Abstract
Candida albicans, a component of the normal flora of the alimentary tract and mucocutaneous membranes, is the leading cause of invasive fungal disease in premature infants, diabetics, and surgical patients and of oropharyngeal disease in AIDS patients. As little is known about the regulation of monocyte/macrophage anti-Candida activity, we sought to determine if fungicidal activity might be regulated by extracellular matrix proteins to which monocytes/macrophages are adherent in vivo. Compared to monocyte/macrophages that adhered to plastic, human monocytes and monocyte-derived macrophages that adhered to type 1 collagen matrices, but not to fibronectin, vitronectin, or laminin, demonstrated a significant increase in candidacidal activity. The enhancement of monocyte fungicidal activity was maintained over a 4-h period, whereas macrophage fungicidal activity was maximum at 1 h. Although adherence of monocytes and macrophages to collagen matrices concomitantly enhanced the production of superoxide anion, only the fungicidal activity of collagen-adherent monocytes was partially blocked by superoxide dismutase and catalase. Remarkably, we found that only 10% of the phagosomes in C. albicans-infected macrophages that adhered to plastic fused with lysosomes. In contrast, 80% of yeast-containing phagosomes of collagen-adherent macrophages fused with lysosomes. These data suggest that nonoxidative mechanisms are critical for human macrophage anti-Candida activity and that C. albicans pathogenicity is mediated, in part, by its ability to inhibit phagolysosomal fusion in macrophages.
Candida albicans is part of the normal microbial flora that colonizes mucocutaneous surfaces of the oral cavity, gastrointestinal tract, and vagina of the healthy human host. Although Candida does not normally cause disease, when immune defenses are compromised or the normal microflora balance is disrupted, C. albicans transforms itself into an opportunistic pathogenic killer. Indeed, Candida is the leading cause of invasive fungal disease in premature infants, diabetics, and surgical patients and of oropharyngeal disease in AIDS patients (1, 5, 7, 9, 13, 17, 23, 34, 45).
Host resistance against infections with C. albicans is mediated predominantly by neutrophils and monocytes/macrophages (Mφ). In the early stages of Candida infection microabscesses contain neutrophils surrounded by a small number of mononuclear cells. As the fungus is eliminated from the lesions, mononuclear cells predominate (32). The importance of neutrophils in host defense against Candida infections is underscored by the high incidence of disseminated disease in individuals with neutropenia (1, 40) and the fact that depletion of neutrophils in mice leads to systemic disease (2, 12). Further, numerous in vitro studies have documented the potent ability of neutrophils from mice, humans, and guinea pigs to kill C. albicans (3, 6, 37), and killing is mediated by both oxidative and nonoxidative mechanisms (8, 21, 22, 38, 46).
While neutrophils clearly are important in host defense against Candida invasion and exhibit potent killing activity against the fungus, the relatively poor candidacidal activity of Mφ is perplexing. Thus, in a comparison of human neutrophils, monocytes, and monocyte-derived Mφ, Mφ clearly were inferior to neutrophils and monocytes in their candidacidal activity. However, unlike neutrophils and monocytes, Mφ killed Candida equally well under aerobic and anaerobic conditions (42). Indeed the loss of candidacidal activity that occurs as monocytes differentiate into Mφ in vitro has been shown to correlate with the loss of the enzyme myeloperoxidase and a decrease in the ability to produce hydroxyl radicals (35). As monocytes and Mφ produced equivalent amounts of superoxide anion, the data suggest that the candidacidal activity of monocytes is mediated by products distal to the superoxide anion. Further, relative to bacteria such as Escherichia coli, Listeria monocytogenes, Salmonella enterica serovar Typhimurium, and Staphylococcus aureus, C. albicans is resistant to toxic oxygen metabolites. Thus, the killing of Candida required 10 times more H2O2, NaI, and Fe2SO4 than the killing of these bacteria (51). Thus, optimum killing by Mφ must rely more on nonoxidative killing mechanisms than on oxidative mechanisms.
Numerous studies suggest that for optimal host protection against C. albicans Mφ are essential, despite their tepid candidacidal activity in vitro, and that, most likely, they require activation by cytokines (2, 4, 33). However, very little is known in this regard. With respect to human cells, granulocyte-Mφ colony stimulating factor and interleukin 3 (IL-3), but not gamma interferon (IFN-γ) have been reported to enhance the growth inhibition and/or killing of C. albicans by monocytes (39, 47). In contrast, IFN-γ modestly boosts the candidacidal activity of monocyte-derived Mφ (25, 26) and alveolar Mφ (44). In addition, IL-1α has been shown to enhance the anti-Candida activity of monocytes and alveolar Mφ (44).
In vivo, monocytes emigrating from the peripheral circulation enter into an extravascular area rich in extracellular matrix (ECM) proteins. It is in this milieu that phagocytes function in host defense against pathogenic microorganisms. In addition, tissue Mφ reside in areas that contain ECM proteins. Previously, we demonstrated that, compared to plastic-adherent monocytes, monocytes adherent to type 1 collagen matrices demonstrated significantly enhanced phagocytic capacity for serum-opsonized E. coli, S. aureus, Streptococcus pyogenes, and Streptococcus pneumoniae and that phagocytic capacity was mediated by activation of complement receptor type 1 (CR1) and CR3 for phagocytosis and augmentation of Fc receptor-mediated phagocytosis (31). Although both collagen- and plastic-adherent monocytes were bactericidal for these microbial pathogens, more bacteria were killed by collagen-adherent monocytes by virtue of their enhanced phagocytic capacity.
More recently, we demonstrated that collagen-adherent Mφ are fungicidal for the dimorphic fungal pathogen Histoplasma capsulatum, whereas plastic-adherent Mφ are permissive for intracellular growth. The mechanism of this activation is the capacity of collagen-adherent Mφ to induce massive phagolysosomal (PL) fusion, whereas minimal PL fusion occurs in plastic-adherent Mφ (29). These results suggest that monocytes/Mφ adherent to type 1 collagen may express a previously unrecognized microbicidal activity that proceeds in the absence of exogenous cytokines. This microbicidal activity may be important with regard to the capacity of Mφ to participate in the inflammatory response and in the induction of cell-mediated immunity in the nonimmune host.
The present study was designed to determine if adherence of human monocytes/Mφ to type 1 collagen matrices or other ECM proteins might augment candidacidal activity and, if so, to determine whether augmentation was caused by oxidative or nonoxidative mechanisms.
