Abstract
Fusobacterium nucleatum is closely associated with human periodontal diseases and may also be a causative agent in other infections, such as pericarditis, septic arthritis, and abscesses of tonsils and liver. Initiation and outcome of infective diseases depend critically on the host cell signaling system altered by the microbe. Production of proteinases by infected cells is an important factor in pericellular tissue destruction and cell migration. We studied binding of F. nucleatum to human epithelial cells (HaCaT keratinocyte line) and subsequent cell signaling related to collagenase 3 expression, cell motility, and cell survival, using a scratch wound cell culture model. F. nucleatum increased levels of 12 protein kinases involved in cell migration, proliferation, and cell survival signaling, as assessed by the Kinetworks immunoblotting system. Epithelial cells of the artificial wound margins were clearly preferential targets of F. nucleatum. The bacterium colocalized with lysosomal structures and stimulated migration of these cells. Of the 13 anaerobic oral bacterial species, F. nucleatum and Fusobacterium necrophorum were among the best inducers of collagenase 3 mRNA levels, a powerful matrix metalloproteinase. Production of collagenase 3 was detected in fusobacterium-infected cells and cell culture medium by immunocytochemistry, immunoblotting, and zymography. The proteinase production involved activation of p38 mitogen-activated protein kinase in the infected cells. The study suggests that F. nucleatum may be involved in the pathogenesis of periodontal diseases (and other infections) by activating multiple cell signaling systems that lead to stimulation of collagenase 3 expression and increased migration and survival of the infected epithelial cells.
Fusobacteria are associated with a wide range of human and animal infections (4). They are gram-negative spindle-shaped anaerobes belonging to the family Bacteroidaceae. Fusobacteria are opportunistic pathogens that may cause numerous inflammatory and necrotic conditions. Their main site of inhabitation is the oropharynx, from where they can be transported to other body parts. Fusobacterium necrophorum may cause necrotizing tonsillitis and stomatitis, root canal infection, otitis, and, in worst cases, septicemia leading to the spread of infection and the development of abscesses in the lung and brain (17, 64, 67). Fusobacterium nucleatum is heterogeneous and currently includes five subspecies. It is the most frequently encountered species in humans and one of the most commonly found bacterial species in periodontal diseases (6, 47, 52). F. nucleatum is present in the dental plaque microflora of both children and adults and is closely associated with both gingivitis and periodontitis (36, 47, 63). Due to its versatile adhesion properties, F. nucleatum can bind several host proteins (3, 12, 45, 74) as well as coaggregate with many other potential oral pathogens, such as Porphyromonas gingivalis, Bacteroides forsythus, Prevotella intermedia, and Campylobacter rectus (7, 35). It is believed that F. nucleatum is an important bridging organism between early and late colonizers of subgingival biofilm (63). Like its relative F. necrophorum, F. nucleatum possesses many virulence factors, such as porin, other outer membrane proteins, and a potent lipopolysaccharide (6, 65). Further, toxic metabolites, e.g., butyrate, propionate, and ammonia, are considered to be important in the virulence of these organisms (33, 50, 57). In concert with other pathogenic bacteria, F. nucleatum appears to play an important part in the destruction of periodontal tissue (7, 18). Indeed, the organism is always present in the microflora of the periodontal sites during an active disease period (48, 16).
The reaction of host cells to microbial infection is a crucially important factor in the process of disease initiation and outcome. For instance, depending on the type and dose of the bacterial factors, infected epithelial cells can either defend themselves and eliminate the bacteria or tolerate the spread of infection or die (46). In any of the scenarios the decisive determinant of cell behavior is the signaling system that is activated in the host cell upon infection (19, 39, 42, 58). For instance, a specific leukotoxin produced by F. necrophorum induces signaling for apoptosis in neutrophils (50). F. nucleatum does not express a true leukotoxin, but it can adhere to epithelial cells and invade them by exploiting the cell signaling and the cytoskeletal elements of the host cells (26, 28, 51, 73).
We have demonstrated that in periodontitis, collagenase 3, a potent matrix metalloproteinase (MMP), is expressed by the gingival pocket epithelial cells growing into the subepithelial connective tissue (70). Epithelial cell motility may thus be associated with local destruction of connective tissue and formation of gingival pockets. We hypothesized that certain oral pathogens may alter epithelial cell signaling and lead to increased production of destructive enzymes. We report here that epithelial cells react to F. nucleatum by activation of multiple cell signaling pathways that lead to production of collagenase 3, increased cell migration, formation of lysosome-related structures, and cell survival.
MATERIALS AND METHODS
Bacterial cultures.
Both clinical isolates and American Type Culture Collection bacterial strains were tested in this study (Table 1). In most studies F. nucleatum subsp. nucleatum ATCC 25586 was used. Bacteria were isolated and identified and samples were prepared at the Anaerobic Reference Laboratory, National Public Health Institute, Helsinki, Finland, using established culture methods for each species (33). After being cultured, the bacteria were washed twice with sterile phosphate-buffered saline (PBS) and stored frozen in PBS. Bacterial stocks were prepared by adjusting the optical density at 600 nm to 0.5 with PBS. An aliquot of the stock solution was added to epithelial cell culture medium to give a bacterial cell number per epithelial cell of 26 (low concentration) or 260 (a high concentration). When added to cultures over 90% of the bacteria were dead.
TABLE 1.
Bacteria used in this study and their sources
| Bacterium | Strain | Source |
|---|---|---|
| Porphyromonas gingivalis | RHI 3610 | |
| (ATCC 33277) | Gingival pocket | |
| Porphyromonas endodontalis | AHN 4610 | Tonsils |
| RHI 3609 | ||
| (ATCC 35406) | Root canal | |
| Prevotella buccae | AHN 10652 | Gingival pocket |
| Prevotella oris | AHN 19550 | Root canal |
| Prevotella intermedia | AHN 18240 | Odontogenic abscess |
| Prevotella denticola | AHN 10695 | Gingival pocket |
| RHI 3606 | ||
| (ATCC 33185) | Maxillary atrium | |
| Prevotella nigrescens | AHN 18826 | Odontogenic abscess |
| Prevotella loeshii | AHN 10628 | Saliva |
| Actinobacillus actinomycetemcomitans | JP-2 | Gingival pocket |
| Fusobacterium nucleatum | AHN 19959 | Odontogenic abscess |
| RHI 4185 | ||
| (ATCC 25586) | Cervicofacial lesion | |
| Fusobacterium necrophorum | AHN 12454 | Odontogenic abscess |
| RHI 3624 | ||
| (ATCC 25286) | Bovine liver abscess | |
| Mitsuokella dentalis | AHN 12573 | Root canal |
| Campylobacter rectus | AHN 19728 | Odontogenic abscess |
Epithelial wound model and immunocytochemistry.
