Abstract
BACKGROUND
Patients with germline BRCA2 gene mutations (BRCA2mut) have more aggressive prostate cancer. Analysis of all reported germline BRCA2mut prostate cancer cases allows better understanding of the clinicopathologic features and survival outcomes of these men.
METHODS
A systematic review was performed with the MEDLINE database to capture articles evaluating clinicopathologic characteristics of men with BRCA2mut associated prostate cancer. Inclusion criteria were at least five subjects, confirmation of BRCA2mut status, and data for at least 2 clinical parameters of disease. Meta-analysis was performed on outcomes data. Chi-squared tests were used to compare disease features among men undergoing formal versus ad hoc screening, as well as an age of diagnosis less than versus greater than 65 years. Rates of metastatic disease among BRCA2mut cases were compared to rates among non-carrier control subjects and the general population using the SEER database.
RESULTS
Twelve out of 289 studies met our inclusion criteria, representing 261 BRCA2mut men. Among carriers, the median age at diagnosis was 62 years and median PSA was 15 ng/dl with 95% of men having a PSA>3. Over 40% of BRCA2mut patients had T3/T4 disease and over 25% were metastatic at presentation. Survival was worse in BRCA2mut men with prostate cancer when compared to non-BRCA2mut subjects. BRCA2mut carriers had significantly higher rates of metastatic disease (18%) versus non-carrier controls (8%) and the SEER population (4%).
CONCLUSIONS
BRCA2mut carriers are more likely to have poor risk of prostate cancer at presentation and exhibit worse oncologic outcomes relative to non-carriers, including a fourfold increase in metastatic disease. Younger men and those undergoing formal screening present with less advanced disease which supports a need for earlier identification and screening protocols. Additionally, this population may benefit from alternative therapeutic paradigms.
Keywords: BRCA2, prostate, metastatic, aggressive, screening
INTRODUCTION
Although prostate cancer is the most common cancer diagnosed among men in the United States, overall cancer specific mortality rates remain at less than 3% [1]. Many research efforts are focused on identifying men harboring disease with high metastatic potential who may benefit from earlier screening and more focused interventions. Somatic and germline alterations in the BRCA2 gene play an important role in human carcinogenesis by preventing homologous recombination of double stranded DNA breaks and have been associated with over 10% of metastatic prostate cancer cases [2]. Associated with hereditary breast and ovarian cancer (HBOC), approximately 1.2% of prostate cancer cases among men less than 65 years of age and 6% of metastatic castration resistant prostate cancer (mCRPC) cases carry germline BRCA2mut [3,4]. Men who carry germline BRCA2mut are up to eight times more likely to develop prostate cancer compared to non-carriers, and develop an aggressive disease variant with advanced stage and grade at presentation and poor survival outcomes [3,5-9]. These findings have been reflected in numerous studies comprised of small samples from disparate populations. While prior reports have qualitatively summarized the clinical features of germline BRCA2mut mutations in prostate cancer, there have been no systematic reviews of the published data [10,11]. The primary objective of this study was to formally characterize the clinicopathologic characteristics upon the diagnosis of prostate cancer in men harboring germline BRCA2mut via a systematic review. Secondarily, outcomes data within included studies underwent a meta-analysis.
METHODS
A systematic review was conducted with the MEDLINE database using the search phrase “BRCA2 prostate” [12]. Inclusion criteria were at least five subjects, genetic confirmation of BRCA2mut mutations, data for at least two clinical parameters such as age, PSA, Gleason’s score (GS), and tumor stage at diagnosis of prostate cancer. The data extracted from each study included year of publication, study type, population of interest, identified mutations, age, PSA level, GS, tumor stage, and outcomes data of overall and cause-specific survival. Tumor stage was determined by clinical [3,5-8,13-15] and surgical [3,6,9,16,17] findings. Chi-squared statistics and P-values were calculated to compare GS and stage among retrospective [3,5-7,9,15-19] and prospective studies [14], as well as by average age groupings (<65 [3,5,7,8,14,15] vs. >65 years [6,13,16,17]). Random effect hazard ratios were calculated for overall and cause-specific survival data with Comprehensive Meta-Analysis Software (Biostat, Inc., Englewood, NJ). A random effect model was chosen as our sample size more appropriately estimated a mean effect size rather than a true effect measure. Tests for heterogeneity included a Q score (Q) with P-value, an I2 statistic, and Tau2 [20]. To compare staging data among BRCA2mut carriers and the general population, both study non-carrier control subjects from included studies and the SEER 18 database were utilized. SEER database selection criteria of age at diagnosis, site, and morphology of prostate, year of diagnosis 2004–2012, and site and morphology of all adenocarcinoma variants (8140/2, 8140/3, 8141/2, 8141/3, 8143/2, 8143/3, 8147/3) was used. Chi-squared statistics were used to determine significant differences in categorical variables between the groups while t-tests were used for discrete variables. Significance was set at a P-value of 0.05. Weighted averages were used for continuous variables.
RESULTS
Study Selection
The MEDLINE database query resulted in 289 studies, of which 12 met inclusion criteria [3,5-9,13-17,21] (Table I). Mitra et al. consisted of preliminary findings that were later reported in the Bancroft et al. study, so it was not included in the analysis [14,18]. Similarly, the Bolton et al. study was excluded as the clinical data were previously reported in the Thorne et al. study [6,22]. Various study types were included in the analysis, including one prospective cohort, a case series, and 10 retrospective cohorts. Publication dates ranged from 1997 to 2015 with subjects from numerous countries, including Portugal, Germany, Canada, United Kingdom, Ireland, Australia, New Zealand, United States, Iceland, Spain, Denmark, Norway, India, Italy, Malaysia, Norway, Slovakia, Slovenia, Sweden, Iceland, Poland, Israel, and the Netherlands (Table I).
