Abstract
Background
Central nervous system (CNS) events are frequently reported among patients with advanced prostate cancer as a consequence of the treatments used in this patient population.
Objective
To assess the incidence of CNS events in patients with advanced prostate cancer who initiated treatment with abiraterone acetate, bicalutamide, enzalutamide, or chemotherapy.
Methods
The Truven Health MarketScan Research databases were used to retrospectively identify patients with prostate cancer who initiated treatment with abiraterone acetate, enzalutamide, bicalutamide, or chemotherapy after September 1, 2012 (ie, the index date). The chemotherapy agents included cabazitaxel, docetaxel, mitoxantrone hydrochloride, and estramustine, and were used as monotherapy or as combination therapy. Patients were followed until December 31, 2014, the end of exposure to treatment, or until loss to follow-up. Kaplan-Meier rates and adjusted Cox proportional hazard models were used to compare the incidence of CNS events between the abiraterone acetate cohort and the other cohorts. A sensitivity analysis of patients with a diagnosis of metastasis was also conducted.
Results
A total of 1067 patients receiving abiraterone acetate, 5524 receiving bicalutamide, 592 receiving enzalutamide, and 256 receiving chemotherapy were identified. After 12 months, patients who received abiraterone acetate were less likely to have a CNS event than patients who received enzalutamide (39.5% vs 46.0%, respectively; P = .0036) or chemotherapy (39.5% vs 51.1%, respectively; P = .0277), and were more likely to have a CNS event than patients who received bicalutamide (39.5% vs 34.2%, respectively; P = .0397). After multivariate adjustment, at 12 months, patients who initiated abiraterone acetate treatment had 20% (P = .0388) reduction in the risk for a CNS event compared with patients who initiated enzalutamide; 8% (P = .3622) versus bicalutamide; and 27% (P = .0456) versus chemotherapy. The sensitivity analysis yielded similar results.
Conclusion
The results of this large observational study suggest that among patients with metastatic prostate cancer, treatment with abiraterone acetate is associated with a significantly lower likelihood of having a CNS event compared with treatment with enzalutamide or chemotherapy, but not with bicalutamide, even when controlling for metastatic disease.
Keywords: abiraterone acetate, advanced prostate cancer, bicalutamide, central nervous system events, chemotherapy, enzalutamide
KEY POINTS
-
▸
With new therapies prolonging the life of patients with prostate cancer, concern about the treatments' effects on the central nervous system (CNS) is growing.
-
▸
This retrospective study compared the impact of treatment initiation with abiraterone acetate, enzalutamide, bicalutamide, or with chemotherapy on CNS events in patients with advanced prostate cancer, using real-world claims data.
-
▸
This is the first study to provide real-world comparison of the incidence of CNS events in patients who received different treatments for advanced prostate cancer.
-
▸
After 12 months, patients receiving abiraterone acetate were less likely to have a CNS event than those receiving enzalutamide (39.5% vs 46.0%) or chemotherapy (39.5% vs 51.1%), but more likely to have a CNS event than patients receiving bicalutamide (39.5% vs 34.2%).
-
▸
After a multivariate adjustment, patients who initiated treatment with abiraterone acetate had a significant reduction in the risk for a CNS event versus patients receiving enzalutamide (20%), or chemotherapy (27%), but a nonsignificant reduction of 8% versus bicalutamide.
The mainstay treatment for prostate cancer consists of lowering the levels of androgens, because androgens have been associated with prostate cancer cell proliferation.1 Advanced prostate cancer is defined as a disease that has spread beyond the prostate gland and the area around the prostate.2 However, for the majority of patients receiving androgen-deprivation therapy, the disease will eventually progress.3 Patients whose disease has progressed while receiving androgen-deprivation therapy are characterized as having castration-resistant prostate cancer (CRPC); patients who, in addition, have metastases are characterized as having metastatic CRPC.4
Bicalutamide, a first-generation antiandrogen, is widely used for the treatment of nonmetastatic and metastatic CRPC.5,6 Historically, the treatment of patients with metastatic CRPC was mostly palliative until 2004, when a chemotherapy taxane agent, docetaxel, demonstrated survival benefits.7 Since then, several new medications using various pathways have also demonstrated improved patient prognosis and provide diversified advanced treatment options for patients with prostate cancer, including cabazitaxel, a taxane approved by the US Food and Drug Administration (FDA) in 2010; sipuleucel-T, an immunotherapy that was also approved by the FDA in 2010; abiraterone acetate plus prednisone (ie, abiraterone), an oral androgen biosynthesis inhibitor approved by the FDA in 2011 for patients who have previously received docetaxel and then in 2012 was approved as first-line therapy; and enzalutamide, an oral androgen receptor inhibitor that was approved by the FDA in 2012 for patients who have received docetaxel and then in 2014 as first-line therapy.8
With the development of new cancer therapies that effectively prolong patients' lives, concern about the effects of prostate cancer and of cancer therapy on the central nervous system (CNS) has grown.9,10 CNS events may present as pain caused by spinal cord compression resulting from bone metastasis or, less often, as headaches or neurologic changes caused by the spread of cancer to the brain.9 In the case of novel prostate cancer therapies specifically, various clinical trials with enzalutamide and abiraterone acetate reported certain CNS adverse events, such as seizure, increased falls, fatigue, and pain.11–17 Because CNS complications may lead to irreversible neurologic consequences and reduced quality of life, or to tradeoffs in cancer treatment, such as dose reductions or treatment interruption, in the hope of decreasing effects on the CNS, it is important that clinicians identify the early signs of neurologic issues and recognize the impact of cancer therapy on the CNS.9,10
Using a large nationwide insurance healthcare claims database, this study aimed to compare the incidence of CNS complications between patients with advanced prostate cancer who initiated abiraterone, enzalutamide, bicalutamide, or chemotherapy, and to identify predictors associated with the occurrence of CNS complications among these patients. Pain and fatigue were specifically evaluated, because they are among the most common CNS events reported for patients after receiving treatment for metastatic CRPC,11–13 although they may not be systematically attributed to the CNS.
Materials and Methods
Healthcare claims from the Truven Health MarketScan Research databases from January 2005 to December 2014 were used to conduct this study. The data elements included health plan enrollment records, patient demographics, inpatient and outpatient medical services, and outpatient prescription drug dispensing records. All data were deidentified in compliance with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act.
A retrospective longitudinal study design was used. Adults who initiated treatment with abiraterone, enzalutamide, bicalutamide, or chemotherapy (ie, cabazitaxel, docetaxel, mitoxantrone hydrochloride, or estramustine used as monotherapy or combination therapy) in 2012, 2013, or 2014 were identified.
The index date was defined as the date of the first dispensing of one of these treatments, with patients attributed to 1 of 4 cohorts according to the index date treatment. Patients were included in the study population if they had at least 6 months of continuous study eligibility before the index date (ie, the baseline period) and at least 1 diagnosis of prostate cancer (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]: 185.xx) during the period of continuous eligibility.
Patients were excluded from the study if they had a healthcare claim with a diagnosis code for specified CNS events (see definition below) or for another cancer in the baseline period (see Appendix A at www.AHDBonline.com).
Patients were followed from the index date until the earliest of the end of continuous eligibility, end of data availability, or index therapy discontinuation (defined as the end of the days of supply of the first fill for which the next fill is more than 90 days later). Therefore, CNS events occurred while the patients were receiving an index treatment.
