound. Specific inhibition of H-ficolin binding could not be examined, since no characterized inhibitor of H-ficolin is known. The experiment was conducted under conditions where no activation via the classical or the alternative pathway occurred.
FIG. 6.
C4 consumption by MBL, L-ficolin, and H-ficolin complexes bound to bacteria. Bacteria were incubated with sera, washed, and incubated with purified C4, and the amount of residual active C4 was estimated. (A) Standard curve used for quantifying residual C4. (B) Percentages of MBL, L-ficolin, and H-ficolin bound to bacteria. (C) C4 consumed after incubation with bacterium-bound complexes.
C4 consumption after incubation with the bacterium-lectin complexes is shown in Fig. 6C. Most of the added C4 was consumed when the sample was incubated with the S. aureus-MBL complex. In the presence of GlcNAc (used as an inhibitor of MBL and L-ficolin binding), no C4 was consumed. A. viridans-H-ficolin complexes displayed the same ability as MBL complexes to activate C4. In contrast, L-ficolin displayed no ability to activate complement in this assay.
DISCUSSION
The efficient clearance of bacteria requires the cooperation of multiple mechanisms from both the innate and the adaptive immune systems. Pathogenic bacteria may evade recognition and escape the immune system through different tactics; e.g., a feature of most bacteria causing invasive diseases is the generation of a capsule. We have studied the influence of capsulation of two gram-positive bacterial species, S. aureus and S. pneumoniae, on recognition by three pattern recognition molecules, MBL, L-ficolin, and H-ficolin, of the innate immune system.
The binding of MBL to various bacteria, viruses, and parasites leads to the activation of the complement system and to the initiation of different kinds of killing mechanisms (11). L-ficolin increase the phagocytosis of Salmonella enterica serovar Typhimurium (19), and H-ficolin inhibit the growth of an A. viridans strain (23).
To validate our assays and to allow for the selection of serum for the binding studies, 97 human plasma samples were analyzed for MBL, L-ficolin, and H-ficolin (Fig. 1). The median concentration of H-ficolin in plasma (18.4 μg/ml) was approximately 5-fold higher than that of L-ficolin (3.3 μg/ml) and more than 20-fold higher than that of MBL (0.76 μg/ml), but significant interindividual differences were observed. The H-ficolin concentration is similar to the value (20 μg/ml) previously reported (44). The L-ficolin concentration is close to the value reported for Caucasian blood donors (3.7 μg/ml) (13); however, this value is approximately threefold lower than the median value reported for a Japanese population (13.7 μg/ml) (35). The observed difference between Caucasian and Japanese populations may be due to polymorphisms in promoters and/or exons present in the two populations, as has been reported for MBL (12).
We examined the potentials of the three proteins to bind to bacteria by using diluted whole serum, whereas purified proteins were used in most other studies (19, 25, 41). Twenty of the most common serotypes (accounting for approximately 85% of invasive pneumococcal infections in humans) (27) of S. pneumoniae and the 13 known capsulated serotypes of S. aureus were used in our experiments. We also included relevant noncapsulated strains of S. pneumoniae (SCR2) and S. aureus (Wood) to examine the influence of capsulation. It is generally accepted that noncapsulated S. pneumoniae and S. aureus strains do not cause invasive infections (31). An E. coli strain and an A. viridans strain were included as positive controls for the binding of MBL and H-ficolin, respectively. Binding to the latter bacteria was previously reported (25, 38).
S. pneumoniae is part of the normal flora of the upper respiratory tract, as all humans probably carry these bacteria at some point during their lifetime. However, pneumococci are serious pathogens causing invasive diseases, such as otitis media, pneumonia, bacterimia, and meningitis.
We found that MBL and H-ficolin were unable to bind to any of the S. pneumoniae serotypes examined and that L-ficolin bound to only 3 of the 20 serotypes examined (11A, 11D, and 11F). None of the three proteins bound to the noncapsulated S. pneumoniae strain (SCR2). These data indicate that L-ficolin specifically binds to capsule constituents of the three serotypes within pneumococcus serogroup 11. In a comparison of the structures of this serogroup (41) with known MBL ligands, i.e., free 3-OH and 4-OH groups in hexose rings, no MBL-binding motifs were obvious. Some of the capsular structures contain N-acetylated carbohydrates, which theoretically constitute ligands for L-ficolin. However, of the serotypes that bound to L-ficolin, only 11F has N-acetylated carbohydrates in its capsular structure. On the other hand, several serotypes that did not display an ability to bind to L-ficolin had N-acetylated carbohydrates in their capsules (40).
