Abstract
Background
Evidence of poor patient adherence to medications for chronic obstructive pulmonary disease (COPD) is well-documented, but its impact on disease exacerbation rates and associated healthcare costs remains unclear.
Objective
To assess the association between adherence levels to different inhaled corticosteroid/long-acting ß2-adrenergic agonist (LABA) and COPD exacerbation rates and costs in a commercially insured population.
Methods
In this observational cohort study, patients with COPD (aged ≥40 years) who were treatment-naïve to inhaled corticosteroid/LABA and were initiating budesonide plus formoterol or fluticasone plus salmeterol between March 1, 2009, and January 31, 2014, were identified in a national representative claims database and were followed for up to 12 months. The date of the first prescription fill for either drug was defined as the index date. Patients were divided into 4 cohorts based on adherence to the index therapy, which was measured by proportion of days covered (PDC); the cohorts were classified as adherent (PDC ≥0.8), mildly nonadherent (0.5 ≤ PDC <0.8), moderately nonadherent (0.3 ≤ PDC <0.5), and highly nonadherent (PDC <0.3). Each nonadherent group was matched in a 1:1 ratio to the adherent group independently, based on prognostically important variables, using propensity score analyses. Exacerbation rates and healthcare costs were analyzed for 1 year after treatment initiation.
Results
During the study period, 13,657 eligible patients with COPD initiated inhaled corticosteroid/LABA; of these, only 1898 (13.9%) patients were adherent during follow-up. Group matching resulted in 1572 patients per group for comparison 1 (adherent vs mildly nonadherent), 1604 patients for comparison 2 (adherent vs moderately nonadherent), and 1755 patients for comparison 3 (adherent vs highly nonadherent). The moderately and highly nonadherent cohorts had higher exacerbation rates than the adherent patients (comparison 2: rate ratio [RR], 1.11; 95% confidence interval [CI], 1.01–1.21; P = .03; comparison 3: RR, 1.11; 95% CI, 1.01–1.21; P = .02). Adherent patients incurred significantly lower healthcare costs than all the nonadherent groups (comparison 1, $22,671 vs $25,545; P <.01; comparison 2, $22,508 vs $24,303; P <.01; comparison 3, $22,460 vs $25,148; P <.01).
Conclusions
Patients adhered to their inhaled corticosteroid/LABA treatments had lower COPD exacerbation rates and lower healthcare costs compared with the moderately and highly nonadherent patients. Better adherence to maintenance therapies may help to reduce the clinical and economic burdens of COPD.
Keywords: adherence, COPD, cost, disease exacerbation, economic burden, inhaled corticosteroid/LABA, nonadherence, propensity score matching, proportion of days covered
Chronic obstructive pulmonary disease (COPD) is characterized by declining lung function attributable to a combination of airway obstruction and inflammation. COPD mainly includes 2 chronic lower respiratory disease disorders—emphysema and chronic bronchitis.1 Approximately 14.8 million people in the United States have been diagnosed with asthma/COPD.2 The condition is associated with substantial disability and was the third leading cause of death in the United States in 2011.3 The annual economic burden of asthma/COPD was estimated to be approximately $68 billion in 2008, including $53.7 billion in direct healthcare and $14.3 billion in indirect mortality costs.2
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines highlight the prevention and treatment of exacerbations as primary objectives for COPD management, underscoring the deleterious effects of exacerbations on patients and the care delivery system.4,5 Recent treatment guidelines (ie, GOLD 2016) suggest initiating controller medications in accordance with patients' history of exacerbations and symptoms.6 For patients with a history of COPD exacerbations, treatment with inhaled corticosteroid/long-acting ß2-adrenergic agonist (LABA) combination therapies is the recommended option; the use of a LABA or an inhaled corticosteroid individually as monotherapy is not recommended.6 To date, the US Food and Drug Administration–approved inhaled corticosteroid/LABA combination medications for COPD maintenance therapy include budesonide plus formoterol (160/4.5 μg),7 fluticasone plus salmeterol (250/50 μg),8 and fluticasone furoate plus vilanterol (100/25 μg).9
The clinical efficacy and safety of inhaled corticosteroid/LABA combination therapy have been demonstrated in several randomized clinical trials (RCTs).10,11 However, one of the characteristics of RCTs is the requirement for high adherence rates among patients who are assigned treatments that are under investigation. This differs substantially from how patients adhere to their prescribed medication regimens in real-world settings, and limits the utility of extracting healthcare utilization data and the resulting economic impact on a system of care within the constraints of most RCTs. Restrepo and colleagues suggested that, based on self-reports, only an average of 40% to 60% of patients with COPD adhere to the prescribed medication.12 Systematic reviews have emphasized the inadequacy of medication adherence among patients with COPD, resulting from reasons such as medication delivery mechanisms, cost burden, and clinicians' preferences.13–16
To date, no retrospective observational study has examined the impact of adherence on outcomes for patients with COPD. The objective of this study was to evaluate the association between different levels of adherence to fixed-dose inhaled corticosteroid/LABA therapy and exacerbation rates and healthcare costs in a cohort of commercially insured patients with COPD receiving usual care in real-world settings in the United States.