The HaCaT cell line, which consists of spontaneously transformed but nonmalignant human skin keratinocytes, was generously provided by Hubert Fusenig, German Cancer Center, Heidelberg, Germany. To create wounds, HaCaT cells were plated at 30,000 cells per cm2 on glow-discharged glass coverslips and cultured at 37°C in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, 2 mM glutamine, 100 IU of penicillin G/ml, and 100 μg of streptomycin/ml for 72 h to form confluent monolayers. The cultures were scratched with a plastic pipette tip to yield “wounds” with a uniform width. F. nucleatum from a frozen stock was added to cultures for an additional 24 to 72 h. To determine the extent of wound closure, the cultures were fixed and stained with 0.1% crystal violet in ethanol and photographed under a light microscope. Other wounded cultures were processed for immunostaining by fixing in PBS containing 4% paraformaldehyde and 5% sucrose followed by permeabilization in 0.5% Triton X-100 for 4 min and blocking in PBS containing 30 mg of bovine serum albumin and 1 mg of glycine/ml, with copious PBS washes between each step. Cultures were incubated with a 1:100 dilution of polyclonal rabbit anti F. nucleatum antibody (a generous gift from Dennis Lopatin, Department of Biologic and Materials Sciences, University of Michigan School of Dentistry). To study colocalization of F. nucleatum and lysosomal membrane structures, the cultures were double stained with anti-F. nucleatum antibody and a monoclonal mouse anti-human lysosomal membrane protein 1 (LAMP-1) antibody (Development Studies Hybridoma Bank, Iowa State University, Ames, Iowa). In other experiments, the relationship of F. nucleatum infection and collagenase 3 (MMP-13) expression was studied by double staining the cultures with anti-F. nucleatum antibody and antiserum against recombinant human collagenase 3 (MMP-13) (1:500 dilution) (21). Primary antibodies were washed five times with PBS containing 1 mg of bovine serum albumin/ml and then incubated with fluorescent secondary antibodies Alexa-488 and Alexa-546 (1:50) (Molecular Probes, Eugene, Oreg.) for 1 h. Cultures were washed twice with PBS and viewed under confocal laser scanning fluorescence microscopy (Bio-Rad, Hercules, Calif.).
Screening of protein kinases.
The levels of 78 protein kinases were analyzed in control and F. nucleatum (ATCC 25586)-treated samples by the commercial Kinetworks protein kinase screening system (KPKS 1.2; Kinexus Bioinformatics Corporation, Vancouver, Canada) as described online at www.kinexus.ca. The reproducibility of the method has been given a standard deviation of 10 to 20%. HaCaT cells were grown to 50% confluence and then deprived of serum for 24 h. After the addition of F. nucleatum for 120 min, cells were collected and centrifuged. The cells were lysed by sonication in extraction buffer (25 mM HEPES, 0.5% Triton X-100, 5 mM EGTA, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 50 mM beta-glycerol phosphate, 10 mM sodium orthovanadate). The cell homogenates were centrifuged for 30 min at 15,000 × g, and the protein concentration of the extracts was determined and adjusted to 1 mg/ml in sample loading buffer. Two bacterium-treated cultures were analyzed.
Collagenase 3 mRNA analysis.
Expression of collagenase 3 in bacterium-treated HaCaT cells was measured as follows. Total cellular RNA was isolated from cultured cells by a single-step method (9). Northern blot hybridization was performed as described previously (67) with MMP-13 cDNAs (a gift from Veli-Matti Kähäri, Centre for Biotechnology, University of Turku, Turku, Finland) labeled with [α32P]dCTP (Amersham, Inc.) by random priming (31). A rat cDNA for glyceraldehyde-3-phosphate dehydrogenase (GAPD) was used to indicate the proportional quantities of cellular RNA loaded to the gels. [32P]cDNA-mRNA hybrids were visualized by autoradiography, and the mRNA levels were quantitated by optical densitometry with NIH Image software (National Institutes of Health, Bethesda, Md.). To study the involvement of mitogen-activated protein (MAP) kinase activity associated with bacterial induction of collagenase 3, HaCaT cells were incubated for 30 min in the presence of 10 μM 1-[3-(amidinothio)propyl-1H-indoyl]-3-(1-methyl-1H-indoyl-3-yl)maleimide methane sulfonate (SB 203580, a specific p38 inhibitor) or 5 μM 2′-amino-3′methoxyflavone (PD 98059, a specific MEK1 inhibitor preventing activation of extracellular signal-regulated kinase [ERK]) prior to addition of F. nucleatum cells to the cultures. Both inhibitors were purchased from Calbiochem (Hornby, Ontario, Canada).
Western blot analysis.
Proteins of the conditioned medium were fractionated on sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked overnight at room temperature in 5% (wt/vol) milk powder-0.1% Tween 20 in PBS. They were subsequently incubated for 1 h with antiserum against recombinant human collagenase 3 (MMP-13) (1:1,000 dilution) (21) in skim milk-0.1% Tween 20-PBS. The membranes were washed once for 15 min and twice for 5 min with 0.1% Tween 20-PBS, and the bound antibodies were detected with the enhanced chemiluminescence detection system (Amersham).
Zymography.
For detection of gelatinolytic MMPs, serum-free conditioned medium samples from cultures exposed to F. nucleatum or F. necrophorum for 3 to 24 h were subjected to discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis, using 12% gels containing 1 mg of gelatin (G-6650; Sigma)/ml. After completion of electrophoresis the gels were washed twice in 50 mM Tris-0.02% NaN3-2.5% Triton X-100 buffer (pH 7.5). The second wash was supplemented with 5 mM CaCl2 and 1 μM ZnCl2. The incubation buffer consisted of 50 mM Tris, 0.02% NaN3, 5 mM CaCl2, and 1 μM ZnCl2 (pH 7.5). After incubation for 20 h at 37°C the gels were fixed and stained with 0.2% Coomassie blue R-250 in 40% methyl hydroxide and 10% acetyl hydroxide and subsequently destained in 7% acetyl hydroxide. The gels were quantified by optical densitometry.
RESULTS
F. nucleatum binds preferentially to the marginal epithelial cells and stimulates their migration
Interaction of epithelial cells and the bacteria was studied by utilizing a wound model that involved creation of scratches of uniform width to confluent keratinocyte cultures. F. nucleatum was observed to bind to selected epithelial cells of the cultures. The migrating cells of the artificial wound margins were clearly preferential targets of the bacteria. At a high bacterial concentration (260 bacteria per cell) almost all of the marginal cells had bound fusobacteria, while only 6% of the confluent cells had one or more F. nucleatum cells bound (Fig. 1A and B). Confocal microscopy revealed that the bacteria were localized both on the surface and inside the cytoplasm of the marginal epithelial cells. These cells remained infected after the wound margins had fused (Fig. 1C). Even at a low bacterial inoculum (26 bacteria per cell) about 50% of the keratinocytes of the wound margin showed clear evidence of multiple bacterial invasion (Fig. 2B).
FIG. 1.
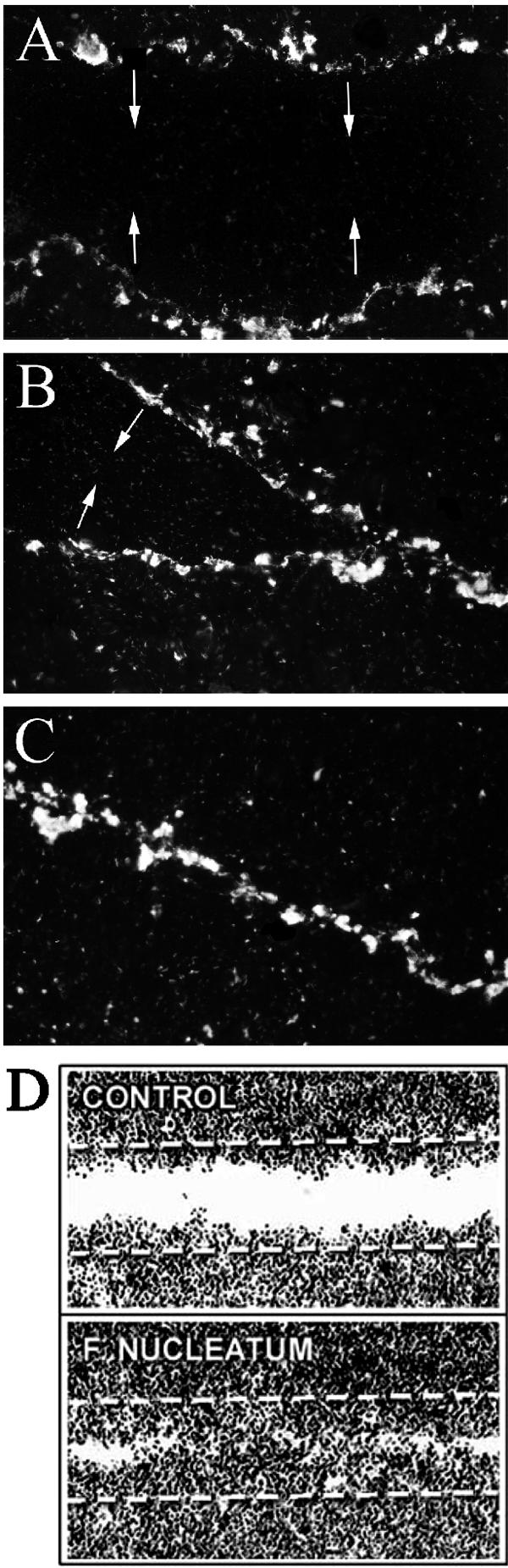
F. nucleatum preferentially binds to marginal cells of artificial epithelial wounds and increases their migration. Scratch wounds were created to confluent HaCaT cell cultures, and F. nucleatum cells were added to the culture medium (260 bacteria per cell) for different time periods. Epifluorescence of cultures detected with anti-F. nucleatum antibody after 6 h (A), 24 h (B) and 48 h (C). Light microscopic images of wounds at 48 h in the presence of TGF-β indicate faster closure of the epithelial wound sheets in F. nucleatum-treated cultures than in control cultures (D).
