Abstract
Pseudomonas aeruginosa is a gram-negative bacterium that causes serious infections in immunocompromised individuals and cystic fibrosis patients. This opportunistic pathogen controls many of its virulence factors and cellular functions through the activity of three cell-to-cell signals, N-(3-oxododecanoyl)-l-homoserine lactone, N-butyryl-l-homoserine lactone, and the Pseudomonas quinolone signal (PQS). The activity of these signals is dependent upon their ability to dissolve in and freely diffuse through the aqueous solution in which P. aeruginosa happens to reside. Despite this, our data indicated that PQS was relatively insoluble in aqueous solutions, which led us to postulate that P. aeruginosa could be producing a PQS-solubilizing factor. In this report, we show that the P. aeruginosa-produced biosurfactant rhamnolipid greatly enhances the solubility of PQS in aqueous solutions. The enhanced solubility of PQS led to an increase in PQS bioactivity, as measured by both a gene induction assay and an apoptosis assay. This is the first demonstration of the importance of a bacterial surfactant in the solubilization and bioactivity of a cell-to-cell signal.
Pseudomonas aeruginosa is an environmental microbe that can cause serious infections when introduced to immunocompromised individuals or those suffering from cystic fibrosis. This opportunistic pathogen uses multiple cell-to-cell signals to communicate with its siblings and thereby control a host of bacterial functions, including virulence (33). One of these signals, the Pseudomonas quinolone signal (PQS), is a unique cell-to-cell signal that was identified as 2-heptyl-3-hydroxy-4-quinolone (34). Although PQS is different from the acyl-homoserine lactone signals produced by P. aeruginosa, it is still a part of the quorum-sensing signaling cascade (23).
The production of PQS is positively controlled by N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) and the las quorum-sensing system, while its bioactivity depends on and can activate the rhl quorum-sensing system (23, 34). Synthesis of PQS requires at least seven genes (6, 12), some of which are regulated in a complex manner. LasR and PqsR positively control the induction of the pqsABCDE operon, which is part of the PQS synthetic gene cluster, while RhlR appears to repress this operon (21). PQS production is also unusual in that it begins during the logarithmic phase of growth (9, 19), but unlike 3-oxo-C12-HSL and N-butyryl-l-homoserine lactone (C4-HSL), it is not produced maximally until late in the stationary phase of growth (23).
Why P. aeruginosa produces PQS is still unknown, but this signal has been shown to control multiple virulence factors (4, 9, 12, 23, 34) and is required for virulence in nematodes, plants, and mice (11, 12, 35). Most interestingly, PQS is produced in the lungs of cystic fibrosis patients infected with P. aeruginosa (5), which implies that the signal is important for adaptation to the lung environment.
The ability of PQS to function as an intercellular signal in culture media and presumably during infections implies that this signal must be soluble in an aqueous environment. However, our studies indicated that PQS was soluble in organic solvents but not in aqueous solutions. In this report, we explore the ability of a P. aeruginosa-produced factor to increase the solubility of PQS. Our results shed light on how PQS could be functioning in seemingly nonoptimal environments.
MATERIALS AND METHODS
Bacterial culture conditions.
P. aeruginosa strain PAO-R1 (lasR) (13) containing plasmid pTS400 (31) was grown in peptone Trypticase soy broth (PTSB) (30) supplemented with carbenicillin (200 μg/ml). Plasmid pTS400 harbors a lasB′-lacZ translational fusion that is activated in a concentration-dependent manner by PQS (34). All experiments began with the use of freshly plated bacteria from −70°C stocks maintained in 10% skim milk (Becton Dickinson). Liquid cultures were grown at 37°C and shaken at 250 rpm.
Examining PQS solubility in aqueous solutions.