TABLE I. Summary of Studies by Author, Country, Rear, Design, Population, Participants, Mutations, and Sample Size.
| Study | Country | Year | Design | n | Population | Participants | BRCA2+ mutations |
|---|---|---|---|---|---|---|---|
| Maia et al. | Portugal | 2015 | Retrospective cohort |
6 | 460 early-onset (≤55 years) and/or familial/hereditary prostate cancer (PrCa) Portuguese probands from a regional cancer registry; 288 male blood donors with no history of PrCa as controls |
n = 6 BRCA2mut, n = 288 men with no history cancer |
c.l56_157insAlu × 6 |
| Maier et al. | Germany | 2014 | Retrospective cohort |
5 | 474 PrCa cases: 382 with FHx from the Prostate Cancer Genetics Project; 92 sporadic, early onset from University Hospital |
n = 5 BRCA2mut, all with FHx, n = 474 all PrCa cases (including BRCA2mut) |
Three frameshifts (1605 fs, V128 fs, T1483 fs, two nonsense (K2013X, Q2499X); VUS excluded |
| Walker et al. | Canada | 2014 | Retrospective cohort |
6 | 106 PrCa naïve men from Prostate Cancer Prevention Clinic (23 with BRCA2mut, 29 with BRCAlmut, and 53 with PrCa FHx) |
n = 6/23 with BRCA2mut and PrCa, n = 11 /53 with FHx of PrCa and PrCa |
c.6137C>A, c.6174delT (×2), c.7757G>A, unknown (×2) |
| Bancroft et al. | Global (≥20 countries) |
2014 | Prospective cohort |
24 | 2,481 PrCa naïve men from IMPACT trial; 731 BRCA2mut carriers and 428 BRCA2 controls, as well as 791 BRCA1 mut carriers and 531 BRCA1 controls |
n = 24/731BRCA2mut carriers and PrCa, n = 7/ 428 non-carriers and PrCa |
Unknown |
| Akbari et al. | Canada | 2014 | Retrospective cohort |
26 | 1,904 men with PrCa from two tertiary care centers |
n = 26 with BRCA2mut, n = 1,878 non-carriers |
Unknown |
| Castro et al. | United Kingdom, Ireland |
2013 | Retrospective cohort |
61 | 2,019 eligible patients from EMBRACE, UKGPC study |
n = 61 BRCA2mut; n = 1,940 non-carrier (n = 18 BRCA1mut) |
Unknown |
| Kote-Jarai et al. |
United Kingdom |
2011 | Retrospective cohort |
19 | 1,621 PrCa cases ≤65 years, 243 PrCa cases >65 with FHx of first degree relative with PrCa from UKGPC study; of which 32 were excluded from the analysis |
n = 19 BRCA2mut, n = 1,813 non-carriers |
1231delA, 1265delA, 1787delATGAAACATCTTA, 1813insA, 2807delAACA, 2836delGA, 3158T>G, 3405C>A, 3847delGT, 4478delAAAG, 4877delAA (×2), 4981delT, 5303delTT< 5645C>A, 6405delCTTAA (×2), 8904delC, 9253insA |
| Thorne et al. | Australia, New Zealand |
2011 | Retrospective cohort |
40 | 137 men with PrCa from kCONFAB cohort |
n = 40 BRCA2mut, n = 97 non-carriers |
24 frameshift, nonsense, missense mutations and 2 Large genomic rearrangements |
| Gallagher et al. |
USA | 2010 | Retrospective cohort |
20 | 832 PrCa cases with localized disease of Ashkenazi Jewish descent at Memorial Sloan Kettering |
n = 20 BRCA2mut, n = 806 non-carriers (n = 6 BRCAlmut) |
BRCA2* 6174delT |
| Mitra et al. | United Kingdom, Ireland |
2008 | Retrospective cohort with nested case control |
16 | PrCa cases from EMBRACE study, IMPACT, cancer genetics outpatient clinic, series of young onset PrCA cases |
n = 16 BRCA2mut, n = 16 controls matched by age, PSA, disease stage of which two were confirmed non-carriers |
6819delTG, 6174delT, 5910C>G, 7771ins A, 6503delTT, 3386T>G (×2) 6503delTT, 7084delAAAAG, 2558insA, 7772insA, 6710delCAA, 5531delTT, 8395G>C, 8205-lG>C, nucleotide variation 2 |
| Tryggvadottir et al. |
Iceland | 2007 | Retrospective cohort with nested case control |
29 | 527 PrCa cases in Icelandic Cancer Registry with female breast cancer relatives |
n = 30 BRCA2mut, n = 497 non-carriers with n = 57 matched controls |
999del5 |
| Sigurdsson et al. |
Iceland | 1997 | Case series | 9 | 12 PrCa cases from BRCA2mut Icelandic families and 77 PrCa diagnosed in 1983 |
n = 9 BRCA2mut, n = 4 non-carriers from BRCA2mut families |
999del5 |
PrCa, prostate cancer; FHx, family history; BRCA2mut, BRCA2 mutation carrier; BRCA1mut, BRCA1 mutation carrier; IMPACT, identification of men with a genetic predisposition to prostate cancer; EMBRACE, epidemiological study of BRCA1/2 Mutation Carrier; UKGPC, United Kingdom Genetic Prostate Cancer study; kCONFAB, Kathleen Cunningham Consortium for Research info Familial Breast Cancer.
Clinical Characteristics
A total of 261 BRCA2mut men were identified among the included studies. Data regarding age at diagnosis were available in 11 studies, with a median age of 62 years (Table II). PSA levels were reported in nine studies, with a median PSA level of 15 ng/ml. PSA at diagnosis was greater than three in 95% of men, greater than 10 ng/ml in 39%, and greater than 100 ng/ml in 12% of men. Gleason score at diagnosis, provided by 11 studies, was 71% with GS ≥ 7. Staging at diagnosis, based on nine studies, revealed 40% of men with cT3/T4 disease [3,5-7,13-15] and 63% with pT3/T4 [3,8,9,14,15,17] (overall, 47% with either clinical or pathologic T3/T4). Metastatic disease was present in 26% of men at diagnosis (Table II).