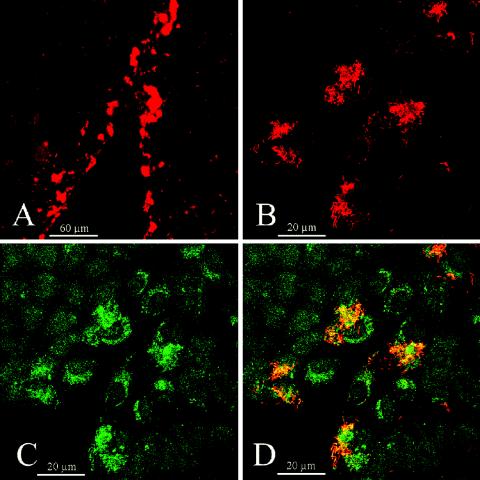
FIG. 2.
Intracellular F. nucleatum colocalizes with lysosomal membrane structures in cells at the leading edge of the epithelial sheets. Scratched HaCaT cell cultures treated for 48 h with F. nucleatum (ATCC 25586; 260 bacteria per cell) were simultaneously immunostained for F. nucleatum and lysosomal membrane protein LAMP-1, followed by two fluorescent secondary antibodies, Alexa-488 and Alexa-546, respectively. Samples were analyzed by laser confocal microscopy, and optical z-axis sections were recorded. One optical section from the basal, mid-, and apical regions of the cells were presented together. Red channel (A and B) corresponding to Alexa-546 fluorescence reveals F. nucleatum invasion, and green channel (C), corresponding to Alexa-488 fluorescence, reveals LAMP-1 expression. Red and green images were merged into an RGB file (D) showing colocalization (yellow/orange) of the two signals.
Bacterial infection may modulate the rate of cell proliferation and migration. Bacteria may also modify the effects of cytokines in infected tissue (76). We studied the effect of F. nucleatum on the rate of closure of the epithelial wound. In the presence of transforming growth factor β (TGF-β), the closure of the wounds was considerably faster in F. nucleatum-treated cultures (Fig. 1D). Most sites along the migrating fronts fused in cultures treated with the bacteria by 48 h, while the wounds remained open in the control cultures. In the absence of transforming growth factor β, F. nucleatum did not accelerate reepithelialization. There was no morphological indication of cell death in the infected epithelial cells.
F. nucleatum alters epithelial cell signaling.
The behavior of infected cells depends on both the direct toxic effects of the bacteria and the cell signaling pathways they turn on. To get an overview of the signaling profile of infected cells, HaCaT cells were exposed to F. nucleatum for 120 min and the cell extracts were analyzed for levels of 78 different protein kinases, utilizing the standardized Kinetworks immunoblotting system. Forty-six known kinases could be detected in the control and bacterium-treated cells. Compared to untreated cultures, F. nucleatum increased levels of 12 kinases and decreased levels of 2 kinases by 50% or more. Many of these kinases are involved in cell proliferation and cell survival signaling (Table 2). A particularly strong increase (about sevenfold) was observed in the cellular levels of DNA-dependent protein kinase, a kinase involved in the detection and repair of damaged DNA (15). An increase in levels of death-associated protein kinase 1 (62) and protein kinase CK-2, an enzyme involved in cell survival (42, 43), was observed. Levels of protein kinase C epsilon and cyclin-dependent kinases 7 and 9 were also increased in epithelial cells by F. nucleatum. Further, the bacteria stimulated levels of Etk/Bmx, S6 kinase p70, and RhoA kinase, enzymes connected to control of cell migration (1, 5, 8, 23, 55, 56). Levels of germinal center kinase, related to stress response (40), and G protein-coupled receptor kinase 2, involved in cell proliferation (2), were decreased by the F. nucleatum challenge. All in all these results indicate that F. nucleatum exerts multiple effects on epithelial cells involving DNA damage and cell activation. As a result, signaling for cell survival, cell proliferation, and cell migration is stimulated in infected cells.
TABLE 2.
Protein kinase profile of epithelial cells treated with F. nucleatuma
| Protein name | Abbreviation | Important signaling function | Mol wt (103) | Relative protein levelb (fold increase)
|
|
|---|---|---|---|---|---|
| Control | F. nucleatum | ||||
| Protein kinase C epsilon | PKCe | Apoptosis (10, 60) | 85 | 169 | 343 (2.0) |
| Cyclin-dependent kinase 7 | CDK7 | Cell division (59) | 36 | 528 | 799 (1.5) |
| Cyclin-dependent kinase 9 | CDK9 | Cell division (59) | 35 | 1,158 | 1,855 (1.6) |
| DNA-activated protein kinase | DNA-PK | DNA repair (15) | 211 | 176 | 1,312 (7.4) |
| Casein kinase 2 (37) | CK2a | Cell survival (42, 43) | 38 | 764 | 1,554 (2.0) |
| Casein kinase 2 (35) | CK2a | Cell survival (42, 43) | 35 | 379 | 1,145 (3.0) |
| Death-associated protein kinase 1 | DAPK | Apoptosis (62) | 148 | 2,151 | 4,693 (2.2) |
| Etk/Bmx kinase | Bmx | Cell survival, migration, signal integration (1, 8, 55) | 64 | 376 | 581 (1.5) |
| S6 kinase p70 | S6k | Cell migration (5, 22) | 55 | 326 | 534 (1.7) |
| RhoA kinase | ROKa | Cell migration (23, 56, 69) | 140 | 50 | 117 (2.3) |
| Germinal center kinase | GCK | Stress response (40) | 78 | 706 | 361 (−2.0) |
| G protein-coupled receptor kinase 2 | GRK-2 | Cell proliferation, signal integration (2) | 74 | 864 | 231 (−3.7) |
Semiconfluent HaCaT cells were cultured in Dulbecco's modified Eagle's medium the absence of serum for 24 h. A suspension of F. nucleatum (ATCC 25586; 260 bacteria per cell) was then added to the culture medium for 2 h Cells were then collected, washed, and extracted for Kinetworks protein kinase profiling, involving Western blotting for 78 kinases.
The values are averages of areas of scanned bands of the blots normalized to correct for differences in the protein content of the samples. The treated samples varied 2 to 28% from each other. Only values showing at least a 50% difference between the control and both of the treated cultures are shown.
