Abstract
Influenza virus causes three to five million severe respiratory infections per year in seasonal epidemics, and sporadic pandemics, three of which occurred in the twentieth century and are a continuing global threat. Currently licensed antivirals exclusively target the viral neuraminidase or M2 ion channel, and emerging drug resistance necessitates the development of novel therapeutics. It is believed that a host-targeted strategy may combat the development of antiviral drug resistance. To this end, a class of molecules known as iminosugars, hydroxylated carbohydrate mimics with the endocyclic oxygen atom replaced by a nitrogen atom, are being investigated for their broad-spectrum antiviral potential. The influenza virus glycoproteins, hemagglutinin and neuraminidase, are susceptible to inhibition of endoplasmic reticulum α-glucosidases by certain iminosugars, leading to reduced virion production or infectivity, demonstrated by in vitro and in vivo studies. In some experiments, viral strain-specific effects are observed. Iminosugars may also inhibit other host and virus targets with antiviral consequences. While investigations of anti-influenza iminosugar activities have been conducted since the 1980s, recent successes of nojirimycin derivatives have re-invigorated investigation of the therapeutic potential of iminosugars as orally available, low cytotoxicity, effective anti-influenza drugs.
Keywords: Influenza, iminosugars, N-glycosylation, hemagglutinin, neuraminidase
Introduction
Influenza viruses (INFVs) are negative-sense RNA viruses of the Orthomyxoviridae family that can be classified as INFV A, B, or C on the basis of nucleoprotein (NP) antigenic specificity (WHO, 1980). INFV A is further classified by hemagglutinin (HA) and neuraminidase (NA) subtype. 18 HA and 11 NA antigenic types are currently described, although only H1N1 and H3N2 INFV As are currently in general circulation in the human population (Centers for Disease Control and Prevention, 2013; Zhang et al., 2015). INFV strain nomenclature follows the conventions of the World Health Organization throughout (WHO, 1980). Birds constitute the major animal reservoir for INFV, as all known INFV A subtypes infect birds except for H17N10 and H18N11, which are solely found in bats (Centers for Disease Control and Prevention, 2013). In contrast, INFV B subtypes are classified based on the derivation from Victoria or Yamagata strains (Rota et al., 1990). INFV A and B cause the majority of human infections during annual epidemics, while INFV A can also cause pandemics. Pandemic viruses originate through antigenic shift, which can occur during co-infection of intermediate hosts, such as pigs, with viruses generated in susceptible species, allowing reassortment between the eight single-stranded RNA segments of each virion (discussed in Kawaoka & Neumann, 2012). INFV C infections are typically asymptomatic (Kawaoka & Neumann, 2012) and will not be considered here. INFV infection of respiratory epithelial cells results in transient tracheo-bronchitis, whilst alveolar viral replication may lead to severe pneumonia and respiratory distress syndrome. Occasionally, complications such as myopathy, myocarditis, and encephalopathy may arise (Kuiken & Taubenberger, 2008). The annual global incidence of infection is estimated at 5–10% in adults and 20–30% in children (WHO, 2014). Hospitalization and deaths are most likely in the very young, the elderly, and patients with comorbidities, with the total burden of epidemic disease estimated at three to five million severe cases and 250,000–500,000 deaths per year (WHO 2014). This broad profile of infection, morbidity, and mortality exists despite prophylactic vaccination and current therapeutics.
Influenza A and B viruses share a similar structure, both possessing the major surface glycoproteins hemagglutinin (HA) and neuraminidase (NA) which are instrumental in the viral replication cycle. HA initiates cellular infection through binding sialic acid (N-acetylneuraminic acid) residues on host cell glycoproteins and glycolipids. Following receptor binding, the virion is endocytosed. Endosomal acidification triggers exposure of the HA fusion peptide and membrane fusion, alongside disruption of protein–protein interactions, releasing ribonucleoprotein (RNP) complexes to the cytosol. Nuclear import of RNPs is followed by viral RNA polymerase-mediated production of mature vRNA and mRNA. HA and NA are translated using host machinery in the endoplasmic reticulum (ER) and modified in the Golgi apparatus, before being directed to the cell membrane for virion packaging and budding. HA tethers the virion at the cell surface until NA sialidase activity cleaves terminal sialic acid residues from cell-surface molecules, mediating virion release. NA sialidase activity also opposes virion aggregation and may enhance infectivity by cleaving mucins, improving access to respiratory epithelia (reviewed in Bouvier & Palese, 2008). Thus HA and NA play a central role in INFV infection.
Influenza infections are targeted using vaccination and antiviral drugs. Vaccine formulations must be updated annually before the flu season due to changes in circulating INFVs. HA is often the most immunogenic vaccine component but is also the most antigenically variable due to the diversity present in the animal reservoir (Ellebedy & Webby, 2009). Incorrect predictions can adversely affect vaccine efficacy, as seen in the Northern hemisphere 2014/2015 winter where vaccine efficacy was lower and the burden of disease higher than expected, with mismatched H3N2 viruses predominating (Centers for Disease Control and Prevention, 2015). A universal INFV vaccine remains elusive, although multiple approaches are being taken towards achieving cross-protective immune responses (Zhang et al., 2015). Even with an effective broadly protective INFV vaccine, a complementary repertoire of therapeutics would be required and particularly important for treating individuals with poor responses to vaccination.
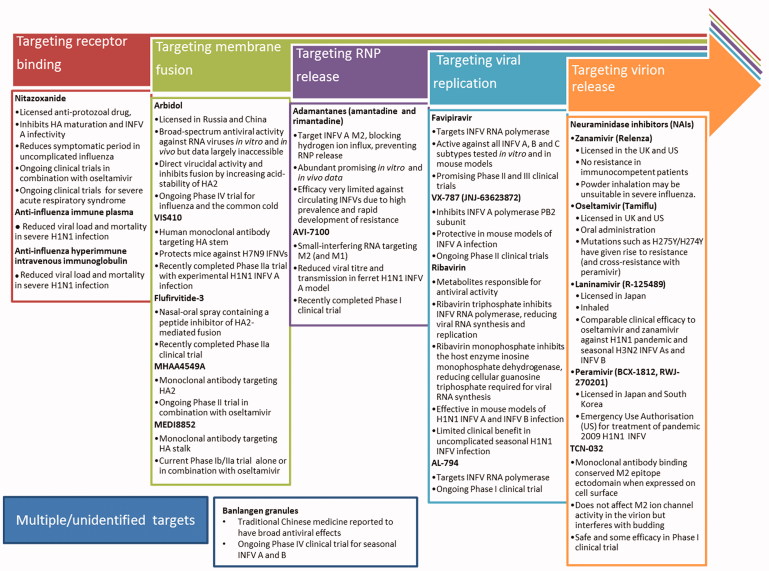
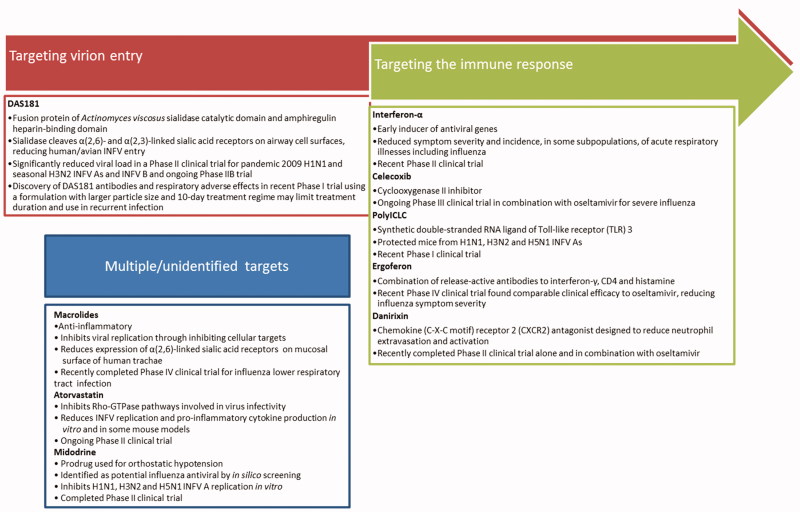
Therapeutics currently in use and under development for influenza infection interfere with multiple stages of the viral life cycle, predominantly directly targeting the virus (Figure 1) and some targeting the host (Figure 2). Given the segmented RNA genome of INFV, and its reliance on the viral RNA-dependent RNA-polymerase which facilitates genomic mutation (Aggarwal et al., 2010), the development of resistance against any direct-acting antiviral will remain a constant challenge. Iminosugars, discussed in this review, target the host glycosylation machinery to give rise to antiviral effects. Their indirect mechanism of action both allows broad-spectrum antiviral activity and provides protection against the development of antiviral drug resistance in circulating viruses. This parallels the aims of vaccine development, inducing broad and robust protection against INFVs. The reliance of INFV on the host glycosylation machinery will be discussed before iminosugars are introduced.
Figure 1.
Licensed and prospective antiviral therapeutics in clinical trials for influenza virus infection. The continual rise of resistance to current therapeutics necessitates the development of further anti-influenza drugs, targeted at multiple stages of the infection and replication cycle. Therapeutics described in Figure 1 are currently or have recently been under investigation in clinical trials registered with Clinicaltrials.gov. Therapeutics targeting receptor binding include nitazoxanide (Rossignol et al., 2009; reviewed in Rossignol, 2014), anti-influenza immune plasma (Luke et al., 2006; NIAID, 2010; Zhou et al., 2007; reviewed in Hui & Lee 2013) and anti-influenza hyperimmune intravenous immunoglobulin (Hung et al., 2013; NIAID, 2014). Therapeutics targeting membrane fusion include arbidol (Gagarinova et al., 1993; reviewed in Blaising et al., 2014), VIS410 (Tharakaraman et al., 2015; Visterra Inc, 2015;), Flufirvitide-3 (Autoimmune Technologies LLC [date unknown]; Autoimmune Technologies LLC, 2015), MHAA4549A (Genentech Inc., 2014; Lim et al., 2016; Nakamura et al., 2013), and MEDI8852 (Kallewaard et al., 2016; MedImmune LLC, 2015). Therapeutics targeting ribonucleoprotein release include the adamantanes, amantadine, and rimantadine (Davies et al., 1964; Wingfield et al., 1969; reviewed in Alves Galvão et al., 2014), and AVI-7100 (NIAID, 2012; reviewed in Dunning et al., 2014). Therapeutics targeting viral replication include favipiravir (Furuta et al., 2002; reviewed in Furuta et al., 2013), VX-787 (Clark et al., 2014; reviewed in Stevaert & Naesens, 2016), ribavirin (Durr et al., 1975; Eriksson et al. 1977; Smith et al., 1980; Smee et al., 2006) and AL-794 (Alios Biopharma Inc., 2015; reviewed in Blair & Cox, 2016). Therapeutics targeting virion release include neuraminidase inhibitors (Babu et al., 2000; Kim et al., 1997; von Itzstein et al., 1993; Yamashita et al., 2009; reviewed in Kamali & Holodniy 2013) and TCN-032 (Ramos et al., 2015). Therapeutics with unidentified mechanisms include banlangen granules (Hutchison Whampoa Guangzhou Baiyunshan Chinese Medicine Company Limited, 2012).
Figure 2.
Host-targeted therapeutics in clinical trials for influenza virus infection. In addition to therapeutics directly targeting the INFV virion, there are many host-targeting drugs in development to combat influenza infection. Therapeutics summarized below are currently or have recently been under investigation in clinical trials registered with Clinicaltrials.gov. DAS181 is a prospective therapeutic targeting virion entry (Ansun Biopharma Inc., 2012; Malakhov et al., 2006; Zenilman et al., 2015; reviewed in Nicholls et al., 2013). Therapeutics targeting the immune response to antiviral effect include interferon-α (Amarillo Biosciences Inc, 2010; Bennett et al., 2013; Solov’ev, 1969), celecoxib (University of Hong Kong, 2014; Zheng et al., 2008; reviewed in Hui & Lee 2013), polyICLC (Wong et al., 1995; reviewed in Wong et al., 2009), ergoferon (Aver'ianov et al., 2012; Verevshchikov et al., 2011), and danirixin (GlaxoSmithKline, 2015). Therapeutics with multiple or unidentified targets include macrolides (Chinese University of Hong Kong, 2013; Cronk & Naumann, 1954; reviewed in Min & Jang, 2012), atorvastatin (Beth Israel Deaconess Medical Center, 2013; Haidari et al., 2007; reviewed in Mehrbod et al., 2014), and midodrine (Hospices Civils de Lyon, 2012; Josset et al., 2010).
Cellular glycosylation pathways: the role of ER α-glucosidases I and II
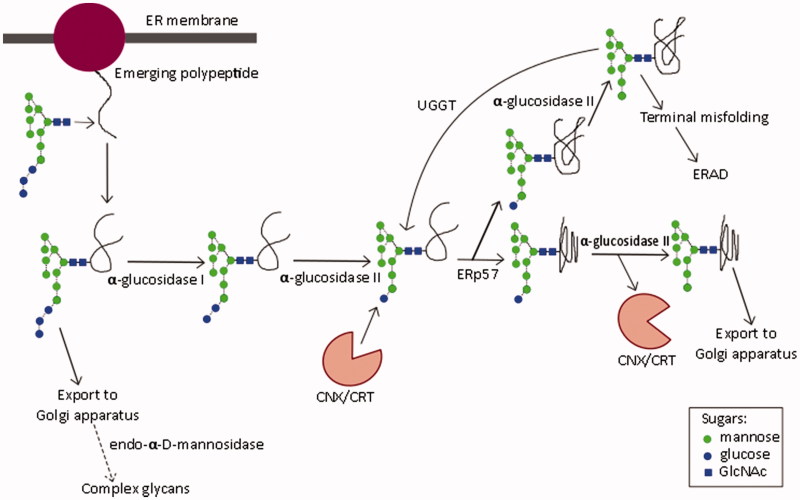
Glycosylation is a fundamental cellular process: most ER-derived proteins are glycosylated (Chang et al., 2013a). The most common form, N-linked glycosylation, is initiated by the addition of the oligosaccharide Glc3Man9GlcNAc2 (Glc, glucose; Man, mannose; GlcNAc, N-acetylglucosamine) to an asparagine residue of the nascent polypeptide in the ER lumen (Kornfeld & Kornfeld, 1985). The asparagine is usually found in the sequon asparagine-X-serine/threonine, where X represents any amino acid except proline (Kasturi et al., 1997). Sequons differ in glycosylation efficiency, with those containing threonine being efficiently glycosylated, and serine, inefficiently so (Kasturi et al., 1997). Sequential processing of the oligosaccharide by α-glucosidases I and II (Figure 3) determines polypeptide interaction with ER chaperones required for correct protein folding. α-Glucosidase I cleaves the α-1,2-glycosidic bond to remove the outermost glucose residue, followed by α-glucosidase II-mediated removal of the two inner α-1,3-linked glucoses (Kornfeld & Kornfeld, 1985). The chaperones calnexin (CNX) (Hammond et al., 1994) and calreticulin (CRT) (Hebert et al., 1995) associate with monoglucosylated glycans, promoting correct disulfide bond formation through interaction with ERp57 (Molinari & Helenius, 1999). Certain iminosugars inhibit ER α-glucosidases, preventing glucose trimming and interaction with CNX/CRT, thus representing a therapeutic target in infections with viruses that require interaction with CNX/CRT for the folding of functional glycoproteins (Norton et al., 2007). If folding is incomplete, UDP-Glc:glycoprotein glucosyltransferase (UGGT) reglucosylates the glycoprotein, enabling cyclical interaction with chaperones until the native conformation is achieved (D’Alessio et al., 2010; Hammond et al., 1994). Alternatively, persistently misfolded proteins enter the ER-associated degradation (ERAD) pathway for proteasomal degradation (reviewed in Benyair et al., 2015). However, the detection of tri-glucosylated viral glycoproteins, produced in vitro and in vivo in the presence of iminosugars, demonstrates a pathway whereby mis- or partially folded glycoproteins can be produced, due to lack of interaction with CNX/CRT (Block et al., 1998; Hussain et al., 2015). In addition, certain iminosugars can enhance secretion of high-mannose glycoproteins (Marcus & Perlmutter, 2000), indicating that ER quality control may be bypassed, such as by Golgi-resident endo-α-d-mannosidase, which cleaves the bond between glucose residues and the polymannose chain of the oligosaccharide (Moore & Spiro, 1990). However, the utilization of this pathway is cell-type specific, and is completely absent in the processing of INFV A/Puerto Rico/8/34 (PR8, H1N1) HA in Chinese hamster ovary (CHO) and Madin–Darby canine kidney (MDCK) cells (Karaivanova et al., 1998). Differential utilization of this pathway complicates the results of iminosugar-mediated ER α-glucosidase inhibition and indicates the importance of using physiologically relevant cell types.