TABLE II. Summary of Clinical Features of Prostate Cancer in BRCA2mut Men by Age, PSA (ng/mL), Gleason’s Score, and Tumor Stage.
| Study | Year | n | Age (years) |
PSA (ng/ml) |
Gleason’s score |
Local tumor stage |
Localized versus non-localized |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤3, n (%) |
>3, n (%) |
Unknown, n (%) |
>10, n (%) |
>100, n (%) |
Average | <7, n (%) |
≥7, n (%) |
Unknown, n (%) |
≤cT2, n (%) |
cT3/T4, n (%) |
≤pT2, n (%) |
pT3/T4, n (%) |
Unknown, n (%) |
M0, n (%) |
M1, n (%) |
||||
| Maia et al. | 2015 | 6 | 61.6 | 1 (17) | 3 (50) | 2 (33) | 1 (17) | 0 | 7.8 | 2 (33) | 3 (50) | 1 (17) | 1 (17) | 1 (17) | 1 (17) | 2 (32) | 1 (17) | 6 (100) |
0 |
| Maier et al. | 2014 | 5 | – | 0 | 5 (100) |
0 | – | – | – | 1 (20) | 4 (80) | – | – | – | 2 (40) | 3 (60) | 0 | – | – |
| Walker et al. | 2014 | 6 | 58.1 | 0 | 5 (83) | 1 (17) | 1 (17) | 0 | 5.9 | 3 (50) | 3 (50) | – | – | – | 3 (50) | 2 (33) | 1 (17) | – | – |
| Bancroft et al. | 2014 | 24 | 58.5 | 1 (4) | 23 (96) |
0 | 2 (8) | 0 | 4.4 | 17 (71) |
6 (25) | 1 (4) | 13 (54) | 3 (12) | 5 (21) | 3 (13) | 0 | 17 (100) |
0 |
| Akbari et al. | 2014 | 26 | 67 | – | – | – | – | – | 56.3 | 1 (4) | 25 (96) |
0 | 13 (50) | 11 (42) | – | – | 2 (8) | 18 (75) |
6 (25) |
| Castro et al. | 2013 | 61 | 57.6 | – | – | – | – | – | 15.1 | 14 (23) |
38 (62) |
9 (15) | 26 (43) | 23 (37) | – | – | 12 (20) | 38 (78) |
11 (22) |
| Kote-Jarai et al. |
2011 | 19 | 56 | – | – | – | – | – | – | 3 (16) | 13 (68) |
3 (16) | 10 (53)a | 7 (37)a | 10 (53)a | 7 (37)a | 2 (10) | 15 (88) |
2 (12) |
| Thorne et al. | 2011 | 40 | 66 | 1 (3) | 28 (70) |
11 (27) | 18 (45) | 4 (10) | – | 2 (5) | 36 (90) |
2 (5) | 21 (52) | 15 (37) | – | – | 2 (5) | 29 (81) |
7 (19) |
| Gallagher et al. |
2010 | 20 | 62 | – | – | – | – | – | 7.0 | 17 (85) |
2 (10) | 1 (5) | – | – | – | – | – | – | – |
| Mitra et al. | 2008 | 16 | 52.5 | 1 (6) | 12 (75) |
3 (19) | 7 (44) | 5 (31) | 24.3 | – | – | – | – | – | – | – | – | – | – |
| Tryggvadottir et al. |
2007 | 29 | 69 | – | – | – | – | 4 (14) | 21 (72) |
4 (14) | – | – | 6 (21) | 23 (79) | 0 | 13 (45) |
16 (55) |
||
| Sigurdsson et al. |
1997 | 9 | 71 | – | – | – | – | 1 (11) | 6 (67) | 2 (22) | – | – | – | – | – | 3 (33) | 6 (67) | ||
| Averages | 61.6b | 4% | 78% | 18% | 39%c | 12%c | 15.1b | 27% | 64% | 9% | 58%c | 42%c | 40%c | 60%c | 9% | 74%c | 26%c | ||
| 61.7c | 5%c | 95%c | 19.6d | 29%c | 71%c | ||||||||||||||
Combined clinical and pathologic staging data.
Median average.
Unknowns removed from denominator.
Weighted mean average.
Clinical features among BRCA2mut cases were compared to non-carrier control subjects from included study populations (Tables I and III) [3,5-9,13-17]. This analysis showed a significantly higher PSA among BRCA2mut men at diagnosis (median of 15.1 ng/dl, mean of 19.5 ng/dl vs. median of 11 ng/dl, mean of 11.1 ng/dl in non-carriers, P<0.001), a significantly higher number of GS ≥ 7 cases among BRCA2mut men (64% vs. 49% of non-carriers, P<0.001), significantly more overall and pathologic T3/T4 disease at presentation (41% vs. 29% of non-carriers, P=0.006, 63% vs. 37%, P < 0.001, respectively) and significantly higher rates of metastatic disease at diagnosis (26% vs. 8% of non-carriers, P < 0.001). No significant difference was seen for age at onset.
TABLE III. Summary of Clinical Features Among BRCA2mut Men, Study Control Subjects (“Non-Carrier Controls”), and the General Population (“SEER Population”).
| BRCA2mut cases, n (%) |
Non-carriers controls, n (%) |
P-value | SEER population, n (%) |
P-value | |
|---|---|---|---|---|---|
| n | 261 | 7,109 | 494,739 | ||
| Median age in years (mean) | 61.6 (61.7) | 65 (62.2) | 0.12 | 66 (66.5) | <0.001a |
| PSA | 0.95 | ||||
| ≤3ng/dl | 4 (5) | 10 (10) | – | ||
| >3ng/dl | 76 (95) | 89 (90) | – | ||
| Unknown | 181 | 7,010 | – | ||
| Median PSA ng/dl (mean) | 15.1 (19.5) | 11 (11.1) | <0.001b | – | |
| Gleason’s score | <0.001b | ||||
| <7 | 65 (29) | 1,972 (46) | – | ||
| ≥7 | 157 (71) | 2,366 (54) | – | ||
| Unknown | 39 | 2,771 | – | ||
| Stage overall | 0.006b | <0.001a | |||
| ≤T2 | 100 (53) | 1,385 (65) | 429,531 (90) | ||
| T3/T4 | 88 (47) | 750 (35) | 45,936 (10) | ||
| Unknown | 73 | 4974 | 19,272 | ||
| Localized | <0.001b | <0.001a | |||
| M0 | 139 (74) | 2,322 (92) | 452,296 (96) | ||
| M1 | 48 (26) | 191 (8) | 18,341 (4) |
Statistically significant difference between BRCA2mut and SEER control subjects.