F. nucleatum induces formation of lysosomal structures in epithelial cells.
Bacterial invasion of host cells may lead to formation of lysosomes or lysosome-related organelles (14, 24). Because proteases and other lysosomal constituents are known to participate in both bacterial killing and other critical functions in a cell-specific manner (11), we double stained the infected epithelial cell cultures with antibodies against LAMP-1 and F. nucleatum. The bacteria markedly induced the formation of lysosomal structures in the perinuclear region of the cells (Fig. 2). LAMP-1 colocalized in areas of F. nucleatum invasion in most cells. In some marginal cells positive for LAMP-1, only weak punctate F. nucleatum staining was evident, suggesting that substances or membrane fragments released from the bacteria are sufficient for signaling formation of lysosome-related structures. Alternatively, bacteria were degraded inside the epithelial cells.
Collagenase 3 (MMP-13) and gelatinase B (MMP-9) expression is stimulated in fusobacterium-treated epithelial cells.
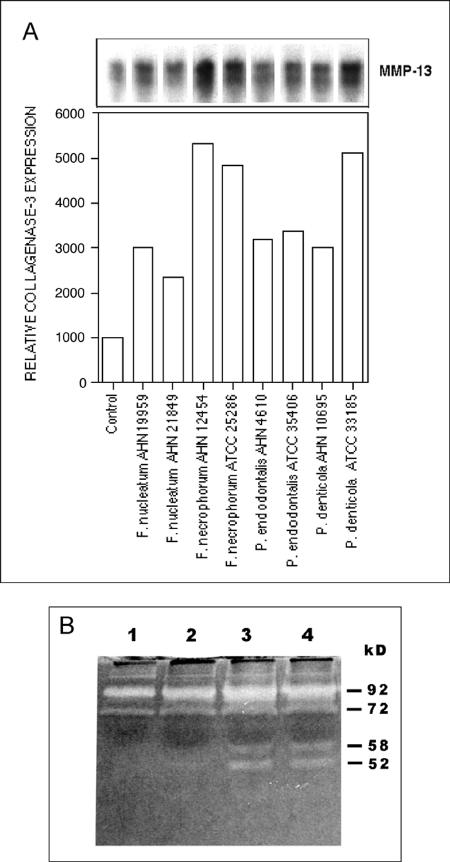
We have previously observed that in the infected pocket epithelium of periodontitis patients cells migrating into the underlying connective tissue express collagenase 3, a wide spectrum MMP (68). We studied, therefore, if bacteria can induce collagenase 3 in epithelial cells. We incubated HaCaT keratinocytes in the presence of 13 anaerobic oral bacteria and measured collagenase 3 mRNA levels in the infected cells. The Northern signal was evident as a double band, as previously reported (31). Collagenase 3 expression was stimulated in F. nucleatum- and F. necrophorum-infected cultures by about three- and fivefold, respectively. Porphyromonas endodontalis and Prevotella denticola also increased collagenase 3 expression (Fig. 3A), while there was little or no stimulation by the other nine bacteria studied (results not shown). A functional MMP assay utilizing zymography of the cell culture medium showed that F. necrophorum induced secretion of two gelatinolytic proteases of about 58 and 52 kDa (Fig. 3B). Similar MMP induction was observed in F. nucleatum-treated cells (data not shown). Solubilized F. necrophorum or F. nucleatum control samples revealed no gelatinolytic bands as measured by a zymogram (data not shown). Western blot analysis confirmed that the 58-kDa protease was pro-MMP-13. pro-MMP-9 (92 kDa) secretion was increased about twofold by F. necrophorum (Fig. 3B). Bacterial exposure of the epithelial cells for 5 or 11 h stimulated MMP production to about the same extent. Western blot analysis indicated that increased secretion of MMP-13 by F. nucleatum could be detected already at 3 h and continued at least for 24 h (Fig. 4). Confocal microscopy of epithelial cell cultures double stained for F. nucleatum and collagenase 3 indicated that the cells at the wound margin that were infected with the bacteria stained markedly more intensely for collagenase 3 (Fig. 5). Collagenase 3 was localized in these cells throughout most of the cytoplasm, and the enzyme did not colocalize with intracellular F. nucleatum.
FIG. 3.
Up-regulation of collagenase 3 (MMP-13) gene and protein expression in epithelial cells cultured in the presence of oral bacteria. Whole bacteria (from a frozen stock solution, optical density at 600 nm of 0.5) of 13 oral anaerobic species were added at a 1:20 dilution to semiconfluent HaCaT cell cultures. (A) After 24 h of culture in the absence of serum, total RNA (20 μg/sample) isolated from the cells was analyzed by Northern hybridization using specific cDNA probes for human collagenase 3 and GAPD (a loading control). The expression levels of collagenase 3 stimulated by eight bacterial strains were quantified by densitometric scanning and adjusted to the GAPD expression level (bar graph). (B) Semiconfluent HaCaT cell cultures were incubated with F. necrophorum (AHN 12454; at 260 bacteria per cell) in the absence of serum for 5 or 11 h, followed by incubation in the absence of the bacteria for 24 h. Aliquots of medium were subjected to gelatin zymography using 12% polyacrylamide gels. Lanes: 1, control culture, no bacteria; 2, F. necrophorum, 5 h, medium collected without further incubation; 3, F. necrophorum, 5 h plus 24-h culture in the absence of bacteria; 4, F. necrophorum 11 h plus 24-h culture in the absence of bacteria.
FIG. 4.
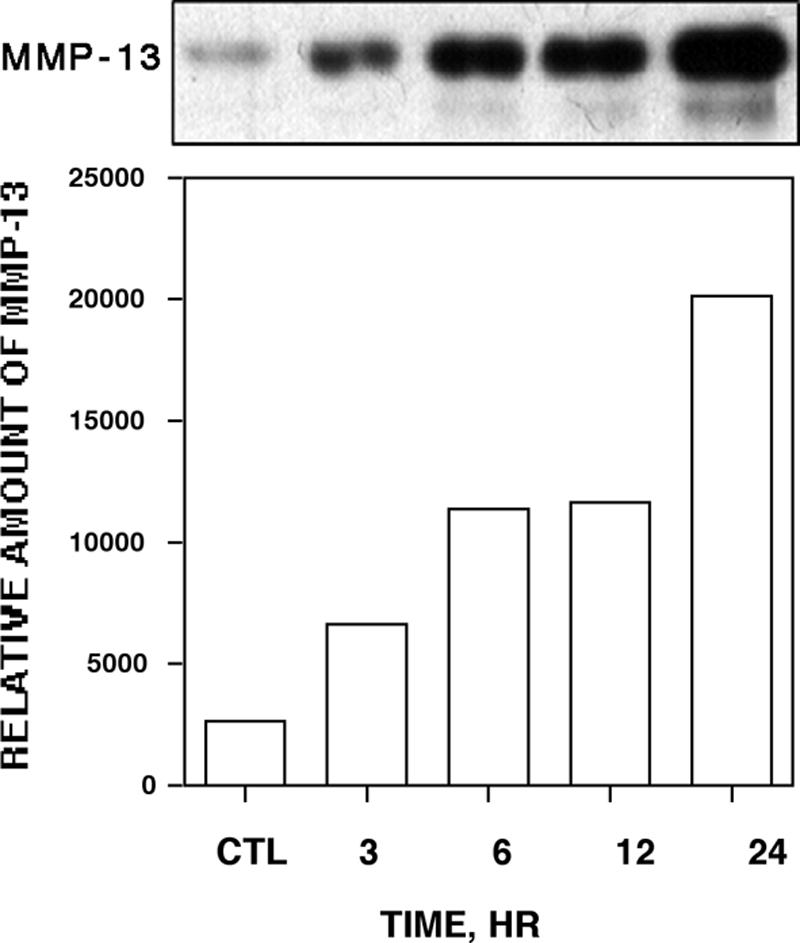
Time dependence of collagenase 3 secretion by epithelial cells treated with F. nucleatum. The bacteria were added to serum-free culture medium of semiconfluent HaCaT cells (260 bacteria per cell). Aliquots of the medium were analyzed for collagenase 3 at different time points by Western blotting. Control cells (CTL) were cultured for 24 h in the absence of the bacteria. Relative levels of pro-collagenase 3 protein were quantified by densitometric scanning.