Figure 3.
N-linked oligosaccharide processing in the endoplasmic reticulum. Glycan structure nomenclature follows the recommendations of the Consortium for Functional Genomics (Consortium for Functional Genomics, 2012).
The importance of N-linked glycosylation for HA and NA
HA
HA determines initial receptor binding and endosomal fusion, thus underlies virion infectivity. The HA precursor, HA0, trimerizes in the ER, and is later enzymatically cleaved to form functional HA1 and HA2, exposing the HA2 fusion peptide (reviewed in Skehel & Wiley, 2000). Membrane-distal residues of HA1 (Tyr98, Trp153, His183, and Tyr195) and secondary structural elements (130-loop, 220-loop, and 190-α-helix) contribute to the receptor binding site (Gamblin & Skehel, 2010). Different HA subtypes vary considerably in both the number and location of N-linked glycosylation sites. An amino acid sequence analysis in 1991 found four glycosylation sites in H4, seven in H9, H11 and H13, eight in H6, nine in H12, and 10 in H8 subtype HA (Nobusawa et al., 1991). However, this represents a snapshot view of the glycosylation status of INFV HA molecules, since the number of glycosylation sites varies both within subtypes and over time, as exemplified by H1 (Sun et al., 2011), H3 (Skehel & Wiley, 2000), H5 (Chen et al., 2012), and H7 (Lebarbenchon & Stallknecht, 2011) INFVs. Despite the extensive variation in glycosylation, iminosugars retain their potential as antivirals for INFV as even a single N-linked glycan can be sufficient to render a glycoprotein susceptible to iminosugar activity (Block et al., 1994).
The receptor linkage specificity of HA determines the host range of the virus. α(2,6)-linked sialic acid is abundant in the human respiratory tract and this is reflected in the binding preference of human INFV HA, while α(2,3)-linkages are more common in the avian intestine and are preferentially bound by avian INFV HA (Baum & Paulson, 1990; Rogers & D'Souza, 1989). In contrast, the porcine respiratory tract contains both linkages, reflected in the promiscuous receptor binding of porcine INFVs (Ito et al., 1998). Despite the glycosylation of sialic acid-linked receptors for INFV, iminosugars are not expected or intended to affect their sialic acid linkages, and thus not impact INFV tropism. Glycosylation is implicated in the determination of host range since HA is divergently glycosylated in INFVs from different species. An analysis of H1 INFVs from ducks, swine and humans (Inkster et al., 1993) found that human viruses contained at least four additional glycosylation sites, some located at the HA head (compared with four and five membrane-proximal sites, respectively). Furthermore, mutations affecting N-glycosylation sites influence the receptor binding specificity and affinity of HA. Comparison of the parental INFV isolate A/USSR/90/77 (H1N1), with HA Asn131, to the MDCK-adapted strain, with HA Asp131 (non-glycosylated), showed that glycosylation at residue 131 interfered with binding to soluble α(2,6)-linked sialic acid-containing receptors, but not to those with an α(2,3)-linkage (Gambaryan et al., 1998). Replication of the parent virus in mice led to loss of the HA glycosylation site at Asn131, or in the presence of mouse serum, at Asn158 (Marinina et al., 2003). The loss of either glycosylation site increased HA affinity for α(2,6)-linked sialic acid, and reduced affinity for the α(2,3)-linkage (Marinina et al., 2003). In contrast, enhanced binding to α(2,3)-linked receptors was observed for chicken egg-adapted H1 INFV A and INFV B, with a loss of glycosylation sites at HA Asn163 and Asn187, respectively (Gambaryan et al., 1999). As such, it appears that different N-linked glycans differentially promote binding to the two sialic acid linkages. Consequently, perturbation of HA glycosylation profiles can have significant consequences for receptor binding and tissue tropism. To clarify, while the passage history of an INFV is known to affect glycosylation, there is no precedent for this changing CNX/CRT dependency and, therefore, iminosugar susceptibility.
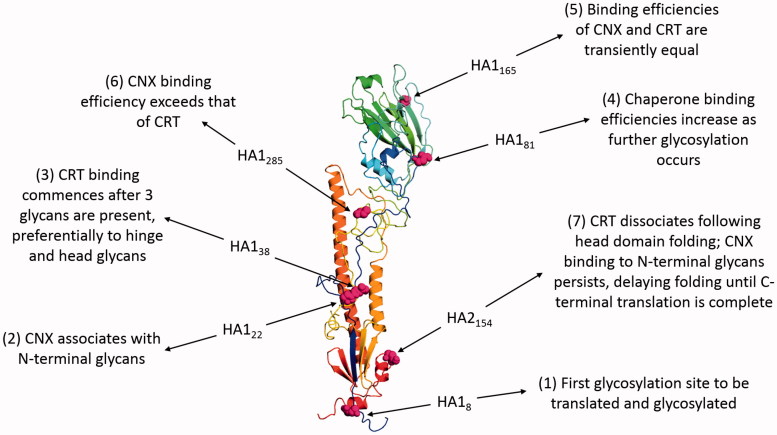
Glycosylation is important for HA folding, providing binding sites for the lectin-like chaperones CNX and CRT in the ER (Daniels et al., 2003). The nascent polypeptide chain is targeted to the ER by the N-terminal signal sequence, which is cleaved as translation commences, allowing cotranslational folding to occur. Chaperone association with HA is dependent on the translation of glycosylation sites and is dynamic (Figure 4).
Figure 4.
The role of N-linked glycosylation in the folding of INFV HA. The figure shows HA of the INFV A/Aichi/68-derived X31 strain (H3N2) with data derived from PDB ID: 1HGF (Sauter et al., 1992b). The polypeptide chain is colored in an N- to C-terminal blue-to-red gradient. The asparagine residues of the seven N-linked glycosylation sites are highlighted in magenta spheres and numbered according to position within mature HA1 or HA2. Labels indicate how the binding of CNX and CRT varies during cotranslational glycosylation.
As translation proceeds, folding commences from the most lumenal region of HA0 and once the top domain is folded, CRT dissociates, potentially due to glycan inaccessibility to UGGT-mediated reglucosylation. CNX binding persists and delays N-terminal processing until the C-terminus is translated, allowing assembly of the HA stem domain. Chaperone binding recruits ERp57 and disulfide bond formation occurs once HA Cys76 enters the ER lumen (Daniels et al., 2003). Thus glycosylation of HA facilitates correct folding, which enables protein progression along the secretory pathway. This is demonstrated by the requirement for at least five of the seven glycosylation sites, in both head and stem regions, of the HA from INFV A/Aichi/68-derived X31 strain (H3N2) for transport of functional HA to the plasma membrane (Gallagher et al., 1992). Binding immunoglobulin protein (BiP), an HSP70-family chaperone, also has a role in HA folding, binding the N-terminal stalk region and helping to prevent the formation of non-native disulfide bonds (Segal et al., 1992). However, the lack of co-immunoprecipitation of BiP with normal X31 HA folding intermediates has led to the suggestion that it may not play a major role in normal HA maturation (Braakman et al., 1992), instead predominantly associating with and retaining misfolded proteins in the ER prior to ERAD (Gallagher et al., 1992; Hurtley et al., 1989).
Folding of HA is clearly glycosylation dependent, but what impact does glycosylation have on INFV virulence? The majority of glycosylation sites are located on the membrane-distal globular head of HA (Vigerust & Shepherd, 2007). Antibodies targeted to this antigenically important site would reduce virulence, but this is prevented by immune evasion mechanisms, such as those contributed by N-glycosylation. Non-immunogenic host-derived oligosaccharides used in glycosylation mask immunogenic viral epitopes, preventing their recognition by the host immune system (Skehel & Wiley, 2000), illustrated by the 1968–1979 invariance of the A/Hong Kong/68 (H3N2) HA in the region masked by the oligosaccharide attached at Asn165 (Wiley & Skehel, 1987). Second, antigenic drift creates new glycosylation sites, generating resistance to antibody binding (Skehel & Wiley, 2000). This is demonstrated by the reversion of 1969 and 1975 INFV A/Hong Kong isolates, which are glycosylated at Asn63, to recognition by an A/Hong Kong/68-targeted antibody when produced in the presence of a glycosylation inhibitor (Skehel et al., 1984). These mechanisms provide a rationale for the increase in HA glycosylation over time, which is seen in the H3N2 Hong Kong INFVs, where the number of glycosylation sites has increased from 6 to 10, with three and seven of these in the antigenically important head region (Skehel & Wiley, 2000; Verhoeyen et al., 1980). It must also be considered that the potential tri-glucosylated HA and NA produced following iminosugar treatment (Block et al., 1998; Hussain et al., 2015) may interact differently with soluble and cell-associated lectins important in INFV interaction with the host (Tate et al., 2014). Such mechanisms indicate that the glycosylation status of HA is an important determinant of INFV virulence. Thus N-linked glycosylation is important for HA production, viral tissue tropism, and virulence.
NA
As described for HA, glycosylation of NA is of multifactorial importance. NA is a tetrameric-stalked molecule with a membrane-distal sialidase active site which binds sialic acid in a different conformation to HA (Gamblin & Skehel, 2010). NA sialidase activity enables virion release from cells and prevents virion aggregation (Seto & Chang, 1969). NA does not affect receptor binding specificity (Rogers & Paulson, 1983), but the cleavage sequence specificity of NA and binding preferences of HA are interlinked (Wagner et al., 2002). Sialidase sequence specificity and activity, at least in N2 subtype NA, have changed over time to reflect adaptation to the human host and the HA binding preference for α(2,6)-linked sialic acid (Baum & Paulson, 1991; Matrosovich et al., 2000). Interestingly, increased HA glycosylation may reduce receptor-binding activity, resulting in inefficient replication if NA receptor-destroying activity remains high (Tsuchiya et al., 2002). Therefore, while NA does not directly affect host and tissue tropism through receptor binding specificity, matching HA and NA sialic acid-linkage specificities are crucial for optimal virulence.
NA subtypes vary in numbers of glycosylation sites, in a manner similar to HA. NA subtypes possess two to four highly conserved glycosylation sites in the stem domain (Asn42, Asn52, Asn63, and Asn66), two conserved and additional middle-low conserved sites in the globular region (Asn87, Asn147, and Asn202, both by N2 numbering), and further sites at the junction of these domains (Chen et al., 2012; Wang et al., 2008). NA glycosylation is a determinant of correct folding. NA derived from the 1918 pandemic INFV is additionally glycosylated on the stalk domain in the tetrameric form, compared with inactive monomeric or dimeric NA, enabling the formation of the higher-order structure. This additional glycosylation also endows resistance to trypsin digestion, which has been hypothesized to increase virulence through contributing to the diverse tissue distribution of this pandemic virus (Wu et al., 2009). Conversely, glycosylation may also reduce virulence. The introduction of Asn130 into the NA of INFV A/WSN/33 (H1N1) reduced NA enzymatic activity 20-fold and attenuated neurovirulence in mice (Li et al., 1993). This glycosylation site (Asn146 by N2 numbering) is conserved across all NA subtypes (Chen et al., 2012), so might represent a virulence determinant of wider significance. Glycosylation of NA is also important for its maturation. Globular head rather than stem domain glycans are essential for maturation of NA from INFV A/tern/Australia/G70C/75 (H1N9) (Wang et al., 2008) and INFV A/duck/Ukraine/1/63 (H3N8) (Saito & Kawano, 1997). Glycosylation can affect NA substrate preference, with mutation of NA Asn130 of INFV A/duck/Ukraine/63 (H3N8) reducing binding to small but not large substrates (Saito & Kawano, 1997). Glycosylation of NA also functions in viral immune evasion as discussed for HA, with multiple glycosylation sites in the N1 and N2 globular domains implicated (Chen et al., 2012). Thus glycosylation impacts NA folding, maturation, and virulence.
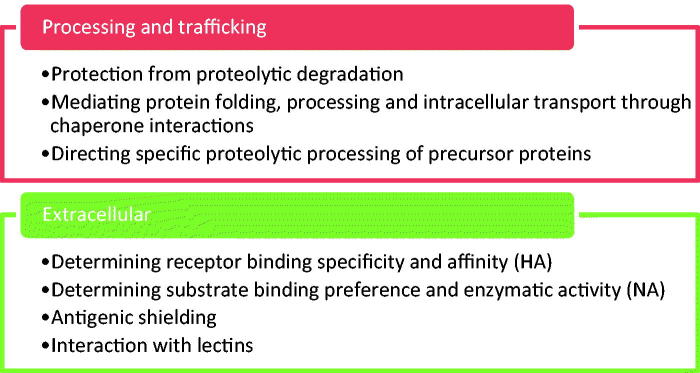
Dependency on N-glycosylation differs between HA and NA: MDCK cells infected with the reassortant virus NWS-duck/Ukraine/1/63 (H1N8) and treated with the glycosylation inhibitor tunicamycin showed an 80% and 97% reduction in HA and NA transport to the cell surface, respectively (Saito & Yamaguchi, 2000). Therefore, N-linked glycosylation is important for both HA and NA function, to different extents (Figure 5).
Figure 5.
Summary of the roles of glycosylation in HA and NA function.