Statistically significant difference between BRCA2mut and non-carrier control subjects.
With the exclusion of studies with recruitment periods preceding 1995 (to minimize bias from pre-PSA era screening regimens) and/or lacking staging information, clinicopathologic data among BRCA2mut cases were compared to SEER-18 population data (Table III) [5,6,13-15]. This analysis confirmed a statistically significant earlier median age of disease onset (62 years in BRCA2mut vs. 66 years from SEER, P < 0.001) and significantly higher stage presentation with cT3/T4 disease in 40% of BRCA2mut carriers versus 9% of SEER prostate cancer cases (P < 0.001). Similarly, metastatic presentation was noted in 18% of BRCA2mut carriers versus 4% of prostate cancer cases from the SEER database (P < 0.001).
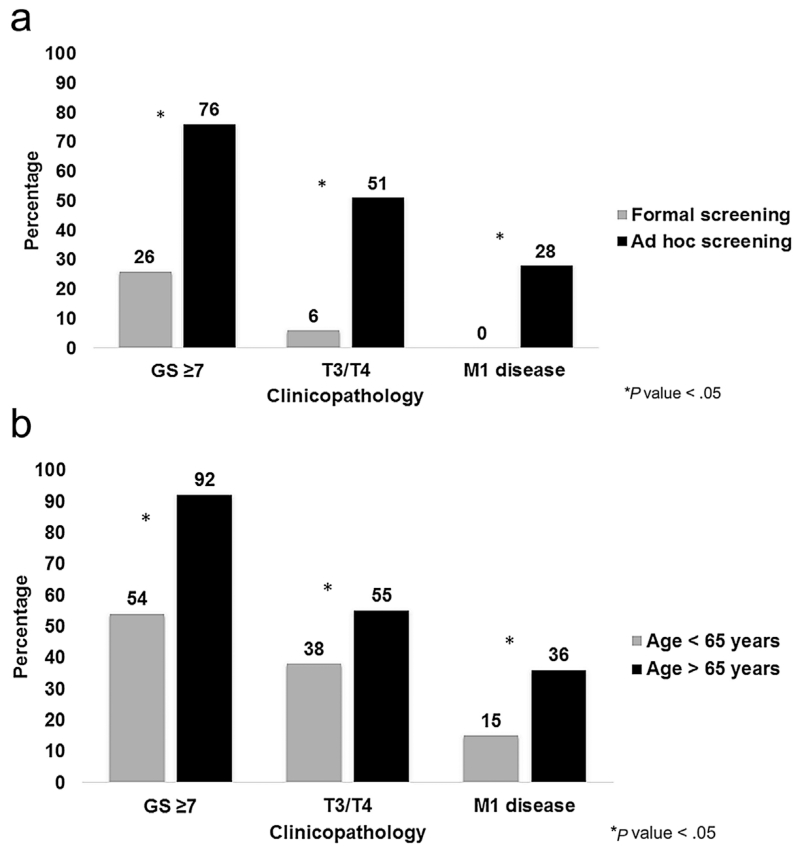
To assess the impact of formal screening protocols, clinicopathologic data from Bancroft et al.’s prospective trial was compared to retrospective studies [3,5-9,13,15-17]. Significantly fewer cases of GS ≥ 7, stage T3/T4, and M1 disease were seen among subjects undergoing systematic screening (P < 0.05) (Fig. 1a). To assess if age of diagnosis impacted how disease presented, we compared studies with an average age >65 years [3,5,7,8,14,15] against those with an average age <65 years [6,13,16,17]. The older populations had significantly more GS ≥ 7, stage T3/T4, and M1 disease (P < 0.05) (Fig. 1b).
Fig. 1.
a: Comparison of GS ≥ 7, T3/T4 stage, and metastasis for subjects from prospective versus retrospective studies. b: Comparison of GS ≥ 7, T3/T4 stage, and metastasis for subjects aged <65 versus >65 years.