MATERIALS AND METHODS
Reagents.
Superoxide dismutase (SOD), catalase, and ferricytochrome c (type 3) were purchased from Sigma-Aldrich, St. Louis, Mo. Cytochrome c (1 mM) was dissolved in Krebs-Ringer phosphate buffer containing 0.2% dextrose and stored at −20°C. All other reagents were prepared in RPMI 1640 and sterile filtered. Human serum prepared from six to eight individual donors was pooled and stored in 200-μl aliquots at −80°C until used.
Preparation of human monocytes and Mφ.
Monocytes were isolated by sequential centrifugation on Ficoll-Hypaque and Percoll gradients (Amersham Pharmacia LKB, Piscataway, N.J.) from buffy coats obtained from the Hoxworth Blood Center, Cincinnati, Ohio, or from blood drawn from healthy adult donors in our laboratory (28). The monocytes were washed, suspended in Hanks balanced salt solution (HBSS) containing 20 mM HEPES and 0.1% autologous serum, and then allowed to adhere to various substrates for 1 h at 37°C in 5% CO2-95% air. The adherent monocytes were washed and then were studied immediately or cultured in M199 (Bio-Whittaker, Walkersville, Md.) containing 10% autologous serum and 10 μg of gentamicin (Sigma)/ml. Medium was replaced on day 3, and Mφ were studied after 7 days of culture.
Alternatively, Mφ were obtained by culture of monocytes at 106/ml in Teflon beakers in RPMI 1640 (Bio-Whittaker) containing 15% human serum, 10 μg of gentamicin/ml, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (Sigma) (28). Mφ were studied after 5 to 7 days in culture.
Yeasts.
C. albicans yeasts (ATCC 18804) were maintained on Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich.). Yeasts were grown to log phase in Sabouraud dextrose broth at 30°C with orbital shaking at 150 rpm. For superoxide anion production assays, yeasts were harvested by centrifugation, washed three times in 0.01 M phosphate buffer, pH 7.2, containing 0.15 M NaCl (PBS), and then heat killed (HK) at 65°C for 1 h. Yeasts were stored at 4°C in PBS containing 0.05% sodium azide.
For studies with viable Candida cells, log-phase yeasts were harvested by centrifugation, washed three times in HBSS containing 0.25% bovine serum albumin (HBSA), and resuspended to 50 ml in HBSA. The yeasts were counted on a hemacytometer and standardized to the appropriate concentration according to the assay protocol.
H. capsulatum strain G217B was maintained as described previously (28). Yeasts were grown in HMM medium (50) at 37°C with orbital shaking at 150 rpm. After 48 h, log-phase yeasts were harvested by centrifugation, washed three times in HBSA, and resuspended to 50 ml in HBSA. Large aggregates were removed by centrifugation at 200 × g for 7 min at 4°C. The top 10 ml was removed, and the single-cell suspension obtained was standardized to the appropriate concentration according to the assay protocol. HK yeasts were prepared as described above for Candida.
S. cerevisiae was inoculated into 50 ml of yeast extract-peptone-dextrose broth and cultured for 24 h at 37°C with orbital shaking at 150 rpm. The yeasts were harvested, washed, and standardized by following the same procedure as that for Candida yeasts.
Preparation of tissue culture plates coated with ECM proteins. (i) Collagen gels.
Type I collagen from rat tails (Sigma) was dissolved in 0.1% acetic acid at 1 mg/ml and dialyzed overnight at 4°C in distilled water. Three hundred microliters of collagen was dispensed into 24-well tissue culture plates and exposed to ammonia fumes for 30 min at 25°C. The gels were washed four times with HBSS prior to adherence of monocytes or Mφ. Alternatively, collagen-coated wells were prepared by dispensing collagen (50 μg/ml in RPMI) into the wells to adhere directly to the plastic for 2 h at 25°C.
(ii) Fibronectin, vitronectin, and laminin.
Fibronectin, vitronectin, and laminin (Sigma) were suspended to 50 μg/ml in RPMI 1640, and 250 μl was aliquoted into the wells of a 24-well tissue culture plate. After 2 h at 25°C, the wells were aspirated and monocytes/Mφ were allowed to adhere for 1 h at 37°C.
Quantitation of monocyte/Mφ fungicidal activity.
Adherent monocytes and Mφ (∼2 × 105) were incubated with C. albicans yeasts (2 × 104) in the presence of 10% pooled human serum for 1, 2, and 4 h at 37°C in 5% CO2-95% air. At each time point, the supernatant was aspirated and the mixtures were resuspended in 1 ml of sterile water to lyse the cells. The contents were vortexed vigorously and then were serially diluted and plated on Sabouraud dextrose agar plates. Inspection of the initial lysate revealed only single colonies, 98% of which were still in the yeast phase. After incubation at 30°C for 48 h, CFU were counted and the percentage of C. albicans cells that were killed was calculated by comparison to the CFU obtained from the original inoculum, which also was quantified by serial dilution and plating.
Quantitation of superoxide anion (O2−) production.
O2− generation by monocytes/Mφ was quantified as the SOD-inhibitable reduction of ferricytochrome c (type 3; Sigma) as described previously (36). Monocytes/Mφ (2 × 105) were incubated for 1 h at 37°C with serum-opsonized Candida yeasts (107) in Krebs-Ringer phosphate buffer with 0.2% dextrose containing 80 μM cytochrome c. Control wells contained cytochrome c without Candida (resting cells) or Candida plus 40 μg of SOD/ml. At the end of the incubation, the supernatants were collected by centrifugation at 4°C. The absorbance of the supernatants at 550 nm was measured in a Spectronic Genesys 5 (Milton Roy, Rochester, N.Y.), and the background absorbance in control tubes containing only buffer and cytochrome c was subtracted. All experiments were performed in duplicate, and results were calculated as nanomoles of O2− from the equation E550 = 2.1 × 104 M−1 cm−1. Results are expressed as nanomoles of cytochrome c reduced per 2 × 105 cells per hour.
Quantitation of PL fusion.