The importance of glycosylation for structure and function of HA and NA indicates that inhibition of ER α-glucosidases might be an effective therapeutic strategy against INFV. Since both endogenous and viral glycoproteins utilize the ER folding apparatus, one might anticipate detrimental effects on host cell protein homeostasis. However, glycoprotein folding is not eliminated by glucosidase inhibitors with antiviral efficacy (Braakman & van Anken, 2000), indicating redundancy in the folding apparatus. Since binding mechanisms to CNX/CRT are the same for host and viral glycoproteins, this suggests that there could be an intrinsic difference in folding requirements. The oligomeric structures of HA and NA necessitate correct folding of multiple interfaces for functional glycoprotein production. Such stringency may confer an amplification effect of susceptibility to iminosugar-mediated glycoprotein misfolding. Furthermore, glycoproteins including INFV HA fold in domains formed from non-continuous regions of the polypeptide chain, requiring a delay in N-terminal folding until the C-terminus has been translated (Braakman & van Anken, 2000), potentially conferring greater reliance on ER chaperones than proteins folding in an N-to-C-terminal fashion. Thus, viral glycoproteins may be more dependent on ER folding machinery than those of the host.
Iminosugars: potential broad-spectrum antivirals
Iminosugars are a structurally diverse class of molecules defined as hydroxylated carbohydrate mimics where the endocyclic oxygen atom is replaced by a nitrogen atom (Nash et al., 2011). Modifications, such as alkyl chain addition, affect biological properties such as uptake by cells and organelles thereby impacting antiviral efficacy and cytotoxicity (Norton et al., 2007; Sayce et al., 2016). Structural mimicry of terminal sugar moieties in natural substrates underlies iminosugar biological activity. Namely, glucose mimics competitively inhibit ER α-glucosidases causing misfolding of viral glycoproteins that may lead to retention or degradation of these products (Asano, 2007). Iminosugars have additional targets: N-alkylated deoxynojirimycin (DNJ) and deoxygalactonojirimycin (DGJ) derivatives inhibit ceramide-specific glucosyltransferase, preventing glycosphingolipid accumulation in lysosomal storage disorders (Dwek et al., 2002). There are other off-target effects of iminosugars, such as inhibition of glucosidases in the gastrointestinal tract, which must be considered for clinical application (Andersson et al., 2000). Despite such activity, iminosugars are already well tolerated in the clinic, with type I Gaucher and Niemann-Pick type C diseases treated with N-butyl-deoxynojirimycin (NB-DNJ, Miglustat), and non-insulin-dependent diabetes with N-hydroxyethyldeoxynojirimycin (Miglitol) (Dwek et al., 2002).
Iminosugar antiviral activity has been demonstrated for a range of viruses. 6-O-Butanoyl castanospermine (BuCAST) reduced murine brain viral load of herpes simplex virus type 1 (HSV-1) following cutaneous infection (Bridges et al., 1995). In studies of bovine viral diarrhea virus (BVDV), DNJ derivatives reduced virion production and infectivity, impairing E1 and E2 glycoprotein folding and heterodimerization (Durantel et al., 2001). DGJ and DNJ derivatives with long alkyl chains additionally increased E2 homodimerization and reduced virion infectivity (Durantel et al., 2001). In HCV-like particles, DNJ derivatives impaired glycoprotein processing and folding (Chapel et al., 2006). DNJ derivatives provided some protection in lethal murine infection models with Ebola and Marburg filoviruses (Chang et al., 2013b). Iminosugar activity against flaviviruses has been demonstrated: N-nonyl-deoxynojirimycin (NN-DNJ) reduced secretion of the glycoproteins E and NS1 and virion production in dengue virus (DENV) and Japanese encephalitis virus (JEV), and reduced mortality rate in a JEV mouse lethal challenge model (Wu et al., 2002). Multiple iminosugars have demonstrated antiviral efficacy against DENV in vitro and in vivo (Chang et al., 2011; Perry et al., 2013; Sayce et al., 2010; Warfield et al., 2015), and celgosivir has recently completed Phase IB clinical trial in DENV infection (Low et al., 2014; Sung et al., 2016). It has been suggested that iminosugars might impact ER-budding viruses, like DENV, and plasma membrane-budding viruses, such as INFV, differently (Norton et al., 2007). However, NB-DNJ reduced glycoprotein maturation, secretion, and function of the human immunodeficiency virus (HIV) gp120 (Fischer et al., 1996), which, like INFV, buds from the plasma membrane. Thus, it is the dependence of viral glycoproteins on CNX/CRT for virion morphogenesis, rather than the cellular structure from which the virus buds, that is hypothesized to underlie antiviral activity mediated by ER α-glucosidase inhibition. Additionally, the host- rather than virus-directed mechanism of action of iminosugars is expected to provide protection against the development of resistance. This has been observed in NB-DNJ treatment of HIV-1 infection in vitro (Pollock et al., 2008), and a high genetic barrier to escape mutants was found in DENV treatment with N-(9-methoxynonyl)-1-deoxynojirimycin (MON-DNJ, UV-4) (Plummer et al., 2015). Therefore, investigation of iminosugar-mediated antiviral activity against INFV is warranted.
Iminosugars: effective therapeutics against INFV?
INFV glycoproteins and iminosugars have been used as tools to analyze cellular glycosylation pathways, with clear evidence of iminosugars affecting INFV glycoproteins in isolation, as summarized in Appendix 1. In some cases, effects on influenza virion production and infectivity were observed, leading to consideration of iminosugar therapeutic application. The following sections illustrate the effects of iminosugars targeting ER α-glucosidases in INFV infection in vitro and in vivo. The impact of iminosugars targeting other host enzymes on INFV infection is detailed in Appendix 2.
Iminosugars have variable efficacy against INFV in vitro
There are variable effects of iminosugars on viral glycoprotein processing and virion release and infectivity (Table 1). In some cases, there are differences in iminosugar antiviral efficacy between INFV strains, such as with BuCAST (Karaivanova et al., 1998; Tyms & Virogen Ltd, 2003), NB-DNJ (Hussain et al., 2015), NN-DNJ (Hussain et al., 2015), and MON-DNJ (Table 2). However, MON-DNJ demonstrated antiviral efficacy independently of strain against H1N1 and H3N2 INFV As and INFV Bs when primary human bronchial epithelial cells were infected rather than MDCK cells (Warfield et al., 2016). To pursue the determinant of strain-specificity, reassortants derived from INFV A/Puerto Rico/8/34, X-181 (with HA and NA sequences 99% identical to A/Lviv/N6/2009 (H1N1)) and X-171b (with HA and NA of A/Brisbane/10/2007 (H3N2)) have been compared, demonstrating that HA was likely to be the determinant of iminosugar susceptibility (Hussain et al., 2015). Furthermore, NA was not deemed responsible due to a lack of strain-specificity in reductions in NA sialidase activity, or plaque size restoration with exogenous NA. In addition, comparison of parental strains and a reassortant encoding NA from INFV A/Lviv/N6/2009 (H1N1) and other RNA segments from INFV A/Brisbane/10/2007 (H3N2) showed that reduced virion infectivity with NN-DNJ treatment was mediated by effects on HA rather than NA (Hussain et al., 2015). Cell type-dependent differences are observed in iminosugar effects, such as with CAST (Ermonval et al., 2000) or MON-DNJ treatment (Warfield et al., 2016 compared with Table 2), despite the same INFV strains being used. Potential explanations for the cell type- and INFV strain-specificity of iminosugar effects are discussed in “Conclusions and perspectives”.
Table 1.
Iminosugars targeting ER α-glucosidases have been tested in vitro against a range of INFV strains, resulting in effects on viral glycoproteins and virion production.
| Iminosugar; concentration | INFV strain | Cell line | Viral glycoprotein processing inhibition | Reference |
|---|---|---|---|---|
| Inhibition of virion production or infectivity | ||||
| Swainsonine | ||||
| 1 μg/ml | A/fowl plague virus/Rostock/34 (H7N1) | Primary calf kidney | Complex glycan formation (10% of control) and high-mannose oligosaccharide processing | Elbein et al. (1982) |
| No effect on infectivity or hemagglutination | ||||
| 0.005–5 μg/ml | A/NWS/33 (H1N1) | MDCK | Glucose trimming | Merkle et al. (1985) |
| No effect on hemagglutination | ||||
| 25, 100 ng/ml | A/NWS/33 (H1N1) | MDCK | Fucosylated hybrid oligosaccharides produced; no effect on sulfation | Schwarz & Elbein (1985) |
| Not tested | ||||
| Castanospermine (CAST) | ||||
| 10 μg/ml | A/NWS/33 (H1N1) | MDCK | Complex glycan formation | Pan et al. (1983) |
| None | ||||
| 10–500 μg/ml | A/NWS/33 (H1N1) | MDCK | Glucose trimming and sulfation | Merkle et al. (1985) |
| No effect on hemagglutination | ||||
| 25, 100 μg/ml | A/NWS/33 (H1N1) | MDCK | Glucose trimming and sulfation | Schwarz & Elbein (1985) |
| Not tested | ||||
| 1 mM | A/HKx31 (H3N2) | CHO 15B | HA glucose trimming and CNX binding | Hammond et al. (1994) |
| Not tested | ||||
| 200 μg/ml | A/NWS/33 (H1N1) | MDCK | HA glucose trimming | Kaushal et al. (1988) |
| Not tested | ||||
| 200 μg/ml | A/HKx31 (H3N2) | CI42 | HA glucose trimming and CNX binding | Ermonval et al. (2000) |
| Not tested | ||||
| 200 μg/ml | A/HKx31 (H3N2) | B3F7 AP2-1 | HA glucose trimming and CNX binding | |
| Not tested | ||||
| 200 μg/ml | A/HKx31 (H3N2) | MadI A214 | No inhibition of HA glucose trimming or CNX binding | |
| Not tested | ||||
| 1 mM | Reassortant virus NWS-duck/Ukraine/1/63 (H1N8) | MDCK | NA secretion 50% of control; HA unaffected. NA activity 50% and HA titer >50% of control | Saito & Yamaguchi (2000) |
| PFU 30% of control | ||||
| 12, 25, 50, 100, 200 μM | A/Hong Kong/11/88 (sic) | MDCK | Not tested | Tyms & Virogen Ltd (2003) |
| IC50 15 μM | ||||
| 6-O-butanoyl-castanospermine (BuCAST) | ||||
| 0.2 mM | A/Puerto Rico/8/34 (H1N1) | MDCK | HA processing by endomannosidase | Karaivanova et al. (1998) |
| No effect on production | ||||
| 6, 12, 25, 50, 100 μM | A/Hong Kong/11/88 | MDCK | Not tested | Tyms and Virogen Ltd (2003) |
| IC50 <6 μM | ||||
| 2,5-dihydroxymethyl-3,4-dihydroxypyrrolidine (DMDP) | ||||
| 5, 50, 250 μg/ml | A/NWS/33 (H1N1) | MDCK | Glucose trimming | Elbein et al. (1984b) |
| Not tested | ||||
| 1-deoxynojirimycin (DNJ) a | ||||
| 2 mM | A/chick/Germany/49 (H10N7) | MDCK | Surface HA expression unaffected | Burke et al. (1984) |
| Infectivity by approximately 30%. No effect on production | ||||
| 10 mM | A/fowl plague virus/Rostock/34 (H7N1) | MDCK | Not tested | Huang et al. (1991) |