KEY POINTS
-
▸
COPD causes substantial disability and death, yet COPD-related exacerbation rates and healthcare costs are not well-documented.
-
▸
This study included 13,657 patients with COPD who were treatment-naïve to inhaled corticosteroid/LABA and initiated budesonide plus formoterol or fluticasone plus salmeterol therapy.
-
▸
Patients were placed into 4 cohorts based on adherence as measured by proportion of days covered—adherent, mildly nonadherent, moderately nonadherent, and highly nonadherent.
-
▸
The moderately and highly nonadherent cohorts had more exacerbations than adherent patients, resulting from increased hospitalizations and emergency department visits.
-
▸
Adherent patients had lower healthcare costs than the nonadherent groups (comparison 1: $22,671 vs $25,545; comparison 2: $22,508 vs $24,303; comparison 3: $22,460 vs $25,148).
-
▸
These findings suggest that there are missed opportunities to obtain optimal clinical benefits from maintenance therapies for COPD, improve adherence, and potentially reduce the economic burden associated with this disease.
Methods
Data Source and Study Design
Data for this observational cohort study were drawn from a commercially insured population residing in 50 states whose administrative claims are curated in the HealthCore Integrated Research Database. The data cover claims from 14 commercial health plans in which Medicare supplemental coverage and commercially managed Medicare Advantage plans are included, which constituted a small portion of the study population. However, federal Medicare and state Medicaid claims are not captured in this database. Medical and pharmacy claims data were queried to identify patients with COPD (≥40 years) who had not received any inhaled corticosteroid/LABA combination therapy during the 12 months before initiating budesonide plus formoterol (160/4.5 μg) or fluticasone plus salmeterol (250/50 μg) therapy between March 1, 2009, and January 31, 2014. Fluticasone furoate plus vilanterol (100/25 μg) therapy was not targeted in this study because of the small sample size during the study period. Strict compliance with applicable Health Insurance Portability and Accountability Act rules was exercised related to the data throughout the study. The study data were kept anonymous to preserve patient confidentiality, and researchers' access did not include individual patient identifiers. This nonexperimental study was conducted under the Research Exception provisions of the Privacy Rule, 45 CFR 164.514(e), and was exempt from Institutional Review Board review.
Inclusion Criteria
The study patients had ≥1 prescription fills for budesonide plus formoterol (160/4.5 μg) or fluticasone plus salmeterol (250/50 μg) between March 1, 2009, and January 31, 2014, and the first observed prescription fill date for either drug was considered the index date. Patients were included in the study if they were aged ≥40 years as of the index date. Inclusion also required a diagnosis of COPD (International Classification of Diseases, Ninth Revision, Clinical Modification codes 491.xx, 492.xx, 496.xx) and ≥1 prescription fills for a short-acting ß2-adrenergic agonist (SABA), short-acting muscarinic antagonist (SAMA), and/or the combination of SABA and SAMA during the 12-month preindex period. All patients were required to have ≥12 months of continuous health plan enrollment before and after the index date.
Exclusion Criteria
Patients with ≥1 prescription fills for any inhaled corticosteroid/LABA combination therapy (including all strength forms of budesonide plus formoterol, fluticasone plus salmeterol, fluticasone furoate plus vilanterol, and mometasone furoate plus formoterol) during the 12-month preindex period were excluded from the analysis. Also excluded were patients who filled budesonide plus formoterol (160/4.5 μg) and fluticasone plus salmeterol (250/50 μg) on the index date, and long-term users for any oral corticosteroids (≥180 days of total length of therapy) during the 12-month preindex period. Patients with ≥2 diagnoses for the same type of cancer within 60 days of each other during the 12-month preindex period were also excluded.
Postindex Follow-Up
The patients were followed for up to 12 months after the index date. If they filled an inhaled corticosteroid/LABA medication different from their index therapy (switching therapy) during the 12-month period, their postindex follow-up was censored at the time of the switch.