S. aureus is a commensal microorganism of humans, as 30 to 50% of healthy adults are colonized, with 10 to 20% showing chronic carriage in the anterior part of the nose. S. aureus is also known to be a common pathogen in humans. Among the 13 known serotypes, T-5 and T-8 account for approximately 75% of human S. aureus infections.
MBL and L-ficolin bound to some strains of S. aureus in our experiments, whereas H-ficolin did not bind to strains of any of the 13 known serotypes. The different strains of S. aureus showed significant variations in their abilities to bind to MBL and L-ficolin (Table 1). L-ficolin bound only to strains of some capsulated serotypes and not to the noncapsulated strain, suggesting that L-ficolin recognizes structures specific to a few capsular polysaccharides. MBL, on the other hand, bound strongly to the noncapsulated control strain (Wood) (Fig. 4 and Table 1) and much less to strains of all of the capsulated serotypes, indicating binding to common cell wall polysaccharides or other cell wall-associated constituents. In agreement with these data, the capsular structures of, e.g., S. aureus T-5 and T-8 strains do not contain potential MBL-binding sites, as the required free 3-OH and/or 4-OH groups are not present. In polysaccharide structures, such groups are involved in glycoside linkage (8, 24). The observed binding to strain Wood may be caused by peptidoglycan in the staphylococcal cell wall consisting of alternating 1,4-beta-linked subunits of GlcNAc and N-acetylmuramic acid. It was recently found that MBL binds to purified peptidoglycan from S. aureus (18). Common cell wall teichoic acids may also be responsible; in these acids, polyribitol phosphate is substituted with GlcNAc, a residue which fulfills the criteria for MBL binding. Alternatively, lipoteichoic acids (LTAs) may mediate binding, as the glycolipid anchor exposes 3-OH and 4-OH groups in a glucose ring (26).
The capacities of serotypes T-7, T-8, T-9, T-11, and T-12 to bind to both MBL and L-ficolin indicate that at least two different antigens are exposed on cultured cells. It seems likely that this scenario is due to the presence of bacteria without capsules as well as capsulated cells in the cultures. Bacterial cultures may thus contain a heterogeneous mixture of cell populations with more or less intact capsules even when optimal conditions have been used for culturing (30), and postincubation processing of bacterial cells may further damage capsulation (2). Heterogeneity among cells in the same culture was demonstrated in the present study by flow cytometric analysis of bacterial cells (Fig. 5).
MBL binding to different kinds of microorganisms has been examined by others (14, 25, 41), but the exact structures were not identified. Our results show the influence of capsulation on binding to bacteria and indicate that some of the binding observed in previous studies may have been due to antigens which may be exposed only when capsulation is incomplete. Variations due to antigens other than capsular polysaccharides are known to occur even among closely related bacteria. Thus, in future studies, homologous noncapsulated mutants may be included in control experiments. The relevance of in vitro binding remains to be evaluated, since common antigens may be masked during an infection. On the other hand, the importance of MBL in antimicrobial defense against S. aureus of Reynolds serotype 5 (equivalent to T-5) was recently illustrated by studies with mice lacking MBL (MBL knockout mice). An increase in the mortality of MBL knockout mice compared to wild-type mice was observed after intravenous administration of S. aureus, and enhanced dissemination of the bacteria was seen after intraperitoneal inoculation (32). In contrast to the growth conditions used in the present study, which induced maximal capsulation, the growth conditions used in the in vivo study may have induced less capsulation.
It has been reported that L-ficolin binds to LTAs purified from gram-positive bacteria (S. aureus, Streptococcus agalactiae, Bifidobacterium animalis, Streptococcus pyogenes, and Bacillus subtilis) (17). The biological relevance of this finding is not clear, since LTAs are located in the plasma membrane of gram-positive bacterial cells and, although LTAs extend into the peptidoglycan layer, it is not known whether they are exposed on intact and fully capsulated bacteria. We did not observe any binding of L-ficolin to cells of noncapsulated staphylococcal strain Wood. The abilities of MBL and L-ficolin to bind to noncapsulated S. aureus, peptidoglycan, and LTAs indicate that these proteins may be involved in the neutralization and removal of bacterial debris; i.e., these proteins may function as scavengers during an infection.