| Hemagglutination and CPE (complete) | ||||
| 1 mM | A/HKx31 (H3N2) | CHO 15B | Glucose trimming and CNX binding | Hammond et al. (1994) |
| Not tested | ||||
| 1 mM | Reassortant virus NWS-duck/Ukraine/1/63 (H1N8) | MDCK | NA activity >40% of control and HA titer >40% of control | Saito & Yamaguchi (2000) |
| PFU 30% of control | ||||
| N-methyl-1-deoxynojirimycin (NM-DNJ) | ||||
| 0.5, 1 mM | A/fowl plague virus/Rostock/34 (H7N1) | CEC | HA cleaved | Romero et al. (1983) |
| No effect on virion release | ||||
| 2 mM | H7 strains | CEC | Glucose trimming | Bosch et al. (1984) |
| No effect on infectivity | ||||
| N-butyl-deoxynojirimycin (NB-DNJ) | ||||
| Titration | A/Udorn/307/72 (H3N2) | MDCK | Not tested | Hussain et al. (2015) |
| HA titer: IC50 21.7 ± 15.9 μM, IC90 280.0 ± 23.6 μM. Infectivity: IC50 34.7 ± 11.2 μM, IC90 296.1 ± 16.1 μM. | ||||
| Titration | A/Brisbane/10/2007 (H3N2) | MDCK | Not tested | |
| HA titer: IC50 43.8 ± 6.5 μM, IC90 207.0 ± 95.3 μM. Infectivity: IC50 43.6 ± 11.8 μM, IC90 250.0 ± 10.4 μM. | ||||
| Titration | A/Lviv/N6/2009 (H1N1) | MDCK | Not tested | |
| HA titer: IC50 51.3 ± 11.3 μM, IC90 >312.5 μM. Infectivity: IC50 46.5 ± 12.2 μM, IC90 >312.5 μM. | ||||
| N-nonyl-deoxynojirimycin (NN-DNJ) | ||||
| Titration for IC50 and IC90 determination, otherwise 62.5 μM | A/Udorn/307/72 (H3N2) | MDCK | HA secretion. NA sialidase activity (by 35–45%). 26.4% triglucosylated HA glycans | Hussain et al. (2015) |
| HA titer 6-8% of control. Infectivity: IC50 0.4 ± 0.2 μM, IC90 16.2 ± 4.7 μM. Plaque number: IC50 >62.5 μM. Plaque size: IC50 6.6 ± 5.5 μM, IC90 >62.5 μM, not restored with exogenous NA | ||||
| Titration for IC50 and IC90 determination, otherwise 62.5 μM | A/Brisbane/10/2007 (H3N2) | MDCK | HA secretion. NA sialidase activity (by 30–40%). 21.8% triglucosylated HA glycans | |
| HA titer 0% of control. Infectivity: IC50 1.73 ± 0.3 μM, IC90 10.3 ± 0.3 μM. Plaque number: IC50 8.2 ± 2.4 μM, IC90 22.0 ± 9.5 μM. Plaque size: IC50 4.1 ± 1.2 μM, IC90 10.9 ± 0.3 μM, not restored with exogenous NA | ||||
| Titration for IC50 and IC90 determination, otherwise 62.5 μM | A/Lviv/N6/2009 (H1N1) | MDCK | No effect on surface HA or NA. NA sialidase activity (by 45–60%). 37.3% triglucosylated HA glycans | |
| HA titer 13–25% of control. Infectivity: IC50 1.9 ± 0.8 μM, IC90 >62.5 μM. Plaque number: IC50 >62.5 μM. Plaque size: IC50 1.8 ± 0.3 μM, IC90 >62.5 μM, not restored with exogenous NA | ||||
| Titration | Reassortant X-181 (H1N1) | MDCK | Not tested | |
| IC50 >62.5 μM | ||||
| Titration | Reassortant X-171b (H3N2) | MDCK | Not tested | |
| IC50 0.4 ± 0.1 μM, IC90 2.4 ± 0.5 μM | ||||
| Titration | Reassortant A/Brisbane/10/2007 (H3N1) with A/Lviv/N6/2009 NA | MDCK | Not tested | |
| Plaque number: IC50 9.2 ± 1.7 μM, IC90 55.0 ± 6.8 μM. Plaque size: IC50 5.8 ± 1.2 μM, IC90 14.3 ± 2.7 μM. Effects greater relative to A/Lviv/N6/2009 and comparable to A/Brisbane/10/2007 | ||||
| N-8′-(2′′-tetrahydrofuranyl)-octyl-deoxynojirimycin (2THO-DNJ, UV-12) | ||||
| Titration <250 μM | A/Texas/36/91 (H1N1) | MDCK | Not tested | Warfield et al. (2015) |
| Infectivity: IC50 >250 μM | ||||
| N-(9-methoxynonyl)-1-deoxynojirimycin (MON-DNJ, UV-4) | ||||
| Titration | A/Texas/36/91 (H1N1) | MDCK | Not tested | Warfield et al. (2016) |
| IC50 >125 μM | ||||
| Titration | A/California/07/2009 (H1N1) | MDCK | Not tested | |
| IC50 >125 μM | ||||
| Titration | A/Mississippi/3/2001 (H1N1) | MDCK | Not tested | |
| IC50 >125 μM | ||||
| Titration | A/Mississippi/3/2001 H275Y (H1N1) | MDCK | Not tested | |
| IC50 >125 μM | ||||
| Titration | A/Hong Kong/68 (H3N2) | MDCK | Not tested | |
| IC50 6.01 μM | ||||
| Titration | A/Perth/16/2009 (H3N2) | MDCK | Not tested | |
| IC50 63.9 μM | ||||
| Titration | A/Victoria/361/2011 (H3N2) | MDCK | Not tested | |
| IC50 3.75 μM | ||||
| Titration | A/Victoria/3/75 (H3N2) | MDCK | Not tested | |
| IC50 >84.9 μM | ||||
| Titration | A/Philippines/2/82 (H3N2) | MDCK | Not tested | |
| IC50 >250 μM | ||||
| Titration | B/Lee/40 | MDCK | Not tested | |
| IC50 >125 μM | ||||
| Titration | B/Brisbane/60/2008 | MDCK | Not tested | |
| IC50 >125 μM | ||||
| Titration | B/Wisconsin/01/2010 | MDCK | Not tested | |
| IC50 >125 μM | ||||
| Titration | A/California/07/2009 (H1N1) | dNHBE | Not tested | |
| IC90 >320 μM | ||||
| Titration | A/California/12/2012 (H1N1) | dNHBE | Not tested | |
| IC90 320 μM; 219 μM | ||||
| Titration | A/Victoria/3/75 (H3N2) | dNHBE | Not tested | |
| IC90 440 μM; 483 μM | ||||
| Titration | A/Texas/50/2012 (H3N2) | dNHBE | Not tested | |
| IC90 82 μM | ||||
| Titration | B/Brisbane/60/2008 | dNHBE | Not tested | |
| IC90 200 μM | ||||
| Titration | B/Florida/4/2006 | dNHBE | Not tested | |
| IC90 150 μM | ||||
| Titration | B/Massachusetts/2/2012 | dNHBE | Not tested | |
| IC90 209 μM; 245 μM | ||||
| Titration | B/Malaysia/2506/2004 | dNHBE | Not tested | |
| IC90 >500 μM | ||||
| N-benzyl-1,5-dideoxy-1,5-imino-d-glucitol | ||||
| 10 mM | A/fowl plague virus/Rostock/34 (H7N1) | MDCK | Not tested | Huang et al. (1991) |
| Hemagglutination (partial) | ||||
| N,2-O-dibenzyl-1,5-dideoxy-1,5-imino-d-glucitol | ||||
| 10 mM | A/fowl plague virus/Rostock/34 (H7N1) | MDCK | Not tested | Huang et al. (1991) |
| Hemagglutination and CPE (complete) | ||||
| Homonojirimycin (HNJ) | ||||
| 100, 200 μg/ml | A/NWS/33 (H1N1) | MDCK | HA high-mannose oligosaccharide processing | Zeng et al. (1997) |
| Not tested | ||||
| Titration | A/Puerto Rico/8/34 (H1N1) | MDCK | Not tested | Zhang, et al. (2013) |
| Infectivity: IC50 10.4 μg/ml in CPE reduction assay | ||||
| N-methyl-α-homonojirimycin (NM-HNJ) | ||||
| 25, 100 μg/ml | A/NWS/33 (H1N1) | MDCK | 25 μg/ml: HA high-mannose oligosaccharide processing (5-fold) 100 μg/ml: HA high-mannose oligosaccharide processing (complete) |
Zeng et al. (1997) |
| Not tested | ||||
B3F7AP2–1 and MadIA214: glycosylation-defective CHO cells; CEC: chicken-embryo cell; CI42: parental CHO cell; CPE: cytopathic effect; dNHBE: differentiated normal human bronchial epithelial; HA titer: haemagglutination titer; IC50 or IC90: drug concentration required to inhibit by 50% or 90%, respectively.
DNJ cannot be considered a specific inhibitor of α-glucosidases since it also inhibits the formation of dolichol-linked oligosaccharides required for N-linked glycosylation (Datema et al., 1984).
Table 2.
Antiviral activity of UV-4B in a virus yield reduction assay format varies with INFV strain in MDCK cells.
| IC50 UV-4B (μM)
a
|
IC50 oseltamivir (μM)
a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Influenza strain | A | B | C | Average | A | B | C | Average |
| A/Texas/36/91 (H1N1) | >125 | >125 | – | >125 | 0.63 | 1.32 | – | 0.975 |
| A/California/07/09 (H1N1) | >125 | >125 | – | >125 | 3.99 | 1.91 | – | 2.95 |
| A/Mississippi/3/2001 (H1N1) | >125 | >125 | – | >125 | 0.173 | 0.069 | – | 0.121 |
| A/Mississippi/3/2001 H275Y (H1N1) | >125 | >125 | – | >125 | >500 | >500 | – | >500 |
| A/Hong Kong/68 (H3N2) | 5.98 | 6.04 | – | 6.01 | 0.673 | 0.423 | – | 0.548 |
| A/Perth/16/2009 (H3N2) | 60.5 | 67.2 | – | 63.9 | 1.55 | 3.83 | – | 2.69 |
| A/Victoria/361/2011 (H3N2) | 2.45 | 5.05 | – | 3.75 | 1.93 | 2.06 | – | 1.995 |
| A/Victoria/3/75 (H3N2) | >250 | 90.9 | 79.0 | >84.9 | 0.151 | 0.137 | 0.131 | 0.140 |
| A/Philippines/2/82 (H3N2) | >250 | >250 | >250 | >250 | 7.27 | 31.3 | 6.22 | 14.9 |
| B/Lee/40 | >125 | >125 | – | >125 | 16.4 | 17.2 | – | 16.8 |
| B/Brisbane/60/2008 | >125 | >125 | – | >125 | 0.657 | 0.391 | – | 0.524 |
| B/Wisconsin/01/2010 | >125 | >125 | – | >125 | 3.20 | 0.98 | – | 2.09 |
Cells were seeded in 24-well plates and incubated with two-fold serial dilutions of UV-4B, starting at 125 μM for 1 h. Cells were infected with INFV for 1 h after which medium was added and cells were incubated for 4 d. Harvested supernatants were stored at −80 °C and thawed for titer evaluation in MDCK cells using a TCID50 assay. Titers for each dilution were plotted against the UV-4B concentration and data points were fitted using a 4-PL algorithm.
IC50 data shown are the results for individual experiments (A–C), each as the average of 2–3 replicates. Results shown as greater than (>) are the highest concentration tested. –: not determined.
Iminosugars have antiviral efficacy in INFV mouse models
Following in vitro studies, investigations in animal models have been conducted. Iminosugars have been tested in BALB/c mice against a range of INFV strains (Table 3). MON-DNJ was tested against lethal doses of INFVs A/Texas/36/91 (H1N1) and A/Perth/261/2009 (H1N1; oseltamivir-resistant) (Stavale et al., 2015). The MEDs (minimum effective dose that is 100% protective) were 100 and 80 mg/kg orally thrice daily, indicating efficacy against both oseltamivir-susceptible and -resistant INFVs. Significant protection was provided when MON-DNJ administration began as late as 72–96 h post-infection (Stavale et al., 2015). In contrast, N-(9′-methoxynonyl)-1,6-dideoxygalactonojirimycin (MON-6-deoxy-DGJ) administration did not protect mice from challenge with INFV A/Texas/36/91 (personal communication from KL Warfield to other authors; unreferenced). This supports the hypothesis that antiviral activity of iminosugars depends on inhibition of ER α-glucosidases, exhibited by MON-DNJ but not MON-6-deoxy-DGJ, rather than on their shared inhibition of ceramide-specific glucosyltransferase. A further study demonstrated the protective effect of MON-DNJ against lethal doses of the H1N1 INFVs A/California/04/2009 and A/New Caledonia/99, the H3N2 INFV A/Pennsylvania/10/2010, and INFV B/Sichuan/379/99 (Warfield et al., 2016). MON-DNJ had no effect on serum haemagglutination inhibition titer following infection with INFV A/Texas/36/91 or vaccination with the 2010/2011 Fluvirin® INFV vaccine, indicating that protective vaccine-induced antibody responses were not disrupted by iminosugar treatment (Stavale et al., 2015). N-8′-(2′′-tetrahydrofuranyl)-octyl-deoxynojirimycin (2THO-DNJ, UV-12) was also tested for efficacy against INFV A/Texas/36/91 (H1N1) and found to have a MED of 100 mg/kg orally thrice daily, and was protective against lethal infection when treatment started 24–48 h post-infection (Warfield et al., 2015). Homonojirimycin (HNJ) has been tested in vivo against PR8 (H1N1), with 1 mg/kg twice per day increasing MSD (mean survival days) by 2.6 days relative to a saline control, reducing lung viral titers and modulating cytokine production (Zhang et al., 2013). BuCAST was also tested against the same viral strain and reduced lung viral titers and tissue mass (Tyms & Virogen Ltd, 2003).
Table 3.
Iminosugars have been tested in vivo against a range of INFV strains, with reduced pathology and increased survival in murine lethal infection models achieved.