Study Cohorts
The study patients were stratified into 4 cohorts based on their medication adherence levels—which was measured by the proportion of days covered (PDC)17,18—to the index therapy during the 12-month postindex period. The PDC was calculated as the number of days a patient had index inhaled corticosteroid/LABA therapy on hand, divided by the number of health plan enrollment days during the postindex follow-up. The PDC ranged from 0 to 1, with a higher number indicating better adherence. A PDC >0.5 means that the patient had at least a 6-month supply for the index medication over 12 months of observation. The patients were classified as adherent (PDC ≥0.8), mildly nonadherent (0.5 ≤ PDC <0.8), moderately nonadherent (0.3 ≤ PDC <0.5), or highly nonadherent (PDC <0.3).
Group Matching
To create comparable cohorts, each nonadherent group was matched to the adherent group independently (1:1) based on demographic and preinitiation clinical characteristics using propensity scores that were calculated from logistic regression models.19,20 The outcome variable in the model was dichotomous, indicating whether a patient was adherent (1) to the index inhaled corticosteroid/LABA combination therapy or nonadherent (0). The goal was to have a similar distribution of patient characteristics between the matched cohorts. Unadjusted bivariate tests were conducted, with α = 0.05, to determine whether the groups were well-balanced. All the following variables were required to be balanced after matching: the number of preindex hospitalizations with a primary diagnosis of COPD; the number of preindex emergency department visits with a primary or secondary diagnosis of COPD; the number of preindex oral corticosteroids, antibiotics, SABA, SAMA, SABA/SAMA, LABA, and long-acting muscarinic antagonist prescription fills; comorbidities; age; sex; and preindex asthma diagnosis.
Outcome Measures
Exacerbation rates and all-cause and COPD-related (medical claims with a COPD diagnosis and pharmacy claims for COPD medication) healthcare costs (adjusted to 2014 US dollars) during the 12-month postindex period were compared between the adherent and nonadherent cohorts, independently. Exacerbation events included hospitalizations with a primary diagnosis of COPD, emergency department visits with a primary or secondary diagnosis of COPD, and outpatient visits with a diagnosis of COPD and oral corticosteroids and/or an antibiotic medication fill on the same day of, or within 10 days after, the outpatient visit.
Statistical Analysis
The exacerbation rates were evaluated with generalized linear models (GLMs) with a negative binomial distribution and log link function. The costs were estimated with GLMs with a gamma distribution and log link weighted by the length of follow-up. The models controlled for all unbalanced preindex factors and the analogous preindex variable (eg, when analyzing the number of COPD-related hospitalizations postindex, the model controlled for the number of preindex COPD-related hospitalizations). All statistical analyses were performed with SAS version 9.4 (SAS Institute, Inc; Cary, NC).
Results
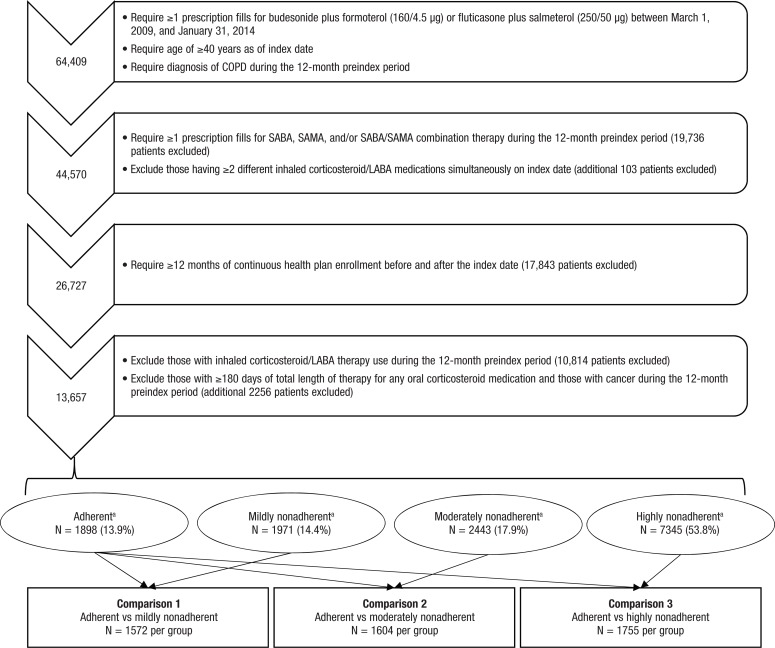
Among the total 13,657 eligible patients with COPD who initiated prespecified inhaled corticosteroid/LABA fixed-dose combination therapy, 1898 (13.9%) patients were adherent to their treatment during the 12-month postindex period. A total of 1971 (14.4%) patients were mildly nonadherent, 2443 (17.9%) patients were moderately nonadherent, and 7345 (53.8%) patients were highly nonadherent. Group matching resulted in 1572 patients per group for comparison 1 (adherent vs mildly nonadherent), 1604 patients per group for comparison 2 (adherent vs moderately nonadherent), and 1755 patients per group for comparison 3 (adherent vs highly nonadherent; Figure 1).