Study Outcome Measures
The outcome measures included the risk for any CNS event, especially fatigue and pain. A CNS event was defined as a postindex healthcare claim containing 1 or more diagnosis codes for amnesia or memory impairment, anxiety, ataxia, cognitive disorders, confusion, convulsions, disturbance in attention, dizziness, falls, fatigue/asthenia, hallucinations, headaches, insomnia, pain, paresthesia, seizures, weakness, or other CNS disorders (see Appendix B at www.AHDBonline.com). All types of pain were considered equivalent. This study reports pain using several ICD-9-CM codes, including 338.xx (pain, not elsewhere classified, which is part of the broader category of diseases of the nervous system and sense organs), 780.96 (generalized pain), 307.80 (psychogenic pain, site unspecified), and 307.89 (other pain disorders related to psychological factors).
Statistical Analysis
Descriptive statistics were used to describe patient demographic and clinical characteristics, as well as the proportions of patients with CNS events during treatment exposure. Means, standard deviations, and medians were used to describe the continuous variables; the frequencies and percentages were reported for categorical variables.
Kaplan-Meier curves and adjusted Cox proportional hazards models (ie, time-to-event analyses) were used to compare the Kaplan-Meier rates and the hazard of having CNS events in patients who initiated abiraterone and patients who initiated enzalutamide, bicalutamide, or chemotherapy. For the Cox analyses, multivariate adjustments accounting for potential imbalances between the cohorts were used to control for the demographic and clinical characteristics that were evaluated, depending on the variable, at baseline or before baseline, and the use of opioids was evaluated at different points in time over the observation period. These characteristics included age; region; year of index date; payment type; Quan-Charlson comorbidity index at baseline; the presence of brain and other metastases at baseline; the presence of specific comorbidities at baseline (ie, diabetes, hypertension, cardiovascular disease, and liver disease); prostate cancer treatment at baseline (ie, bicalutamide and chemotherapy, in the case of comparisons, where these agents were not the index treatment, other chemotherapy treatments, and hormonal treatments, except bicalutamide, and sipuleucel-T); the use of corticosteroids at baseline; and the presence of any CNS event at any time before the baseline period.
Because patients receiving bicalutamide might have received their treatment as first-line androgen-deprivation therapy and might have had earlier-stage disease, we also conducted a sensitivity analysis restricting the study population to the subset of patients with a diagnosis for metastases (ICD-9-CM: 196.xx-199.xx) at any time before the index date.
Cox multivariate regression analyses, using the same covariates as described above and an additional variable controlling for the presence of abiraterone at baseline, were also used to identify the key factors associated with any CNS, fatigue, and pain events among the study population. Of note, the time-varying use of opioids was not considered as a covariate for the identification of key factors associated with pain events.
The rates and hazards in the cohort receiving abiraterone versus the other cohorts were compared through log-rank tests, hazard ratios (HRs), 95% confidence intervals (CIs), and P values. Kaplan-Meier rates, as well as the results of the Cox analyses, were reported at 3, 6, 9, and 12 months after the index date. The key factors associated with the presence of CNS events were assessed using HRs, 95% CIs, and P values. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc; Cary, NC).
Results
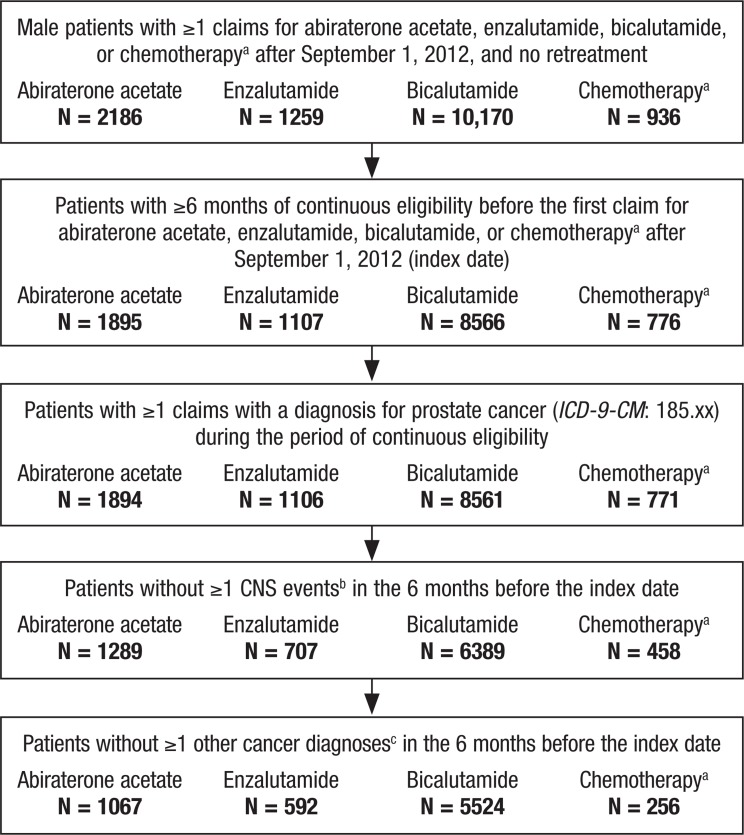
A total of 14,551 patients initiated treatment with abiraterone, enzalutamide, bicalutamide, or chemotherapy after September 1, 2012. Among those, 7439 patients who met the inclusion and exclusion criteria were identified, of whom 1067 initiated abiraterone, 592 enzalutamide, 5524 bicalutamide, and 256 chemotherapy. Figure 1 details the patient selection flowchart, and Table 1 shows the demographics and baseline characteristics of the study population.
Figure 1. Patient Selection.
aChemotherapy includes cabazitaxel, docetaxel, mitoxantrone hydrochloride, and estramustine.
bCNS events include amnesia/memory impairment, anxiety, ataxia, cognitive disorders, confusion, convulsions, disturbance in attention, dizziness, falls, fatigue/asthenia, hallucinations, headaches, insomnia, pain, paresthesia, seizures, weakness, and other CNS disorders.
cOther cancer diagnoses are identified with the following ICD-9-CM: 140.xx-184.xx, 186.xx–195.xx, 200.xx-209.xx.
CNS indicates central nervous system; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
Table 1.