| Dose | ||||
|---|---|---|---|---|
| Response | ||||
| Iminosugar; INFV | Model | Study size | Control(s) | Reference |
| 6-O-butanoyl-castanospermine (BuCAST) | ||||
| 103–104 PFU A/Puerto Rico/8/34 (H1N1), intranasal | Female BALB/c mice weighing 15–20 g | n = 5/group | 200 or 400 mg/kg/d orally BID from 2 h p.i. for 72 h | Tyms & Virogen Ltd (2003) |
| 200 mg/kg: 20% less increase in lung tissue mass and 2.8-fold reduction in lung PFU 400 mg/kg: 15% less increase in lung tissue mass and 10-fold reduction in lung PFU | ||||
| PBS | ||||
| Homonojirimycin (HNJ) | ||||
| 5 ID50 A/Puerto Rico/8/34 (H1N1), intranasal | Female BALB/c mice weighing 18–22 g | n = 12/group | 0.5, 1, and 2 mg/kg orally BID from 2 d pre-challenge, for 6 d | Zhang et al. (2013) |
| 1 mg/kg: MSD 12.3 ± 1.5 d. 2 mg/kg: MSD 11.7 ± 0.9 d | ||||
| Saline: MSD 9.7 ± 3.2 d. 70 mg/kg ribavirin: 70% survival at day 15 | ||||
| n = 6/group | 1 mg/kg orally BID from 2 d pre-challenge, for 6 d | |||
| Significant reduction in lung viral titer at days 4 and 6 p.i. | ||||
| Saline. 70 mg/kg ribavirin: significantly reduced lung viral titer | ||||
| n = 18/group | 1 mg/kg orally BID beginning 2 d pre-challenge, for 6 d | |||
| Increased serum IFN-γ increased at days 4 and 6 p.i., and IL-10 at days 2, 4 and 6 p.i. Reduced serum IL-6 at days 4 and 6 p.i., and TNF-α at days 2, 4, and 6 p.i. Similar effects in lung tissue | ||||
| Saline | ||||
| N-(9-methoxynonyl)-1-deoxynojirimycin (MON-DNJ, UV-4) | ||||
| 1 LD90 mouse-adapted A/Texas/36/91 (H1N1), intranasal | 6–8 week old female BALB/c mice | n = 10/group | 10 or 100 mg/kg orally from 1 h pre-challenge, and for 7 d p.i. BID or TID | Stavale et al. (2015) |
| 10 mg/kg: no effect. 100 mg/kg: BID 60% survival; TID 100% survival | ||||
| Water: 0% survival | ||||
| n = 10/group | 10, 20, 40, 60, 80, or 100 mg/kg orally TID from 1 h pre-challenge, for 10 d | |||
| 80 mg/kg: 60% survival at day 14. 100 mg/kg (the MED): 100% survival at day 14 | ||||
| Water: 0% survival. 20 mg/kg oseltamivir phosphate orally BID for 5 d: 100% survival at day 14 | ||||
| n = 10/group | 100 mg/kg orally TID starting at −1, 24, 48, 72, 96, or 120 h relative to challenge, for 7 or 10 d | |||
| Significant protection when given 72–96 hp.i. for 7 or 10 d | ||||
| Water: 20% survival. 20 mg/kg oseltamivir phosphate BID for 5 d: protection <120 h p.i. | ||||
| n = 5/group | 100 mg/kg orally TID from 1 h pre-challenge, for 10 d | |||
| No significant increase in lung tissue mass; lower mean lung viral titer per gram by TCID50 assay at days 2, 4, and 7 p.i. | ||||
| Water: increase in lung tissue mass; approximately 1 log higher lung viral titers per gram than treated mice. 20 mg/kg oseltamivir phosphate: similar mass and titers to MON-DNJ-treated mice | ||||
| n = 20/group, treated; n = 10/group, control | 100 mg/kg orally TID from 1 h pre-challenge, for 10 d | |||
| 100% survival; average serum HAI titers were 62, 43, and 174 on days 15, 30, and 120, respectively | ||||
| Water: 10% survival; similar HAI titers | ||||
| 1 LD90 mouse-adapted oseltamivir-resistant A/Perth/261/2009 (H1N1), intranasal | 6–8 week old female BALB/c mice | n = 10/group | 40, 60, 80, 100, 150, or 200 mg/kg orally TID from 1 h pre-challenge, for 10 d | |
| 40 mg/kg: 40% survival. 60 mg/kg: 70% survival. 80, 100, 150, and 200 mg/kg: 100% survival | ||||
| Water: 20% survival. 20 mg/kg oseltamivir phosphate BID for 5 d: 10% survival | ||||
| 50 μl 2010/2011 Fluvirin® INFV vaccine, intramuscular, on days 0, 14, and 28 | 6–8 week old female BALB/c mice | n = 20/group, control; n = 10/group, treated | 100 mg/kg orally TID for 10 d post-vaccination | |
| No effect on serum HAI titer on days 0, 14, 30, and 42 | ||||
| 50 μl PBS intramuscular | ||||
| 1 LD90 mouse-adapted A/California/04/2009 (H1N1), intranasal | 6–8 week old female BALB/c mice | n = 10/group, treated; n = 15/group, control | 50, 75, 100, or 150 mg/kg orally TID from 1 h pre-challenge, for 10 d | Warfield et al. (2016) |
| 50 or 150 mg/kg: 80% survival. 75 mg/kg (the MED): 100% survival. 100 mg/kg: 100% survival | ||||
| Water: 7% survival. 20 mg/kg oseltamivir phosphate BID for 5 d: 100% survival | ||||
| 1 LD90 mouse-adapted A/New Caledonia/99 (H1N1), intranasal | 17 g female BALB/c mice | n = 10/group, treated; n = 14/group, control | 50, 75, 100, or 150 mg/kg orally TID from 1 h pre-challenge, for 7 d | |
| 50 mg/kg: 50% survival. 75 mg/kg: 75% survival. 100 mg/kg: 67% survival. 150 mg/kg: 89% survival | ||||
| Water: 0% survival. 30 mg/kg oseltamivir phosphate: 78% survival | ||||
| 1 LD90 A/Pennsylvania/10/2010 (H3N2) swine variant, intranasal | 17–20 g female BALB/c mice | n = 10/group, treated; n = 14/group, control | 50, 75, 100, or 150 mg/kg orally TID from 1 h pre-challenge, for 7 d | |
| 50 mg/kg: 30% survival. 75 mg/kg: 90% survival. 100 mg/kg: 50% survival. 150 mg/kg: 70% survival | ||||
| Water: 7% survival. 20 mg/kg oseltamivir phosphate BID for 5 d: 50% survival | ||||
| 1 LD90 B/Sichuan/379/99, intranasal | 18 g female BALB/c mice | n = 10/group, treated; n = 15/group, control | 50, 75, 100, or 150 mg/kg orally TID from 1 h pre-challenge, for 10 d | |
| 50 mg/kg: 0% survival. 75 mg/kg: 20% survival. 100 mg/kg: 50% survival. 150 mg/kg (the MED): 100% survival | ||||
| Water TID for 8 d: 0% survival. 10 mg/kg oseltamivir phosphate TID for 8 d: 100% survival | ||||
| N-8′-(2′′-tetrahydrofuranyl)-octyl-deoxynojirimycin (2THO-DNJ, UV-12) | ||||
| 1 LD90 mouse-adapted A/Texas/36/91 (H1N1), intranasal | 6–8 week old female BALB/c mice | n = 10/group | 20, 40, 60, 80, or 100 mg/kg orally TID from 1 h pre-challenge, for 10 d | Warfield et al. (2015) |
| 20 mg/kg: 0% survival. 40 mg/kg: 50% survival at day 14. 60–80 mg/kg: 90% survival at day 14. 100 mg/kg (the MED): 100% survival at day 14 | ||||
| Water: 0% survival | ||||
| n = 10/group | 100 mg/kg orally TID starting at −1, 24, 48, or 72 h relative to challenge, for 10 d | |||
| 1 h relative to challenge: 100% survival. 24 h p.i.: 70% survival. 48–72 h p.i.: 0% survival | ||||
| Water: 0% survival | ||||
| n = 10/group | 60 mg/kg orally TID starting at -1, 24, 48, or 72 h relative to challenge, for 10 d | |||
| −1 h relative to challenge: 80% survival. 24 h p.i: 60% survival. 48 h p.i.: 80% survival. 72 h p.i.: 40% survival | ||||
| Water: 0% survival | ||||
BID: bis in die (twice daily); HAI: hemagglutination inhibition; ID50: dose that is infectious in 50% cases; LD90: lethal dose in 90% cases; MED: minimum effective dose that is 100% protective; MSD: mean survival days; PBS: phosphate buffered saline; PFU: plaque forming units; p.i.: post infection; TCID50: amount of pathogen inducing pathological change in 50% inoculated cell cultures; TID: ter in die (thrice daily).
Conclusions and perspectives
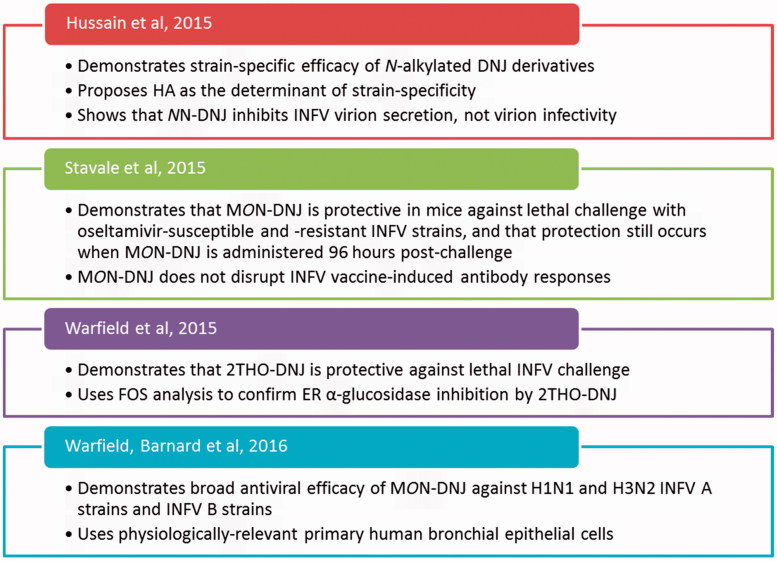
Iminosugars with glucostereochemistry present the opportunity to target a host process: modifying N-linked glycosylation and thus glycoprotein production, through inhibiting ER α-glucosidases. This may be harnessed as a broad-spectrum antiviral strategy, and antiviral efficacy has previously been shown against viruses including BVDV (Durantel et al., 2001), HCV (Chapel et al., 2006), JEV (Wu et al., 2002), DENV (Chang et al., 2011; Perry et al., 2013; Sayce et al., 2010; Warfield et al., 2015; Wu et al., 2002), and HIV (Fischer et al., 1996). Iminosugar antiviral activity is thought to be mediated by ER α-glucosidase inhibition; however, due to the ubiquity of glucose, iminosugars with glucostereochemistry exhibit off-target effects, which may or may not impact antiviral efficacy. Here, evidence for antiviral efficacy against INFVs has been presented, considering effects on INFVs in cell culture (Tables 1 and 2 and Appendix 2) and in mouse models (Table 3). In summary, iminosugar antiviral efficacy is observed in vitro and also in mouse models with MON-DNJ, HNJ and BuCAST, and in mouse models with 2THO-DNJ (Table 3). In particular, MON-DNJ was protective against lethal doses of H1N1 and H3N2 INFV As and INFV B (Stavale et al., 2015; Warfield et al., 2016), and reduced lung viral titers with both oseltamivir-sensitive and -resistant INFV strains (Stavale et al., 2015), suggesting potential efficacy in difficult-to-treat infections. Recent publications have re-invigorated the investigation of iminosugars as anti-influenza therapeutics, and their contributions are highlighted in Figure 6.
Figure 6.
The key findings of recent papers to have advanced the field.
INFV strain-dependent effects
One intriguing observation is that iminosugar efficacy varies between INFV strains. This is repeatedly seen for iminosugars that inhibit ER α-glucosidases, such as DNJ, NB-DNJ, NN-DNJ, and MON-DNJ in INFV-infected MDCK cells (Table 1). The molecular basis for this is poorly understood, but may be composed of cell type-specific and INFV strain-specific effects.
Cell type-dependent iminosugar efficacy may occur for a number of reasons, despite iminosugars having a common target. Iminosugar uptake by different cell types may vary. Iminosugars must cross both the plasma and ER membranes to access their ER α-glucosidase targets, which impose different permeability barriers to iminosugar entry (Tan et al., 1994), and have variable compositions in different cell types. While there is evidence for iminosugars entering cells by non-facilitated diffusion (Bollen & Stalmans, 1989; Neefjes et al., 1989), it is possible that iminosugar uptake may be affected by transporters (Mellor et al., 2004b). Iminosugar design has sought to improve antiviral potency by increasing cellular uptake, through N-alkylation of DNJ and DGJ derivatives (Zitzmann et al., 1999), although very long N-alkyl chains limit iminosugar accessibility to the ER lumen (Mellor et al., 2004a). Iminosugar uptake can be confirmed through free oligosaccharide (FOS) analysis, whereby inhibition of ER α-glucosidases can be quantified through detecting the accumulation of diagnostic FOS on enzyme inhibition – for ER α-glucosidase I, Glc3Man5GlcNAc1, and for ER α-glucosidase II, Glc1Man4GlcNAc1 (Alonzi et al., 2008). This technique has been used previously in studies of iminosugar efficacy in INFV infection (Warfield et al., 2015), but could be more widely adopted to confirm that iminosugars have accessed ER α-glucosidases to aid interpretation of antiviral efficacy data. Second, glycosylation pathways vary in different cell types. Expression levels of glycosylation pathway components may vary between cell types, alongside glycoprotein production levels. This means that iminosugars may be able to compete more or less successfully with N-linked glycoproteins for the ER α-glucosidase active site. Iminosugar inhibition of oligosaccharide processing by ER α-glucosidases can be bypassed by endo-α-d-mannosidase, but this pathway is not active in all cell types, and for example, does not contribute to HA processing in MDCK or CHO cells (Karaivanova et al., 1998). Cell types also show variation in glycoprotein trafficking pathways (Zurzolo et al., 1992). These mechanisms indicate that iminosugar antiviral efficacy with the same INFV strain can be affected by the cell type used in studies. This illustrates the importance of using physiologically relevant cell types when studying glycosylation in viral infection, and of performing experiments in the same cell types. It is encouraging that broad antiviral efficacy was observed with MON-DNJ against H1N1 and H3N2 INFV As and INFV Bs using human bronchial epithelial cells (Warfield et al., 2016), a more physiologically relevant system than the historically favored MDCK cell.
However, cell-type differences alone cannot account for the observed variation in antiviral efficacy, since iminosugars tested in the same in vitro model against different INFV strains display divergent efficacy. This implicates a viral determinant. HA has been proposed to underlie this strain-specificity by Hussain et al. (2015), who used reassortant viruses to analyze the strain-dependent effects of NN-DNJ. Since glycoproteins from different INFV strains have evolved with different glycosylation sites (Chen et al., 2012; Nobusawa et al., 1991), their reliance on glucose trimming and dependence on CNX/CRT for correct folding may vary. While a single N-linked glycan can be sufficient to endow susceptibility to iminosugars (Block et al., 1994), glycoprotein misfolding will only occur with iminosugar-mediated inhibition of ER α-glucosidases if folding is dependent on the CNX/CRT cycle. Different INFV glycoprotein subtypes may vary in dependency on CNX/CRT for folding, perhaps relying to a greater or lesser extent on other ER chaperones such as BiP, or requiring less chaperoning to fold correctly. INFVs may also have strain-specific requirements for the positioning of glycans to determine disulfide bond formation and for the extent of glucose trimming needed for glycoprotein stability and transport, leading to a variable impact of iminosugar treatment on virulence.
But how does the impact of iminosugars on INFV compare with other viruses? The consequences of iminosugar-induced glycoprotein misfolding vary between viruses, possibly because misfolding may be detrimental for a certain life-cycle stage, such as virion formation or receptor binding and membrane fusion. Iminosugar treatment can reduce virion formation and secretion rather than infectivity of individual virions, such as with DENV (Sayce et al., 2016; Warfield et al., 2016) and HBV (Block et al., 1994). Alternatively, iminosugars can predominantly affect the infectivity of virions produced rather than total virion secretion, such as with HIV (Fischer et al., 1995). In INFV infection, NN-DNJ reduces virion secretion rather than specific infectivity, to an extent that is dependent on INFV strain (Hussain et al., 2015). This suggests that the overarching impact of iminosugars on INFV is similar to that seen with DENV. However, the striking strain-specificity seen with INFV is not recapitulated with DENV. DENV has four established serotypes, each expressing antigenically distinct N-linked glycoproteins prM and E on the virion surface, which confer susceptibility to iminosugars (alongside NS1) (reviewed in Sayce et al., 2010). Unlike with INFV, MON-DNJ treatment of Vero cells infected with several isolates of each DENV serotype resulted in a similar antiviral efficacy between serotypes (Warfield et al., 2016). This suggests that the strain-specificity of iminosugar effects observed with INFV is not a generalized feature of viruses possessing subtypes with distinct surface glycoproteins. Further work is certainly required to investigate the strain-dependence of iminosugar anti-influenza effects, enabling progress towards clinical development.
In vitro data may not predict in vivo iminosugar efficacy against INFVs
It is notable that iminosugars targeting ER α-glucosidases have consistently weaker effects on viral infection in vitro (Table 1) when H1 subtype INFVs are considered relative to other subtypes, in experiments mainly utilizing MDCK cells. Although HA trimming and processing is affected in some of these experiments, the evidence points to low efficacy against H1-bearing INFVs. However, in contrast to the MDCK-based in vitro data, mouse models consistently indicate potent antiviral efficacy of iminosugars against H1N1 INFVs, alongside a single report of efficacy against an H3N2 INFV (Table 3). Further in vivo investigation of iminosugar efficacy against H3N2 INFVs is required to better understand iminosugar strain-specificity, as it is possible that antiviral efficacy could be more pronounced than in H1 INFVs given the in vitro results. However, it is also possible that the observed disconnect in antiviral efficacy against H1N1 INFVs may be a function of the difference in the biology of the cells used for in vitro studies and mice, reinforcing the importance of using relevant cell types as a foundation for mouse models.
Iminosugars: promising influenza antivirals
Taken together, the data presented in this review indicate promise for iminosugars as future therapeutics against influenza A and B. An antiviral drug active against the diversity of INFVs would be of significant clinical benefit, particularly enabling the treatment of infections resistant to alternative drugs. Targeting a host process, the approach of iminosugars directed against the glycosylation pathway, endows resilience against the development of resistance and enables broad-spectrum antiviral efficacy. Several other host-acting antivirals are currently in development for INFV (Figure 2), largely targeting the immune response or virus entry, including DAS-181, which has sialidase activity, cleaving α(2,6)- and α(2,3)-linked sialic acid receptors for human and avian INFVs (Malakhov et al., 2006), and macrolides, which reduce α(2,6)-linked sialic acid receptor expression (Min & Jang, 2012). Resistance to DAS-181 should prove difficult to generate, as both classical sialic acid linkage chemistries are targeted. However, there have been reports of some INFV strains, including H3N2 clinical isolates, entering, and undergoing multicycle replication in desialylated cells (Stray et al., 2000), as well as the identification of a second ligand-binding site in X31 HA (H3N2) (Sauter et al., 1992a). One could imagine that escape from DAS-181 efficacy, requiring receptor binding properties that have already been identified in some INFV isolates, might occur more readily than escape from iminosugars, which would require loss of the requirement for glycosylation or evolution of a novel glycosylation mechanism.