Survival Outcomes
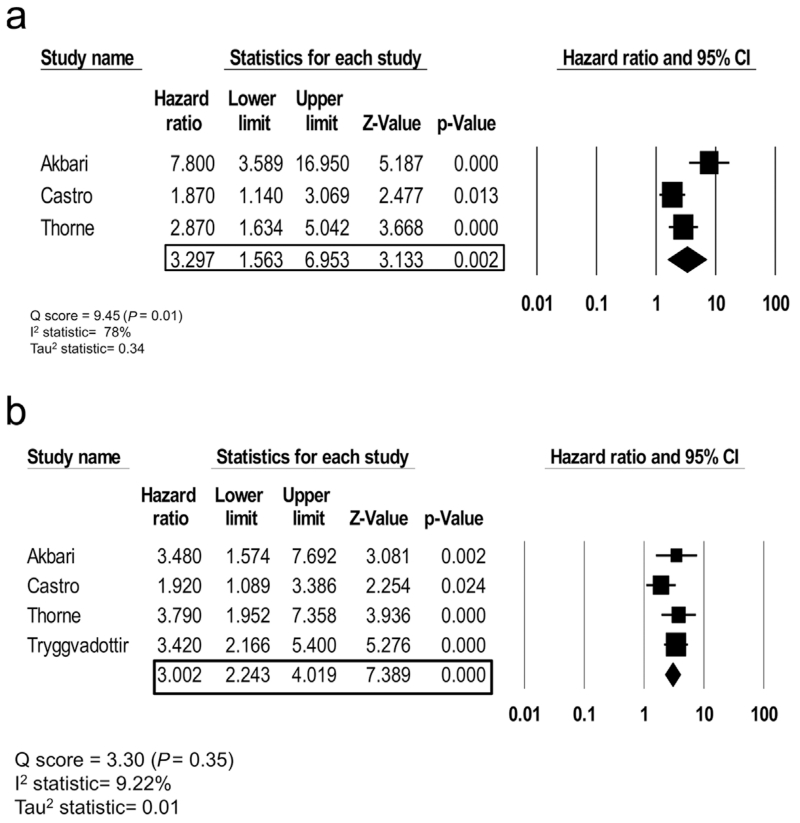
Overall survival (OS) hazard ratios were reported in three studies, while cause-specific survival (CSS) outcomes were reported in four studies. For all BRCA2mut carriers, the OS random effect hazard ratio is 3.30 (95%CI, 1.56–6.95) with a Q-score of 9.24 (P-value=0.01), an I2 statistic of 78%, and a Tau2 of 0.34 which suggests increased heterogeneity among studies (Fig. 2a). The CSS random effect hazard ratio is 3.00 (95%CI, 2.20–4.09) with a Q-score of 3.30 (P-value = 0.35), an I2 statistic of 9.22%, and a Tau2 of 0.01 which implies decreased heterogeneity among studies (Fig. 2b).
Fig. 2.
a: Random effect model meta-analysis for OS hazard ratio with measures of heterogeneity (Q score, I2 statistic, and Tau2 statistic). b: Random effect model meta-analysis for CSS hazard ratio with measures of heterogeneity (Q score, I2 statistic, and Tau2 statistic).
DISCUSSION
This systematic review allows a comprehensive overview of the clinical features of BRCA2mut prostate cancer. At diagnosis, greater than two-thirds of carriers have GS ≥ 7 disease and nearly half have cT3/T4 disease. Most concerning, over one quarter had metastatic disease at presentation. When comparing disease in BRCA2mut cases to the general population, carriers are four times more likely to present with both metastatic and higher stage (T3/T4) disease. When compared to non-carrier men from similar geographical and/or higher risk backgrounds, they are three times more likely to develop metastatic disease. They also have worse overall survival and are three times more likely to die from their prostate cancer than their counterparts. Recent genetic testing of patients with mCRPC has revealed high rates of mutations to DNA repair genes, including BRCA2 [2,23]. This suggests that BRCA2mut disease has a tendency for early spread and therapeutic resistance so early detection of mutations is believed to be important. The NCCN recommends genetic testing in men with advanced prostate cancer (GS ≥ 7) in the context of a family history of breast, ovarian, pancreatic, or aggressive prostate cancer (GS ≥ 7), or a similar family history even without a personal history of prostate cancer [24]. Integration of genetic testing into the screening paradigm should be considered [25].
Upon identification of BRCA2mut, specific surveillance strategies have not been established. In the Identification of Men with a genetic predisposition to ProstAte Cancer Trial (IMPACT) screening protocol, men aged 40–59 years from families with known BRCA mutations were recruited and underwent an annual PSA assay that utilized a biopsy threshold of 3 ng/dl [14]. The trial showed that a PSA level of 3 ng/dl had a 48% positive predictive value (PPV) in BRCA2mut patients versus 33% in controls. Ongoing follow-up analyses from IMPACT, including an optional end of study biopsy in men with PSA levels < 3 ng/dl, will offer insights regarding negative predictive values (NPV) and optimal PSA thresholds.
Other markers, such as PCA3, Ki-67, and microseminoprotein (MSP), have been explored but have not been shown to provide additional diagnostic value in BRCA2mut men [14,18,26]. Newer strategies of prognostication, such as prostate multiparametric MRI (mpMRI), with a reported PPV of 65% for detection and localization of cancer in a non-BRCA2mut specific population, offers potential improved diagnostic certainty with a greater confidence of disease exclusion among BRCA2mut men [27]. Further studies to specifically compare diagnostic performance of mpMRI among BRCA2mut men are currently underway [28,29].
The median age of prostate cancer onset among BRCA2mut men was found to be 61.7 years, which is 5 years less than the general population (per SEER data). Interestingly, no significant difference in age was found between BRCA2mut and non-carrier controls. Given that most studies were retrospective in nature, this allowed for age-matched controls [17,21], a focus on study populations comprised of early-onset disease [5], or populations with a known or suspected oncogenic predisposition [6]. Based on this information, we consider age to be mostly a controlled variable when comparing carriers versus non-carriers. This is further supported by the IMPACT trial findings of an average age of onset among BRCA2mut men of 58.5 versus 65 years in non-carriers. Limited by a small sample size, a second prospective study, among BRCA2mut male carriers aged 40–70 in Israel, is being conducted to assess screening methods and will offer more data regarding average age of onset [30]. Ideally, these prospective studies may also assess optimal PSA dynamics and thresholds for diagnosis. These prospective studies will offer greater insights on how best to design and when to initiate screening algorithms.
Kote-Jarai et al. showed worse 5 year outcomes (as assessed by metastatic free survival [MFS] and CSS) in similarly treated BRCA2mut patients with M0 disease versus controls with M0 disease [5]. Given the strong metastatic potential of BRCA2mut disease, this suggests a need for alternative treatment algorithms. Castro et al. showed significantly better outcomes (as assessed by MFS and CSS) among men who underwent radical prostatectomy versus radiation (± androgen deprivation therapy [ADT]) for localized disease [5,31]. This study was confounded by the fact that subjects who received radiation had more advanced localized disease. Nevertheless, these findings mimic the pattern seen among women with BRCAmut associated breast cancer who do significantly better with mastectomy versus lumpectomy with radiation [32]. Interestingly, outcomes are equalized when women undergoing lumpectomy with radiation also receive chemotherapy [32]. There is a need to explore the optimal initial radical therapy for localized disease, as well as the potential for adjuvant or neoadjuvant chemotherapy [33].