Mφ were allowed to adhere in 24-well tissue culture plates to 12-mm-diameter round glass coverslips or to coverslips coated with collagen gels. Monolayers then were incubated with 200 nM Lysotracker red (Molecular Probes, Eugene, Oreg.) in RPMI 1640 containing 5% fetal calf serum for 2 h to label lysosomes. After two washes, the Mφ were infected with viable S. cerevisiae (2 × 106 cells) or HK and viable C. albicans (1 × 105 cells) or H. capsulatum (2 × 106 cells) for 1 h at 37°C. HK C. albicans and H. capsulatum were labeled with fluorescein isothiocyanate as described previously (30). After an additional two washes, the Mφ were cultured in 200 nM Lysotracker red for an additional 2 h. After being washed, the monolayers were fixed in 3.75% paraformaldehyde for 20 min at 25°C. The Mφ then were covered in Dulbecco's PBS (DPBS) containing 5% glucose. Mφ that adhered to collagen matrices were incubated overnight at 4°C. The buffer was aspirated, and the gels were allowed to desiccate at 30°C for 24 h. The monolayers then were covered in DPBS-glucose. Mφ that adhered to the coverslips were counted immediately. Coverslips were mounted cell side down in 90% glycerol in phosphate-buffered saline onto microscope slides. One hundred yeast-containing phagosomes were scored for lysosomal fusion or no fusion. The data are expressed as the means ± standard errors of the means (SEM) of the percentages of PL fusion in three or more experiments performed in duplicate.
Alternatively, Mφ adhered to plastic or collagen gels in six-well plates were incubated for 2 h at 37°C in M199 containing 10% human serum, 10 μg of gentamicin/ml, and 18-nm colloidal gold stabilized with horseradish peroxidase (HRP-Au18) (29). The Mφ were washed three times with medium and then incubated for an additional 2 h at 37°C to insure that the HRP-Au18 entered the lysosomal compartments. After the second incubation, 106 viable or HK Candida yeasts were added, and phagocytosis was allowed to proceed for 1 h at 37°C. After 1 h the Mφ were washed three times with ice-cold 0.1 M sodium cacodylate buffer, pH 7.4, and then processed for electron microscopy. Mφ PL fusion was quantified by counting the 18-nm gold particles in phagosomes containing Candida yeasts (29). The data are expressed as the means ± SEM of the average numbers of gold particles per yeast-containing phagosome.
Statistics.
Statistical analysis of the data was performed using Sigma Stat (Jandel Scientific, San Rafael, Calif.). Student's t test or the Mann-Whitney rank sum test was used to analyze the data for statistical significance, and results were considered significant at P values <0.05.
RESULTS
Fungicidal activity of monocytes/Mφ adherent to collagen matrices, fibronectin, vitronectin, and laminin.
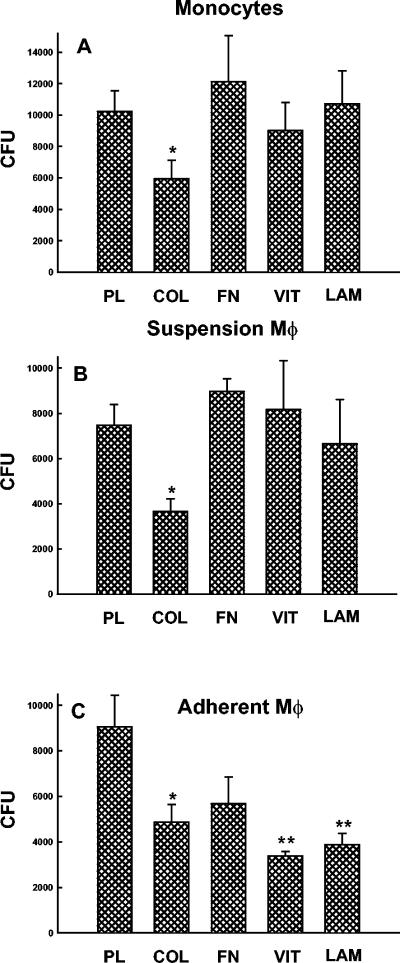
In initial experiments we sought to determine if adherence of freshly isolated monocytes or monocyte-derived Mφ to ECM proteins regulated the phagocytes' capacity to kill C. albicans yeasts. Therefore, monocytes and suspension-cultured Mφ were allowed to adhere to plastic or the various substrates for 1 h and were then incubated with 2 × 104 yeasts for an additional 1 h. Candidacidal activity then was quantified by determining the remaining CFU as described in Materials and Methods. After 1 h, monocytes killed an average of 48% of the initial inoculum and Mφ killed 57% (Fig. 1A and B). In contrast, monocytes and Mφ that adhered to collagen matrices killed 70 and 79%, respectively, of the C. albicans yeasts. This significant enhancement of monocyte/Mφ candidacidal activity was not observed when the phagocytes were allowed to adhere to fibronectin, vitronectin, or laminin (Fig. 1A and B). In addition, adherence of monocytes/Mφ to collagen-coated wells did not enhance fungicidal activity (data not shown), indicating that the enhanced killing required that the collagen be in the form of a three-dimensional matrix, as is found in vivo.
FIG. 1.
Collagen-adherent monocytes and Mφ have enhanced fungicidal activity against C. albicans. Freshly isolated monocytes and suspension-cultured Mφ were allowed to adhere to plastic (PL), type 1 collagen matrices (COL), fibronectin (FN), vitronectin (VIT), or laminin (LAM) for 1 h at 37°C. Alternatively, monocytes were cultured for 7 days on the various ECM components (adherent Mφ). After the monolayers were washed, the cells were incubated with 2 × 104 C. albicans yeasts in the presence of 10% pooled human serum for 1 h at 37°C. At the end of the incubation period, the remaining CFU were quantified as described in Materials and Methods. The data are the means ± SEM of the remaining CFU from five experiments with monocytes and suspension-cultured Mφ and seven experiments with adherently cultured Mφ. *, P < 0.05 compared to plastic (t test); **, P < 0.05 compared to plastic (Mann-Whitney rank sum test).
We also sought to determine if monocyte differentiation into Mφ on the different ECM proteins would regulate phagocyte fungicidal activity. Figure 1C shows that, when monocytes were cultured for 7 days on ECM proteins, enhanced candidacidal activity was observed with vitronectin and laminin, as well as collagen, matrices. The enhanced fungicidal activity observed after 7 days of culture on fibronectin was consistent but did not reach statistical significance.
Enhanced fungicidal activity of monocytes/Mφ adherent to type 1 collagen matrices.