Synthetic PQS (10 μg) was evaporated to dryness under nitrogen in 13-ml polystyrene culture tubes. Subsequently, 0.5 ml of either PTSB, distilled water, or acidified ethyl acetate was added, and the tubes were vortexed for 30 min. A 250-μl aliquot of each aqueous solvent was then removed and extracted with 500 μl of acidified ethyl acetate. One half of the resulting organic phase was evaporated to dryness and reconstituted in 50 μl of 1:1 acidified ethyl acetate-acetonitrile. The tube in which acidified ethyl acetate was used as a solvent was not extracted, but 125 μl was removed after vortexing, dried, and reconstituted in 50 μl of 1:1 acidified ethyl acetate-acetonitrile. This sample was used to demonstrate the maximum potential recovery of PQS in the assay. Samples were analyzed by thin-layer chromatography (TLC) and photographed under long-wave UV light as described previously (5).
Determining PQS solubility in the presence of rhamnolipids.
P. aeruginosa rhamnolipids were obtained from Jeneil Biosurfactant Co., Saukville, Wis. The rhamnolipids are 99.9% pure and are a mixture of mono-rhamnolipid (l-rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate) and di-rhamnolipid (l-rhamnosyl-l-rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate). Rhamnolipids were dissolved in acidified ethyl acetate, and appropriate amounts were evaporated to dryness in 13-ml polystyrene culture tubes which already contained 5 μg of dried PQS. Next, 0.5 ml of distilled water, PTSB, or phosphate-buffered saline (PBS) was added, and the tubes were incubated at 37°C with shaking (250 rpm) for 2 h. After this, a 200-μl aliquot was removed and extracted with 600 μl of acidified ethyl acetate. The organic phase was evaporated to dryness and reconstituted in 50 μl of 1:1 acidified ethyl acetate-acetonitrile. Samples were analyzed by TLC as described above. Quantification of PQS was completed with the use of computer densitometry to compare unknowns to synthetic standards as described previously (3). All data are reported as the mean ± standard deviation (σn−1) of three separate experiments.
Effect of rhamnolipids on PQS bioactivity.
Freshly plated cells of P. aeruginosa strain PAO-R1(pTS400) were used to inoculate 10-ml overnight cultures. After 20 h, overnight cultures were washed and used to inoculate 10-ml subcultures to an absorbance of 0.05 at 660 nm. Subcultures were grown until the mid-logarithmic phase, washed with fresh medium, and 1-ml aliquots (starting A660 = 0.05) were added to tubes containing synthetic PQS and P. aeruginosa rhamnolipids. After 18 h of growth, β-galactosidase activity was measured in duplicate samples. All data are reported in Miller units (24) as the mean ± standard deviation (σn−1) of three separate experiments.
Examining PQS-induced apoptosis.
Induction of apoptosis and cell viability were measured with the annexin V and propidium iodide binding assays as described previously (37). Cell cultures were maintained in a humidified 5% CO2 incubator. The interleukin-3-dependent murine FDC-P1 (8) and FL5.12 (22) cell lines were cultured in the absence of growth factors in RPMI medium (Gibco) with 5% fetal bovine serum (Atlanta Biologicals) supplemented with 10% WEHI-3B(D−) conditioned medium (20) as a source of interleukin-3. These cell lines were chosen because they are sensitive indicators of cell viability and are regularly used to determine a compound's ability to induce apoptosis (37).
For assaying PQS effects on viability and apoptosis, synthetic PQS (5.2, 2.6, and 0.52 μg) dissolved in 1:1 ethyl acetate-acetonitrile was evaporated to dryness in the wells of a six-well polystyrene tissue culture plate. Approximately 106 cells in 2 ml of complete cell culture medium were added to each well, and the plates were incubated for 3 days. Cells were then collected by gentle pipetting, and viability and the extent of apoptosis were measured as described above.
Determining whether rhamnolipids enhance PQS-induced apoptosis.
For examining the effect of rhamnolipids on PQS-induced apoptosis, suspensions of synthetic PQS (13.0 μg) and/or P. aeruginosa rhamnolipids (200 μg) were evaporated in 1.6-ml microcentrifuge tubes. Subsequently, 0.5 ml of distilled water was added, and the mixtures were incubated at 37°C with shaking (250 rpm) for 2 h. A 200-μl sample from each tube was immediately added to a well of a six-well culture plate that contained 2 ml of complete cell culture medium with approximately 106 cells, and the cultures were incubated for 2 days. (If 100% of the PQS was resuspended in 0.5 ml of water, then the final concentration of PQS in each cell culture would be 10 μM.) The final concentration of rhamnolipid in each cell culture was 63 μM. Cell viability and apoptosis induction were measured as described above. Data for cell culture experiments were normalized by assigning a value of 1 to appropriate controls (see the figure legends). Results are presented as the mean ± standard deviation from at least three separate experiments.