Despite targeting the host, iminosugars display remarkable non-toxicity, and are already successfully utilized in the clinic in the treatment of lysosomal storage disorders and non-insulin-dependent diabetes (Dwek et al., 2002). This, in combination with the recent Phase IB clinical trial of celgosivir in DENV infection (Low et al., 2014; Sung et al., 2016), and the recently completed trial of MON-DNJ for safety, tolerability, and pharmacokinetics in healthy individuals (Unither Virology, 2014) indicates the clinical potential of iminosugars for the treatment of viral disease. In addition, the significant protection afforded by MON-DNJ administration up to 96 h post-infection (Stavale et al., 2015) or 2THO-DNJ up to 48 h post-infection (Warfield et al., 2015) with INFV A/Texas/36/91 (H1N1) suggests that as well as being efficacious as prophylaxis, iminosugars have potential as post-infection therapeutics. In summary, the possibilities for iminosugars as antivirals are exciting and timely: in this era of multiple emerging and potentially devastating viruses, broad-spectrum antivirals could prove instrumental in the future arsenal of control strategies for viral disease.
Acknowledgements
We would like to thank John McCauley and Saira Hussain for discussion on influenza and iminosugars, and John Kiappes for discussion on the chemistry of iminosugars. We would also like to thank Raymond Dwek for support throughout. Contributions: BET wrote the first draft and all authors contributed to revisions. BET prepared the Figures and Tables except for Figure 4, prepared by ACS and BET, and Table 2, for which data was generated by Sven Enterlein and Urban Ramstedt.
Appendices
Appendix 1. Iminosugars tested against cell-free INFV glycoproteins can impact HA glycosylation and NA sialidase activity, providing a rationale for investigation of their antiviral efficacy. GPI: glycosylphosphatidylinositol; WT: wild type.
| Iminosugar; concentration | Viral target | Effect on virion component | Reference |
|---|---|---|---|
| 3-episiastatin B | |||
| 100 μM | NA from A/Fort Monmouth/1/47 (H1N1) | 53.1% inhibition of enzymatic activity | Nishimura et al. (1993) |
| 100 μM | NA from A/Kayano/57 (H2N2) | 25.68% inhibition of enzymatic activity | |
| 100 μM | NA from B/Lee/40 | 67.2% inhibition of enzymatic activity | |
| 3,4-diepisiastatin B | |||
| 100 μM | NA from A/Fort Monmouth/1/47 (H1N1) | No inhibition of enzymatic activity | Nishimura et al. (1993) |
| 100 μM | NA from A/Kayano/57 (H2N2) | 2% inhibition of enzymatic activity | |
| 100 μM | NA from B/Lee/40 | 19.8% inhibition of enzymatic activity | |
| 1-deoxymannojirimycin (DMJ) | |||
| 0.25 mM | WT and GPI-linked HA from A/HKx31 (H3N2) | High-mannose HA formed; restored erythrocyte-binding ability of GPI-linked HA | Kemble et al. (1993) |
| Deoxynojirimycin (DNJ) | |||
| 1 mM | HA from A/HKx31 (H3N2) | Prevented binding to CNX and inhibited glucose trimming | Hebert et al. (1995) |
| N-methyl-1-deoxynojirimycin (NM-DNJ) | |||
| 1 mM | HA from A/HKx31 (H3N2) | Prevented binding to CNX and inhibited glucose trimming | Hebert et al. (1995) |
| Castanospermine (CAST) | |||
| 1 mM | HA from A/HKx31 (H3N2) | Prevented binding to CNX and inhibited glucose trimming | Hebert et al. (1995) |
| Reduced folding efficiency and increased degradation | Hebert et al. (1996) | ||
| 1 mM | HA truncations derived from A/Aichi/68 (X31, H3N2) | HA secretion reduced to 2% of control with puromycin, increased to 10% with CAST due to reduced HA association with CNX/CRT | Zhang et al. (1997) |
Appendix 2. Iminosugars with targets other than ER α-glucosidases have variable effects against INFV in vitro. CEC: chicken-embryo cell; LLC-PK1: pig kidney epithelial cell line.
| Viral glycoprotein processing inhibition |
||||
|---|---|---|---|---|
| Iminosugar and target; concentration | INFV strain | Cell line | Virion production or infectivity inhibition | Reference |
| 1-deoxymannojirimycin (DMJ), targeting mannosidase I | ||||
| 1 mM | A/chick/Germany/49 (H10N7) | MDCK | HA expression unaffected | Burke et al. (1984) |
| Infectivity (slight). No effect on production | ||||
| 2, 10, 25 μg/ml | A/NWS/33 (H1N1) | MDCK | High-mannose oligosaccharide processing and complex chain formation | Elbein et al. (1984a) |
| No effect on hemagglutination | ||||
| 25 μg/ml plus 100 μg/ml CAST | A/NWS/33 (H1N1) | MDCK | No inhibition of oligosaccharide processing (CAST concentration too low to inhibit α-glucosidases) | |
| Not tested | ||||
| 0.25, 0.5, 1, 2, 4 mM | A/Puerto Rico/8/34 (H1N1) | MDCK | Mannose incorporation (50% at >2 mM) | |
| Not tested | ||||
| 0.05, 0.1, 0.25, 0.5, 1, 2, 4 mM | A/Puerto Rico/8/34 (H1N1) | CEC | Mannose incorporation | |
| Not tested | ||||
| 1, 10, 50 μg/ml | Unknown | MDCK | Unaffected | Elbein et al. (1990) |
| Not tested | ||||
| 1,4-dideoxy-1,4-imino-d-mannitol (DIM), targeting mannosidase I and II | ||||
| 10, 100, 250 μg/ml | A/NWS/33 (H1N1) | MDCK | High-mannose oligosaccharide processing | Palamarczyk et al. (1985) |
| Not tested | ||||
| Kifunensine, targeting mannosidase I | ||||
| 10, 100 ng/ml 1, 10 μg/ml | Unknown | MDCK | High-mannose oligosaccharide processing and complex chain formation | Elbein et al. (1990) |
| Not tested | ||||
| 0.18 mM | A/Puerto Rico/8/34/MS (H1N1) | MDCK | HA sulfation | Karaivanova & Spiro (1998) |
| Not tested | ||||
| 0.18 mM | A/Puerto Rico/8/34/MS (H1N1) | LLC-PK1 | HA sulfation | |
| Not tested | ||||
| N-benzyl-1,5-dideoxy-1,5-imino-d-mannitol, targeting mannosidases | ||||
| 10 mM | A/fowl plague virus/Rostock/34 (H7N1) | MDCK | Not tested | Huang et al. (1991) |
| Haemagglutination and CPE | ||||
| N-benzyl-1,5-dideoxy-1,5-imino-4,6-O-isopropylidene-d-mannitol, targeting mannosidases | ||||
| 10 mM | A/fowl plague virus/Rostock/34 (H7N1) | MDCK | Not tested | Huang et al. (1991) |
| Haemagglutination and CPE | ||||
| 3-episiastatin B, targeting NA | ||||
| 10, 20, 40, 100 μM | A/Fort Monmouth/1/47 (H1N1) | MDCK | IC50 = 74 μM for NA | Nishimura et al. (1993) |
| PFU 11.1% of control at 40 μM | ||||
| 100 μM | A/Kayano/57 (H2N2) | MDCK | IC50 > 10 μM for NA | |
| Not tested | ||||
| 100 μM | B/Lee/40 | MDCK | IC50 = 42 μM for NA | |
| Not tested | ||||
| N-nonyl-deoxygalactonojirimycin (NN-DGJ), targeting ceramide-specific glucosyltransferase | ||||
| Titration | A/Udorn/307/72 (H3N2) | MDCK | Not tested | Hussain et al. (2015) |
| HA titer 117 ± 19%, viral titer 97 ± 20%, plaque number 74 ± 16% and plaque size 88 ± 20% of control at 62.5 μM | ||||
| Titration | A/Brisbane/10/2007 (H3N2) | MDCK | Not tested | |
| HA titer 75 ± 15%, viral titer 71 ± 21%, plaque number 60 ± 10% and plaque size 76 ± 4% of control at 62.5 μM | ||||
| Titration | A/Lviv/N6/2009 (H1N1) | MDCK | Not tested | |
| HA titer 60 ± 10%, viral titer 85 ± 16% and plaque number 73 ± 8% of control at 62.5 μM. No effect on plaque size | ||||
Disclosure statement
Beatrice Ellen Tyrrell is supported by the Wellcome Trust [105402/Z/14/Z]. Andrew Cameron Sayce and Joanna Louise Miller are supported by the Oxford Glycobiology Institute Endowment. Andrew Cameron Sayce is also supported by the Clarendon Fund. Kelly Lyn Warfield is an employee of Emergent BioSolutions. Nicole Zitzmann is a consultant to Emergent BioSolutions. Nicole Zitzmann and Kelly Lyn Warfield are authors on pending and issued patents related to the use of iminosugars as treatments for viral disease. Nicole Zitzmann is a Fellow of Merton College, Oxford.
References
- Aggarwal S, Bradel-Tretheway B, Takimoto T, et al. (2010). Biochemical characterization of enzyme fidelity of influenza A virus RNA polymerase complex. PLoS One 5:e10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alios Biopharma Inc [Internet] A study of AL-794 to evaluate the safety, tolerability, and pharmacokinetics of single and multiple doses, and the antiviral activity of multiple doses in an influenza challenge study. 2015. [updated 2016 Jan 20; cited 2016 Jul 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT02588521. [Google Scholar]
- Alonzi DS, Neville DCA, Lachmann RH, et al. (2008). Glucosylated free oligosaccharides are biomarkers of endoplasmic-reticulum alpha-glucosidase inhibition. Biochem J 409:571–80. [DOI] [PubMed] [Google Scholar]
- Alves Galvão MG, Santos MARC, Alves da Cunha AJL. (2014). Amantadine and rimantadine for influenza A in children and the elderly. Cochrane Database of Systematic Review. doi: 10.1002/14651858.CD002745.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarillo Biosciences Inc [Internet] Interferon alpha lozenges plus oseltamivir for influenza treatment. 2010. [updated 2013 Aug 19; last accessed 25 Jul 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01146535. [Google Scholar]
- Andersson U, Butters TD, Dwek RA, Platt FM. (2000). N-butyldeoxygalactonojirimycin: a more selective inhibitor of glycosphingolipid biosynthesis than N-butyldeoxynojirimycin, in vitro and in vivo . Biochem Pharmacol (Amsterdam, Neth) 59:821–9. [DOI] [PubMed] [Google Scholar]
- Ansun Biopharma Inc [Internet] Phase 2B study on safety and therapeutic efficacy of DAS181 in adult subjects with naturally acquired influenza. 2012. [updated 2013 Jul 11; last accessed 5 Jun 2015]. Available from: https://clinicaltrials.gov/show/NCT01740063. [Google Scholar]
- Asano N. (2007). Naturally occurring iminosugars and related alkaloids: structure, activity and applications In: Compain P, Martin OR, eds. Iminosugars: from synthesis to therapeutic applications. Chichester: John Wiley & Sons, Ltd, 7–24. [Google Scholar]
- Autoimmune Technologies LLC [Internet] FF-3, the first cell-entry-inhibiting influenza drug. [date unknown; last accessed 29 Apr 2016]. Available from: http://www.autoimmune.com/FF-3.html. [Google Scholar]
- Autoimmune Technologies LLC [Internet] Safety and protective efficacy of FF-3 dry powder in healthy subjects infected with influenza challenge strain. 2015. [updated 2016 Jun 21; last accessed 8 Jul 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02423577. [Google Scholar]
- Aver'ianov A, Babkin A, Bart B, et al. (2012). [Ergoferon and oseltamivir in treatment of influenza: results of multicentre randomized comparative clinical trial]. [abstract] Antibiot Khimioter. 57:23–30. Russian. [PubMed] [Google Scholar]
- Babu YS, Chand P, Bantia S, et al. (2000). BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem 43:3482–6. [DOI] [PubMed] [Google Scholar]
- Baum LG, Paulson JC. (1990). Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem 40:35–8. [PubMed] [Google Scholar]
- Baum LG, Paulson JC. (1991). The N2 neuraminidase of human influenza virus has acquired a substrate specificity complementary to the hemagglutinin receptor specificity. Virology 180:10–15. [DOI] [PubMed] [Google Scholar]
- Bennett AL, Smith DW, Cummins MJ, et al. (2013). Low-dose oral interferon alpha as prophylaxis against viral respiratory illness: a double-blind, parallel controlled trial during an influenza pandemic year. Influenza Other Respir Viruses 7:854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyair R, Ogen-Shtern N, Lederkremer GZ. (2015). Glycan regulation of ER-associated degradation through compartmentalization. Semin Cell Dev Biol 41:99–109. [DOI] [PubMed] [Google Scholar]
- Beth Israel Deaconess Medical Center [Internet] Statin therapy in acute influenza. 2013. [updated 2016 Jan 18; last accessed 29 Apr 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02056340. [Google Scholar]
- Blair W, Cox C. (2016). Current landscape of antiviral drug discovery. F1000Research 5:F1000 Faculty Rev-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaising J, Polyak SJ, Pécheur E-I. (2014). Arbidol as a broad-spectrum antiviral: an update. Antiviral Res 107:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block TM, Lu X, Mehta AS, et al. (1998). Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nat Med 4:610–14. [DOI] [PubMed] [Google Scholar]
- Block TM, Lu X, Platt FM, et al. (1994). Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin . Proc Natl Acad Sci USA 91:2235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M, Stalmans W. (1989). The antiglycogenolytic action of 1-deoxynojirimycin results from a specific inhibition of the alpha-1,6-glucosidase activity of the debranching enzyme. Eur J Biochem 181:775–80. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Orlich M, Legler G, et al. (1984). Effect of inhibitors of glycosylation on proteolytic activation of avian influenza virus hemagglutinins: discrimination between tryptic cleavage and elimination of the connecting peptide. Virology 132:199–204. [DOI] [PubMed] [Google Scholar]
- Bouvier NM, Palese P. (2008). The biology of influenza viruses. Vaccine 26:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I, Helenius J, Helenius A. (1992). Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J 11:1717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I, van Anken E. (2000). Folding of viral envelope glycoproteins in the endoplasmic reticulum. Traffic (Oxford, U K) 1:533–9. Epub 2001 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CG, Ahmed SP, Kang MS, et al. (1995). The effect of oral treatment with 6-O-butanoyl castanospermine (MDL 28,574) in the murine zosteriform model of HSV-1 infection. Glycobiology 5:249–53. [DOI] [PubMed] [Google Scholar]
- Burke B, Matlin K, Bause E, et al. (1984). Inhibition of N-linked oligosaccharide trimming does not interfere with surface expression of certain integral membrane proteins. EMBO J 3:551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [Internet] Influenza type A viruses and subtypes. 2013. [last accessed 5 Jan 2015]. Available from: http://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm. [Google Scholar]
- Centers for Disease Control and Prevention [Internet] What you should know for the 2014-2015 influenza season. 2015. [last accessed 23 Apr 2015]. Available from: http://www.cdc.gov/flu/about/season/flu-season-2014-2015.htm. [Google Scholar]
- Chang J, Block TM, Guo JT. (2013a). Antiviral therapies targeting host ER alpha-glucosidases: current status and future directions. Antiviral Res 99:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Schul W, Yip A, et al. (2011). Competitive inhibitor of cellular α-glucosidases protects mice from lethal dengue virus infection. Antiviral Res 92:369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Warren TK, Zhao X, et al. (2013b). Small molecule inhibitors of ER α-glucosidases are active against multiple hemorrhagic fever viruses. Antiviral Res 98:432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapel C, Garcia C, Roingeard P, et al. (2006). Antiviral effect of alpha-glucosidase inhibitors on viral morphogenesis and binding properties of hepatitis C virus-like particles. J Gen Virol 87:861–71. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhong Y, Qin Y, et al. (2012). The evolutionary pattern of glycosylation sites in influenza virus (H5N1) hemagglutinin and neuraminidase. PLoS One 7:e49224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese University of Hong Kong [Internet] Anti-inflammatory effects of macrolide treatment in influenza lower respiratory tract infections. 2013. [updated 2016 Jul 25; last accessed 1 Sep 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT01779570. [Google Scholar]