Currently, alternative therapies are being explored within the sub-population of mCRPC BRCA2mut disease. The poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors, acting via synthetic lethality with possible BRCA2mut cell targeting, were recently granted breakthrough therapy designation for mCRPC in the setting of BRCA1/2 or ATM mutations [34]. Among eight of the nine BRCA2mut mCRPC men, PARP inhibitors decreased PSA by at least 50% from baseline; all seven BRCA2mut men with complete BRCA2 loss had a noted decrease in PSA [4]. Among the six BRCA2mut subjects with measurable disease at baseline, a radiologic partial response was elicited [4]. A recently published case report described the first patient with BRCA2mut mCRPC disease to have a complete response with PARP therapy [35]. In addition to PARP inhibitors, alkylating platinum therapy is being explored in mCRPC due to its efficacy in BRCAmut associated epithelial ovarian cancer [36]. A recent study showed a partial to complete response for at least 6 months among three men with mCRPC BRCA2mut disease, of whom two previously had biochemical recurrence in the setting of ADT with taxanes [37]. Given the tendency for metastatic disease and the possibility for more efficacious therapeutics, the significance of genetic testing and earlier detection on BRCA2mut status is further emphasized.
Limitations of this study include a reliance on retrospective studies that comprised disparate cohorts. Our literature search limitation to the MEDLINE database may have missed relevant studies in other aggregate databases. Our inclusion criteria requirement to report data for at least two clinical parameters and a sample of five subjects may have prevented identification of some BRCA2mut cases. Study samples may be geographically or ethnically biased (i.e., Gallagher et al. with Ashkenazi Jewish patients, Tryggvadottir et al. and Sigurdsson et al. with the 999del5 Icelandic mutation) which could influence screening paradigms, access to healthcare, and the manner in which particular germline mutations manifest clinically. Another limitation, common to all systematic reviews, is the lack of access to full data sets, which led to several unreported values in our analysis. With regard to our meta-analysis, a small number of studies were included which limits the reliability of our outcomes and leads to increased sampling error. Given the significant heterogeneity for the overall survival model, it is unclear if the included studies are truly representative of the BRCA2mut population. A more robust analysis is needed to assess survival outcomes among BRCA2-mut carriers. While the SEER population highlighted a deviation from more typical disease presentation, it is important to consider the likely earlier and more vigorous screening regimens among study candidates representing BRCA2mut carriers which limits a direct comparison. The comparison with non-carrier control subjects with similar high risk features (family history, early onset disease, overlap in geography), however, controls for some ascertainment bias allowing us to better appreciate our findings. Additionally, it is important to note that the SEER data may contain BRCA2mut men.
CONCLUSION
This systematic review quantitatively characterizes the clinical characteristics of prostate cancer among BRCA2mut men. Prostate disease in BRCA2mut carriers is more aggressive with early systemic spread and poorer survival outcomes. Given that no formal screening recommendations exist for this high-risk subset of men, ongoing studies exploring various screening tools and survival outcomes from earlier detection will be highly beneficial. Additionally, ongoing studies to explore the impact of alternative treatment regimens and innovative therapeutics hold much promise.
ACKNOWLEDGMENTS
Supported in part by National Institutes of Health grant R01 CA161018, PI: Leszek Kotula.
Footnotes
Conflicts of interest: The authors have nothing to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng FY, Tomlins SA, Cooney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu EY, Mostaghel EA, Cheng HH, Mulcahy H, True LD, Plymate SR, Dvinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson BD, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PS, Garraway LA, Sawyers CL, Chinnaiyan AM. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kote-Jarai Z, Leongamornlert D, Saunders E, Tymrakiewicz M, Castro E, Mahmud N, Guy M, Edwards S, O’Brien L, Sawyer E, Hall A, Wilkinson R, Dadaev T, Goh C, Easton D, Collaborators U, Goldgar D, Eeles R. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: Implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230–1234. doi: 10.1038/bjc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A’Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, Mahmud N, Dadaev T, Govindasami K, Guy M, Sawyer E, Wilkinson R, Ardern-Jones A, Ellis S, Frost D, Peock S, Evans DG, Tischkowitz M, Cole T, Davidson R, Eccles D, Brewer C, Douglas F, Porteous ME, Donaldson A, Dorkins H, Izatt L, Cook J, Hodgson S, Kennedy MJ, Side LE, Eason J, Murray A, Antoniou AC, Easton DF, Kote-Jarai Z, Eeles R. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorne H, Willems AJ, Niedermayr E, Hoh IM, Li J, Clouston D, Mitchell G, Fox S, Hopper JL, C Kathleen Cunningham Consortium for Research in Familial Breast Cancer. Bolton D. Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res (Phila) 2011;4(7):1002–1010. doi: 10.1158/1940-6207.CAPR-10-0397. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher DJ, Gaudet MM, Pal P, Kirchhoff T, Balistreri L, Vora K, Bhatia J, Stadler Z, Fine SW, Reuter V, Zelefsky M, Morris MJ, Scher HI, Klein RJ, Norton L, Eastham JA, Scardino PT, Robson ME, Offit K. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16(7):2115–2121. doi: 10.1158/1078-0432.CCR-09-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker R, Louis A, Berlin A, Horsburgh S, Bristow RG, Trachtenberg J. Prostate cancer screening characteristics in men with BRCA1/2 mutations attending a high-risk prevention clinic. Can Urol Assoc J. 2014;8(11-12):E783–E788. doi: 10.5489/cuaj.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maier C, Herkommer K, Luedeke M, Rinckleb A, Schrader M, Vogel W. Subgroups of familial and aggressive prostate cancer with considerable frequencies of BRCA2 mutations. Prostate. 2014;74(14):1444–1451. doi: 10.1002/pros.22860. [DOI] [PubMed] [Google Scholar]
- 10.Alanee SR, Glogowski EA, Schrader KA, Eastham JA, Offit K. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed) 2014;6:15–30. doi: 10.2741/e686. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute [Accessed June 20, 2015];PDQ® Genetics of Prostate Cancer. 2015 http://www.cancer.gov/types/prostate/hp/prostate-genetics-pdq#section/_981.