Patient Demographics and Clinical Characteristics, by Treatment Group
| Characteristics | Abiraterone (N = 1067) | Enzalutamide (N = 592) | Bicalutamide (N = 5524) | Chemotherapya (N = 256) |
|---|---|---|---|---|
| Age, mean ± SD (median) | 73.7 ± 10.1 (74.0) | 73.1 ± 10.0 (74.0) | 71.4 ± 10.5 (71.0) | 68.1 ± 9.4 (67.0) |
| Age category, N (%) | ||||
| 35–44 yrs | 2 (0.2) | 0 (0.0) | 11 (0.2) | 0 (0.0) |
| 45–54 yrs | 23 (2.2) | 18 (3.0) | 223 (4.0) | 16 (6.3) |
| 55–64 yrs | 204 (19.1) | 120 (20.3) | 1458 (26.4) | 94 (36.7) |
| 65–74 yrs | 313 (29.3) | 166 (28.0) | 1617 (29.3) | 70 (27.3) |
| ≥75 yrs | 525 (49.2) | 288 (48.6) | 2215 (40.1) | 76 (29.7) |
| Region, N (%) | ||||
| Northeast | 258 (24.2) | 130 (22.0) | 1362 (24.7) | 39 (15.2) |
| North Central | 288 (27.0) | 154 (26.0) | 1405 (25.4) | 74 (28.9) |
| South | 318 (29.8) | 189 (31.9) | 1588 (28.7) | 97 (37.9) |
| West | 198 (18.6) | 113 (19.1) | 1107 (20.0) | 43 (16.8) |
| Unknown | 5 (0.5) | 6 (1.0) | 62 (1.1) | 3 (1.2) |
| Year of index date, N (%) | ||||
| 2012 | 210 (19.7) | 218 (36.8) | 986 (17.8) | 106 (41.4) |
| 2013 | 615 (57.6) | 230 (38.9) | 2520 (45.6) | 94 (36.7) |
| 2014 | 242 (22.7) | 144 (24.3) | 2018 (36.5) | 56 (21.9) |
| Insurance type, N (%) | ||||
| Commercial | 244 (22.9) | 146 (24.7) | 1778 (32.2) | 113 (44.1) |
| Medicare | 823 (77.1) | 446 (75.3) | 3746 (67.8) | 143 (55.9) |
| Quan-Charlson comorbidity index,b mean ± SD (median) | 5.4 ± 2.3 (6.0) | 5.6 ± 2.1 (6.0) | 3.2 ± 2.1 (3.0) | 5.9 ± 1.9 (6.0) |
| Patients with metastases,b N (%) | 707 (66.3) | 440 (74.3) | 904 (16.4) | 212 (82.8) |
| Brain metastases | 18 (1.7) | 14 (2.4) | 13 (0.2) | 4 (1.6) |
| Other metastases | 706 (66.2) | 439 (74.2) | 901 (16.3) | 212 (82.8) |
| Comorbidities,b N (%) | ||||
| Hypertension | 469 (44.0) | 254 (42.9) | 2762 (50.0) | 116 (45.3) |
| Cardiovascular disease | 319 (29.9) | 163 (27.5) | 1583 (28.7) | 66 (25.8) |
| Diabetes | 228 (21.4) | 122 (20.6) | 1239 (22.4) | 50 (19.5) |
| Liver disease | 61 (5.7) | 29 (4.9) | 299 (5.4) | 17 (6.6) |
| Treatments during the baseline period, N (%) | ||||
| Abiraterone | 0 (0.0) | 226 (38.2) | 1 (0.0) | 18 (7.0) |
| Enzalutamide | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bicalutamide | 394 (36.9) | 90 (15.2) | 0 (0.0) | 82 (32.0) |
| Chemotherapy | 81 (7.6) | 108 (18.2) | 4 (0.1) | 0 (0.0) |
| Other chemotherapy treatments | 20 (1.9) | 21 (3.5) | 83 (1.5) | 9 (3.5) |
| Hormone treatmentsc (except bicalutamide) | 829 (77.7) | 421 (71.1) | 1182 (21.4) | 201 (78.5) |
| Sipuleucel-T | 77 (7.2) | 29 (4.9) | 8 (0.1) | 23 (9.0) |
| Radium 223 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Opioids | 382 (35.8) | 267 (45.1) | 1468 (26.6) | 131 (51.2) |
| Corticosteroids | 536 (50.2) | 384 (64.9) | 821 (14.9) | 169 (66.0) |
| Prednisone | 421 (39.5) | 317 (53.5) | 309 (5.6) | 111 (43.4) |
| Dexamethasone | 109 (10.2) | 129 (21.8) | 151 (2.7) | 95 (37.1) |
| Other corticosteroids | 132 (12.4) | 66 (11.1) | 483 (8.7) | 32 (12.5) |
| Hormone treatments before the index date,c,d N (%) | 1022 (95.8) | 557 (94.1) | 1787 (32.3) | 237 (92.6) |
| LhRH/GnRH | 914 (85.7) | 503 (85.0) | 1575 (28.5) | 224 (87.5) |
| Estrogen | 6 (0.6) | 8 (1.4) | 1 (0.0) | 3 (1.2) |
| Antiandrogens | 840 (78.7) | 420 (70.9) | 37 (0.7) | 178 (69.5) |
| Adrenal androgen blockers | 188 (17.6) | 136 (23.0) | 276 (5.0) | 42 (16.4) |
| Hormonal treatment excluding bicalutamide | 961 (90.1) | 526 (88.9) | 1787 (32.3) | 231 (90.2) |
| Orchiectomy,d,e N (%) | 23 (2.2) | 9 (1.5) | 22 (0.4) | 11 (4.3) |
| Time from insurance eligibility start to index date, mo, mean ± SD (median) | 67 ± 34 (66) | 64 ± 35 (60) | 62 ± 37 (58) | 62 ± 34 (60) |
| Any CNS events before baseline period,f N (%) | 544 (51.0) | 322 (54.4) | 2279 (41.3) | 101 (39.5) |
| Time between last CNS event and index date, mo, mean ± SD (median) | 26 ± 21 (19) | 25 ± 19 (20) | 27 ± 22 (20) | 27 ± 21 (18) |
| Use of opioids in the follow-up period, N (%) | 558 (52.3) | 359 (60.6) | 2,122 (38.4) | 160 (62.5) |
| Length of exposure to index treatment, days, mean ± SD (median)g | 246 ± 186 (200) | 184 ± 165 (120) | 141 ± 167 (74) | 121 ± 90 (106) |
Chemotherapy includes cabazitaxel, docetaxel, mitoxantrone hydrochloride, and estramustine.
Evaluated during the 6-month baseline period.
Hormone therapy includes LhRH/GnRH (leuprolide, histrelin, triptorelin, goserelin, buserelin, nafarelin, and degarelix), estrogen (estrogen and diethylstilbestrol), antiandrogens (flutamide, nilutamide, cyproterone, and bicalutamide), and adrenal androgen blockers (ketoconazole and aminoglutethimide).
Identified at any time before the index date.
Identified with CPT codes 54520, 54522, 54530, 54535, and 54690, and with ICD-9-CM procedure codes 62.3 and 62.4.
Identified at any time before the 6-month baseline period.
Exposure spans from index date through the earliest of (1) end of data availability, (2) end of insurance coverage, or (3) treatment nonpersistence (ie, the end of the days of supply of the first fill for which the next fill of the index treatment, if any, is >90 days later). Exposure was not adjusted for early refills.
CNS indicates central nervous system; CPT, Current Procedural Terminology; GnRH, gonadotropin-releasing hormone; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; LhRH, luteinizing hormone-releasing hormone; SD, standard deviation.
Occurrence of CNS Events
Table 2 presents the occurrence of CNS events among patients after 3, 6, 9, and 12 months of treatment exposure. Fatigue and pain were the most reported conditions affecting patients during treatment.
Table 2.