Figure 1. Medication Nonadherence in Patients with COPD.
aAdherent, proportion of days covered (PDC) ≥0.8; mildly nonadherent, 0.5 ≤ PDC <0.8; moderately nonadherent, 0.3 ≤ PDC <0.5; highly nonadherent, PDC <0.3.
COPD indicates chronic obstructive pulmonary disease; LABA, long-acting ß2-adrenergic agonist; SABA, short-acting ß2-adrenergic agonist; SAMA, short-acting muscarinic antagonist.
The cohorts were well-balanced in age (mean, 67 years), sex (51%–53% female), previous COPD-related medication use, healthcare utilization, and comorbid conditions (Table).
Table.
Baseline Demographics and Clinical Characteristics of Patients with COPD Initiating Budesonide plus Formoterol (160/4.5 μg) or Fluticasone plus Salmeterol (250/50 μg)
| Comparison 1 (N = 1572 each) | Comparison 2 (N = 1604 each) | Comparison 3 (N = 1755 each) | ||||
|---|---|---|---|---|---|---|
| Parameter | Adherenta | Mildly nonadherenta | Adherent | Moderately nonadherent | Adherent | Highly nonadherenta |
| Demographics | ||||||
| Age, mean (SD) | 67.0 (11.0) | 67.1 (11.0) | 67.2 (10.9) | 67.2 (11.1) | 67.2 (10.9) | 67.3 (11.1) |
| Female, N (%) | 833 (53.0) | 821 (52.2) | 838 (52.2) | 841 (52.4) | 898 (51.2) | 900 (51.3) |
| COPD severity | ||||||
| Previous COPD inpatient visits, mean (SD) | 0.2 (0.4) | 0.2 (0.4) | 0.2 (0.4) | 0.2 (0.4) | 0.2 (0.4) | 0.2 (0.5) |
| Previous COPD emergency department visits, mean (SD) | 0.2 (0.6) | 0.2 (0.6) | 0.2 (0.6) | 0.2 (0.6) | 0.2 (0.6) | 0.3 (0.7) |
| Previous oral corticosteroid fills, mean (SD) | 1.4 (1.7) | 1.3 (1.7) | 1.3 (1.7) | 1.3 (1.7) | 1.3 (1.7) | 1.3 (1.8) |
| Previous antibiotic fills, mean (SD) | 2.6 (2.8) | 2.7 (3.0) | 2.7 (3.0) | 2.6 (2.8) | 2.7 (3.0) | 2.8 (3.1) |
| COPD medications | ||||||
| Previous SABA, SAMA, or SABA/SAMA fills, mean (SD) | 5.3 (5.7) | 5.2 (5.7) | 4.8 (4.9) | 4.7 (5.1) | 5.2 (5.3) | 5.1 (5.8) |
| Previous LABA fills, mean (SD) | 0.3 (1.6) | 0.3 (1.6) | 0.2 (1.3) | 0.2 (1.3) | 0.3 (1.5) | 0.3 (1.4) |
| Previous LAMA fills, mean (SD) | 1.8 (3.3) | 1.8 (3.3) | 1.6 (3.1) | 1.7 (3.1) | 1.7 (3.2) | 1.7 (3.1) |
| Comorbid conditions, N (%) | ||||||
| Hypertension | 1095 (69.7) | 1104 (70.2) | 1139 (71.0) | 1142 (71.2) | 1244 (70.9) | 1243 (70.8) |
| Depression or psychotropic drug use | 813 (51.7) | 795 (50.6) | 822 (51.2) | 813 (50.7) | 890 (50.7) | 896 (51.1) |
| Asthma | 541 (34.4) | 550 (35.0) | 548 (34.2) | 538 (33.5) | 601 (34.2) | 605 (34.5) |