- Clark MP, Ledeboer MW, Davies I, et al. (2014). Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem 57:6668–78. [DOI] [PubMed] [Google Scholar]
- Consortium for Functional Genomics [Internet] Symbol and text nomenclature for representation of glycan structure. 2012. [last accessed 26 Apr 2015]. Available from: http://www.functionalglycomics.org/static/consortium/Nomenclature.shtml. [Google Scholar]
- Cronk GA, Naumann DE. (1954). Ilotycin (erythromycin) in the treatment of A prime influenza. N Y State J Med 54:373–5. [PubMed] [Google Scholar]
- D’Alessio C, Caramelo JJ, Parodi AJ. (2010). UDP-Glc: glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Semin Cell Dev Biol 21:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R, Kurowski B, Johnson AE, Hebert DN. (2003). N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol Cell 11:79–90. [DOI] [PubMed] [Google Scholar]
- Datema R, Romero PA, Rott R, Schwarz RT. (1984). On the role of oligosaccharide trimming in the maturation of Sindbis and influenza virus. Arch Virol 81:25–39. [DOI] [PubMed] [Google Scholar]
- Davies WL, Grunert RR, Haff RF, et al. (1964). Antiviral activity of 1-adamantanamine (amantadine). Science 144:862–3. [DOI] [PubMed] [Google Scholar]
- Dunning J, Baillie JK, Cao B, Hayden FG. On behalf of the International Severe Acute R, Emerging Infection C (2014). Antiviral combinations for severe influenza. Lancet Infect Dis 14:1259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durantel D, Branza-Nichita N, Carrouee-Durantel S, et al. (2001). Study of the mechanism of antiviral action of iminosugar derivatives against bovine viral diarrhea virus. J Virol 75:8987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr FE, Lindh HF, Forbes M. (1975). Efficacy of 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide against influenza virus infections in mice. Antimicrob Agents Chemother 7:582–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwek RA, Butters TD, Platt FM, Zitzmann N. (2002). Targeting glycosylation as a therapeutic approach. Nat Rev Drug Discov 1:65–75. [DOI] [PubMed] [Google Scholar]
- Elbein AD, Dorling PR, Vosbeck K, Horisberger M. (1982). Swainsonine prevents the processing of the oligosaccharide chains of influenza virus hemagglutinin. J Biol Chem 257:1573–6. [PubMed] [Google Scholar]
- Elbein AD, Legler G, Tlusty A, et al. (1984a). The effect of deoxymannojirimycin on the processing of the influenza viral glycoproteins. Arch Biochem Biophys 235:579–88. [DOI] [PubMed] [Google Scholar]
- Elbein AD, Mitchell M, Sanford BA, et al. (1984b). The pyrrolidine alkaloid, 2,5-dihydroxymethyl-3,4-dihydroxypyrrolidine, inhibits glycoprotein processing. J Biol Chem 259:12409–13. [PubMed] [Google Scholar]
- Elbein AD, Tropea JE, Mitchell M, Kaushal GP. (1990). Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem 265:15599–605. [PubMed] [Google Scholar]
- Ellebedy AH, Webby RJ. (2009). Influenza vaccines. Vaccine 27:D65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson B, Helgstrand E, Johansson NG, et al. (1977). Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother 11:946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermonval M, Duvet S, Zonneveld D, et al. (2000). Truncated N-glycans affect protein folding in the ER of CHO-derived mutant cell lines without preventing calnexin binding. Glycobiology 10:77–87. [DOI] [PubMed] [Google Scholar]
- Fischer PB, Collin M, Karlsson GB, et al. (1995). The alpha-glucosidase inhibitor N-butyldeoxynojirimycin inhibits human immunodeficiency virus entry at the level of post-CD4 binding. J Virol 69:5791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer PB, Karlsson GB, Dwek RA, Platt FM. (1996). N-Butyldeoxynojirimycin-mediated inhibition of human immunodeficiency virus entry correlates with impaired gp120 shedding and gp41 exposure. J Virol 70:7153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Gowen BB, Takahashi K, et al. (2013). Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Fukuda Y, et al. (2002). In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother 46:977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagarinova VM, Ignatyeva GS, Sinitskaya LV, et al. (1993). New chemopreparation arbidol: its prophylactic effectiveness during influenza epidemics. [abstract] Zhurnal Mikrobiologii Epidemiologii I Immunobiologii 70:40–3 (Russian). [PubMed] [Google Scholar]
- Gallagher PJ, Henneberry JM, Sambrook JF, Gething MJ. (1992). Glycosylation requirements for intracellular transport and function of the hemagglutinin of influenza virus. J Virol 66:7136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambaryan AS, Marinina VP, Tuzikov AB, et al. (1998). Effects of host-dependent glycosylation of hemagglutinin on receptor-binding properties on H1N1 human influenza A virus grown in MDCK cells and in embryonated eggs. Virology 247:170–7. [DOI] [PubMed] [Google Scholar]
- Gambaryan AS, Robertson JS, Matrosovich MN. (1999). Effects of egg-adaptation on the receptor-binding properties of human influenza A and B viruses. Virology 258:232–9. [DOI] [PubMed] [Google Scholar]
- Gamblin SJ, Skehel JJ. (2010). Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem 285:28403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genentech Inc [Internet] A study of MHAA4549A in combination with oseltamivir versus oseltamivir in patients with severe influenza A infection. 2014. [updated 2016 Jul 1; last accessed 8 Jul 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02293863. [Google Scholar]
- GlaxoSmithKline [Internet] Safety, tolerability and clinical effect of danirixin in adults with influenza. 2015. [updated 2016 Jun 27; last accessed 8 Jul 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02469298. [Google Scholar]
- Haidari M, Ali M, Casscells SW, Madjid M. (2007). Statins block influenza infection by down-regulating Rho/rho kinase pathway. Circulation 116:Abstract 147. [Google Scholar]
- Hammond C, Braakman I, Helenius A. (1994). Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control . Proc Natl Acad Sci U S A 91:913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. (1995). Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell 81:425–33. [DOI] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. (1996). Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J 15:2961–8. [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Simons JF, Peterson JR, Helenius A. (1995). Calnexin, calreticulin, and Bip/Kar2p in protein folding. Cold Spring Harb Symp Quant Biol 60:405–15. [DOI] [PubMed] [Google Scholar]
- Hospices Civils de Lyon [Internet] Confirmation of the antiviral effects of midodrine identified with a gene expression signature-based screening of inluenza A virus infected cells (FLUMED). 2012. [updated 2015 Jan 7; last accessed 29 Jul 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01546506. [Google Scholar]
- Huang R, Dietsch E, Lockhoff O, et al. (1991). Antiviral activity of some natural and synthetic sugar analogues. FEBS Lett 291:199–202. [DOI] [PubMed] [Google Scholar]
- Hui DSC, Lee N. (2013). Adjunctive therapies and immunomodulating agents for severe influenza. Influenza Other Respir Viruses 7:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IFN, To KKW, Lee CK, et al. (2013). Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 144:464–73. [DOI] [PubMed] [Google Scholar]
- Hurtley SM, Bole DG, Hoover-Litty H, et al. (1989). Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol 108:2117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Miller JL, Harvey DJ, et al. (2015). Strain-specific antiviral activity of iminosugars against human influenza A viruses. J Antimicrob Chemother 70:136–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison Whampoa Guangzhou Baiyunshan Chinese Medicine Company Limited [Internet] Banlangen granules anti-seasonal influenza study (BLG). 2012. [updated 2016 Mar 2; last accessed 29 Jul 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT02232945. [Google Scholar]
- Inkster MD, Hinshaw VS, Schulze IT. (1993). The hemagglutinins of duck and human H1 influenza viruses differ in sequence conservation and in glycosylation. J Virol 67:7436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Couceiro JN, Kelm S, et al. (1998). Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol 72:7367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josset L, Textoris J, Loriod B, et al. (2010). Gene expression signature-based screening identifies new broadly effective influenza A antivirals. PLoS One 5:e13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallewaard N, Corti D, Collins PJ, et al. (2016). Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166:596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali A, Holodniy M. (2013). Influenza treatment and prophylaxis with neuraminidase inhibitors: a review. Infect Drug Resist 6:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaivanova VK, Luan P, Spiro RG. (1998). Processing of viral envelope glycoprotein by the endomannosidase pathway: evaluation of host cell specificity. Glycobiology 8:725–30. [DOI] [PubMed] [Google Scholar]
- Karaivanova VK, Spiro RG. (1998). Sulphation of N-linked oligosaccharides of vesicular stomatitis and influenza virus envelope glycoproteins: host cell specificity, subcellular localization and identification of substituted saccharides. Biochem J 329:511–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi L, Chen H, Shakin-Eshleman SH. (1997). Regulation of N-linked core glycosylation: use of a site-directed mutagenesis approach to identify Asn-Xaa-Ser/Thr sequons that are poor oligosaccharide acceptors. Biochem J 323:415–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal GP, Pan YT, Tropea JE, et al. (1988). Selective inhibition of glycoprotein-processing enzymes. Differential inhibition of glucosidases I and II in cell culture. J Biol Chem 263:17278–83. [PubMed] [Google Scholar]
- Kawaoka Y, Neumann G. (2012). Influenza viruses: an introduction In: Kawaoka Y, Neumann G, eds. Influenza virus: methods and protocols. New York: Humana Press, 1–9. [Google Scholar]
- Kemble GW, Henis YI, White JM. (1993). GPI-and transmembrane-anchored influenza hemagglutinin differ in structure and receptor binding activity. J Cell Biol 122:1253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CU, Lew W, Williams MA, et al. (1997). Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc 119:681–90. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. (1985). Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem 54:631–64. [DOI] [PubMed] [Google Scholar]
- Kuiken T, Taubenberger J. (2008). Pathology of human influenza revisited. Vaccine 26:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebarbenchon C, Stallknecht DE. (2011). Host shifts and molecular evolution of H7 avian influenza virus hemagglutinin. Virol J 8:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Schulman J, Itamura S, Palese P. (1993). Glycosylation of neuraminidase determines the neurovirulence of influenza A/WSN/33 virus. J Virol 67:6667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JJ, Deng R, Derby MA, et al. (2016). Two phase 1, randomized, double-blind, placebo-controlled, single-ascending-dose studies to investigate the safety, tolerability, and pharmacokinetics of an anti-influenza A virus monoclonal antibody, MHAA4549A, in healthy volunteers. Antimicrob Agents Chemother 60:5437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low JG, Sung C, Wijaya L, et al. (2014). Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect Dis 14:706–15. [DOI] [PubMed] [Google Scholar]
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. (2006). Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 145:599–609. [DOI] [PubMed] [Google Scholar]
- Malakhov MP, Aschenbrenner LM, Smee DF, et al. (2006). Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother 50:1470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus NY, Perlmutter DH. (2000). Glucosidase and mannosidase inhibitors mediate increased secretion of mutant alpha1 antitrypsin Z. J Biol Chem 275:1987–92. [DOI] [PubMed] [Google Scholar]
- Marinina VP, Gambaryan AS, Bovin NV, et al. (2003). The effect of losing glycosylation sites near the receptor-binding region on the receptor phenotype of the human influenza virus H1N1. Mol Biol 37:468–72. [PubMed] [Google Scholar]
- Matrosovich M, Tuzikov A, Bovin N, et al. (2000). Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J Virol 74:8502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MedImmune LLC [Internet] A phase 1b/2a to evaluate the safety of MEDI8852 in adults with uncomplicated influenza (MEDI8852). 2015. [updated 2016 Mar 8; last accessed 8 Jul 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02603952. [Google Scholar]
- Mehrbod P, Omar AR, Hair-Bejo M, et al. (2014). Mechanisms of action and efficacy of statins against influenza. BioMed Res Int 2014:872370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor HR, Neville DCA, Harvey DJ, et al. (2004a). Cellular effects of deoxynojirimycin analogues: inhibition of N-linked oligosaccharide processing and generation of free glucosylated oligosaccharides. Biochem J 381:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor HR, Neville DCA, Harvey DJ, et al. (2004b). Cellular effects of deoxynojirimycin analogues: uptake, retention and inhibition of glycosphingolipid biosynthesis. Biochem J 381:861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle RK, Elbein AD, Heifetz A. (1985). The effect of swainsonine and castanospermine on the sulfation of the oligosaccharide chains of N-linked glycoproteins. J Biol Chem 260:1083–9. [PubMed] [Google Scholar]
- Min JY, Jang YJ. (2012). Macrolide therapy in respiratory viral infections . Mediators Inflamm 2012:649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Helenius A. (1999). Glycoproteins form mixed disulphides with oxidoreductases during folding in living cells. Nature 402:90–3. [DOI] [PubMed] [Google Scholar]
- Moore SE, Spiro RG. (1990). Demonstration that Golgi endo-alpha-d-mannosidase provides a glucosidase-independent pathway for the formation of complex N-linked oligosaccharides of glycoproteins. J Biol Chem 265:13104–12. [PubMed] [Google Scholar]
- Nakamura G, Chai N, Park S, et al. (2013). An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe 14:93–103. [DOI] [PubMed] [Google Scholar]
- Nash RJ, Kato A, Yu CY, Fleet GW. (2011). Iminosugars as therapeutic agents: recent advances and promising trends. Future Med Chem 3:1513–21. [DOI] [PubMed] [Google Scholar]
- [NIAID] National Institute of Allergy and Infectious Diseases [Internet] Safety and efficacy of investigational anti-influenza immune plasma in treating influenza (IRC002). 2010. [updated 2016 Aug 12; last accessed 15 Aug 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT01052480. [Google Scholar]