Patients with CNS Events, by Drug Therapy, at 3-Month Intervals
| CNS events | Abiraterone (N = 1067) | Enzalutamide (N = 592) | Bicalutamide (N = 5524) | Chemotherapya (N = 256) | CNS events | Abiraterone (N = 1067) | Enzalutamide (N = 592) | Bicalutamide (N = 5524) | Chemotherapya (N = 256) |
|---|---|---|---|---|---|---|---|---|---|
| Patients with 3 months of exposure, N (%) | 665 (62.3) | 326 (55.1) | 2422 (43.8) | 133 (52.0) | Patients with 9 months of exposure, N (%) | 284 (26.6) | 89 (15.0) | 784 (14.2) | 9 (3.5) |
| Any CNS event | 78 (11.7) | 54 (16.6) | 244 (10.1) | 23 (17.3) | Any CNS event | 80 (28.2) | 29 (32.6) | 194 (24.7) | 3 (33.3) |
| Fatigue/asthenia | 25 (3.8) | 27 (8.3) | 94 (3.9) | 12 (9.0) | Fatigue/asthenia | 31 (10.9) | 11 (12.4) | 87 (11.1) | 0 (0.0) |
| Pain | 19 (2.9) | 15 (4.6) | 41 (1.7) | 6 (4.5) | Pain | 20 (7.0) | 6 (6.7) | 32 (4.1) | 1 (11.1) |
| Dizziness | 9 (1.4) | 6 (1.8) | 39 (1.6) | 1 (0.8) | Dizziness | 17 (6.0) | 2 (2.2) | 44 (5.6) | 0 (0.0) |
| Weakness | 6 (0.9) | 5 (1.5) | 14 (0.6) | 3 (2.3) | Weakness | 10 (3.5) | 6 (6.7) | 21 (2.7) | 2 (22.2) |
| Headaches | 6 (0.9) | 4 (1.2) | 24 (1.0) | 2 (1.5) | Headaches | 10 (3.5) | 1 (1.1) | 19 (2.4) | 1 (11.1) |
| Paresthesia | 5 (0.8) | 1 (0.3) | 21 (0.9) | 2 (1.5) | Paresthesia | 5 (1.8) | 1 (1.1) | 18 (2.3) | 1 (11.1) |
| Insomnia | 5 (0.8) | 3 (0.9) | 12 (0.5) | 1 (0.8) | Insomnia | 6 (2.1) | 0 (0.0) | 8 (1.0) | 1 (11.1) |
| Amnesia/memory impairment | 5 (0.8) | 2 (0.6) | 8 (0.3) | 0 (0.0) | Amnesia/memory impairment | 3 (1.1) | 2 (2.2) | 3 (0.4) | 0 (0.0) |
| Cognitive disorders | 2 (0.3) | 0 (0.0) | 4 (0.2) | 0 (0.0) | Cognitive disorders | 2 (0.7) | 0 (0.0) | 2 (0.3) | 0 (0.0) |
| Seizures | 2 (0.3) | 1 (0.3) | 5 (0.2) | 0 (0.0) | Seizures | 1 (0.4) | 2 (2.2) | 4 (0.5) | 0 (0.0) |
| Anxiety | 3 (0.5) | 2 (0.6) | 17 (0.7) | 1 (0.8) | Anxiety | 4 (1.4) | 1 (1.1) | 16 (2.0) | 1 (11.1) |
| Ataxia | 1 (0.2) | 0 (0.0) | 10 (0.4) | 1 (0.8) | Ataxia | 3 (1.1) | 1 (1.1) | 5 (0.6) | 1 (11.1) |
| Convulsions | 1 (0.2) | 0 (0.0) | 4 (0.2) | 0 (0.0) | Convulsions | 4 (1.4) | 2 (2.2) | 4 (0.5) | 1 (11.1) |
| Falls | 1 (0.2) | 2 (0.6) | 3 (0.1) | 0 (0.0) | Falls | 3 (1.1) | 1 (1.1) | 4 (0.5) | 0 (0.0) |
| Confusion | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | Confusion | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hallucinations | 0 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | Hallucinations | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Disturbance in attention | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | Disturbance in attention | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other CNS disorders | 5 (0.8) | 7 (2.1) | 19 (0.8) | 2 (1.5) | Other CNS disorders | 5 (1.8) | 3 (3.4) | 13 (1.7) | 1 (11.1) |
| Patients with 6 months of exposure, N (%) | 448 (42.0) | 170 (28.7) | 1319 (23.9) | 41 (16.0) | Patients with 12 months of exposure, N (%) | 160 (15.0) | 42 (7.1) | 478 (8.7) | 4 (1.6) |
| Any CNS event | 89 (19.9) | 43 (25.3) | 255 (19.3) | 9 (22.0) | Any CNS event | 59 (36.9) | 19 (45.2) | 142 (29.7) | 1 (25.0) |
| Fatigue/asthenia | 32 (7.1) | 19 (11.2) | 104 (7.9) | 2 (4.9) | Fatigue/asthenia | 24 (15.0) | 8 (19.0) | 63 (13.2) | 1 (25.0) |
| Pain | 21 (4.7) | 9 (5.3) | 36 (2.7) | 3 (7.3) | Pain | 15 (9.4) | 5 (11.9) | 23 (4.8) | 1 (25.0) |
| Dizziness | 15 (3.3) | 8 (4.7) | 52 (3.9) | 1 (2.4) | Dizziness | 8 (5.0) | 2 (4.8) | 32 (6.7) | 0 (0.0) |
| Weakness | 10 (2.2) | 5 (2.9) | 18 (1.4) | 2 (4.9) | Weakness | 11 (6.9) | 5 (11.9) | 16 (3.3) | 1 (25.0) |
| Headaches | 9 (2.0) | 4 (2.4) | 28 (2.1) | 4 (9.8) | Headaches | 6 (3.8) | 1 (2.4) | 16 (3.3) | 0 (0.0) |
| Paresthesia | 7 (1.6) | 2 (1.2) | 27 (2.0) | 2 (4.9) | Paresthesia | 5 (3.1) | 1 (2.4) | 15 (3.1) | 0 (0.0) |
| Insomnia | 6 (1.3) | 1 (0.6) | 13 (1.0) | 2 (4.9) | Insomnia | 3 (1.9) | 0 (0.0) | 8 (1.7) | 0 (0.0) |
| Amnesia/memory impairment | 4 (0.9) | 2 (1.2) | 6 (0.5) | 0 (0.0) | Amnesia/memory impairment | 3 (1.9) | 1 (2.4) | 4 (0.8) | 0 (0.0) |
| Cognitive disorders | 2 (0.4) | 0 (0.0) | 2 (0.2) | 0 (0.0) | Cognitive disorders | 2 (1.3) | 0 (0.0) | 3 (0.6) | 0 (0.0) |
| Seizures | 1 (0.2) | 1 (0.6) | 4 (0.3) | 0 (0.0) | Seizures | 0 (0.0) | 1 (2.4) | 5 (1.0) | 0 (0.0) |
| Anxiety | 3 (0.7) | 2 (1.2) | 17 (1.3) | 1 (2.4) | Anxiety | 2 (1.3) | 1 (2.4) | 12 (2.5) | 0 (0.0) |
| Ataxia | 2 (0.4) | 0 (0.0) | 8 (0.6) | 1 (2.4) | Ataxia | 5 (3.1) | 1 (2.4) | 4 (0.8) | 1 (25.0) |
| Convulsions | 3 (0.7) | 1 (0.6) | 3 (0.2) | 1 (2.4) | Convulsions | 3 (1.9) | 1 (2.4) | 4 (0.8) | 1 (25.0) |
| Falls | 2 (0.4) | 2 (1.2) | 1 (0.1) | 0 (0.0) | Falls | 1 (0.6) | 0 (0.0) | 4 (0.8) | 0 (0.0) |
| Confusion | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | Confusion | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hallucinations | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | Hallucinations | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) |
| Disturbance in attention | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | Disturbance in attention | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other CNS disorders | 4 (0.9) | 4 (2.4) | 16 (1.2) | 0 (0.0) | Other CNS disorders | 2 (1.3) | 3 (7.1) | 9 (1.9) | 0 (0.0) |
Chemotherapy includes cabazitaxel, docetaxel, mitoxantrone hydrochloride, and estramustine.