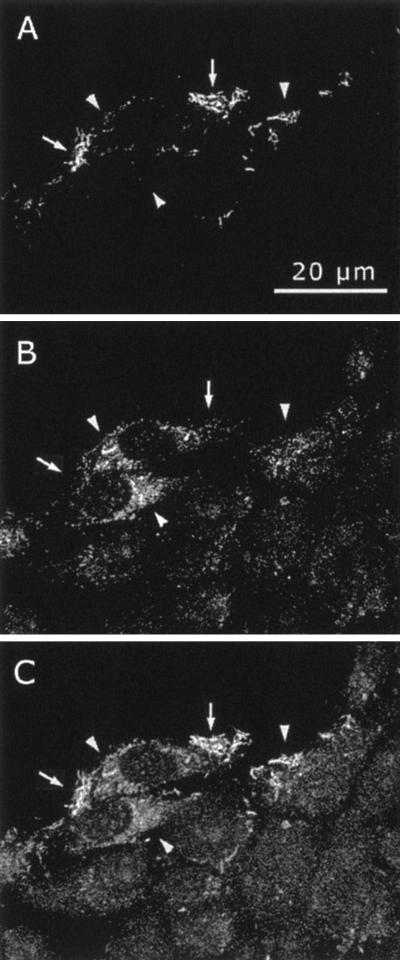
FIG. 5.
Up-regulation of epithelial cell-associated collagenase 3 expression by F. nucleatum. HaCaT cells were cultured to full confluence and then wounded by scratching with a pipette tip. They were then cultured in the presence of F. nucleatum (ATCC 25586; 260 bacteria per cell) for 12 h. The cells were washed and simultaneously immunostained for F. nucleatum and collagenase 3, followed by two fluorescent secondary antibodies, Alexa-488 and Alexa-546, respectively. Alexa-546 fluorescence reveals F. nucleatum invasion in certain cells in the wound margin (A, arrows). Alexa-488 fluorescence reveals collagenase 3 staining in the infected cells (B, arrowheads). Combination of the two images shows no clear colocalization of the signals (C).
Collagenase 3 expression by F. nucleatum involves p38 MAP kinase activity.
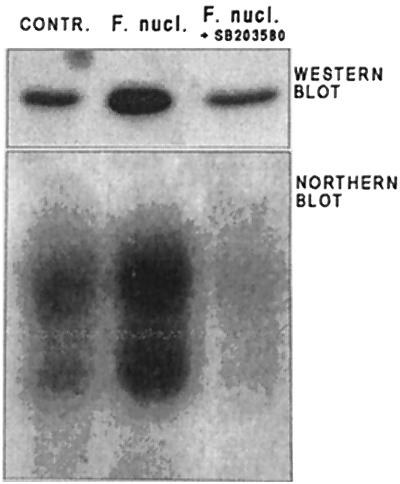
It was shown previously that expression of collagenase 3 by cytokine-treated keratinocytes is dependent on the activity of p38 MAP kinase (32). Further, the membrane fraction of F. nucleatum activates p38 in epithelial cells (37). We therefore tested if p38 or ERK MAP kinase activity is involved also in the induction of collagenase 3 by F. nucleatum. SB 203580, an inhibitor of p38, almost totally inhibited collagenase 3 mRNA expression and reduced secretion of the proteinase to the control level, as assessed by Northern and Western blot analysis (Fig. 6). The ERK pathway inhibitor PD 98059 did not have any effect on collagenase 3 induction (data not shown).
FIG. 6.
Effect of p38 MAP kinase inhibitor on F. nucleatum-induced collagenase 3 expression in epithelial cells. HaCaT cells were preincubated for 30 min with 10 μM SB203580 (inhibitor of p38) and then cultured in the presence of F. nucleatum (ATCC 25586; 160 bacteria per cell) for 24 h. Culture medium was analyzed by Western blotting, using anti-collagenase 3 antibodies, and RNA extracted from the cells (20 μg) was analyzed for collagenase 3 mRNA levels by Northern blotting.
DISCUSSION
Our study demonstrated that F. nucleatum selectively adheres to epithelial cells in culture. In the epithelial wound model central cells surrounded by other cells had relatively small amounts of surface-bound or intracellular F. nucleatum. In contrast, migrating cells in the wound margins were preferentially and heavily associated with the bacteria. We had earlier observed the same phenomenon with Treponema denticola invasion into cultured epithelial cells (71). Marginal cells with a free edge have more adhesive surface than more mature cells that are in contact with their neighbor cells (72). This appears to be due to different distributions of cell surface receptors during different physiological cell stages. For instance, we have observed a dramatic increase in KGF-1 receptor levels in wound edge epithelial cells compared to confluent cells (20). Interestingly, the cells remained infected after the margins of the epithelial wounds had fused. There was no clear morphological indication that the infected cells had impaired function. In fact, closure of the wounds took place much faster in F. nucleatum-treated cultures, indicating that F. nucleatum is able to stimulate cell growth or migration. These findings suggest that in ulcerative epithelium with increased cell proliferation and migration certain bacteria are able to invade a subpopulation of cells and stay inside them without interfering with host cell viability. This kind of situation exists in inflamed periodontal pockets that have a chronic condition of microulcerations, loss of epithelial cell contacts, altered integrin expression, and increased cell growth (27, 66).
The infected wound edge cells were found to produce lysosome-related structures upon F. nucleatum invasion. Lysosome membrane-specific protein LAMP-1 colocalized with intracellular F. nucleatum. It is unclear if this reflects an attempt by the cells to eliminate the bacteria or if F. nucleatum exploits the epithelial membrane system for living inside the epithelial cells or both. Cells with free margin are able to perform active phagocytosis (72). Because in our culture system most of the bacteria were dead it is not likely that the bacteria actively invaded the cells (28). Rather, the epithelial cells at the wound edge internalized the bacteria. Indeed, cytochalasin D, which disrupts actin function required for phagocytosis, totally blocks the uptake of F. nucleatum (28). It has been shown that the membrane fraction of F. nucleatum stimulates cultured epithelial cells to produce β-defensin 2, a potent antimicrobial peptide (38). Further, of the studied putative oral pathogens, F. nucleatum is one of the best inducers of tumor necrosis factor alpha (TNF-α) and interleukin-8, a neutrophil chemotactic cytokine (28, 38, 61). These findings suggest that epithelial cells attempt to eliminate the infecting bacteria. However, F. nucleatum appears to resist the antimicrobial effects of the cells. In our culture model system F. nucleatum persisted inside epithelial cells even after the wound edges had fused. Possibly F. nucleatum is able to interfere with the phagosome-endosome system and prevent normal fusion of lysosomes with phagosomes. This survival strategy is utilized by some intracellular bacteria, e.g., Salmonella and Brucella (13, 14, 19, 24). These bacteria reside inside intracellular vacuoles that contain lysosomal membrane proteins but not the normal content of lysosomes, such as cathepsins.