RESULTS
PQS solubility in aqueous solutions is low.
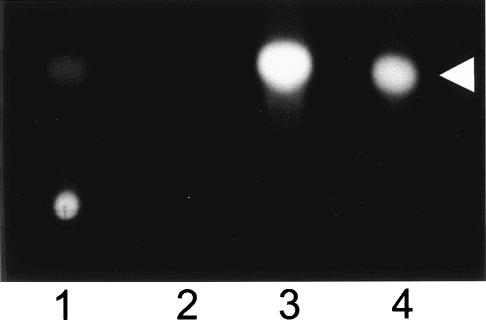
During our studies of PQS, we noticed that this signal had low solubility in aqueous solvents. To demonstrate this, PQS was dried in polystyrene culture tubes and various solvents were added. (Note that stock solutions of synthetic PQS are in 1:1 acidified ethyl acetate-acetonitrile.) After vortexing these solutions for 30 min, an aliquot of each aqueous solution was removed and extracted with acidified ethyl acetate. The organic layer of the extract was removed, dried, and analyzed by TLC as described in Materials and Methods. The results of this experiment showed that PQS did not dissolve in distilled water and that only a very small amount of PQS dissolved in PTSB (bacterial culture medium) (Fig. 1, lanes 1 and 2). As we have seen before, PQS easily dissolved in acidified ethyl acetate, which was included as a control to show maximal PQS resuspension (Fig. 1, lane 3). These results left us with a very interesting question. If PQS is not soluble in sterile culture medium, how does it act as a signal which activates a bioassay in the presence of growing P. aeruginosa cells?
FIG. 1.
PQS solubility is low in aqueous solutions. PQS (10 μg) was dried in 13-ml polystyrene tubes, and 0.5 ml of PTSB (lane 1), distilled water (lane 2), or acidified ethyl acetate (lane 3) was added to each tube. Mixtures were vortexed at high speed for 30 min, and aliquots were removed for PQS extraction. Samples were analyzed by TLC as described in Materials and Methods. The figure is a representative TLC plate photographed under UV light. Lane 4 contains 50 ng of synthetic PQS and was included as a standard. The arrowhead indicates PQS.
Rhamnolipids increase the solubility of PQS.
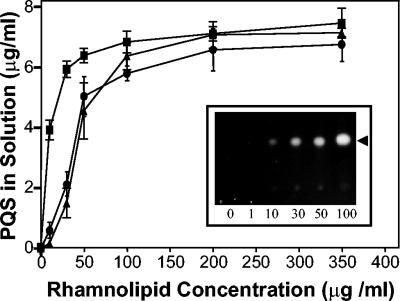
We felt that the most likely answer to our question was that P. aeruginosa produces a factor that increases PQS solubility. With this in mind, the effects of P. aeruginosa-produced rhamnolipids on PQS solubility were examined. Rhamnolipids are a quorum-sensing-controlled biosurfactant which increases the solubility of long-chain hydrocarbons to facilitate their use as a carbon source by P. aeruginosa (25, 28). To determine the effects of rhamnolipids on PQS solubility, increasing amounts of P. aeruginosa rhamnolipids were added to tubes containing dried PQS, and either distilled water, PBS, or PTSB was used as a solvent. After 2 h of mixing, aliquots were removed and ethyl acetate extractions were performed to recover the PQS that was in solution. The organic layer of each extract was then analyzed by TLC as described above.