CNS indicates central nervous system.
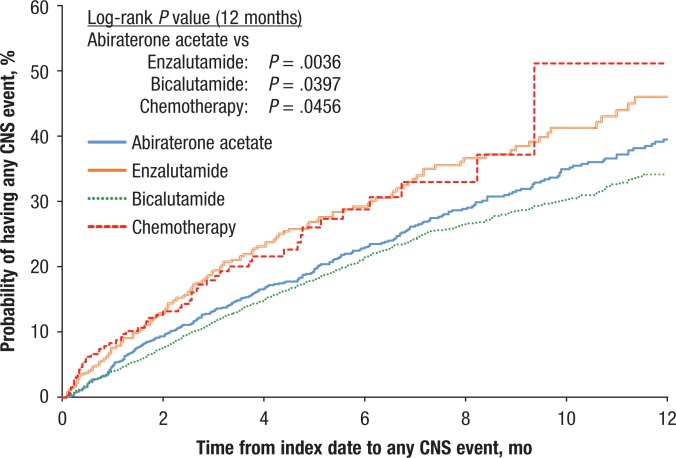
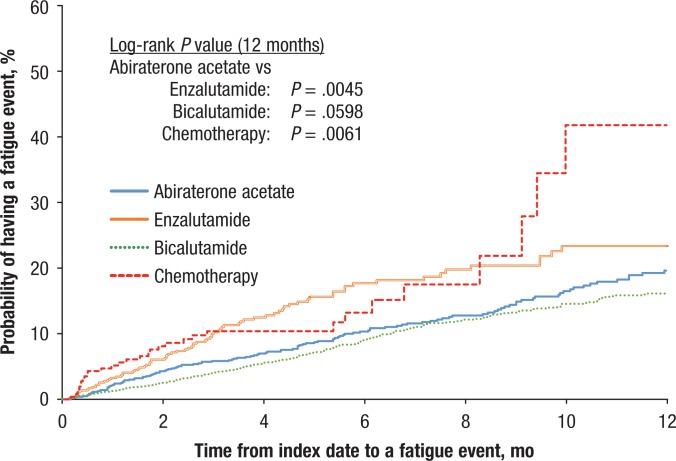
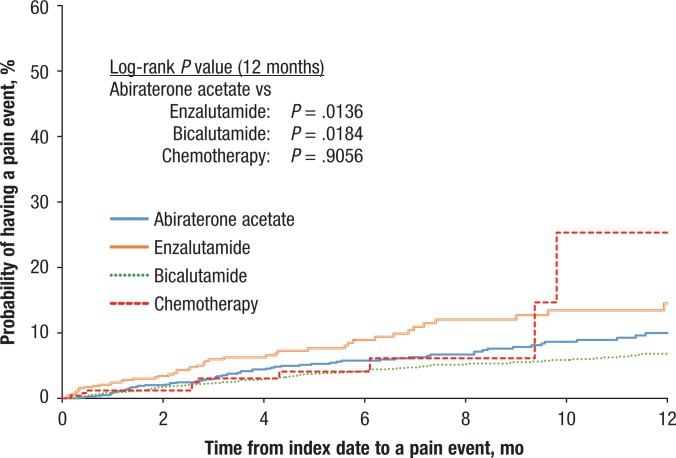
Figures 2, 3, and 4 display the Kaplan-Meier curves for the unadjusted incidence of any CNS, fatigue, and pain events, respectively, for patients who initiated abiraterone compared with patients who initiated treatment with enzalutamide, bicalutamide, or chemotherapy. The figures show that the rates of CNS, pain, and fatigue events were lower in patients using abiraterone than in patients using enzalutamide or chemotherapy.
Figure 2. Time to Any CNS Event.
CNS indicates central nervous system.
Figure 3. Time to Fatigue Events.
Figure 4. Time to Pain Events.
Table 3 compares the Kaplan-Meier rates and the Cox adjusted HRs of having any CNS event at 3, 6, 9, and 12 months between patients receiving abiraterone versus patients from the other cohorts. Compared with patients using enzalutamide or chemotherapy, patients receiving abiraterone were less likely to have CNS events (at 12 months; enzalutamide: 39.5% vs 46.0%, P = .0036; chemotherapy: 39.5% vs 51.1%, P = .0277) and more likely compared with patients receiving bicalutamide (at 12 months; 39.5% vs 34.2%, P = .0397), although differences in Kaplan-Meier rates between patients receiving abiraterone or bicalutamide were not significant before 12 months.
Table 3.
Comparing Time to CNS Events in Patients Receiving Abiraterone, Enzalutamide, Bicalutamide, or Chemotherapy
| Probability of any CNS event, by treatment | Overall population | Sensitivity analysis Population with metastases before the index date | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survival function (Kaplan-Meier estimates) | HRs for CNS conditionsa | Survival function (Kaplan-Meier estimates) | HRs for CNS conditionsa | ||||||||
| Abiraterone cohort N = 1067 | Control cohort N = 592 | P valueb | Abiraterone vs control cohort (95% CI) | P valuec | Abiraterone cohort N = 924 | Control cohort N = 531 | P valueb | Abiraterone vs control cohort (95% CI) | P valuec | ||
| Enzalutamide | 3 months | 13.3% | 19.5% | .0019 | 0.65 (0.49–0.86) | .0028 | 13.5% | 19.7% | .0036 | 0.68 (0.50–0.91) | .0104 |
| 6 months | 23.0% | 29.3% | .0033 | 0.75 (0.59–0.95) | .0169 | 23.4% | 30.5% | .0030 | 0.75 (0.58–0.96) | .0230 | |
| 9 months | 31.7% | 38.5% | .0030 | 0.77 (0.62–0.96) | .0223 | 31.8% | 40.4% | .0018 | 0.75 (0.60–0.95) | .0173 | |
| 12 months | 39.5% | 46.0% | .0036 | 0.80 (0.65–0.99) | .0388 | 40.4% | 49.1% | .0018 | 0.77 (0.62–0.97) | .0246 | |
| N = 1067 | N = 5524 | N = 924 | N = 1917 | ||||||||
| Bicalutamide | 3 months | 13.3% | 11.6% | .1190 | 0.88 (0.69–1.14) | .3408 | 13.5% | 15.9% | .1440 | 0.84 (0.64–1.12) | .2356 |
| 6 months | 23.0% | 21.5% | .2054 | 0.85 (0.69–1.05) | .1263 | 23.4% | 26.8% | .0830 | 0.81 (0.65–1.03) | .0817 | |
| 9 months | 31.7% | 28.6% | .1009 | 0.89 (0.74–1.08) | .2295 | 31.8% | 33.9% | .1440 | 0.85 (0.68–1.04) | .1190 | |
| 12 months | 39.5% | 34.2% | .0397 | 0.92 (0.77–1.10) | .3622 | 40.4% | 41.5% | .2051 | 0.86 (0.71–1.05) | .1472 | |
| N = 1067 | N = 256 | N = 924 | N = 247 | ||||||||
| Chemotherapy | 3 months | 13.3% | 18.0% | .0450 | 0.64 (0.43–0.94) | .0213 | 13.5% | 18.0% | .0645 | 0.64 (0.43–0.95) | .0256 |
| 6 months | 23.0% | 28.8% | .0407 | 0.73 (0.52–1.02) | .0624 | 23.4% | 29.1% | .0568 | 0.71 (0.51–1.00) | .0499 | |
| 9 months | 31.7% | 37.2% | .0431 | 0.73 (0.53–1.00) | .0513 | 31.8% | 37.5% | .0590 | 0.72 (0.52–1.00) | .0470 | |
| 12 months | 39.5% | 51.1% | .0277 | 0.73 (0.53–0.99) | .0456 | 40.4% | 51.4% | .0398 | 0.72 (0.52–0.99) | .0404 | |
The Cox model controls for the following covariates: age, region, year of index date, payment type, Quan-Charlson comorbidity index at baseline, presence of brain metastases at baseline, presence of other metastases at baseline, comorbidities at baseline (ie, diabetes, hypertension, cardiovascular disease, and liver disease), prostate cancer treatment at baseline (ie, bicalutamide and chemotherapy [cabazitaxel, docetaxel, mitoxantrone hydrochloride, and estramustine], other chemotherapy treatments, hormonal treatments [except bicalutamide], and sipuleucel), use of corticosteroids at baseline, presence of any CNS conditions at any time before the 6-month baseline period, and opioid use as a time-varying covariate.