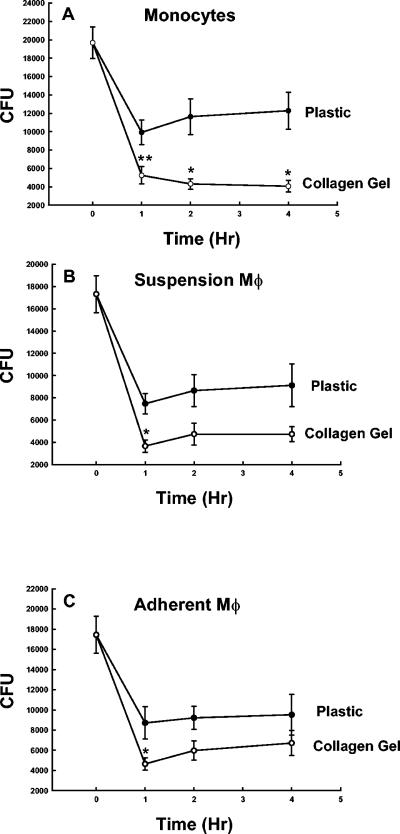
We next sought to determine the kinetics of the enhanced fungicidal activity exhibited by monocytes/Mφ adherent to collagen matrices. Therefore, monocytes/Mφ were allowed to adhere to plastic or collagen gels for 1 h and candidacidal activity was quantified over a 4-h period. Figure 2A shows that collagen-adherent monocytes continued to kill C. albicans yeasts over the 4-h incubation period, whereas there was an increase in Candida CFU at 2 and 4 h with plastic-adherent monocytes. In contrast, with both suspension-cultured Mφ adherent to collagen gels for 1 h and Mφ cultured for 7 days on a collagen matrix, maximum fungal killing was observed at 1 h. Thereafter, there was a slight increase in CFU, as was observed with Mφ adherent to plastic.
FIG. 2.
Time course of monocyte/Mφ fungicidal activity on plastic versus collagen matrices. Freshly isolated monocytes and suspension-cultured Mφ were allowed to adhere to plastic or type 1 collagen matrices for 1 h at 37°C, or monocytes were cultured for 7 days on plastic or collagen. After the monolayers were washed, the cells were incubated with 2 × 104 C. albicans yeasts in the presence of 10% pooled human serum for 1, 2, and 4 h at 37°C. Remaining CFU then were quantified as described in Materials and Methods. The data are the means ± SEM of the remaining CFU from eight experiments with monocytes and adherently cultured Mφ and five experiments with suspension-cultured Mφ. * and **, P < 0.01 and < 0.05, respectively, compared to plastic (t test).
Mechanism of monocyte/Mφ fungicidal activity against C. albicans.
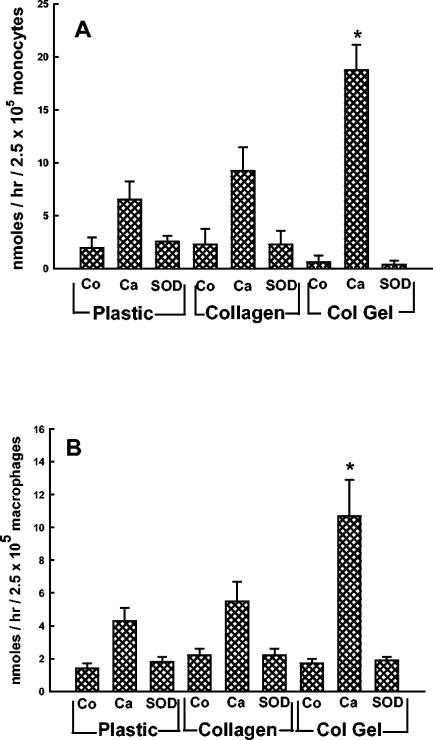
Data from numerous laboratories suggest that neutrophils kill C. albicans predominantly via the production of toxic oxygen metabolites (8, 42, 46), whereas Mφ fungicidal activity is mediated by both oxidative and nonoxidative mechanisms. Therefore, we next sought to determine if collagen-adherent monocytes and Mφ stimulated the production of greater amounts of superoxide anion than plastic-adherent cells. Monocytes and Mφ were incubated for 1 h with opsonized C. albicans yeasts, and superoxide anion production was quantified by measuring the SOD-inhibitable reduction of cytochrome c. The data in Fig. 3A show that monocytes that adhered to collagen matrices, but not nongelled collagen, produced significantly more SOD-inhibitable superoxide anion than plastic-adherent monocytes. Similar results were obtained with Mφ adherent to collagen matrices (Fig. 3B).
FIG. 3.
Adherence of monocytes/Mφ to collagen matrices enhances the production of superoxide anion. Monocytes (A) or Mφ (B) adherent to plastic, collagen, or collagen gels (Col Gel) were incubated in buffer alone (Co) or with opsonized C. albicans (Ca) (107 yeasts) for 1 h at 37°C in the absence or presence of SOD (40 μg/ml), and the production of superoxide anion was quantified by measuring the reduction of cytochrome c. The data are the means ± SEM of three experiments with monocytes (A) and six experiments with Mφ (B). *, P < 0.05, compared to plastic (t test).
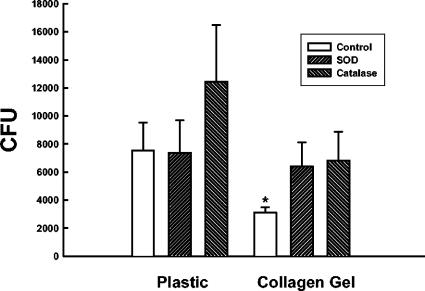
To determine if an enhanced respiratory burst was responsible for the increased candidacidal activity of collagen-adherent monocytes/Mφ, the effect of respiratory burst inhibitors on monocyte/Mφ killing of C. albicans was quantified. Monocytes and Mφ adherent to plastic, collagen or collagen matrices were incubated with Candida yeasts in the presence or absence of SOD or catalase, and the remaining CFU were quantified after 1 h at 37°C. The fungicidal activity of plastic-adherent monocytes was partially reversed by catalase, but not SOD, but the effect of catalase was not statistically significant (Fig. 4). Likewise, the fungicidal activity of collagen-adherent monocytes was partially reversed by both SOD and catalase, but these results also were not statistically significant (Fig. 4). In identical experiments performed with Mφ, neither SOD nor catalase had any effect on the fungicidal activity of Mφ regardless of the substrate to which the cells adhered (data not shown).
FIG. 4.