Most interestingly, our data showed that P. aeruginosa rhamnolipids increased the solubility of PQS in all three aqueous solutions, and this increased solubility was dependent on the rhamnolipid concentration (Fig. 2). In distilled water or PBS, the addition of 50 μg of rhamnolipid per ml resulted in solubilization of approximately 50% of the total PQS added to the sample tube (Fig. 2). Exposing PQS in distilled water or PBS to a rhamnolipid concentration of 200 μg/ml resulted in a maximum recovery of approximately 65% of the total PQS added to the assay (Fig. 2). In PTSB, a slightly lower concentration of rhamnolipid solubilized 50% of the PQS, and maximum solubilization was reached when 100 μg of rhamnolipid per ml was present (Fig. 2). Overall, these data show that P. aeruginosa-produced rhamnolipids greatly increased the solubility of the cell-to-cell signal PQS.
FIG. 2.
Rhamnolipids increase PQS solubility in aqueous solutions. Synthetic PQS (5 μg) and increasing amounts of rhamnolipids were dried in 13-ml polystyrene tubes. Distilled water (circles), phosphate-buffered saline (triangles), or PTSB (squares) (0.5 ml of each) was then added to produce the indicated concentrations of rhamnolipid, and the mixture was incubated at 37°C with vigorous shaking (250 rpm) for 2 h. Aliquots removed from each tube were then organically extracted and analyzed as described in Materials and Methods. The amount of PQS in solution in each mixture was calculated and is presented as the mean ± standard deviation from three separate experiments. The insert is a representative TLC plate photographed under UV light. Numbers within the inset indicate rhamnolipid concentration, and the arrowhead indicates PQS. The PQS solubility assay was performed as in Fig. 1, and the photograph is included to visually show the effects of lower rhamnolipid concentrations on PQS solubility in distilled water.
Rhamnolipids enhance the ability of PQS to activate a P. aeruginosa bioassay.
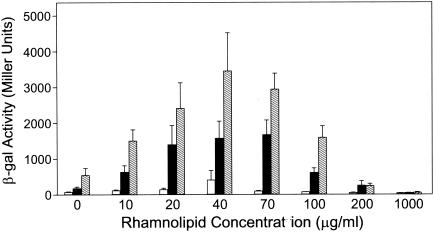
The discovery that PQS solubility increased in the presence of rhamnolipids led us to hypothesize that this surfactant would also increase the bioactivity of PQS. To test this, we used our PQS bioassay (34) to determine the effect of increasing rhamnolipid concentrations on three physiological concentrations of PQS. These results showed that the ability of PQS to induce a lasB′-lacZ fusion in P. aeruginosa was greatly enhanced by the presence of rhamnolipids (Fig. 3). This effect was maximized as the rhamnolipid concentration increased to 40 μg/ml (69 μM) and then the effect decreased as higher rhamnolipid concentrations were tested (Fig. 3). [A P. aeruginosa wild-type strain produced rhamnolipids at a concentration of approximately 22 μg/ml (38 μM) and 35 μg/ml (61 μM) after 24 and 48 h of growth, respectively (data not shown)]. At a concentration of 1 mg of rhamnolipid per ml (1.7 mM), nearly all bioactivity was inhibited (Fig. 3). The mechanism of this inhibition is not known, but we speculate that a high concentration of rhamnolipid could produce an excess of micelles that sequester PQS from bioassay cells and thereby lower the concentration of available PQS.
FIG. 3.
Rhamnolipids enhance a PQS bioassay. P. aeruginosa strain PAO-R1(pTS400) (lasR) was grown in the presence of 10 μM (white bars), 20 μM (black bars), or 30 μM (hatched bars) synthetic PQS and increasing amounts of rhamnolipids for 18 h. β-Galactosidase activity was subsequently assayed, and data are presented as the mean in Miller units ± standard deviation of duplicate assays from three separate experiments.
Rhamnolipids augment PQS-induced apoptosis.