| Coronary artery disease | 466 (29.6) | 474 (30.2) | 476 (29.7) | 489 (30.5) | 531 (30.3) | 518 (29.5) |
| Pneumonia | 347 (22.1) | 364 (23.2) | 375 (23.4) | 363 (22.6) | 409 (23.3) | 420 (23.9) |
| Diabetes | 333 (21.2) | 323 (20.5) | 341 (21.3) | 335 (20.9) | 368 (21.0) | 356 (20.3) |
| Congestive heart failure | 304 (19.3) | 290 (18.4) | 306 (19.1) | 302 (18.8) | 325 (18.5) | 326 (18.6) |
| Anxiety | 238 (15.1) | 227 (14.4) | 239 (14.9) | 226 (14.1) | 258 (14.7) | 283 (16.1) |
| Pulmonary hypertension | 108 (6.9) | 107 (6.8) | 107 (6.7) | 103 (6.4) | 120 (6.8) | 123 (7.0) |
| Chronic respiratory failure | 95 (6.0) | 91 (5.8) | 94 (5.9) | 95 (5.9) | 108 (6.2) | 112 (6.4) |
| Stroke | 51 (3.2) | 50 (3.2) | 48 (3.0) | 49 (3.1) | 51 (2.9) | 60 (3.4) |
| Left ventricular failure | 23 (1.5) | 27 (1.7) | 28 (1.7) | 31 (1.9) | 28 (1.6) | 29 (1.7) |
Adherent, proportion of days covered (PDC) ≥0.8; mildly nonadherent, 0.5 ≤ PDC <0.8; moderately nonadherent, 0.3 ≤ PDC <0.5; highly nonadherent, PDC <0.3.
COPD indicates chronic obstructive pulmonary disease; LABA, long-acting ß2-adrenergic agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting ß2-adrenergic agonist; SAMA, short-acting muscarinic antagonist; SD, standard deviation.
Exacerbation Rates
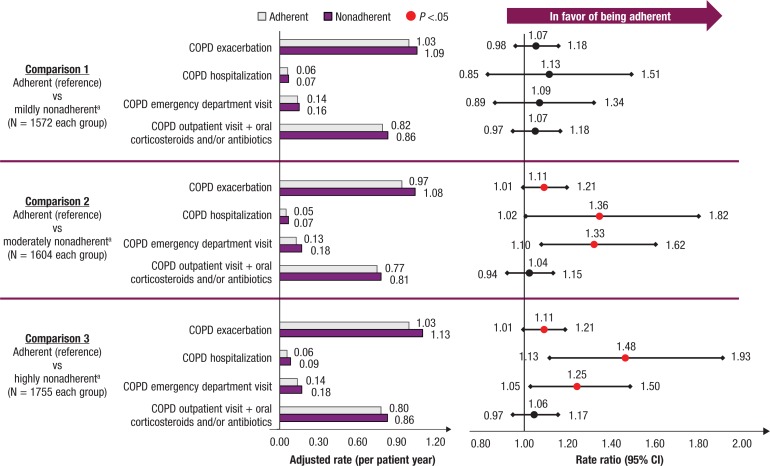
During follow-up, no significant difference in COPD exacerbation rate was seen between the adherent and mildly nonadherent cohorts (rate ratio [RR], 1.07; 95% confidence interval [CI], 0.98–1.18; P = .14; Figure 2). The moderately nonadherent and highly nonadherent cohorts had significantly higher rates of COPD exacerbations compared with the adherent cohort (comparison 2: RR, 1.11; 95% CI, 1.01–1.21; P = .03; comparison 3: RR, 1.11; 95% CI, 1.01–1.21; P = .02), which was mainly driven by higher rates of COPD-related hospitalizations (comparison 2: RR, 1.36; 95% CI, 1.02–1.82; P = .03; comparison 3: RR, 1.48; 95% CI, 1.13–1.93; P <.01) and COPD-related emergency department visits (comparison 2: RR, 1.33; 95% CI, 1.10–1.62; P <.01; comparison 3: RR, 1.25; 95% CI, 1.05–1.50; P = .01).
Figure 2. Association Between Nonadherence to First-Year Fixed-Dose Combination Inhaled Corticosteroid/LABA Therapy and COPD Exacerbations Rate.
aAdherent, proportion of days covered (PDC) ≥0.8; mildly nonadherent, 0.5 ≤ PDC <0.8; moderately nonadherent, 0.3 ≤ PDC <0.5; highly nonadherent, PDC <0.3.
CI indicates confidence interval; COPD, chronic obstructive pulmonary disease; LABA, long-acting ß2-adrenergic agonist.