Reversal of monocyte fungicidal activity by SOD and catalase. Monocytes were allowed to adhere to plastic or type 1 collagen matrices for 1 h at 37°C. After being washed, the monocytes were incubated with 2 × 104 C. albicans yeasts for 1 h at 37°C in the presence or absence of SOD or catalase. Remaining CFU then were quantified as described in the legend to Fig. 1. The data are the means ± SEM of five experiments. *, P < 0.05 compared to plastic control (Mann-Whitney rank sum test).
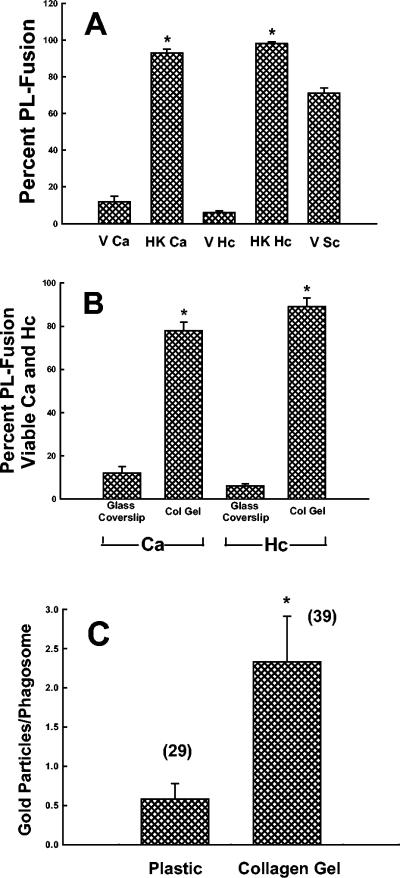
As the production of toxic oxygen radicals did not appear to be involved in Mφ fungicidal activity, we sought to compare the amounts of PL fusion mediated by plastic- versus collagen-adherent Mφ upon phagocytosis of C. albicans. Mφ were allowed to adhere to plastic or collagen matrices, loaded with Lysotracker red, and then incubated with viable or HK Candida yeasts for 1 h at 37°C. For comparison, we quantified Mφ PL fusion after phagocytosis of viable H. capsulatum and S. cerevisiae yeasts and HK H. capsulatum yeasts. In Mφ that ingested either HK Candida or HK Histoplasma, over 80% of yeast-containing phagosomes demonstrated PL fusion (Fig. 5A). Mφ phagosomes that contained H. capsulatum showed about 5% PL fusion, confirming our previous data (29). Remarkably, only 10% of phagosomes containing C. albicans demonstrated PL fusion. There was about 70% PL fusion in Mφ that had phagocytosed viable S. cerevisiae.
FIG. 5.
Adherence of Mφ to collagen matrices enhances PL fusion. Glass- or collagen-adherent Mφ were loaded with Lysotracker red and then incubated for 1 h with viable (V) or HK C. albicans (Ca) or H. capsulatum (Hc) or viable S. cerevisiae (V Sc). The percentage of lysosomal fusion was quantified as described in Materials and Methods. The data are the means ± SEM of four to nine experiments with C. albicans and H. capsulatum and seven experiments with S. cerevisiae. (A) Data comparing levels of PL fusion for viable and HK C. albicans and H. capsulatum and viable S. cerevisiae when the Mφ were allowed to adhere to glass coverslips. *, P < 0.001 compared to viable fungi (t test). (B) Data comparing levels of PL fusion with viable fungi for Mφ adherent to glass coverslips versus collagen gels. *, P < 0.001 compared to glass-adherent Mφ (t test). (C) Plastic- or collagen-adherent Mφ were labeled with HRP-Au18 and then incubated with C. albicans for 1 h at 37°C. After fixation and processing for electron microscopy, the number of gold particles in yeast-containing phagosomes was quantified. The data are the means ± SEM of the numbers of gold particles per phagosome. The number above each bar on the graph denotes the number of phagosomes counted for each fungus. *, P < 0.001 compared to plastic (Mann-Whitney rank sum test).
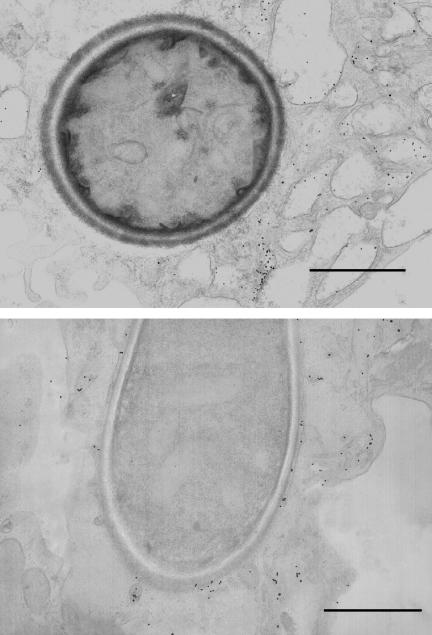
When Mφ were allowed to adhere to collagen matrices, phagosomes containing either viable C. albicans or viable H. capsulatum demonstrated about 80% PL fusion (Fig. 5B). These results were confirmed for C. albicans with HRP-Au18. Figure 5C shows that, after 1 h of phagocytosis, plastic-adherent Mφ containing C. albicans had an average of 0.58 gold particles per phagosome, whereas collagen-adherent Mφ had an average of 2.3 gold particles per phagosome, a fourfold increase. In contrast, Mφ that had ingested HK yeasts had an average of about 12 gold particles per phagosome, regardless of the substrate to which the cells were adherent (data not shown). These results are illustrated in Fig. 6. The top panel of Fig. 6 shows that, despite numerous gold particles nearby in the cytoplasm, no gold particles are in the yeast-containing phagosome of a Mφ adherent to plastic. In contrast, in the bottom panel, numerous gold particles can be seen adjacent to the yeast in a Mφ adherent to collagen.
FIG. 6.
Adherence of Mφ to collagen matrices enhances PL fusion. Plastic- or collagen-adherent Mφ were labeled with HRP-Au18 and then incubated with C. albicans for 1 h at 37°C and then fixed and processed for electron microscopy. (Top) Electron micrograph showing a C. albicans yeast within the phagosome of a Mφ adherent to plastic. Note that there are numerous gold particles in the surrounding cytoplasm but none in the phagosome. (Bottom) Collagen-adherent Mφ with numerous gold particles within the yeast-containing phagosome. Bars, 1 μm.