Upon exposure to pathogenic bacteria the epithelial cell has to make a decision whether to try to eliminate the invader through its own antimicrobial systems, signal professional defense cells for help, repair the damages, or commit suicide through apoptosis. We studied if the cell signaling profile provides evidence for the direction taken by F. nucleatum-treated epithelial cells. The Kinetworks method involves standardized immunoblotting utilizing validated, specific commercial antibodies (www.kinexus.ca). Analysis of the protein kinase profile showed that levels of several kinases were altered within 2 h of F. nucleatum exposure. Activation of death-associated protein kinase (62) and protein kinase C epsilon, which is known to increase TNF-α levels in macrophages (10, 60), suggests that some of the cells are proapoptotic. F. nucleatum has been shown to cause apoptosis of mononuclear cells and neutrophils (30). On the other hand, several kinases connected to cell repair and survival were also increased in the F. nucleatum-treated cells. The biggest increase (sevenfold) was observed in DNA-activated protein kinase, which is a key player in signaling of DNA damage, leading to repair of DNA double strand breaks and preventing apoptosis through activation of the NF-κB transcription factor (15). Etk/Bmx kinase, which was increased in the treated cells, is also related to cytoprotection and adaptive responses against environmental stress (1, 8). Another important regulator of cell survival that was increased by F. nucleatum is casein kinase 2. The enzyme resides in many cellular compartments and participates in the phosphorylation of a broad array of cellular target proteins, thereby participating in regulation of a complex series of cellular functions, including the control of cell cycle progression and the maintenance of cell viability. Casein kinase 2 also has an antiapoptotic role by protecting regulatory proteins from caspase-mediated degradation (42, 43). Other kinases whose levels were elevated in cells treated with F. nucleatum are important in the control of cell proliferation (cyclin-dependent kinases 7 and 9) and cell migration (59). Etk/Bmx is known to induce membrane ruffling and cell motility through regulation of the actin cytoskeleton (1, 8). RhoA kinase and S6K (p70), which were also increased by F. nucleatum, are other important players in the complex regulation of cytoskeletal organization, focal adhesions, and cell migration (22, 23, 69). Even though analysis of protein kinase levels as performed in this study gives only a limited view of cell signaling and does not take into account activation of the kinases, it points to the conclusion that pathways involved in cell survival, cell proliferation, and cell migration are prevalent in F. nucleatum-treated cells. This view is supported by our cell culture experiments, which showed an increased rate of epithelial migration and no morphological evidence of impairment of cell function upon exposure to the bacteria.
MMPs are a family of 25 neutral proteinases involved in many aspects of tissue remodeling and cell motility (34, 49, 53). We have found previously that gingival pocket epithelial cells invading the subepithelial connective tissue are producing collagenase 3, which is able to degrade a variety of proteins, including interstitial collagens (70). Collagenase 3 appears to be critical in normal tissue metabolism and homeostasis, but it also has an important role in inflammation, tumor invasion, and metastasis (21, 29, 31, 34, 41). We have shown that collagenase 3 is stimulated by cytokines, such as TNF-α, keratinocyte growth factor, TGF-α, and TGF-β, through activation of p38 MAP kinase (32, 70). We wanted to study if some potentially pathogenic bacteria can directly activate collagenase 3 production in epithelial cells. Of the 13 oral anaerobes, F. necrophorum and F. nucleatum were among the species increasing collagenase 3 mRNA levels in cells. Even though F. necrophorum and F. nucleatum are similar, they differ in their virulence and association with different diseases. It appears, however, that they share the ability to induce MMP production in host cells. Stimulated collagenase 3 protein production could be observed by Western blotting and zymography of the cell culture medium. Furthermore, in the epithelial wound assay, collagenase 3 staining was markedly more increased in infected cells of the wound margin. Interestingly, collagenase 3 induction by F. nucleatum also involved p38 MAP kinase activation. Previously, p38 activation has been found to be involved in β-defensin 2 and interleukin-8 production in epithelial cells treated with F. nucleatum (37). Because collagenase 3 secretion was clearly elevated in the culture medium by 3 h following F. nucleatum challenge, it is unlikely that the induction is due to production of MMP-inducing cytokines in the epithelial cells. In addition to degrading extracellular proteins, collagenase 3 may be related to migration of epithelial cells. It is one of the enzymes shown to cleave laminin 5 and convert it to a form conducive for cell migration (25, 54). In gingival pocket epithelium, collagenase 3 induction was observed in cells staining for laminin 5 (70). Other MMPs, such as MMP-2 (gelatinase A), MMP-7 (matrilysin) and MMP-9 (gelatinase B), that we found stimulated by F. necrophorum have been shown to be involved in cell migration (44, 49, 53, 69). Analogously, Helicobacter pylori has been found to stimulate MMP-7 production and thereby increase migration of gastric epithelial cells (75).
This study shows that F. nucleatum is able to adhere to epithelial cells with free edges and turn on signal transduction pathways related to cell survival and cell migration. Cell migration stimulated by F. nucleatum may be related to induction of collagenase 3. Through stimulation of collagenase 3 and cell migration F. nucleatum may be involved directly in gingival pocket formation during periodontal disease.
Acknowledgments
Fruitful collaboration with the late Hannele Jousimies-Somer will be truly missed.
This study was supported by grants from CIHR, Canada, and the Academy of Finland.
Editor: F. C. Fang
REFERENCES
- 1.Abassi, Y. A., M. Rehn, N. Ekman, K. Alitalo, and K. Vuori. 2003. p130Cas couples the tyrosine kinase Bmx/Etk with regulation of the actin cytoskeleton and cell migration. J. Biol. Chem. 278:35636-35643. [DOI] [PubMed] [Google Scholar]
- 2.Aragay, A. M., A. Ruiz-Gomez, P. Penela, S. Sarnago, A. Elorza, M. C. Jimenez-Sainz, and F. Mayor, Jr. 1998. G protein-coupled receptor kinase 2 (GRK2): mechanisms of regulation and physiological functions. FEBS Lett. 430:37-40. [DOI] [PubMed] [Google Scholar]
- 3.Babu, J. P., J. W. Dean, and M. J. Pabst. 1995. Attachment of Fusobacterium nucleatum to fibronectin immobilized on gingival epithelial cells or glass coverslips. J. Periodontol. 66:285-290. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, K. W., and A. Eley. 1993. Fusobacteria: new taxonomy and related diseases. J. Med. Microbiol. 39:246-254. [DOI] [PubMed] [Google Scholar]
- 5.Berven, L. A., and M. F. Crouch. 2000. Cellular function of p70S6K: a role in regulating cell motility. Immunol. Cell Biol. 78:447-451. [DOI] [PubMed] [Google Scholar]
- 6.Bolstad, A. I., H. B. Jensen, and V. Bakken. 1996. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin. Microbiol. Rev. 9:55-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook, I., and I. Walker. 1986. The relationship between Fusobacterium species and other flora in mixed infection. J. Med. Microbiol. 21:93-100. [DOI] [PubMed] [Google Scholar]