Healthcare Costs
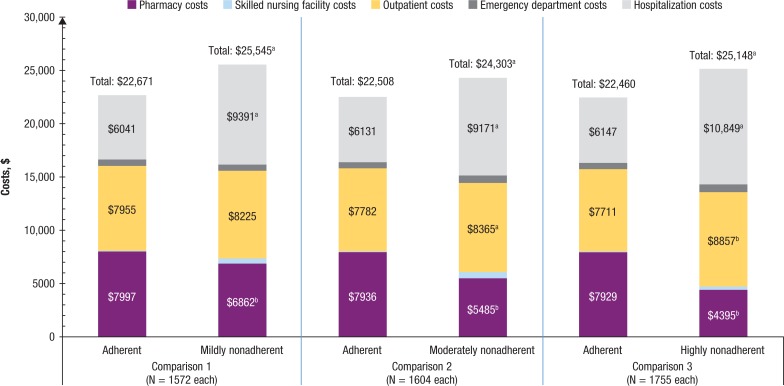
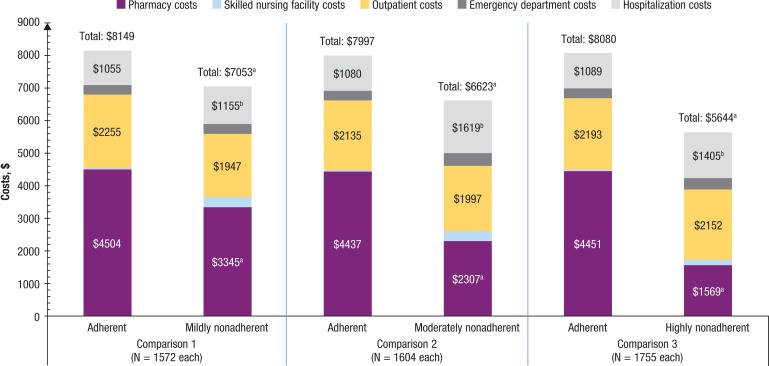
As shown in Figure 3, during the 12-month follow-up, adherent patients incurred significantly lower mean all-cause healthcare costs than nonadherent patients for the 3 comparisons (comparison 1, unadjusted mean $22,671 vs $25,545; adjusted mean difference, $1653; P <.01; comparison 2, $22,508 vs $24,303; adjusted mean difference, $2001; P <.01; comparison 3, $22,460 vs $25,148; adjusted mean difference, $1854; P <.01), which was mainly driven by lower hospitalization and outpatient costs, despite the higher pharmacy costs. Although COPD-related hospitalization costs were lower in adherent patients, as shown in Figure 4, the higher COPD-related pharmacy costs resulted in significantly higher mean total COPD-related healthcare costs for adherent patients (comparison 1, unadjusted mean, $8149 vs $7053; adjusted mean difference −$712; P <.01; comparison 2, $7997 vs $6623; adjusted mean difference −$1282; P <.01; comparison 3, $8080 vs $5644; adjusted mean difference −$2639; P <.01).
Figure 3. Postindex All-Cause Healthcare Costs Among Patients with COPD.
NOTE: Values for emergency department costs and SNF costs are not shown in the figure because of their relatively small amount. Adherent, proportion of days covered (PDC) ≥0.8; mildly nonadherent, 0.5 ≤ PDC <0.8; moderately nonadherent, 0.3 ≤ PDC <0.5; highly nonadherent, PDC <0.3.
aP <.05, in favor of being adherent.
bP <.05, in favor of being nonadherent.
COPD indicates chronic obstructive pulmonary disease.
Figure 4. Postindex COPD-Related Healthcare Costs Among Patients with COPD.
NOTE: Values for emergency department costs and skilled nursing facility costs are not shown in the figure because of their relatively small amount. Adherent, proportion of days covered (PDC) ≥0.8; mildly nonadherent, 0.5 ≤ PDC <0.8; moderately nonadherent, 0.3 ≤ PDC <0.5; highly nonadherent, PDC <0.3.
aP <.05, in favor of being nonadherent.
bP <.05, in favor of being adherent.
COPD indicates chronic obstructive pulmonary disease.
Discussion
The disease exacerbation rates were significantly higher among moderately and highly nonadherent patients compared with adherent patients, although they were not different between the adherent and mildly nonadherent cohorts in this study. This finding points to an association between poor adherence and increased rates of COPD exacerbations during the 12-month follow-up period in this cohort of patients with COPD. Consistent with previous literature that showed suboptimal adherence to COPD medication,12,14–16 we found that the majority of patients with COPD had poor adherence to fixed-dose combination inhaled corticosteroid/LABA therapy, which suggests lost opportunities in usual care settings to optimize the benefits of this maintenance therapy.