DISCUSSION
Studies of murine models of disseminated candidiasis demonstrate that Mφ play an important role in host resistance (2, 4, 33). However, in vitro, Mφ candidacidal activity is underwhelming, particularly compared to that of neutrophils (reviewed in reference 43). Part of the explanation for the low killing capacity lies in the fact that, as monocytes differentiate into mature Mφ, they lose the enzyme myeloperoxidase and, therefore, are not able to generate potent toxic oxygen radicals distal to H2O2 (24, 35). Thus, for protection of the host, Mφ must either rely on PL fusion and lysosomal enzymes or be activated by T-cell cytokines, such as IFN-γ. However, IFN-γ alone does not turn Mφ into potent killers of C. albicans (25, 26, 47).
We hypothesized that part of the inability of Mφ to efficiently kill C. albicans might be caused by a deficit in PL fusion. Previously we had demonstrated that adherence of human Mφ to type 1 collagen gels stimulated the Mφ to kill H. capsulatum cells via the induction of PL fusion. Therefore, we sought to determine if adherence to collagen or other ECM proteins might enhance Mφ candidacidal activity by the same mechanism.
Indeed, we found that both monocytes and monocyte-derived Mφ demonstrated significantly increased candidacidal activity in cells that adhered to type 1 collagen matrices compared to cells that adhered to plastic. Activation of Mφ occurred immediately upon adherence and did not require additional time in culture. Enhanced candidacidal activity was not observed when monocytes or Mφ adhered to fibronectin, vitronectin, laminin, or nongelled collagen. However, when monocytes were allowed to differentiate into Mφ while adherent to ECM proteins, culture on vitronectin and laminin, but not fibronectin, also induced a significant increase in Mφ candidacidal activity. We have focused here on collagen, as it was the most consistent ECM protein in enhancing Mφ fungicidal activity.
We hypothesize that optimum Mφ anti-Candida activity probably requires some combination of ECM proteins and cytokines and occurs only upon the induction of CMI. However, it also is possible that monocytes emigrating into an inflammatory site where type 1 collagen and other ECM proteins are present may exhibit sufficient fungicidal activity to help prevent or slow the dissemination of Candida, particularly when neutrophils are present (16). Thus, we suggest that, in the healthy human host, during the inflammatory response to Candida the influx of neutrophils and monocytes, in conjunction with the ECM, might be sufficient to prevent Candida from causing disease. Clearly, when neutrophils are removed from the equation, inflammatory monocytes alone are insufficient to prevent dissemination and disease (1, 12, 18, 20, 40).
It is generally accepted that the respiratory burst accounts for the majority of the candidacidal activity of human neutrophils, and both oxygen-dependent and -independent mechanisms have been promoted for monocyte/Mφ candidacidal activity (reviewed in reference 43). In a previous study with human Mφ and dendritic cells (DC), we were unable to obtain any evidence that either cell type killed C. albicans through the generation of toxic oxygen metabolites (30). In fact, Mφ killing and DC killing of Candida were equivalent under aerobic and anaerobic conditions, confirming earlier studies with Mφ by Thompson and Wilton (42).
The data in the present study also do not support a significant role for toxic oxygen radicals in the candidacidal activity of monocytes or Mφ. Remarkably, we found that Candida-infected plastic-adherent Mφ demonstrated very little PL fusion compared to the amount of PL fusion induced by phagocytosis of the nonpathogenic yeast S. cerevisiae or HK Candida or Histoplasma. In contrast, when Mφ were allowed to adhere to collagen matrices and then infected with C. albicans, 80% of yeast-containing phagosomes demonstrated PL fusion, similar to that seen when the Mφ were infected with H. capsulatum (29). Interestingly, and in contrast to our observations with human Mφ, PL fusion has been reported to occur normally in mouse peritoneal Mφ (19, 27, 48).
The fact that PL fusion is inhibited upon ingestion of Candida cells by Mφ and the fact that Mφ do not generate toxic oxygen radicals distal to H2O2 (24, 35) may explain why human Mφ mediate such poor candidacidal activity. The data are surprising considering the facts that Candida is an opportunistic pathogen and that the capacity to inhibit PL fusion generally is considered to be a property of intracellular pathogens.
The mechanism by which C. albicans inhibits PL fusion is unknown. However, it is not likely to be related to signal transduction through the mannose receptor, as PL fusion occurs normally after ingestion of S. cerevisiae and HK Candida. It also is not clear if filamentation is involved in inhibition of PL fusion. Most likely, the yeasts respond to phagocytosis by secreting an unknown agent that inhibits the fusion process.
It also is unknown how Mφ adherence to a collagen matrix stimulates the Mφ to undergo PL fusion, overriding the negative signal from ingested C. albicans cells. The “very late antigen” (VLA) receptor family are cell surface glycoproteins that promote cell-matrix adhesion and belong to the integrin family of adhesion molecules (14). Of the VLA molecules that have been described, VLA-2 has been shown to be specific for collagen and VLA-3 has been shown to be specific for collagen, laminin, and fibronectin (41, 49). Of these, small amounts of only VLA-2 have been detected on human monocytes/Mφ (15). Thus, we hypothesize that adherence to collagen provides an additional signal to the Mφ through VLA-2 that promotes PL fusion that enhances Mφ candidacidal activity.
However, inhibition of PL fusion seems only to be part of the strategy by which C. albicans survives in Mφ. Adherence of Mφ to collagen induced PL fusion in 80% of phagosomes containing Candida cells. Therefore, it is curious that Mφ candidacidal activity was not more robust. Indeed, adherence of human Mφ to collagen matrices leads to significant killing of H. capsulatum cells, which also survive intracellularly, in part, by inhibiting PL fusion (29). It is possible that the reason that Mφ Candida killing on collagen is not more efficient is because the yeasts also regulate intraphagosomal pH. Thus, C. albicans may modulate the pH in the phagosome so that the pH is suboptimal for killing by lysosomal hydrolases, even in the presence of PL fusion. This is similar to the strategy utilized by H. capsulatum to survive in murine Mφ (10, 11).