Very little is known about the binding of H-ficolin to microorganisms. We did not detect the binding of H-ficolin to any of the S. pneumoniae, S. aureus, or E. coli strains examined. On the other hand, our results confirm the previous observations that H-ficolin binds to A. viridans (38). However, only one of the two strains that we tested showed binding to H-ficolin. The function of H-ficolin is still unknown, and future studies concerning H-ficolin should also examine binding to other types of microorganisms, i.e., viruses, fungi, and parasites.
In agreement with previous reports (25), we found that MBL activated complement upon binding to the surface of bacteria. This was also true for H-ficolin. L-ficolin was previously found to activate complement when immobilized on anti-L-ficolin antibodies as well as when bound to Salmonella serovar Typhimurium (19) or LTAs (17), in agreement with the finding of MASP-2 in association with L-ficolin (20). We did not observe L-ficolin activating complement when bound to an S. pneumoniae 11F strain. Our experimental conditions included 0.5 M NaCl in the binding step to avoid the classical and alternative pathways of complement activation, and this condition may influence the ability of L-ficolin to activate complement. On the other hand, complexes between MASP-2 and MBL are not disrupted at this ionic strength (28). This is also true for L-ficolin and H-ficolin (unpublished observations). Another possibility is that the complement-activiting potential of L-ficolin-MASP complexes is significantly lower than that of the corresponding MBL-H-ficolin complexes or that the observed C4 activation is below the detection limit.
Our results demonstrate the importance of identifying the structures involved in the binding of MBL, L-ficolin, and H-ficolin to bacterial cells and suggest that caution is warranted during extrapolation from in vitro observations to the in vivo situation. It may be speculated that the role of these three proteins is to prevent infection by commensal bacteria, since many pathogenic bacteria seem to circumvent the action of the proteins through capsulation. Another important function of these proteins may be their action as scavengers.
ADDENDUM IN PROOF
Since the acceptance of our manuscript, a paper describing the specificity of L-ficolin for the acetyl groups has been published (A. Krarup, S. Thiel, A. Hansen, T. Fujita, and J. C. Jensenius, J. Biol. Chem. 279:47513-47519, 2004). The paper describes specificities of L-ficolin on a molecular level as opposed to the specificities toward bacteria described in the present paper.
Acknowledgments
This work was supported by The Danish Medical Research Council.
We thank Mihaela Gadjeva and Hanne Jacobsen for assistance with the flow cytometry experiments, Teizo Fujita for supplying the antificolin antibodies, and Mogens Kilian for commenting on the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Akaiwa, M., Y. Yae, R. Sugimoto, S. O. Suzuki, T. Iwaki, K. Izuhara, and N. Hamasaki. 1999. Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J. Histochem. Cytochem. 47:777-786. [DOI] [PubMed] [Google Scholar]
- 2.Arizono, T., A. Umeda, and K. Amako. 1991. Distribution of capsular materials on the cell wall surface of strain Smith diffuse of Staphylococcus aureus. J. Bacteriol. 173:4333-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colley, K. J., M. C. Beranek, and J. U. Baenziger. 1988. Purification and characterization of the core-specific lectin from human serum and liver. Biochem. J. 256:61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunnion, K. M., J. C. Lee, and M. M. Frank. 2001. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect. Immun. 69:6796-6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagnaes-Hansen, F., M. Kilian, and K. Fuursted. 2004. Septicaemia associated with an Aerococcus viridans infection in immunodeficient mice. Lab. Anim. 38:321-325. [DOI] [PubMed] [Google Scholar]
- 6.Dahl, M. R., S. Thiel, M. Matsushita, T. Fujita, A. C. Willis, T. Christensen, T. Vorup-Jensen, and J. C. Jensenius. 2001. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity 15:127-135. [DOI] [PubMed] [Google Scholar]