This study's findings show that better treatment adherence to fixed-dose combination inhaled corticosteroid/LABA therapy was associated with significantly lower all-cause healthcare costs during the 12-month period after treatment initiation, which was mainly driven by the lower all-cause hospitalization and outpatient costs. Notably, patients adherent to treatment had fewer all-cause hospitalizations and intensive care unit stays relative to each of the 3 nonadherent groups.
One explanation for this observation is that adherence to fixed-dose combination inhaled corticosteroid/LABA therapy could indicate better adherence to medications for comorbid conditions. Although not assessed in this study, overall medication adherence might have contributed to the reduced healthcare utilization for conditions other than COPD and might have subsequently led to lower overall healthcare costs. With regard to COPD-specific healthcare costs, we found that adherent patients incurred significantly higher COPD-related pharmacy costs that were not completely offset by savings from reduced COPD-related inpatient hospitalization costs, thereby leading to higher overall COPD-related healthcare costs compared with nonadherent patients. However, this study was not designed to evaluate the impact of COPD maintenance therapy nonadherence on COPD-related costs beyond 1 year.
In a review of some of the important factors contributing to medication nonadherence, Restrepo and colleagues noted that progress in COPD management has been stymied by poor medication adherence.12 The authors reported that adherence rates among patients with COPD averaged 40% to 60%. Treatment efficacy, side effects, ease of medication administration, medication cost, clinician's preference, and patient's belief were potential factors contributing to suboptimal medication adherence. Assigning responsibility to patients and to providers, Restrepo and colleagues suggest that current attempts to educate patients are not yielding the desired results.12
The findings in our study have important implications for all stakeholders—patients, providers, and payers—who are engaged with the management of COPD. With the potential to realize substantial clinical and economic benefits from the effective use of maintenance therapies, initiatives, including greater emphasis on patient education and on the benefits of medication adherence among patients with COPD, should be developed. These initiatives could include educational programs for physicians to raise awareness and encourage the assessment of adherence to medications, and to counsel patients on the importance of complying with the recommendations regarding prescribed treatment regimens. Finally, technologies could be better utilized to facilitate real-time communication between patients and providers to proactively collect patient feedback, monitor medication adherence, and send refill reminders.
We also acknowledge that there is a fundamental difference between patient self-reported outcomes and claims-based measures. The adherence rate reported by Restrepo and colleagues was measured using medication adherence report scales,12 which, like other frequently used patient self-reporting techniques, tend to overestimate adherence.21 By contrast, claims-based measures tend to underestimate adherence, because prescriptions paid for by cash over the counter, those administered in the hospital, and free samples are usually not captured in claims. Therefore, each technique has its limitation.
In addition, the adherence reported by Restrepo and colleagues refers to the overall COPD medications,12 whereas our study only focused on inhaled corticosteroid/LABA combination therapy, which may have lower adherence because of the complexity of the delivery device and the high cost.
Limitations
This study has some limitations that should be considered. One of the most salient limitations is that adherence to therapy and outcomes were measured during the same time period. It was unclear whether poor therapy adherence led to the outcomes, or the occurrence of outcomes led to poor adherence. We recognize that adding and analyzing another year of data after the 1-year postindex data could provide the basis to reexamine this relationship; however, that step was outside the scope of this study. As a result, no causal implication can be concluded in this study, and it is not possible to claim that greater adherence to fixed-dose combination inhaled corticosteroid/LABA therapy leads to lower rates of COPD exacerbation or reduced costs over time.
The prescription claim date is the date a medication was filled, and is not necessarily the date of treatment administration, as is assumed in this study. Furthermore, this study was not designed to indicate how patients took their medication, as is typical in studies using secondary data sources. Thus, prescription refill is only a surrogate marker for medication adherence. Any inpatient-administered medications were not present in these claims data and were not included in any of the analyses.
It was also not possible to determine the primary reason for outpatient visits, including emergency department visits, via claims data. Although a COPD diagnosis code was indicated for visits, these visits could have been for routine follow-up or non–COPD-related reasons and not necessarily because of a COPD exacerbation. Important risk factors, such as smoking status, were not captured in the administrative claims data; thus, this study was not able to address such unmeasured confounding factors. Furthermore, these results may have limited generalizability to a non–commercially insured population.