This idea is supported by studies of murine resident peritoneal Mφ (48). In these studies, PL fusion in unactivated Mφ was equivalent to that in IFN-γ-activated Mφ. However, there was a major difference in levels of phagosomal acidification. Only 3% of Candida-infected resident peritoneal Mφ acidified phagolysosomes to a pH of <4.0, whereas in IFN-γ-activated Mφ, 42% of infected Mφ demonstrated acidified phagolysosomes. Further, the enhanced candidacidal activity of IFN-γ-activated murine peritoneal Mφ directly correlated with phagosomal acidification, but not the production of toxic oxygen radicals. Thus, C. albicans clearly uses more than a single strategy for intracellular survival in Mφ and appears to use slightly different strategies to survive within human versus murine Mφ. A better understanding of these survival strategies should help clarify the pathogenic properties of this dimorphic fungus and could possibly lead to the discovery of novel therapies.
Acknowledgments
This work was supported by Public Health Service grants AI-37639 from the National Institute of Allergy and Infectious Diseases and HL-55948 from the National Heart, Lung, and Blood Institute.
We thank Georgianne Ciraolo for technical assistance with electron microscopy.
Editor: T. R. Kozel
REFERENCES
- 1.Anaissie, E. J., J. H. Rex, O. Uzun, and S. Vartivarian. 1998. Predictors of adverse outcome in cancer patients with candidemia. Am. J. Med. 104:238-245. [DOI] [PubMed] [Google Scholar]
- 2.Bistoni, F., M. Baccarini, E. Blasi, P. Marconi, P. Puccetti, and E. Garaci. 1983. Correlation between in vivo and in vitro studies of modulation of resistance to experimental Candida albicans infection by cyclophosphamide in mice. Infect. Immun. 40:46-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bistoni, F., M. Baccarini, E. Blasi, P. Puccetti, and P. Marconi. 1982. A radiolabel release microassay for phagocytic killing of Candida albicans. J. Immunol. Methods 52:369-377. [DOI] [PubMed] [Google Scholar]
- 4.Bistoni, F., A. Vecchiarelli, E. Cenci, P. Puccetti, P. Marconi, and A. Cassone. 1986. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 51:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, K. M., and C. J. Baker. 1988. Candida: an increasingly important pathogen in the nursery. Pediatr. Clin. North Am. 35:543-563. [DOI] [PubMed] [Google Scholar]
- 6.Cutler, J. E., and B. D. Thompson. 1984. A simple and inexpensive method for assessing in vitro candidacidal activity of leukocytes. J. Immunol. Methods 66:27-33. [DOI] [PubMed] [Google Scholar]
- 7.Dean, D. A., and K. W. Burchard. 1998. Surgical perspective on invasive Candida infections. World J. Surg. 22:127-134. [DOI] [PubMed] [Google Scholar]
- 8.Diamond, R. D., R. A. Clark, and C. C. Haudenschild. 1980. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J. Clin. Investig. 66:908-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dromer, F., L. Improvisi, B. Dupont, M. Eliaszewicz, G. Pialoux, S. Fournier, and V. Feuillie. 1997. Oral transmission of Candida albicans between partners in HIV-infected couples could contribute to dissemination of fluconazole-resistant isolates. AIDS 11:1095-1101. [DOI] [PubMed] [Google Scholar]
- 10.Eissenberg, L. G., W. E. Goldman, and P. H. Schlesinger. 1993. Histoplasma capsulatum modulates the acidification of phagolysosomes. J. Exp. Med. 177:1605-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eissenberg, L. G., P. H. Schlesinger, and W. E. Goldman. 1988. Phagosome-lysosome fusion in P388D1 macrophages infected with Histoplasma capsulatum. J. Leukoc. Biol. 43:483-491. [DOI] [PubMed] [Google Scholar]
- 12.Fulurija, A., R. B. Ashman, and J. M. Papadimitriou. 1996. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology 142:3487-3496. [DOI] [PubMed] [Google Scholar]
- 13.Guggenheimer, J., P. A. Moore, K. Rossie, D. Myers, M. B. Mongelluzzo, H. M. Block, R. Weyant, and T. Orchard. 2000. Insulin-dependent diabetes mellitus and oral soft tissue pathologies. II. Prevalence and characteristics of Candida and candidal lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 89:570-576. [DOI] [PubMed] [Google Scholar]
- 14.Hemler, M. E. 1988. Adhesive protein receptors on hematopoietic cells. Immunol. Today 9:109-113. [DOI] [PubMed] [Google Scholar]
- 15.Hemler, M. E., C. Huang, and L. Schwarz. 1987. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J. Biol. Chem. 262:3300-3309. [PubMed] [Google Scholar]
- 16.Hermann, M., M. E. Jaconi, C. Dahlgren, F. A. Waldvogel, O. Stendahl, and D. P. Lew. 1990. Neutrophil bactericidal activity against Staphylococcus aureus adherent on biological surfaces. Surface-bound extracellular matrix proteins activate intracellular killing by oxygen-dependent and -independent mechanisms. J. Clin. Investig. 86:942-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, Y. C., T. Y. Lin, R. I. Lien, Y. H. Chou, C. Y. Kuo, P. H. Yang, and W. S. Hsieh. 2000. Candidaemia in special care nurseries: comparison of albicans and parapsilosis infection. J. Infect. 40:171-175. [DOI] [PubMed] [Google Scholar]
- 18.Jensen, J., T. Warner, and E. Balish. 1993. Resistance of SCID mice to Candida albicans administered intravenously or colonizing the gut: role of polymorphonuclear leukocytes and macrophages. J. Infect. Dis. 167:912-919. [DOI] [PubMed] [Google Scholar]
- 19.Kaposzta, R., L. Marodi, M. Hollinshead, S. Gordon, and R. P. da Silva. 1999. Rapid recruitment of late endosomes and lysosomes in mouse macrophages ingesting Candida albicans. J. Cell Sci. 112(Pt. 19):3237-3248. [DOI] [PubMed] [Google Scholar]
- 20.Lal, S., M. Mitsuyama, M. Miyata, N. Ogata, K. Amako, and K. Nomoto. 1986. Pulmonary defence mechanism in mice. A comparative role of alveolar macrophages and polymorphonuclear cells against infection with Candida albicans. J. Clin. Lab. Immunol. 19:127-133. [PubMed] [Google Scholar]
- 21.Lehrer, R. I. 1975. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J. Clin. Investig. 55:338-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrer, R. I., D. Szklarek, T. Ganz, and M. E. Selsted. 1985. Correlation of binding of rabbit granulocyte peptides to Candida albicans with candidacidal activity. Infect. Immun. 49:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus, J., M. E. Grossman, M. J. Yunakov, and F. Rappaport. 1992. Disseminated candidiasis, Candida arthritis, and unilateral skin lesions. J. Am. Acad. Dermatol. 26:295-297. [DOI] [PubMed] [Google Scholar]