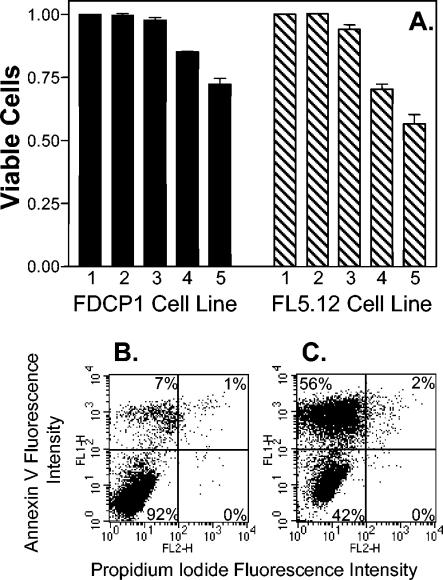
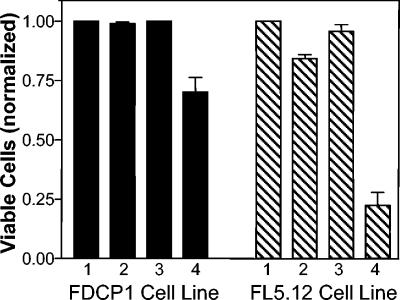
Although PQS acts as a bacterial cell-to-cell signal, its production during infections led us to wonder whether this compound could also effect eukaryotic cells. To begin such studies, two murine cell lines which are sensitive indicators of cell viability and apoptosis induction were grown in the presence of various physiological concentrations of PQS. After 3 days of growth, cells were harvested, and viability and the extent of apoptosis were measured as described in Materials and Methods. These data showed that PQS caused the induction of apoptosis and a decrease in cell viability for both cell lines (Fig. 4). This effect was dose dependent, with increasing PQS concentrations causing lower viability (Fig. 4).
FIG. 4.
PQS induces apoptosis. The indicated cell lines were exposed to various physiological concentrations of PQS for 3 days, and the extent of apoptosis was determined by staining with annexin V and propidium iodide. (A) The percentage of viable cells was normalized to 1 for the no-addition control (lane 1) of each cell line. This represents cells which did not stain with either annexin V or propidium iodide. Lane 2 contained 5.2 μl (the maximum amount of solvent used in PQS-containing wells) of dried 1:1 acidified ethyl acetate-acetonitrile as a solvent control. Lanes 3, 4, and 5 contained dried PQS that would produce final concentrations of 1 μM, 5 μM, and 10 μM PQS, respectively, when 2 ml of cell culture was added. Results are presented as the mean ± standard deviation from at least three separate experiments. (B and C) Representative dot plots are included to show the apoptotic shift induced by PQS in the FL5.12 cell line. Panel B shows cells that were grown without PQS (no addition), and panel C shows cells grown in the presence of 10 μM PQS. The percentages given within each panel indicate the percentage of cells in that panel. The lower left panel of each dot plot contains viable cells, as indicated by the lack of staining with either annexin V or propidium iodide. The upper left and upper right panels contain apoptotic cells, which stained with annexin V, and the lower right panels contain dead cells, which stained with propidium iodide only.
The ability of rhamnolipids to augment PQS-induced cell killing was then examined. To do this, dried PQS was suspended in distilled water in the presence or absence of rhamnolipids. This suspension was then added to cell cultures which were incubated for 2 days before measuring apoptosis induction and cell viability. The results of this experiment showed that when resuspended in water, neither rhamnolipids nor PQS alone affected cells (Fig. 5, lanes 2 and 3). This would presumably be due to the small amount of PQS that was solubilized in the solution (see Fig. 1) added to the cells. However, when PQS was resuspended in water in the presence of rhamnolipids, this solution caused apoptosis induction with a concurrent decrease in cell viability (Fig. 5, lane 4). Taken together, these data suggest that PQS and rhamnolipids could be a potent virulence team during P. aeruginosa infections.
FIG. 5.
Rhamnolipids enhance PQS-induced apoptosis. PQS and/or rhamnolipids were dried in polystyrene tubes and resuspended in distilled water as described in Material and Methods. The final concentration of rhamnolipid in each tube was 40 μg/ml, and if all PQS became soluble, the final concentration of PQS would be 10 μM. A 200-μl aliquot of suspension (or distilled water as a control) was then added to the indicated cell cultures, which were incubated for 2 days before assaying for apoptosis induction and cell viability as in Fig. 4. Lanes: 1, water; 2, rhamnolipid suspension; 3, PQS suspension; 4, rhamnolipid and PQS suspension. Data were normalized by assigning a value of 1 to the percentage of viable cells found in the distilled-water control (lane 1).