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Med. 2009;3(3):123–130. [PMC free article] [PubMed] [Google Scholar]
- 13.Akbari MR, Wallis CJ, Toi A, Trachtenberg J, Sun P, Narod SA, Nam RK. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer. Br J Cancer. 2014;111(6):1238–1240. doi: 10.1038/bjc.2014.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bancroft EK, Page EC, Castro E, Lilja H, Vickers A, Sjoberg D, Assel M, Foster CS, Mitchell G, Drew K, Maehle L, Axcrona K, Evans DG, Bulman B, Eccles D, McBride D, van Asperen C, Vasen H, Kiemeney LA, Ringelberg J, Cybulski C, Wokolorczyk D, Selkirk C, Hulick PJ, Bojesen A, Skytte AB, Lam J, Taylor L, Oldenburg R, Cremers R, Verhaegh G, van Zelst-Stams WA, Oosterwijk JC, Blanco I, Salinas M, Cook J, Rosario DJ, Buys S, Conner T, Ausems MG, Ong KR, Hoffman J, Domchek S, Powers J, Teixeira MR, Maia S, Foulkes WD, Taherian N, Ruijs M, Helderman-van den Enden AT, Izatt L, Davidson R, Adank MA, Walker L, Schmutzler R, Tucker K, Kirk J, Hodgson S, Harris M, Douglas F, Lindeman GJ, Zgajnar J, Tischkowitz M, Clowes VE, Susman R, Ramon y, Cajal T, Patcher N, Gadea N, Spigelman A, van Os T, Liljegren A, Side L, Brewer C, Brady AF, Donaldson A, Stefansdottir V, Friedman E, Chen-Shtoyerman R, Amor DJ, Copakova L, Barwell J, Giri VN, Murthy V, Nicolai N, Teo SH, Greenhalgh L, Strom S, Henderson A, McGrath J, Gallagher D, Aaronson N, Ardern-Jones A, Bangma C, Dearnaley D, Costello P, Eyfjord J, Rothwell J, Falconer A, Gronberg H, Hamdy FC, Johannsson O, Khoo V, Kote-Jarai Z, Lubinski J, Axcrona U, Melia J, McKinley J, Mitra AV, Moynihan C, Rennert G, Suri M, Wilson P, Killick E, Collaborators I, Moss S, Eeles RA. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: Results from the initial screening round of the IMPACT study. Eur Urol. 2014;66(3):489–499. doi: 10.1016/j.eururo.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maia S, Cardoso M, Paulo P, Pinheiro M, Pinto P, Santos C, Pinto C, Peixoto A, Henrique R, Teixeira MR. The role of germline mutations in the BRCA1/2 and mismatch repair genes in men ascertained for early-onset and/or familial prostate cancer. Fam Cancer. 2016;15(1):111–121. doi: 10.1007/s10689-015-9832-x. [DOI] [PubMed] [Google Scholar]
- 16.Sigurdsson S, Thorlacius S, Tomasson J, Tryggvadottir L, Benediktsdottir K, Eyfjord JE, Jonsson E. BRCA2 mutation in Icelandic prostate cancer patients. J Mol Med (Berl) 1997;75(10):758–761. doi: 10.1007/s001090050162. [DOI] [PubMed] [Google Scholar]
- 17.Tryggvadottir L, Vidarsdottir L, Thorgeirsson T, Jonasson JG, Olafsdottir EJ, Olafsdottir GH, Rafnar T, Thorlacius S, Jonsson E, Eyfjord JE, Tulinius H. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(12):929–935. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 18.Mitra AV, Bancroft EK, Barbachano Y, Page EC, Foster CS, Jameson C, Mitchell G, Lindeman GJ, Stapleton A, Suthers G, Evans DG, Cruger D, Blanco I, Mercer C, Kirk J, Maehle L, Hodgson S, Walker L, Izatt L, Douglas F, Tucker K, Dorkins H, Clowes V, Male A, Donaldson A, Brewer C, Doherty R, Bulman B, Osther PJ, Salinas M, Eccles D, Axcrona K, Jobson I, Newcombe B, Cybulski C, Rubinstein WS, Buys S, Townshend S, Friedman E, Domchek S, Ramon T, Cajal Y, Spigelman A, Teo SH, Nicolai N, Aaronson N, Ardern-Jones A, Bangma C, Dearnaley D, Eyfjord J, Falconer A, Gronberg H, Hamdy F, Johannsson O, Khoo V, Kote-Jarai Z, Lilja H, Lubinski J, Melia J, Moynihan C, Peock S, Rennert G, Schroder F, Sibley P, Suri M, Wilson P, Bignon YJ, Strom S, Tischkowitz M, Liljegren A, Ilencikova D, Abele A, Kyriacou K, van Asperen C, Kiemeney L, Collaborators IS, Easton DF, Eeles RA. Targeted prostate cancer screening in men with mutations in BRCA1 and BRCA2 detects aggressive prostate cancer: Preliminary analysis of the results of the IMPACT study. BJU Int. 2011;107(1):28–39. doi: 10.1111/j.1464-410X.2010.09648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Institute [Accessed April 1, 2015];BRCA1 and BRCA2: Cancer Risk and Genetic Testing. 2015 http://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet.