- 8.Chau, C. H., K. Y. Chen, H. T. Deng, K. J. Kim, K. Hosoya, T. Terasaki, H. M. Shih, and D. K. Ann. 2002. Coordinating Etk/Bmx activation and VEGF upregulation to promote cell survival and proliferation. Oncogene 21:8817-8829. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 10.Comalada, M., J. Xaus, A. F. Valledor, C. Lopez-Lopez, D. J. Pennington, and A. Celada. 2003. PKC epsilon is involved in JNK activation that mediates LPS-induced TNF-alpha, which induces apoptosis in macrophages. Am. J. Cell Physiol. 285:C1235-C1245. [DOI] [PubMed] [Google Scholar]
- 11.Cuervo, A. M., and J. F. Dice. 1998. Lysosomes, a meeting point of proteins, chaperones, and proteases. J. Mol. Med. 76:6-12. [DOI] [PubMed] [Google Scholar]
- 12.Darenfed, H., D. Grenier, and D. Mayrand. 1999. Acquisition of plasmin activity by Fusobacterium nucleatum subsp. nucleatum and potential contribution to tissue destruction during periodontitis. Infect. Immun. 67:6439-6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg, M. S., J. B. Kaper, and B. B. Finlay. 1997. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 5:109-114. [DOI] [PubMed] [Google Scholar]
- 14.Duclos, S., and M. Desjardins. 2000. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell Microbiol. 2:365-377. [DOI] [PubMed] [Google Scholar]
- 15.Durocher, D., and S. P. Jackson. 2001. DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr. Opin. Cell Biol. 13:225-231. [DOI] [PubMed] [Google Scholar]
- 16.Dzink, J. L., S. S. Socransky, and A. D. Haffajee. 1988. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J. Clin. Periodontol. 15:316-323. [DOI] [PubMed] [Google Scholar]
- 17.Falkler, W. A., Jr., C. O. Enwonwu, and E. O. Idigbe. 1999. Isolation of Fusobacterium necrophorum from cancrum oris (noma). Am. J. Trop. Med. Hyg. 60:150-156. [DOI] [PubMed] [Google Scholar]
- 18.Feuille, F., L. Kesavalu, M. J. Steffen, S. C. Holt, and J. L. Ebersole. 1994. Synergistic tissue destruction induced by P. gingivalis and F. nucleatum. J. Dent. Res. 73:159-164. [Google Scholar]
- 19.Finlay, B. B. Interactions of enteric pathogens with human epithelial cells. Bacterial exploitation of host processes. 1997. Adv. Exp. Med. Biol. 412:289-293. [DOI] [PubMed] [Google Scholar]
- 20.Firth, J. D., and E. E. Putnins. 2004. Keratinocyte growth factor-1 inhibits wound edge epithelial cell apoptosis in vitro. J. Investig. Dermatol. 122:222-231. [DOI] [PubMed] [Google Scholar]
- 21.Freije, J. M. P., I. Diéz-Itza, M. Balbín, L. M. Sánchez, R. Blasco, J. Tolivia, and C. López-Otín. 1994. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J. Biol. Chem. 296:16766-16773. [PubMed] [Google Scholar]
- 22.Frodin, M., and S. Gammeltoft. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell Endocrinol. 151:65-77. [DOI] [PubMed] [Google Scholar]
- 23.Fukata, Y., N. Oshiro, N. Kinoshita, Y. Kawano, Y. Matsuoka, V. Bennett, Y. Matsuura, and K. Kaibuchi. 1999. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J. Cell Biol. 145:347-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-del Portillo, F., M. B. Zwick, K. Y. Leung, and B. B. Finlay. 1993. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc. Natl. Acad. Sci. USA 90:10544-10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannelli, G., J. Falk-Marzillier, O. Schiraldi, W. G. Stetler-Stevenson, and V. Quaranta. 1997. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277:225-228. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons, R. J., and J. van Houte. 1971. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect. Immun. 3:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haapasalmi, K., M. Mäkelä, O. Oksala, J. Heino, K. M. Yamada, V. J. Uitto, and H. Larjava. 1995. Expression of epithelial adhesion proteins and integrins in chronic inflammation. Am. J. Pathol. 147:193-206. [PMC free article] [PubMed] [Google Scholar]
- 28.Han, Y. W., W. Shi, G. T. Huang, S. Kinder Haake, N. H. Park, H. Kuramitsu, and R. J. Genco. 2000. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect. Immun. 68:3140-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Impola, U., V. J. Uitto, J. Hietanen, L. Häkkinen, L. Zhang, H. Larjava, K. Isaka, and U. Saarialho-Kere. 2004. Differential expression of matrilysin-1 (MMP-7), 92 kDa gelatinase (MMP-9) and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J. Pathol. 202:14-22. [DOI] [PubMed] [Google Scholar]
- 30.Jewett, A., W. R. Hume, H. Le, T. N. Huynh, Y. W. Han, G. Cheng, and W. Shi. 2000. Induction of apoptotic cell death in peripheral blood mononuclear and polymorphonuclear cells by an oral bacterium, Fusobacterium nucleatum. Infect. Immun. 68:1893-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson, N., K. Airola, R. Grenman, A. L. Kariniemi, U. Saarialho-Kere, and V. M. Kähäri. 1997. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am. J. Pathol. 151:499-508. [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson, N., R. Ala-aho, V. Uitto, R. Grenman, N. E. Fusenig, C. Lopez-Otin, and V. M. Kähäri. 2000. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J. Cell Sci. 113(Part 2):227-235. [DOI] [PubMed] [Google Scholar]
- 33.Jousimies-Somer, H., P. H. Summanen, and S. M. Finegold. 1995. Bacteroides, Porphyromonas, Prevotella, Fusobacterium, and other anaerobic gram-negative bacteria, p. 603-620. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. L. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D.C.
- 34.Kähäri, V. M., and U. Saarialho-Kere. 1999. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann. Med. 31:34-45. [DOI] [PubMed] [Google Scholar]
- 35.Kinder, S. A., and S. C. Holt. 1993. Localization of the Fusobacterium nucleatum T18 adhesin activity mediating coaggregation with Porphyromonas gingivalis T22. J. Bacteriol. 175:840-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Könönen, E. 2000. Development of oral bacterial flora in young children. Ann. Med. 32:107-112. [DOI] [PubMed] [Google Scholar]
- 37.Krisanaprakornkit, S., J. R. Kimball, and B. A. Dale. 2002. Regulation of human beta-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-kappaB transcription factor family. J. Immunol. 168:316-324. [DOI] [PubMed] [Google Scholar]
- 38.Krisanaprakornkit, S., J. R. Kimball, A. Weinberg, R. P. Darveau, B. W. Bainbridge, and B. A. Dale. 2000. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 68:2907-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyburz, D., J. Rethage, R. Seibl, R. Lauener, R. E. Gay, D. A. Carson, and S. Gay. 2003. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by toll-like receptor signaling. Arthritis Rheum. 48:642-650. [DOI] [PubMed] [Google Scholar]
- 40.Kyriakis, J. M. 1999. Signaling by the germinal center kinase family of protein kinases. J. Biol. Chem. 274:5259-5262. [DOI] [PubMed] [Google Scholar]
- 41.Leeman, M. F., S. Curran, and G. I. Murray. 2002. The structure, regulation, and function of human matrix metalloproteinase-13. Crit. Rev. Biochem. Mol. Biol. 37:149-166. [DOI] [PubMed] [Google Scholar]
- 42.Litchfield, D. W. 2003. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 369:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loizou, J. I., S. F. El-Khamisy, A. Zlatanou, D. J. Moore, D. W. Chan, J. Qin, S. Sarno, F. Meggio, L. A. Pinna, and K. W. Caldecott. 2004. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell 117:17-28. [DOI] [PubMed] [Google Scholar]