Finally, the intention of excluding long-term users for any oral corticosteroids was to exclude “very sick” patients who may be managed differently with various adherence behaviors. These patients were likely to be the cost outliers that would confound the study results. Because we did not compare the adherence rate between these sicker patients with other healthier patients, we do not know if the exclusion could lead to a bigger or smaller differential between the adherence subtypes. However, it is an appropriate topic for future researchers to explore.
Conclusions
This study shows the presence of suboptimal adherence to fixed-dose combination inhaled corticosteroid/LABA therapy in a majority of patients with COPD. Poor adherence was associated with an increased rate of COPD exacerbations and higher overall all-cause healthcare costs. These findings point to missed opportunities to realize optimal clinical benefits from these maintenance therapies. Furthermore, improving adherence may help to reduce the economic burden associated with COPD.
Acknowledgment
Bernard B. Tulsi, MSc, provided writing and editorial support for this study.
Source of Funding
Funding for this study was provided by AstraZeneca.
Author Disclosure Statement
Dr Trudo is a shareholder/stockholder of AstraZeneca. Ms Davis, Mr Wu, Dr Kern, Mr Tunceli, Dr Fox, Mr Horton, and Dr Legg reported no conflicts of interest.
Contributor Information
Jill R. Davis, Director, Health Economics and Outcomes Research, AstraZeneca R&D, Wilmington, DE.
Bingcao Wu, Associate Director, RWE Design & Analytics, Janssen Pharmaceuticals, and was Associate Director, HealthCore, Wilmington, DE, at the time of the study.
David M. Kern, Director of Research, HealthCore.
Ozgur Tunceli, Director, RWE Design & Analytics, Janssen Pharmaceuticals, and was Director of Research, HealthCore, at the time of the study.
Kathleen M. Fox, Director of Health Economics Outcomes Research, AstraZeneca, and is currently with Strategic Healthcare Solutions.
John Horton, Employees of AstraZeneca R&D at the time of the study.
Randall F. Legg, Employees of AstraZeneca R&D at the time of the study.
Frank Trudo, Medical Lead – Respiratory, AstraZeneca US Medical Affairs.
References
- 1.National Heart, Lung, and Blood Institute. COPD: Learn More Breathe Better. NIH Publication No 07–5840. September 2013. www.nhlbi.nih.gov/files/docs/public/lung/copd-atrisk.pdf. Accessed March 15, 2017.
- 2.National Heart, Lung, and Blood Institute. Morbidity and Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases February 2012. www.nhlbi.nih.gov/files/docs/research/2012_ChartBook.pdf. Accessed March 15, 2017.
- 3.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012; 61: 1–51. [PubMed] [Google Scholar]
- 4.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 5.Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. MMWR Surveill Summ. 2002; 51: 1–16. [PubMed] [Google Scholar]
- 6.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2016. http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed January 22, 2016.
- 7.Symbicort (budesonide and formoterol fumarate dihydrate) inhalation aerosol [prescribing information]. Wilmington, DE: AstraZeneca; February 2016.
- 8.Advair Diskus (fluticasone propionate and salmeterol) inhalation powder [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; April 2016.
- 9.Breo Ellipta (fluticasone furoate and vilanterol) inhalation powder [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; April 2015.
- 10.Dong YH, Lin HH, Shau WY, et al. Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trials. Thorax. 2013; 68: 48–56. [DOI] [PubMed] [Google Scholar]
- 11.Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014:CD010844. [DOI] [PMC free article] [PubMed]
- 12.Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008; 3: 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albertson TE, Harper R, Murin S, Sandrock C. Patient considerations in the treatment of COPD: focus on the new combination inhaler umeclidinium/vilanterol. Patient Prefer Adherence. 2015; 9: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ágh T, Inotai A, Mészáros Á. Factors associated with medication adherence in patients with chronic obstructive pulmonary disease. Respiration. 2011; 82: 328–334. [DOI] [PubMed] [Google Scholar]
- 15.Bryant J, McDonald VM, Boyes A, et al. Improving medication adherence in chronic obstructive pulmonary disease: a systematic review. Respir Res. 2013; 14: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lareau SC, Yawn BP. Improving adherence with inhaler therapy in COPD. Int J Chron Obstruct Pulmon Dis. 2010; 5: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006; 40: 1280–1288. [DOI] [PubMed] [Google Scholar]
- 18.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005; 11: 449–457. [PubMed] [Google Scholar]
- 19.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008; 27: 2037–2049. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010; 172: 1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999; 21: 1074–1090. [DOI] [PubMed] [Google Scholar]