- 20.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. updated March 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
- 21.Mitra A, Fisher C, Foster CS, Jameson C, Barbachanno Y, Bartlett J, Bancroft E, Doherty R, Kote-Jarai Z, Peock S, Easton D, Impact, Collaborators E. Eeles R. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer. 2008;98(2):502–507. doi: 10.1038/sj.bjc.6604132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolton D, Cheng Y, Willems-Jones AJ, Li J, Niedermeyr E, Mitchell G, Clouston D, Lawrentschuk N, Sliwinski A, Fox S, Thorne H. Altered significance of D’Amico risk classification in patients with prostate cancer linked to a familial breast cancer (kConFab) cohort. BJU Int. 2015;116(2):207–212. doi: 10.1111/bju.12792. [DOI] [PubMed] [Google Scholar]
- 23.Jiang R, Lu YT, Ho H, Li B, Chen JF, Lin M, Li F, Wu K, Wu H, Lichterman J, Wan H, Lu CL, OuYang W, Ni M, Wang L, Li G, Lee T, Zhang X, Yang J, Rettig M, Chung LW, Yang H, Li KC, Hou Y, Tseng HR, Hou S, Xu X, Wang J, Posadas EM. A comparison of isolated circulating tumor cells and tissue biopsies using whole-genome sequencing in prostate cancer. Oncotarget. 2015;6(42):44781–44793. doi: 10.18632/oncotarget.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Network [Accessed May 18, 2015];Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 2.2015) http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
- 25.Lynch HT, Kosoko-Lasaki O, Leslie SW, Rendell M, Shaw T, Snyder C, D’Amico AV, Buxbaum S, Isaacs WB, Loeb S, Moul JW, Powell I. Screening for familial and hereditary prostate cancer. Int J Cancer. 2016;138(11):2579–2591. doi: 10.1002/ijc.29949. [DOI] [PubMed] [Google Scholar]
- 26.Cremers RG, Eeles RA, Bancroft EK, Ringelberg-Borsboom J, Vasen HF, Van Asperen CJ, Committee IS, Schalken JA, Verhaegh GW, Kiemeney LA. The role of the prostate cancer gene 3 urine test in addition to serum prostate-specific antigen level in prostate cancer screening among breast cancer, early-onset gene mutation carriers. Urol Oncol. 2015;33(5):202.e19–202.e28. doi: 10.1016/j.urolonc.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Bjurlin MA, Meng X, Le Nobin J, Wysock JS, Lepor H, Rosenkrantz AB, Taneja SS. Optimization of prostate biopsy: The role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol. 2014;192(3):648–658. doi: 10.1016/j.juro.2014.03.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toronto Sunnybrook Regional Cancer Centre . Prostate Screening Study Using MRI in BRCA Carriers. National Library of Medicine (US); Bethesda (MD): 2000. ClinicalTrials.gov [Internet] [cited 2015 June 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT01990521 NLM Identifier: NCT01990521. [Google Scholar]
- 29.Rabin Medical Center . Prostate Cancer Screening Among Men With High Risk Genetic Predisposition. National Library of Medicine (US); Bethesda (MD): 2000. ClinicalTrials.gov [Internet] [cited 2015 June 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT02053805 NLM Identifier: NCT02053805. [Google Scholar]
- 30.Margel D, Benjaminov O, Ozalvo R, Shavit-Grievink L, Kedar I, Yerushalmi R, Ben-Aharon I, Neiman V, Yossepowitch O, Kedar D, Levy Z, Shohat M, Brenner B, Baniel J, Rosenbaum E. Personalized prostate cancer screening among men with high risk genetic predisposition—Study protocol for a prospective cohort study. BMC Cancer. 2014;14:528. doi: 10.1186/1471-2407-14-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro E, Goh C, Leongamornlert D, Saunders E, Tymrakiewicz M, Dadaev T, Govindasami K, Guy M, Ellis S, Frost D, Bancroft E, Cole T, Tischkowitz M, Kennedy MJ, Eason J, Brewer C, Evans DG, Davidson R, Eccles D, Porteous ME, Douglas F, Adlard J, Donaldson A, Antoniou AC, Kote-Jarai Z, Easton DF, Olmos D, Eeles R. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68(2):186–193. doi: 10.1016/j.eururo.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Pierce LJ, Phillips KA, Griffith KA, Buys S, Gaffney DK, Moran MS, Haffty BG, Ben-David M, Kaufman B, Garber JE, Merajver SD, Balmana J, Meirovitz A, Domchek SM. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: Comparison of breast conservation and mastectomy. Breast Cancer Res Treat. 2010;121(2):389–398. doi: 10.1007/s10549-010-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cambridge University Hospitals NHS Foundation Trust . Studying the Effects of Olaparib Given to Men With Intermediate/High Risk Prostate Cancer Before Radical Prostatectomy (CaN-CaP03) National Library of Medicine (US); Bethesda (MD): 2000. ClinicalTrials.gov [Internet] [cited 2016 Feb 09]. Available from: https://clinicaltrials.gov/ct2/show/NCT02324998 NLM Identifier: NCT02324998. [Google Scholar]
- 34.AstraZeneca Lynparza™ (olaparib) granted Breakthrough Therapy designation by US FDA for treatment of BRCA1/2 or ATM gene mutated metastatic Castration Resistant Prostate Cancer [news release] 2016 Retrieved from: https://www.astrazeneca.com/media-centre/press-releases/2016/Lynparza-Olaparib-granted-Breakthrough-Therapy-Designation-by-US-FDA-for-treatment-of-BRCA1-2-or-ATM-gene-mutated-metastatic-Castration-Resistant-Prostate-Cancer-28012016.html.
- 35.VanderWeele DJ, Paner GP, Fleming GF, Szmulewitz RZ. Sustained complete response to cytotoxic therapy and the PARP inhibitor veliparib in metastatic castration-resistant prostate cancer—A case report. Front Oncol. 2015;5:169. doi: 10.3389/fonc.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, Ardern-Jones A, Norman A, Kaye SB, Gore ME. “BRCAness” syndrome in ovarian cancer: A case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26(34):5530–5536. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 37.Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol. 2015;69(6):992–995. doi: 10.1016/j.eururo.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]