Calculated using the log-rank test with Kaplan-Meier estimates.
Calculated using a Cox proportional hazard model.
CI indicates confidence interval; CNS, central nervous system; HR, hazard ratio.
After multivariate adjustment and at 12 months, patients receiving abiraterone had a 20% reduction in the hazard of having CNS events compared with patients receiving enzalutamide (HR, 0.80; 95% CI, 0.65–0.99), an 8% reduction compared with patients receiving bicalutamide (HR, 0.92; 95% CI, 0.77–1.10), and a 27% reduction compared with patients receiving chemotherapy (HR, 0.73; 95% CI, 0.53–0.99). When restricted to patients with metastatic prostate cancer, the sensitivity analysis yielded similar results (Table 3).
Predictors of CNS Events
Table 4 presents the predictors of having any CNS event, including fatigue and pain events, which were estimated using a Cox proportional hazard model.
Table 4.
Predictors of CNS Events in the Study Population
| Potential predictors | Any CNS condition | Fatigue | Pain | |||
|---|---|---|---|---|---|---|
| HRa (95% CI) | P valueb | HRa (95% CI) | P valueb | HRa (95% CI) | P valueb | |
| Index treatment (reference: abiraterone) | ||||||
| Enzalutamide | 1.26 (1.00–1.58) | .0478 | 1.47 (1.07–2.02) | .0190 | 1.38 (0.88–2.16) | .1653 |
| Bicalutamide | 1.10 (0.91–1.32) | .3321 | 1.08 (0.82–1.41) | .5923 | 1.29 (0.88–1.88) | .1951 |
| Chemotherapy | 1.36 (1.01–1.83) | .0399 | 1.71 (1.14–2.55) | .0091 | 0.85 (0.44–1.63) | .6208 |
| Age at index date | 1.02 (1.01–1.03) | <.0001 | 1.02 (1.01–1.04) | <.0001 | 1.01 (0.99–1.03) | .2822 |
| Region (reference: West) | ||||||
| Northeast | 0.92 (0.80–1.07) | .2958 | 0.78 (0.63–0.98) | .0309 | 0.82 (0.60–1.12) | .2219 |
| South | 0.82 (0.71–0.94) | .0062 | 0.82 (0.67–1.02) | .0711 | 0.51 (0.37–0.71) | <.0001 |
| Midwest | 0.99 (0.84–1.16) | .8661 | 0.94 (0.74–1.18) | .5841 | 0.89 (0.65–1.23) | .4793 |
| Unknown | 1.43 (0.85–2.41) | .1752 | 0.74 (0.28–2.01) | .5615 | 1.88 (0.81–4.34) | .1399 |
| Year of index date (reference: 2012) | ||||||
| 2013 | 0.95 (0.82–1.09) | .4302 | 0.86 (0.70–1.05) | .1354 | 1.13 (0.84–1.51) | .4145 |
| 2014 | 1.01 (0.85–1.19) | .9291 | 0.94 (0.73–1.19) | .5931 | 0.96 (0.67–1.38) | .8318 |
| Commercial insurance (reference: Medicare) | 1.38 (1.13–1.67) | .0012 | 1.16 (0.86–1.56) | .3320 | 1.44 (0.98–2.13) | .0662 |
| Presence of metastases at baseline (reference: no metastases) | ||||||
| Brain metastases | 1.12 (0.65–1.96) | .6795 | 0.57 (0.21–1.54) | .2691 | 0.59 (0.18–1.89) | .3735 |
| Other metastases | 0.98 (0.76–1.26) | .8574 | 0.78 (0.54–1.13) | .1847 | 1.62 (0.92–2.86) | .0957 |
| Quan-Charlson comorbidity index at baseline | 1.09 (1.03–1.15) | .0012 | 1.14 (1.06–1.23) | .0004 | 1.06 (0.94–1.19) | .3380 |
| Comorbidities at baseline (reference: no comorbidity) | ||||||
| Diabetes | 0.94 (0.81–1.09) | .3778 | 0.80 (0.64–1.00) | .0463 | 1.05 (0.77–1.45) | .7446 |
| Hypertension | 1.09 (0.97–1.22) | .1283 | 1.13 (0.96–1.34) | .1423 | 1.05 (0.82–1.34) | .6871 |
| Cardiovascular disease | 1.23 (1.08–1.39) | .0014 | 1.33 (1.11–1.60) | .0019 | 1.17 (0.89–1.53) | .2533 |
| Liver disease | 0.82 (0.63–1.07) | .1446 | 0.82 (0.56–1.21) | .3196 | 0.71 (0.39–1.29) | .2594 |
| Prostate cancer treatment at baseline (reference: no prostate cancer treatment) | ||||||
| Abiraterone | 0.94 (0.68–1.30) | .7025 | 1.11 (0.72–1.71) | .6452 | 0.74 (0.40–1.34) | .3180 |
| Enzalutamide | — | — | — | — | — | — |
| Bicalutamide | 1.06 (0.87–1.28) | .5925 | 1.22 (0.93–1.60) | .1525 | 0.85 (0.56–1.29) | .4447 |
| Chemotherapy | 1.20 (0.89–1.63) | .2271 | 0.87 (0.54–1.41) | .5796 | 1.08 (0.64–1.83) | .7606 |
| Other chemotherapy treatments | 1.13 (0.79–1.62) | .5165 | 0.95 (0.53–1.69) | .8584 | 1.17 (0.59–2.29) | .6524 |
| Other hormonal treatment | 0.88 (0.78–1.00) | .0495 | 1.02 (0.85–1.23) | .8214 | 0.86 (0.66–1.12) | .2509 |
| Sipuleucel-T | 1.16 (0.84–1.60) | .3724 | 0.90 (0.54–1.51) | .6989 | 1.46 (0.81–2.63) | .2083 |
| Corticosteroids | 1.00 (0.87–1.14) | .9826 | 1.05 (0.86–1.28) | .6155 | 1.34 (1.02–1.76) | .0362 |
| Time-varying opioid use (reference: no opioid use) | 2.54 (2.21–2.93) | <.0001 | 1.72 (1.38–2.15) | <.0001 | 8.08 (6.33–10.30) | <.0001 |
| Any CNS conditions at any time before the 6-month baseline period (reference: no CNS condition) | 1.55 (1.39–1.73) | <.0001 | 1.44 (1.22–1.70) | <.0001 | 1.20 (0.95–1.51) | .1331 |
HR >1 indicates a higher hazard of CNS events than the reference.