- [NIAID] National Institute of Allergy and Infectious Diseases [Internet] Testing the AVI-7100 flu drug in healthy volunteers. 2012. [updated 2016 July 6; last accessed 8 Jul 2016]. Available from: https://clinicaltrials.gov/show/NCT01747148. [Google Scholar]
- [NIAID] National Institute of Allergy and Infectious Diseases [Internet] Evaluating the safety and efficacy of anti-influenza intravenous hyperimmune immunoglobulin (IVIG) in adults hospitalized with influenza. 2014. [updated 2016 Jan 13; last accessed 7 Jul 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02287467. [Google Scholar]
- Nicholls JM, Moss RB, Haslam SM. (2013). The use of sialidase therapy for respiratory viral infections. Antiviral Res 98:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes JJ, Lindhout J, Broxterman HJ, et al. (1989). Non-carrier-mediated uptake of the mannosidase I inhibitor 1-deoxymannojirimycin by K562 erythroleukemic cells. J Biol Chem 264:10271–5. [PubMed] [Google Scholar]
- Nishimura Y, Umezawa Y, Kondo S, et al. (1993). Synthesis of 3-episiastatin B analogues having anti-influenza virus activity. J Antibiot 46:1883–9. [DOI] [PubMed] [Google Scholar]
- Nobusawa E, Aoyama T, Kato H, et al. (1991). Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology 182:475–85. [DOI] [PubMed] [Google Scholar]
- Norton PA, Gu B, Block TM. (2007). Iminosugars as antiviral agents In: Compain P, Martin OR, eds. Iminosugars: from synthesis to therapeutic applications. Chichester: John Wiley & Sons, Ltd, 209–24. [Google Scholar]
- Palamarczyk G, Mitchell M, Smith PW, et al. (1985). 1,4-Dideoxy-1,4-imino-d-mannitol inhibits glycoprotein processing and mannosidase. Arch Biochem Biophys 243:35–45. [DOI] [PubMed] [Google Scholar]
- Pan YT, Hori H, Saul R, et al. (1983). Castanospermine inhibits the processing of the oligosaccharide portion of the influenza viral hemagglutinin. Biochemistry 22:3975–84. [DOI] [PubMed] [Google Scholar]
- Perry ST, Buck MD, Plummer EM, et al. (2013). An iminosugar with potent inhibition of dengue virus infection in vivo . Antiviral Res 98:35–43. [DOI] [PubMed] [Google Scholar]
- Plummer E, Buck MD, Sanchez M, et al. (2015). Dengue virus evolution under a host-targeted antiviral. J Virol 89:5592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock S, Dwek RA, Burton DR, Zitzmann N. (2008). N-Butyldeoxynojirimycin is a broadly effective anti-HIV therapy significantly enhanced by targeted liposome delivery. AIDS 22:1961–9. [DOI] [PubMed] [Google Scholar]
- Ramos EL, Mitcham JL, Koller TD, et al. (2015). Efficacy and safety of treatment with an anti-m2e monoclonal antibody in experimental human influenza. J Infect Dis 211:1038–44. [DOI] [PubMed] [Google Scholar]
- Rogers GN, D'Souza BL. (1989). Receptor binding properties of human and animal H1 influenza virus isolates. Virology 173:317–22. [DOI] [PubMed] [Google Scholar]
- Rogers GN, Paulson JC. (1983). Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127:361–73. [DOI] [PubMed] [Google Scholar]
- Romero PA, Datema R, Schwarz RT. (1983). N-Methyl-1-deoxynojirimycin, a novel inhibitor of glycoprotein processing, and its effect on fowl plague virus maturation. Virology 130:238–42. [DOI] [PubMed] [Google Scholar]
- Rossignol JF. (2014). Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res 110:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol JF, La Frazia S, Chiappa L, et al. (2009). Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem 284:29798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota PA, Wallis TR, Harmon MW, et al. (1990). Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 175:59–68. [DOI] [PubMed] [Google Scholar]
- Saito T, Kawano K. (1997). Loss of glycosylation at Asn144 alters the substrate preference of the N8 influenza A virus neuraminidase. J Vet Med Sci 59:923–6. [DOI] [PubMed] [Google Scholar]
- Saito T, Yamaguchi I. (2000). Effect of glycosylation and glucose trimming inhibitors on the influenza A virus glycoproteins. J Vet Med Sci 62:575–81. [DOI] [PubMed] [Google Scholar]
- Sauter NK, Glick GD, Crowther RL, et al. (1992a). Crystallographic detection of a second ligand binding site in influenza virus hemagglutinin . Proc Natl Acad Sci U S A 89:324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter NK, Hanson JE, Glick GD, et al. (1992b). Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: analysis by proton nuclear magnetic resonance spectroscopy and X-ray crystallography. Biochemistry 31:9609–21. [DOI] [PubMed] [Google Scholar]
- Sayce AC, Alonzi DS, Killingbeck SS, et al. (2016). Iminosugars inhibit dengue virus production via inhibition of ER alpha-glucosidases – not glycolipid processing enzymes. PLoS Negl Trop Dis 10:e0004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayce AC, Miller JL, Zitzmann N. (2010). Targeting a host process as an antiviral approach against dengue virus. Trends Microbiol 18:323–30. [DOI] [PubMed] [Google Scholar]
- Schwarz PM, Elbein AD. (1985). The effect of glycoprotein-processing inhibitors on fucosylation of glycoproteins. J Biol Chem 260:14452–8. [PubMed] [Google Scholar]
- Segal MS, Bye JM, Sambrook JF, Gething MJH. (1992). Disulfide bond formation during the folding of influenza virus hemagglutinin. J Cell Biol 118:227–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto JT, Chang FS. (1969). Functional significance of sialidase during influenza virus multiplication: an electron microscope study. J Virol 4:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Stevens DJ, Daniels RS, et al. (1984). A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody . Proc Natl Acad Sci U S A 81:1779–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Wiley DC. (2000). Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 69:531–69. [DOI] [PubMed] [Google Scholar]
- Smee DF, Wong MH, Bailey KW, Sidwell RW. (2006). Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza A (H1N1) and B viruses. Antiviral Chem Chemother 17:185–92. [DOI] [PubMed] [Google Scholar]
- Smith CB, Charette RP, Fox JP, et al. (1980). Lack of effect of oral ribavirin in naturally occurring influenza A virus (H1N1) infection. J Infect Dis 141:548–54. [DOI] [PubMed] [Google Scholar]
- Solov’ev VD. (1969). The results of controlled observations on the prophylaxis of influenza with interferon. Bull World Health Organ 41:683–8. [PMC free article] [PubMed] [Google Scholar]
- Stavale EJ, Vu H, Sampath A, et al. (2015). In vivo therapeutic protection against influenza A (H1N1) oseltamivir-sensitive and resistant viruses by the iminosugar UV-4. PLoS One 10:e0121662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevaert A, Naesens L. (2016). The influenza virus polymerase complex: an update on its structure, functions, and significance for antiviral drug design. Med Res Rev. doi: 10.1002/med.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stray SJ, Cummings RD, Air GM. (2000). Influenza virus infection of desialylated cells. Glycobiology 10:649–58. [DOI] [PubMed] [Google Scholar]
- Sun S, Wang Q, Zhao F, et al. (2011). Glycosylation site alteration in the evolution of influenza A (H1N1) viruses. PLoS One 6:e22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C, Wei Y, Watanabe S, et al. (2016). Extended evaluation of virological, immunological and pharmacokinetic endpoints of CELADEN: a randomized, placebo-controlled trial of celgosivir in dengue fever patients. PLoS Negl Trop Dis 10:e0004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, van den Broek L, Bolscher J, et al. (1994). Introduction of oxygen into the alkyl chain of N-decyl-dNM decreases lipophilicity and results in increased retention of glucose residues on N-linked oligosaccharides. Glycobiology 4:141–9. [DOI] [PubMed] [Google Scholar]
- Tate MD, Job ER, Deng YM, et al. (2014). Playing hide and seek: how glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses 6:1294–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharakaraman K, Subramanian V, Viswanathan K, et al. (2015). A broadly neutralizing human monoclonal antibody is effective against H7N9 . Proc Natl Acad Sci USA 112:10890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya E, Sugawara K, Hongo S, et al. (2002). Effect of addition of new oligosaccharide chains to the globular head of influenza A/H2N2 virus haemagglutinin on the intracellular transport and biological activities of the molecule. J Gen Virol 83:1137–46. [DOI] [PubMed] [Google Scholar]
- Tyms AS., Virogen Ltd (2003). Use of certain castanospermine esters in the treatment of influenza virus infections. African Intellectual Property Organization, African Regional Intellectual Property Organization, Eurasian Patent Organization, European Patent Office, United States Patent Office. Patent WO2003006017. [Google Scholar]
- Unither Virology [Internet] Study to determine the safety, tolerability and pharmacokinetics of UV-4B solution administered orally in healthy subjects. 2014. [updated 2016 Jan 28; last accessed 6 Jul 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02061358. [Google Scholar]
- University of Hong Kong [Internet] Treatment of severe influenza A infection. 2014. [updated 2015 Nov 30; last accessed 8 Jul 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02108366. [Google Scholar]
- Verevshchikov VK, Borzunov VM, Shemiakina EK. (2011). Ergoferon and improvement of etiopathogenetic therapy of influenza and acute respiratore viral infection in adults. [abstract] Antibiot Khimioter 56:23–6 (Russian). [PubMed] [Google Scholar]
- Verhoeyen M, Fang R, Jou WM, et al. (1980). Antigenic drift between the haemagglutinin of the Hong Kong influenza strains A/Aichi/2/68 and A/Victoria/3/75. Nature 286:771–6. [DOI] [PubMed] [Google Scholar]
- Vigerust DJ, Shepherd VL. (2007). Virus glycosylation: role in virulence and immune interactions. Trends Microbiol 15:211–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visterra Inc [Internet] Influenza challenge study of VIS410 in healthy volunteers. 2015. [updated 2016 Apr 4; last accessed 8 Jul 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02468115. [Google Scholar]
- von Itzstein M, Wu WY, Kok GB, et al. (1993). Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–23. [DOI] [PubMed] [Google Scholar]
- Wagner R, Matrosovich M, Klenk H-D. (2002). Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol 12:159–66. [DOI] [PubMed] [Google Scholar]
- Wang N, Glidden EJ, Murphy SR, et al. (2008). The cotranslational maturation program for the type II membrane glycoprotein influenza neuraminidase. J Biol Chem 283:33826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Barnard DL, Enterlein SG, et al. (2016). The iminosugar UV-4 is a broad inhibitor of influenza A and B viruses ex vivo and in mice. Viruses 8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Plummer E, Alonzi DS, et al. (2015). A novel iminosugar UV-12 with activity against the diverse viruses influenza and dengue (novel iminosugar antiviral for influenza and dengue). Viruses 7:2404–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Plummer EM, Sayce AC, et al. (2016). Inhibition of endoplasmic reticulum glucosidases is required for in vitro and in vivo dengue antiviral activity by the iminosugar UV-4. Antiviral Res 129:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley DC, Skehel JJ. (1987). The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem 56:365–94. [DOI] [PubMed] [Google Scholar]
- Wingfield WL, Pollack D, Grunert RR. (1969). Therapeutic efficacy of amantadine HCl and rimantadine HCl in naturally occurring influenza A2 respiratory illness in man. N Engl J Med 281:579–84. [DOI] [PubMed] [Google Scholar]
- Wong JP, Christopher ME, Viswanathan S, et al. (2009). Antiviral role of Toll-like receptor-3 agonists against seasonal and avian influenza viruses. Curr Pharm Des 15:1269–74. [DOI] [PubMed] [Google Scholar]
- Wong JP, Saravolac EG, Sabuda D, et al. (1995). Prophylactic and therapeutic efficacies of poly(IC.LC) against respiratory influenza A virus infection in mice. Antimicrob Agents Chemother 39:2574–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [WHO] World Health Organisation (1980). A revision of the system of nomenclature for influenza viruses: a WHO Memorandum. Bull W H O. 58:585-591. [PMC free article] [PubMed] [Google Scholar]
- [WHO] World Health Organisation [Internet] Influenza (Seasonal) Fact sheet Number 211. 2014 [last accessed 4 Jan 2015]. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/. [Google Scholar]
- Wu SF, Lee CJ, Liao CL, et al. (2002). Antiviral effects of an iminosugar derivative on flavivirus infections. J Virol 76:3596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZL, Ethen C, Hickey GE, Jiang W. (2009). Active 1918 pandemic flu viral neuraminidase has distinct N-glycan profile and is resistant to trypsin digestion. Biochem Biophys Res Commun 379:749–53. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Tomozawa T, Kakuta M, et al. (2009). CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob Agents Chemother 53:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng YC, Pan YT, Asano N, et al. (1997). Homonojirimycin and N-methyl-homonojirimycin inhibit N-linked oligosaccharide processing. Glycobiology 7:297–304. [DOI] [PubMed] [Google Scholar]
- Zenilman JM, Fuchs EJ, Hendrix CW, et al. (2015). Phase 1 clinical trials of DAS181, an inhaled sialidase, in healthy adults. Antiviral Res 123:114–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GB, Tian LQ, Li YM, et al. (2013). Protective effect of homonojirimycin from Commelina communis (dayflower) on influenza virus infection in mice. Phytomedicine 20:964–8. [DOI] [PubMed] [Google Scholar]
- Zhang GB, Zhang B, Zhang XX, Bing FH. (2013). Homonojirimycin, an alkaloid from dayflower inhibits the growth of influenza A virus in vitro . Acta Virol (Engl Ed) 57:85–6. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Braakman I, Matlack KE, Helenius A. (1997). Quality control in the secretory pathway: the role of calreticulin, calnexin and BiP in the retention of glycoproteins with C-terminal truncations. Mol Biol Cell 8:1943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Zheng B-J, Lu L, et al. (2015). Advancements in the development of subunit influenza vaccines. Microbes Infect 17:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng BJ, Chan KW, Lin YP, et al. (2008). Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci U S A 105:8091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Zhong N, Guan Y. (2007). Treatment with convalescent plasma for influenza A (H5N1) infection . N Engl J Med 357:1450–1. [DOI] [PubMed] [Google Scholar]
- Zitzmann N, Mehta AS, Carrouée S, et al. (1999). Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents . Proc Natl Acad Sci U S A 96:11878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C, Polistina C, Saini M, et al. (1992). Opposite polarity of virus budding and of viral envelope glycoprotein distribution in epithelial cells derived from different tissues. J Cell Biol 117:551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]