DISCUSSION
In this report, we show that the solubility of a P. aeruginosa cell-to-cell signal is increased in aqueous solutions by rhamnolipids, a P. aeruginosa-produced biosurfactant. The increase in solubility of the signal led to a concurrent increase in signal bioactivity in two different assays. This is the first report of a cell-to-cell signal being solubilized and made more active by the organism which produced it.
Rhamnolipids are natural surfactants produced by P. aeruginosa and are widely used for industrial purposes, such as bioremediation and biotransformation (26). By forming micelles, rhamnolipids are able to increase the solubility of many hydrophobic compounds. Such is apparently the case with the cell-to-cell signal PQS (Fig. 2). In addition, rhamnolipids increase the uptake of hydrophobic compounds by P. aeruginosa (25). This is believed to occur because rhamnolipids can extract lipopolysaccharide from the P. aeruginosa outer membrane and thereby increase the hydrophobicity of the cell surface (1). Our results suggest that rhamnolipids are also facilitating the uptake of PQS by P. aeruginosa, as evidenced by the rhamnolipid-associated increase in PQS bioactivity in our P. aeruginosa bioassay (Fig. 3).
PQS and rhamnolipids have both been shown to act as virulence factors (11, 12, 35, 36), and both are known to be produced in the lungs of P. aeruginosa-infected cystic fibrosis patients (5, 18). Our data indicate that PQS alone caused apoptosis to be induced in eukaryotic cells (Fig. 4). This suggests that PQS may act independently as a virulence factor that affects eukaryotic cells, as seen with another P. aeruginosa cell-to-cell signal, 3-oxo-C12-HSL (10, 38, 39). Some fluoroquinolone compounds with antibiotic activity have cytotoxic effects on eukaryotic cells (2, 15). This activity is believed to occur through effects on topoisomerase II, which is homologous to DNA gyrase, the bacterial target of fluoroquinolone antibiotics (2). Whether PQS, which has not exhibited antibiotic activity (34), is acting similarly is not known.
The data from the eukaryotic cell experiments also imply that PQS must be at least partially soluble in the absence of rhamnolipids, since the experiments in Fig. 4 amounted to placing eukaryotic cell cultures onto dried PQS. Only 2% of the PQS dried in the well became soluble after 3 days of incubation in cell culture medium without cells (data not shown). This implies that eukaryotic cells themselves are allowing enough PQS to become soluble for bioactivity (apoptosis/cell death) to be seen. Most importantly, when PQS was mixed with rhamnolipids in water before being added to eukaryotic cells, apoptosis was induced and cell viability was greatly decreased (Fig. 5). PQS in water alone had no effect on cell viability (Fig. 5), presumably due to the low solubility of PQS in water (Fig. 1). Overall, our data suggest that PQS and rhamnolipids are acting in concert to increase P. aeruginosa virulence.
The control of rhamnolipid production is strongly influenced by multiple cell-to-cell signals. P. aeruginosa rhamnolipids are produced through the action of the RhlA/RhlB rhamnosyltransferases (27). The operon that encodes these enzymes, rhlAB, is positively controlled at the transcriptional and translational levels by RhlR and C4-HSL (28, 29, 32, 40), and the small RNA RsmA (16), respectively. In addition, PQS can induce the gene which encodes the C4-HSL synthase (rhlI) (23). This is probably why a PQS mutant makes less rhamnolipid than the wild-type strain (9). While the effect of PQS on the rhl quorum-sensing system has not been completely defined, it appears that PQS does increase the ability of RhlR to induce at least some of the genes that it controls (9, 23, 34). Taking all of these factors into consideration with the data presented here, it can be suggested that PQS may have a positive effect on the rhl quorum-sensing system, and thus rhamnolipid production, in order to ensure its own solubility in the extracellular environment.