Calculated using a Cox proportional hazard model.
CI indicates confidence interval; CNS, central nervous system; HR, hazard ratio.
Beginning treatment with enzalutamide or chemotherapy was a strong predictor of having CNS events relative to treatment initiation with abiraterone. The patients starting with enzalutamide were 1.26-fold (26%; 95% CI, 1.00–1.58) more likely to have a CNS event than patients starting with abiraterone. Patients initiating treatment with chemotherapy were 1.36-fold (36%; 95% CI, 1.01–1.83) more likely to have a CNS event than patients starting with abiraterone.
The use of opioids during treatment and the diagnosis of CNS events before the baseline period were also strong predictors of CNS events. Patients receiving opioids during treatment were 2.54-fold (154%; 95% CI, 2.21–2.93) more likely to have CNS events than patients not receiving opioids, and patients presenting with CNS events before the baseline period were 1.55-fold (55%; 95% CI, 1.39–1.73) more likely to have some CNS events during treatment with abiraterone, enzalutamide, bicalutamide, or chemotherapy compared with similar patients who had no evidence of CNS events before treatment initiation with any of these medications.
Exposure to hormonal treatment other than bicalutamide during the baseline period was found to decrease the hazard of CNS events (HR, 0.88; 95% CI, 0.78–1.00). A baseline diagnosis for metastases was not associated with the risk for CNS events (HR for brain metastases, 1.12; 95% CI, 0.65–1.96; HR for other metastases, 0.98; 95% CI, 0.76–1.26). The predictors of fatigue and pain events overall had similar trends.
Discussion
This large retrospective observational study of more than 7000 patients with advanced prostate cancer shows that treatment with abiraterone is associated with a lower risk for CNS events than treatment with enzalutamide or with chemotherapy.
In the literature, advanced prostate cancer has often been associated with CNS events, such as fatigue, pain, or spinal cord compression, which can be symptomatic of disease progression, such as metastases to the spine or brain, or a side effect of treatment.18,19
Our study did not identify the presence of brain or other metastases at baseline as predictors of CNS events. In addition, the sensitivity analysis restricting the study population to patients with a previous diagnosis for metastasis did not affect the study results. These findings suggest that the treatments compared in the study had an impact on the incidence of CNS events independent of the presence of metastases. CNS complications occurring in relation to treatment need to be recognized so they could be addressed, and so that potential complications stemming from irreversible nerve damage could be prevented.10
Chemotherapy has been shown to affect the CNS negatively,20,21 but little is known about the causality. It was initially postulated that cytotoxic chemotherapy agents also damaged nervous system cells that, like tumor cells, have a fast cellular genesis.21 However this would not explain the relationship identified between more targeted novel therapies and the development of CNS complications.10 In 2008, Dietrich and Wen reported, “Recent studies suggest that the cause of neurotoxicity is far more complex than simply toxic effects on proliferating cells.”21 Moreover, prescribing information for chemotherapy agents also report CNS complications, such as headaches, anxiety, insomnia, and paresthesia among others.22–25 The findings of the present study, which show higher rates of CNS complications in patients receiving chemotherapy than in those receiving abiraterone, concur with the previous finding that chemotherapy may negatively affect the CNS.20
The results of the present study suggest that enzalutamide could be a predictor of the occurrence of any CNS event, in particular, fatigue. This is in alignment with the results of various trials that reported an association between enzalutamide and several CNS complications, particularly fatigue.11–13 A phase 1/2 trial suggested a dose-related incidence of fatigue in patients receiving enzalutamide.11 The phase 3 clinical trial AFFIRM, of patients who have received chemotherapy, showed a higher incidence of fatigue, musculoskeletal pain, seizure, and headache in patients who received enzalutamide than in the control cohort.12 The phase 3 clinical trial PREVAIL, of chemotherapy-naïve patients, also found an increased incidence of fatigue, back pain, asthenia, and falls in patients receiving enzalutamide compared with patients in the control group.13 Enzalutamide is known to penetrate the blood–brain barrier, which may at least in part explain its effect on the CNS.26
Little has been published about bicalutamide and CNS events. Nonetheless, one study found that patients who received bicalutamide had less fatigue than patients receiving leuprolide.27 The results from the recent phase 2 clinical trial TERRAIN,28 which compared the impact of enzalutamide and bicalutamide in patients with metastatic CRPC, showed that fatigue, back pain, and hot flashes (thought to be related to changes in the CNS29), were among the side effects occurring more frequently in patients receiving enzalutamide than in those receiving bicalutamide. In the phase 2 clinical trial STRIVE, which compared the efficacy and safety of enzalutamide versus bicalutamide in patients with nonmetastatic or metastatic CRPC, adverse events, including fatigue, back pain, hot flashes, falls, and dizziness, were more common in patients receiving enzalutamide than in patients receiving bicalutamide.30 Our present study showed that qualitatively fewer patients receiving bicalutamide had CNS events relative to patients receiving enzalutamide.
Finally, the phase 3 clinical trial COU-AA-301 reported an improvement in fatigue for patients receiving abiraterone compared with those receiving prednisone alone in a population of patients who had received docetaxel treatment.14 This could suggest a lower CNS toxicity of abiraterone, which may be indirectly supported by the findings in our study. Furthermore, in the same trial, fewer patients discontinued treatment because of toxicities in the abiraterone cohort than in the control group.15
To our knowledge, this is the first study to compare the incidence of CNS complications among patients with advanced prostate cancer who received various therapies. The findings suggest that abiraterone is less toxic to the CNS than chemotherapy and enzalutamide, and they are consistent with previous studies, prescribing information for the therapies, and controlled clinical trials that individually reported CNS complications for enzalutamide and for chemotherapy, and less so for abiraterone; these findings further suggest that physicians should be on the lookout for these complications when prescribing therapies for patients with advanced prostate cancer.
Limitations
This study is subject to several limitations. As is the case with claims databases, the Truven Health MarketScan Research databases may contain inaccuracies or omissions in procedures, diagnoses, or costs. Also, the lack of coding of some events, such as pain or fatigue, may happen because the occurrence of such events is expected with prostate cancer. The diagnostic criteria for CNS events used in this study may not be causally related to CNS dysfunction, but they may be associated with other physical or disease manifestations. Moreover, the CNS events identified in this study are very broad and numerous; some are very serious (requiring healthcare resource utilization) and some may be less serious (ie, minor annoyance).