- 7.Dodds, A. W. 1993. Small-scale preparation of complement components C3 and C4. Methods Enzymol. 223:46-61. [DOI] [PubMed] [Google Scholar]
- 8.Fournier, J. M., W. F. Vann, and W. W. Karakawa. 1984. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect. Immun. 45:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbert, S., S. W. Newell, C. Lee, K. P. Wieland, B. Dassy, J. M. Fournier, C. Wolz, and G. Doring. 2001. Regulation of Staphylococcus aureus type 5 and type 8 capsular polysaccharides by CO2. J. Bacteriol. 183:4609-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack, D. L., N. J. Klein, and M. W. Turner. 2001. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol. Rev. 180:86-99. [DOI] [PubMed] [Google Scholar]
- 11.Jack, D. L., and M. W. Turner. 2003. Anti-microbial activities of mannose-binding lectin. Biochem. Soc. Trans. 31:753-757. [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick, D. C. 2002. Mannan-binding lectin: clinical significance and applications. Biochim. Biophys. Acta 1572:401-413. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick, D. C., T. Fujita, and M. Matsushita. 1999. P35, an opsonic lectin of the ficolin family, in human blood from neonates, normal adults, and recurrent miscarriage patients. Immunol. Lett. 67:109-112. [DOI] [PubMed] [Google Scholar]
- 14.Kuipers, S., P. C. Aerts, and H. van Dijk. 2003. Differential microorganism-induced mannose-binding lectin activation. FEMS Immunol. Med. Microbiol. 36:33-39. [DOI] [PubMed] [Google Scholar]
- 15.Le, Y., S. H. Lee, O. L. Kon, and J. Lu. 1998. Human L-ficolin: plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 425:367-370. [DOI] [PubMed] [Google Scholar]
- 16.Lee, J. C., S. Takeda, P. J. Livolsi, and L. C. Paoletti. 1993. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect. Immun. 61:1853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch, N. J., S. Roscher, T. Hartung, S. Morath, M. Matsushita, D. N. Maennel, M. Kuraya, T. Fujita, and W. J. Schwaeble. 2004. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of gram-positive bacteria, and activates the lectin pathway of complement. J. Immunol. 172:1198-1202. [DOI] [PubMed] [Google Scholar]
- 18.Ma, Y. G., M. Y. Cho, M. Zhao, J. W. Park, M. Matsushita, T. Fujita, and B. L. Lee. 2004. Human mannose-binding lectin and L-ficolin function as specific pattern recognition proteins in the lectin activation pathway of complement. J. Biol. Chem. 279:25307-25312. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita, M., Y. Endo, and T. Fujita. 2000. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J. Immunol. 164:2281-2284. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita, M., Y. Endo, N. Hamasaki, and T. Fujita. 2001. Activation of the lectin complement pathway by ficolins. Int. Immunopharmacol. 1:359-363. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita, M., Y. Endo, S. Taira, Y. Sato, T. Fujita, N. Ichikawa, M. Nakata, and T. Mizuochi. 1996. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J. Biol. Chem. 271:2448-2454. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita, M., and T. Fujita. 1992. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J. Exp. Med. 176:1497-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushita, M., M. Kuraya, N. Hamasaki, M. Tsujimura, H. Shiraki, and T. Fujita. 2002. Activation of the lectin complement pathway by H-ficolin (Hakata antigen). J. Immunol. 168:3502-3506. [DOI] [PubMed] [Google Scholar]
- 24.Moreau, M., J. C. Richards, J. M. Fournier, R. A. Byrd, W. W. Karakawa, and W. F. Vann. 1990. Structure of the type 5 capsular polysaccharide of Staphylococcus aureus. Carbohydr. Res. 201:285-297. [DOI] [PubMed] [Google Scholar]
- 25.Neth, O., D. L. Jack, A. W. Dodds, H. Holzel, N. J. Klein, and M. W. Turner. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen, S. V., and J. Henrichsen. 1992. Capsular types of Streptococcus pneumoniae isolated from blood and CSF during 1982-1987. Clin. Infect. Dis. 15:794-798. [DOI] [PubMed] [Google Scholar]
- 28.Petersen, S. V., S. Thiel, L. Jensen, T. Vorup-Jensen, C. Koch, and J. C. Jensenius. 2000. Control of the classical and the MBL pathway of complement activation. Mol. Immunol. 37:803-811. [DOI] [PubMed] [Google Scholar]