- 24.Marodi, L., J. R. Forehand, and R. B. Johnston, Jr. 1991. Mechanisms of host defense against Candida species. II. Biochemical basis for the killing of Candida by mononuclear phagocytes. J. Immunol. 146:2790-2794. [PubMed] [Google Scholar]
- 25.Marodi, L., and R. B. Johnston, Jr. 1993. Enhancement of macrophage candidacidal activity by interferon-gamma. Immunodeficiency 4:181-185. [PubMed] [Google Scholar]
- 26.Marodi, L., S. Schreiber, D. C. Anderson, R. P. MacDermott, H. M. Korchak, and R. B. Johnston, Jr. 1993. Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J. Clin. Investig. 91:2596-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mor, N., and M. B. Goren. 1987. Discrepancy in assessment of phagosome-lysosome fusion with two lysosomal markers in murine macrophages infected with Candida albicans. Infect. Immun. 55:1663-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman, S. L., C. Bucher, J. Rhodes, and W. E. Bullock. 1990. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J. Clin. Investig. 85:223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman, S. L., L. Gootee, C. Kidd, G. M. Ciraolo, and R. Morris. 1997. Activation of human macrophage fungistatic activity against Histoplasma capsulatum upon adherence to type 1 collagen matrices. J. Immunol. 158:1779-1786. [PubMed] [Google Scholar]
- 30.Newman, S. L., and A. Holly. 2001. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect. Immun. 69:6813-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman, S. L., and M. A. Tucci. 1990. Regulation of human monocyte/macrophage function by extracellular matrix. Adherence of monocytes to collagen matrices enhances phagocytosis of opsonized bacteria by activation of complement receptors and enhancement of Fc receptor function. J. Clin. Investig. 86:703-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearsall, N. N., and D. Lagunoff. 1974. Immunological responses to Candida albicans. I. Mouse thigh lesion as a model for experimental candidiasis. Infect. Immun. 9:999-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian, Q., M. A. Jutila, N. Van Rooijen, and J. E. Cutler. 1994. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J. Immunol. 152:5000-5008. [PubMed] [Google Scholar]
- 34.Rantala, A. 1993. Postoperative candidiasis. Ann. Chir. Gynaecol. Suppl. 205:1-52. [PubMed] [Google Scholar]
- 35.Sasada, M., A. Kubo, T. Nishimura, T. Kakita, T. Moriguchi, K. Yamamoto, and H. Uchino. 1987. Candidacidal activity of monocyte-derived human macrophages: relationship between Candida killing and oxygen radical generation by human macrophages. J. Leukoc. Biol. 41:289-294. [DOI] [PubMed] [Google Scholar]
- 36.Schnur, R. A., and S. L. Newman. 1990. The respiratory burst response to Histoplasma capsulatum by human neutrophils. Evidence for intracellular trapping of superoxide anion. J. Immunol. 144:4765-4772. [PubMed] [Google Scholar]
- 37.Schuit, K. E. 1979. Phagocytosis and intracellular killing of pathogenic yeasts by human monocytes and neutrophils. Infect. Immun. 24:932-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selsted, M. E., D. Szklarek, T. Ganz, and R. I. Lehrer. 1985. Activity of rabbit leukocyte peptides against Candida albicans. Infect. Immun. 49:202-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, P. D., C. L. Lamerson, S. M. Banks, S. S. Saini, L. M. Wahl, R. A. Calderone, and S. M. Wahl. 1990. Granulocyte-macrophage colony-stimulating factor augments human monocyte fungicidal activity for Candida albicans. J. Infect. Dis. 161:999-1005. [DOI] [PubMed] [Google Scholar]
- 40.Swerdloff, J. N., S. G. Filler, and J. E. Edwards, Jr. 1993. Severe candidal infections in neutropenic patients. Clin. Infect. Dis. 17(Suppl. 2):S457-S467. [DOI] [PubMed] [Google Scholar]
- 41.Takada, Y., E. A. Wayner, W. G. Carter, and M. E. Hemler. 1988. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J. Cell. Biochem. 37:385-393. [DOI] [PubMed] [Google Scholar]
- 42.Thompson, H. L., and J. M. Wilton. 1992. Interaction and intracellular killing of Candida albicans blastospores by human polymorphonuclear leucocytes, monocytes and monocyte-derived macrophages in aerobic and anaerobic conditions. Clin. Exp. Immunol. 87:316-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez-Torres, A., and E. Balish. 1997. Macrophages in resistance to candidiasis. Microbiol. Mol. Biol. Rev. 61:170-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vecchiarelli, A., T. Todisco, M. Puliti, M. Dottorini, and F. Bistoni. 1989. Modulation of anti-Candida activity of human alveolar macrophages by interferon-gamma or interleukin-1-alpha. Am. J. Respir. Cell Mol. Biol. 1:49-55. [DOI] [PubMed] [Google Scholar]
- 45.Verduyn-Lunel, F. M., J. F. Meis, and A. Voss. 1999. Nosocomial fungal infections: candidemia. Diagn. Microbiol. Infect. Dis. 34:213-220. [DOI] [PubMed] [Google Scholar]
- 46.Wagner, D. K., C. Collins-Lech, and P. G. Sohnle. 1986. Inhibition of neutrophil killing of Candida albicans pseudohyphae by substances which quench hypochlorous acid and chloramines. Infect. Immun. 51:731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, M., H. Friedman, and J. Y. Djeu. 1989. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. J. Immunol. 143:671-677. [PubMed] [Google Scholar]
- 48.Watanabe, K., K. Kagaya, T. Yamada, and Y. Fukazawa. 1991. Mechanism for candidacidal activity in macrophages activated by recombinant gamma interferon. Infect. Immun. 59:521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wayner, E. A., and W. G. Carter. 1987. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J. Cell Biol. 105:1873-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Worsham, P. L., and W. E. Goldman. 1988. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J. Med. Vet. Mycol. 26:137-143. [PubMed] [Google Scholar]
- 51.Yamada, Y., H. Saito, H. Tomioka, and J. Jidoi. 1987. Relationship between the susceptibility of various bacteria to active oxygen species and to intracellular killing by macrophages. J. Gen. Microbiol. 133:2015-2021. [DOI] [PubMed] [Google Scholar]