- 44.Mäkelä, M., H. Larjava, E. Pirilä, P. Maisi, T. Salo, T. Sorsa, and V. J. Uitto. 1999. Matrix metalloproteinase 2 (gelatinase A) is related to migration of keratinocytes. Exp. Cell Res. 251:67-78. [DOI] [PubMed] [Google Scholar]
- 45.Mangan, D. F., M. J. Novak, S. A. Vora, J. Mourad, and P. S. Kriger. 1989. Lectinlike interactions of Fusobacterium nucleatum with human neutrophils. Infect. Immun. 57:3601-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menaker R. J., and N. L. Jones. 2003. Fascination with bacteria-triggered cell death: the significance of Fas-mediated apoptosis during bacterial infection in vivo. Microbes Infect. 5:1149-1158. [DOI] [PubMed] [Google Scholar]
- 47.Moore, W. E., and L. V. Moore. 1994. The bacteria of periodontal diseases. Periodontol. 2000. 5:66-77. [DOI] [PubMed] [Google Scholar]
- 48.Moore, W. E. C., L. H. Moore, R. R. Ranney, R. M. Smibert, J. A. Burmeister, and H. A. Schenkein. 1991. The microflora of periodontal sites showing active destructive progression. J. Clin. Periodontol. 18:729-739. [DOI] [PubMed] [Google Scholar]
- 49.Murphy, G., and J. Gavrilovic. 1999. Proteolysis and cell migration: creating a path? Curr. Opin. Cell Biol. 11:614-621. [DOI] [PubMed] [Google Scholar]
- 50.Narayanan, S., G. C. Stewart, M. M. Chengappa, L. Willard, W. Shuman, M. Wilkerson, and T. G. Nagaraja 2002. Fusobacterium necrophorum leukotoxin induces activation and apoptosis of bovine leukocytes. Infect. Immun. 70:4609-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozaki, M., Y. Miyake, M. Shirakawa, T. Takemoto, H. Okamoto, and H. Suginaka. 1990. Binding specificity of Fusobacterium nucleatum to human erythrocytes, polymorphonuclear leukocytes, fibroblasts, and HeLa cells. J. Periodont. Res. 25:129-134. [DOI] [PubMed] [Google Scholar]
- 52.Papapanou, P. N., A. Sellen, J. L. Wennstrom, and G. Dahlen. 1993. An analysis of the subgingival microflora in randomly selected subjects. Oral Microbiol. Immunol. 8:24-29. [DOI] [PubMed] [Google Scholar]
- 53.Parks, W. C. 1999. Matrix metalloproteinases in repair. Wound Repair Regen. 7:423-432. [DOI] [PubMed] [Google Scholar]
- 54.Pirilä, E., A. Sharabi, T. Salo, V. Quaranta, H. Tu, R. Heljasvaara, N. Koshikawa, T. Sorsa, and P. Maisi. 2003. Matrix metalloproteinases process the laminin-5 gamma 2-chain and regulate epithelial cell migration. Biochem. Biophys. Res. Commun. 303:1012-1017. [DOI] [PubMed] [Google Scholar]
- 55.Qiu, Y., and H. J. Kung. 2000. Signaling network of the Btk family kinases. Oncogene 19:5651-5661. [DOI] [PubMed] [Google Scholar]
- 56.Raftopoulou, M., and A. Hall. 2004. Cell migration: Rho GTPases lead the way. Dev. Biol. 265:23-32. [DOI] [PubMed] [Google Scholar]
- 57.Rogers, A. H., P. S. Zilm, N. J. Gully, A. L. Pfennig, and P. D. Marsh. 1991. Aspects of the growth and metabolism of Fusobacterium nucleatum ATCC 10953 in continuous culture. Oral Microbiol. Immunol. 6:250-255. [DOI] [PubMed] [Google Scholar]
- 58.Sancar, A., L. A. Lindsey-Boltz, K. Unsal-Kaccmaz, and S. Linn. 2004. Molecular mechanism of mammalian DNA repair and the DNA damage check points. Annu. Rev. Biochem. 73:39-85. [DOI] [PubMed] [Google Scholar]
- 59.Senderowicz, A. M. 2003. Small-molecule cyclin-dependent kinase modulators. Oncogene 22:6609-6620. [DOI] [PubMed] [Google Scholar]
- 60.Shapira, L., V. L. Sylvia, A. Halabi, W. A. Soskolne, T. E. Van Dyke, D. D. Dean, B. D. Boyan, and Z. Schwartz. 1997. Bacterial lipopolysaccharide induces early and late activation of protein kinase C in inflammatory macrophages by selective activation of PKC-epsilon. Biochem. Biophys. Res. Commun. 240:629-634. [DOI] [PubMed] [Google Scholar]
- 61.Sheikhi, M., A. Gustafsson, and C. Jarstrand. 2000. Cytokine, elastase and oxygen radical release by Fusobacterium nucleatum-activated leukocytes: a possible pathogenic factor in periodontitis. J. Clin. Periodontol. 27:758-762. [DOI] [PubMed] [Google Scholar]
- 62.Shohat, G., G. Shani, M. Eisenstein, and A. Kimchi. 2002. The DAP-kinase family of proteins: study of a novel group of calcium-regulated death-promoting kinases. Biochim. Biophys. Acta 1600:45-50. [DOI] [PubMed] [Google Scholar]
- 63.Socransky, S. S., and A. D. Haffajee. 2002. Dental biofilms: difficult therapeutic targets. Periodontol. 2000. 28:12-55. [DOI] [PubMed] [Google Scholar]
- 64.Sundquist, G. 1992. Associations between microbial species in dental root canal infections. Oral Microbiol. Immunol. 7:257-262. [DOI] [PubMed] [Google Scholar]
- 65.Takada, H., T. Ogawa, F. Yoshimura, K. Otsuka, S. Kokeguchi, K. Kato, T. Umemoto, and S. Kotani. 1988. Immunobiological activities of a porin fraction isolated from Fusobacterium nucleatum ATCC 10953. Infect. Immun. 56:855-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takarada, H., M. Cattoni, A. Sugimoto, and G. G. Rose. 1974. Ultrastructural studies of human gingiva. II. The lower part of the pocket epithelium in chronic periodontitis. J. Periodontol. 45:155-169. [DOI] [PubMed] [Google Scholar]
- 67.Tan, Z. L., T. G. Nagaraja, and M. M. Chengappa. 1996. Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism and control measures. Vet. Res. Commun. 20:113-140. [DOI] [PubMed] [Google Scholar]
- 68.Thomas, P. S. 1983. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 100:255-266. [DOI] [PubMed] [Google Scholar]
- 69.Turchi, L., A. A. Chassot, I. Bourget, C. Baldescchi, J. P. Ortonne, G. Meneguzzi, E. Lemichez, and G. Ponzio. 2003. Cross-talk between RhoGTPases and stress activated kinases for matrix metalloproteinase-9 induction in response to keratinocytes injury. J. Investig. Dermatol. 121:1291-1300. [DOI] [PubMed] [Google Scholar]
- 70.Uitto, V. J., K. Airola, M. Vaalamo, N. Johansson, E. E. Putnins, J. D. Firth, J. Salonen, C. Lopez-Otin, U. Saarialho-Kere, and V. M. Kähäri. 1998. Collagenase-3 (matrix metalloproteinase-13) expression is induced in oral mucosal epithelium during chronic inflammation. Am. J. Pathol. 152:1489-1499. [PMC free article] [PubMed] [Google Scholar]
- 71.Uitto, V. J., Y. M. Pan, W. K. Leung, H. Larjava, R. P. Ellen, B. B. Finlay, and B. C. McBride. 1995. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect. Immun. 63:3401-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vasiliev, J. M., I. M. Gelfand, L. V. Domnina, O. S. Zacharova, and A. V. Ljubimov. 1975. Contact inhibition of phagocytosis in epithelial sheets: alterations of cell surface properties induced by cell-cell contacts. Proc. Natl. Acad. Sci. USA 72:719-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weiss, E. I., B. Shaniztki, M. Dotan, N. Ganeshkumar, P. E. Kolenbrander, and Z. Metzger. 2000. Attachment of Fusobacterium nucleatum PK1594 to mammalian cells and its coaggregation with periodontopathogenic bacteria are mediated by the same galactose-binding adhesin. Oral Microbiol. Immunol. 15:371-377. [DOI] [PubMed] [Google Scholar]
- 74.Winkler, J. R., S. R. John, R. H. Kramer, C. I. Hoover, and P. A. Murray. 1987. Attachment of oral bacteria to a basement-membrane-like matrix and to purified matrix proteins. Infect. Immun. 55:2721-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wroblewski, L. E., P. J. Noble, A. Pagliocca, D. M. Pritchard, C. A. Hart, F. Campbell, A. R. Dodson, G. J. Dockray, and A. Varro. 2003. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J. Cell Sci. 116:3017-3026. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, L., S. Pelech, and V. J. Uitto. 2004. Long-term effect of heat shock protein 60 from Actinobacillus actinomycetemcomitans on epithelial cell viability and mitogen-activated protein kinases. Infect. Immun. 72:38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]