- 29.Poutrel, B., F. B. Gilbert, and M. Lebrun. 1995. Effects of culture conditions on production of type 5 capsular polysaccharide by human and bovine Staphylococcus aureus strains. Clin. Diagn. Lab. Immunol. 2:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poutrel, B., P. Rainard, and P. Sarradin. 1997. Heterogeneity of cell-associated CP5 expression on Staphylococcus aureus strains demonstrated by flow cytometry. Clin. Diagn. Lab. Immunol. 4:275-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins, J. B., R. Schneerson, S. C. Szu, A. Fattom, Y. Yang, T. Lagergard, C. Chu, and U. S. Sorensen. 1989. Prevention of invasive bacterial diseases by immunization with polysaccharide-protein conjugates. Curr. Top. Microbiol. Immunol. 146:169-180. [DOI] [PubMed] [Google Scholar]
- 32.Shi, L., K. Takahashi, J. Dundee, S. Shahroor-Karni, S. Thiel, J. C. Jensenius, F. Gad, M. R. Hamblin, K. N. Sastry, and R. A. Ezekowitz. 2004. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 199:1379-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skov Sorensen, U. B., J. Blom, A. Birch-Andersen, and J. Henrichsen. 1988. Ultrastructural localization of capsules, cell wall polysaccharide, cell wall proteins, and F antigen in pneumococci. Infect. Immun. 56:1890-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto, R., Y. Yae, M. Akaiwa, S. Kitajima, Y. Shibata, H. Sato, J. Hirata, K. Okochi, K. Izuhara, and N. Hamasaki. 1998. Cloning and characterization of the Hakata antigen, a member of the ficolin/opsonin p35 lectin family. J. Biol. Chem. 273:20721-20727. [DOI] [PubMed] [Google Scholar]
- 35.Taira, S., N. Kodama, M. Matsushita, and T. Fujita. 2000. Opsonic function and concentration of human serum ficolin/P35. Fukushima J. Med. Sci. 46:13-23. [DOI] [PubMed] [Google Scholar]
- 36.Thiel, S., T. Bjerke, D. Hansen, L. K. Poulsen, P. O. Schiotz, and J. C. Jensenius. 1995. Ontogeny of human mannan-binding protein, a lectin of the innate immune system. Pediatr. Allergy Immunol. 6:20-23. [DOI] [PubMed] [Google Scholar]
- 37.Thiel, S., T. Vorup-Jensen, C. M. Stover, W. Schwaeble, S. B. Laursen, K. Poulsen, A. C. Willis, P. Eggleton, S. Hansen, U. Holmskov, K. B. Reid, and J. C. Jensenius. 1997. A second serine protease associated with mannan-binding lectin that activates complement. Nature 386:506-510. [DOI] [PubMed] [Google Scholar]
- 38.Tsujimura, M., T. Miyazaki, E. Kojima, Y. Sagara, H. Shiraki, K. Okochi, and Y. Maeda. 2002. Serum concentration of Hakata antigen, a member of the ficolins, is linked with inhibition of Aerococcus viridans growth. Clin. Chim. Acta 325:139-146. [DOI] [PubMed] [Google Scholar]
- 39.Turner, M. W., and R. M. Hamvas. 2000. Mannose-binding lectin: structure, function, genetics and disease associations. Rev. Immunogenet. 2:305-322. [PubMed] [Google Scholar]
- 40.van Dam, J. E., A. Fleer, and H. Snippe. 1990. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Leeuwenhoek 58:1-47. [DOI] [PubMed] [Google Scholar]
- 41.van Emmerik, L. C., E. J. Kuijper, C. A. Fijen, J. Dankert, and S. Thiel. 1994. Binding of mannan-binding protein to various bacterial pathogens of meningitis. Clin. Exp. Immunol. 97:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vorup-Jensen, T., E. S. Sorensen, U. B. Jensen, W. Schwaeble, T. Kawasaki, Y. Ma, K. Uemura, N. Wakamiya, Y. Suzuki, T. G. Jensen, K. Takahashi, R. A. Ezekowitz, S. Thiel, and J. C. Jensenius. 2001. Recombinant expression of human mannan-binding lectin. Int. Immunopharmacol. 1:677-687. [DOI] [PubMed] [Google Scholar]
- 43.Weis, W. I., K. Drickamer, and W. A. Hendrickson. 1992. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature 360:127-134. [DOI] [PubMed] [Google Scholar]
- 44.Yae, Y., S. Inaba, H. Sato, K. Okochi, F. Tokunaga, and S. Iwanaga. 1991. Isolation and characterization of a thermolabile beta-2 macroglycoprotein (‘thermolabile substance’ or ‘Hakata antigen’) detected by precipitating (auto) antibody in sera of patients with systemic lupus erythematosus. Biochim. Biophys. Acta 1078:369-376. [DOI] [PubMed] [Google Scholar]