Several converging lines of research indicate that rhamnolipid activity is multifaceted. First, as mentioned above, it will solubilize hydrophobic molecules such as long-chain hydrocarbons and allow their use as a carbon source by P. aeruginosa. Second, it is apparently important for interactions between cells. This is supported by the findings that the addition of rhamnolipids can cause cells to aggregate, or “clump” (14), and a rhamnolipid mutant no longer exhibits swarming motility (17). In addition, rhamnolipid mutants form thick biofilms without typical fluid channels (7). Our findings add another function for rhamnolipids and indicate that this surfactant is not only controlled by cell-to-cell communication, but is important for the function of at least one cell-to-cell signal, PQS. While previous studies have shown that P. aeruginosa rhamnolipids have activities associated with virulence, our results indicate a novel mechanism whereby rhamnolipids assist another virulence factor and enhance its activity.
Acknowledgments
This work was supported by research grants from the National Institute of Allergy and Infectious Diseases (grant R01-AI46682) to E.C.P. and the National Cancer Institute (grant R01-CA98185) to J.A.M.
We thank J. Coleman, C. Jordan, E. Ling, J. Whelan, D. Wade, and C. Pesci for help in manuscript preparation and thoughtful insight.
Editor: F. C. Fang
REFERENCES
- 1.Al-Tahhan, R. A., T. R. Sandrin, A. A. Bodour, and R. M. Maier. 2000. Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates. Appl. Environ. Microbiol. 66:3262-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromberg, K. D., A. B. Burgin, and N. Osheroff. 2003. Quinolone action against human topoisomerase IIalpha: stimulation of enzyme-mediated double-stranded DNA cleavage. Biochemistry 42:3393-3398. [DOI] [PubMed] [Google Scholar]
- 3.Calfee, M. W., J. P. Coleman, and E. C. Pesci. 2001. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 98:11633-11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, H., G. Krishnan, B. Goumnerov, J. Tsongalis, R. Tompkins, and L. G. Rahme. 2001. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc. Natl. Acad. Sci. USA 98:14613-14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier, D. N., L. Anderson, S. L. McKnight, T. L. Noah, M. Knowles, R. Boucher, U. Schwab, P. Gilligan, and E. C. Pesci. 2002. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett. 215:41-46. [DOI] [PubMed] [Google Scholar]
- 6.D'Argenio, D. A., M. W. Calfee, P. B. Rainey, and E. C. Pesci. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davey, M. E., N. C. Caiazza, and G. A. O'Toole. 2003. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 185:1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dexter, T. M., J. Garland, D. Scott, E. Scolnick, and D. Metcalf. 1980. Growth of factor-dependent hemopoietic precursor cell lines. J. Exp. Med. 152:1036-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diggle, S. P., K. Winzer, S. R. Chhabra, K. E. Worrall, M. Camara, and P. Williams. 2003. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 50:29-43. [DOI] [PubMed] [Google Scholar]
- 10.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher, L. A., and C. Manoil. 2001. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J. Bacteriol. 183:6207-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher, L. A., S. L. McKnight, M. S. Kuznetsova, E. C. Pesci, and C. Manoil. 2002. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 184:6472-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman, D. C., Y. Zhang, and R. M. Miller. 1997. Rhamnolipid (biosurfactant) effects on cell aggregation and biodegradation of residual hexadecane under saturated flow conditions. Appl. Environ. Microbiol. 63:3622-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herold, C., M. Ocker, M. Ganslmayer, H. Gerauer, E. G. Hahn, and D. Schuppan. 2002. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br. J. Cancer 86:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heurlier, K., F. Williams, S. Heeb, C. Dormond, G. Pessi, D. Singer, M. Camara, P. Williams, and D. Haas. 2004. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol. 186:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohler, T., L. K. Curty, F. Barja, C. van Delden, and J. C. Pechere. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kownatzki, R., B. Tummler, and G. Doring. 1987. Rhamnolipid of Pseudomonas aeruginosa in sputum of cystic fibrosis patients. Lancet i:1026-1027. [DOI] [PubMed] [Google Scholar]