Furthermore, adherence to treatment was assumed in this study, and claims-based analyses of oral therapies cannot confirm adherence. Similarly, this study could not specifically confirm the stage of the disease. Hence, some patients in our study who received bicalutamide may have received their treatment as first-line androgen-deprivation therapy, and thus have been at an earlier disease stage. The sensitivity analysis was restricted to patients with a previous diagnosis of metastasis to address this limitation.
In addition, the study design evaluated patients who were naïve to their index treatment, but not necessarily to the other treatments (but all patients were naïve to enzalutamide, because it was not available before September 1, 2012); notably, 38.2% of patients who received enzalutamide had received abiraterone acetate at baseline. Further research is warranted to better understand the impact that multiple treatment sequence may have on the CNS.
This study excluded patients with CNS events in the 6 months preceding the index date. Although this criterion reduced the cohorts by 26% to 41% depending on the treatment, which might have affected the overall generalizability of the results, the goal of the study was to investigate new CNS events rather than capture recurring events in the follow-up period that might have been caused by something other than the initiated treatment. Additional research would be necessary to evaluate the recurrence of CNS events.
Finally, abiraterone acetate is approved in the United States for use in combination with prednisone for the treatment of metastatic CRPC. However, the abiraterone cohort used in this analysis was limited to providing data only on patients receiving abiraterone acetate.
Despite these limitations, observational studies with health insurance claims data that use statistical techniques to adjust for multiple potential observed confounding factors provide valuable real-world information and high generalizability.
Conclusion
This large retrospective observational study, using real-world data, shows that patients with advanced prostate cancer who received abiraterone acetate were less likely to have CNS events than those receiving enzalutamide or chemotherapy, and had similar likelihood of having CNS events as patients who received bicalutamide treatment, even when controlling for metastatic disease. Future prospective studies are warranted to evaluate the impact of CNS events on treatment duration, dosing variation, and overall survival in patients who receive treatment for metastatic CRPC. Furthermore, additional studies controlling for the severity of CNS are needed to understand the impact of CNS events on healthcare costs, resource utilization, and therapy discontinuations in patients with advanced prostate cancer.
Source of Funding
This research was funded by Janssen Scientific Affairs.
Author Disclosure Statement
Mr Pilon is an employee of Groupe d'analyse, which received research grants from Janssen for this study. Dr Behl is an employee of Janssen Scientific Affairs and a stockholder of Johnson & Johnson. Dr Ellis is an employee of Janssen Scientific Affairs and a stockholder of Johnson & Johnson. Ms Robitaille was an employee of Analysis Group at the time of this study. Mr Lefebvre is an employee of Groupe d'analyse. Dr Dawson is on the Speaker's Bureaus of Janssen and of Astellas.
Contributor Information
Dominic Pilon, Senior Economist, Groupe d'analyse, Ltée, Montréal, Quebec, Canada.
Ajay S. Behl, Director, US Health Economics and Outcomes Research, Janssen Scientific Affairs, Horsham, PA.
Lorie A. Ellis, Director, US Health Economics and Outcomes Research, Janssen Scientific Affairs.
Marie-Noëlle Robitaille, Economist, Groupe d'analyse, during this study.
Patrick Lefebvre, Managing Principal, Groupe d'analyse.
Nancy A. Dawson, Oncologist, Georgetown University Lombardi Comprehensive Cancer Center, Washington, DC..
References
- 1.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006; 66: 7783–7792. [DOI] [PubMed] [Google Scholar]
- 2.Dawson NA. Patient education: treatment for advanced prostate cancer (Beyond the Basics). UpToDate. Updated February 2016. www.uptodate.com/contents/treatment-for-advanced-prostate-cancer-beyond-the-basics. Accessed November 21, 2016.
- 3.Kantoff PW, Higano CS, Shore ND, et al; for the IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010; 363: 411–422. [DOI] [PubMed] [Google Scholar]
- 4.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011; 65: 1180–1192. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Prostate Cancer. Version 2.2017. February 21, 2017. www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed April 7, 2015.
- 6.Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010; 17(suppl 2): S72–S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004; 351: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 8.Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015; 26: 1589–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamin R. Neurologic complications of prostate cancer. Am Fam Physician. 2002; 65: 1834–1840. [PubMed] [Google Scholar]
- 10.Rinne ML, Lee EQ, Wen PY. Central nervous system complications of cancer therapy. J Support Oncol. 2012; 10: 133–141. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Beer TM, Higano CS, et al; for the Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010; 375: 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher HI, Fizazi K, Saad F, et al; for the AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012; 367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 13.Beer TM, Armstrong AJ, Rathkopf DE, et al; for the PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternberg CN, Molina A, North S, et al. Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann Oncol. 2013; 24: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 15.de Bono JS, Logothetis CJ, Molina A, et al; for the COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan CJ, Smith MR, Fizazi K, et al; for the COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015; 16: 152–160. [DOI] [PubMed] [Google Scholar]
- 17.Ryan CJ, Smith MR, de Bono JS, et al; for the COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368: 138–148. Erratum in: N Engl J Med. 2013; 368:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langston B, Armes J, Levy A, et al. The prevalence and severity of fatigue in men with prostate cancer: a systematic review of the literature. Support Care Cancer. 2013; 21: 1761–1771. [DOI] [PubMed] [Google Scholar]
- 19.Smith JA, Jr, Soloway MS, Young MJ. Complications of advanced prostate cancer. Urology. 1999; 54(6A suppl): 8–14. [DOI] [PubMed] [Google Scholar]
- 20.Soussain C, Ricard D, Fike JR, et al. CNS complications of radiotherapy and chemotherapy. Lancet. 2009; 374: 1639–1651. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich J, Wen PY. Neurologic complications of chemotherapy. In: Schiff D, Kesari S, Wen PY, eds. Cancer Neurology in Clinical Practice: Neurologic Complications of Cancer and Its Treatment. 2nd ed. Totowa, NJ: Humana Press; 2008:287–326.
- 22.Xtandi (enzalutamide) capsules [prescribing information]. Northbrook, IL: Astellas Pharma US; October 2016.
- 23.Jevtana (cabazitaxel) injection [prescribing information]. Bridgewater, NJ: sanofi-aventis US; September 2016.
- 24.Novantrone (mitoxantrone for injection concentrate) [prescribing information]. Rockland, MA: EMD Serono; March 2012.
- 25.Emcyt (estramustine phosphate sodium) capsules [prescribing information]. New York, NY: Pfizer; June 2007.
- 26.Vogelzang NJ. Enzalutamide—a major advance in the treatment of metastatic prostate cancer. N Engl J Med. 2012; 367: 1256–1257. [DOI] [PubMed] [Google Scholar]
- 27.Smith MR, Goode M, Zietman AL, et al. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004; 22: 2546–2553. [DOI] [PubMed] [Google Scholar]
- 28.Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016; 17: 153–163. [DOI] [PubMed] [Google Scholar]
- 29.Archer DF, Sturdee DW, Baber R, et al. Menopausal hot flushes and night sweats: where are we now? Climacteric. 2011; 14: 515–528. [DOI] [PubMed] [Google Scholar]
- 30.Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016; 34: 2098–2106. [DOI] [PubMed] [Google Scholar]