- 19.Lepine, F., E. Deziel, S. Milot, and L. G. Rahme. 2003. A stable isotope dilution assay for the quantification of the Pseudomonas quinolone signal in Pseudomonas aeruginosa cultures. Biochim. Biophys. Acta 1622:36-41. [DOI] [PubMed] [Google Scholar]
- 20.McCubrey, J., J. P. McKearn, and G. Kohler. 1985. Transformation of B and non-B cell lines with the 2,4,6,-trinitrophenyl (TNP)-specific immunoglobulin genes. Eur. J. Immunol. 15:1117-1124. [DOI] [PubMed] [Google Scholar]
- 21.McGrath, S., D. S. Wade, and E. C. Pesci. 2004. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS). FEMS Microbiol. Lett. 230:27-34. [DOI] [PubMed] [Google Scholar]
- 22.McKearn, J. P., J. McCubrey, and B. Fagg. 1985. Enrichment of hematopoietic precursor cells and cloning of multipotential B-lymphocyte precursors. Proc. Natl. Acad. Sci. USA 82:7414-7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKnight, S. L., B. H. Iglewski, and E. C. Pesci. 2000. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 182:2702-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Noordman, W. H., and D. B. Janssen. 2002. Rhamnolipid stimulates uptake of hydrophobic compounds by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 68:4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochsner, U., J. Reiser, A. Fiechter, and B. Witholt. 1995. Production of Pseudomonas aeruginosa rhamnolipid biosurfactants in heterologous hosts. Appl. Environ. Microbiol. 61:3503-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochsner, U. A., A. Fiechter, and J. Reiser. 1994. Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. J. Biol. Chem. 269:19787-19795. [PubMed] [Google Scholar]
- 28.Ochsner, U. A., A. K. Koch, A. Fiechter, and J. Reiser. 1994. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 176:2044-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochsner, U. A., and J. Reiser. 1995. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:6424-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohman, D. E., S. J. Cryz, and B. H. Iglewski. 1980. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J. Bacteriol. 142:836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 32.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pesci, E. C., and B. H. Iglewski. 2003. Quorum sensing, p. 55-65. In D. L. Burns, J. T. Barbieri, B. H. Iglewski, and R. Rappuoli (ed.), Bacterial protein toxins. ASM Press, Washington, D.C.
- 34.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahme, L. G., M. W. Tan, L. Le, S. M. Wong, R. G. Tompkins, S. B. Calderwood, and F. M. Ausubel. 1997. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 94:13245-13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Read, R. C., P. Roberts, N. Munro, A. Rutman, A. Hastie, T. Shryock, R. Hall, W. McDonald-Gibson, V. Lund, and G. Taylor. 1992. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J. Appl. Physiol. 72:2271-2277. [DOI] [PubMed] [Google Scholar]
- 37.Shelton, J. G., L. S. Steelman, J. T. Lee, S. L. Knapp, W. L. Blalock, P. W. Moye, R. A. Franklin, S. C. Pohnert, A. M. Mirza, M. McMahon, and J. A. McCubrey. 2003. Effects of the RAF/MEK/ERK and PI3K/AKT signal transduction pathways on the abrogation of cytokine-dependence and prevention of apoptosis in hematopoietic cells. Oncogene 22:2478-2492. [DOI] [PubMed] [Google Scholar]
- 38.Smith, R. S., S. G. Harris, R. Phipps, and B. Iglewski. 2002. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 184:1132-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams, S. C., E. K. Patterson, N. L. Carty, J. A. Griswold, A. N. Hamood, and K. P. Rumbaugh. 2004. Pseudomonas aeruginosa autoinducer enters and functions in mammalian cells. J. Bacteriol. 186:2281-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winson, M. K., M. Camara, A. Latifi, M. Foglino, S. R. Chhabra, M. Daykin, M. Bally, V. Chapon, G. P. Salmond, and B. W. Bycroft. 1995. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:9427-9431. [DOI] [PMC free article] [PubMed] [Google Scholar]