Abstract
Lung cancer is the most common cause of cancer-related death worldwide, less than 7% of patients survive 10 years following diagnosis across all stages of lung cancer. Late stage of diagnosis and lack of effective and personalized medicine reflect the need for a better understanding of the mechanisms that underlie lung cancer progression. Quantitative proteomics provides the relative different protein abundance in normal and cancer patients which offers the information for molecular interactions, signaling pathways, and biomarker identification. Here we introduce both theoretical and practical applications in the use of quantitative proteomics approaches, with principles of current technologies and methodologies including gel-based, label free, stable isotope labeling as well as targeted proteomics. Predictive markers of drug resistance, candidate biomarkers for diagnosis, and prognostic markers in lung cancer have also been discovered and analyzed by quantitative proteomic analysis. Moreover, construction of protein networks enables to provide an opportunity to interpret disease pathway and improve our understanding in cancer therapeutic strategies, allowing the discovery of molecular markers and new therapeutic targets for lung cancer.
Keywords: Quantitative proteomics, Lung cancer, Biomarkers, Drug targets, Functional network
Background
Lung cancer is the most common cancer-related mortality worldwide, with approximately 27% of all cancer deaths per year [1]. Lung cancer divided into two main types including small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). 10–15% of lung cancer cases are SCLC which is responsive to chemotherapy and radiation treatment [2]. However, more than eighty percent of lung cancer is NSCLC, which has become resistant to anticancer drugs [3]. Regardless of subtypes, the overall survival rate of lung cancer patients is still disappointing; less than 7% of patients survive 10 years following diagnosis across all stages of lung cancer [4]. Current treatments and therapies are not sufficient to reduce the mortality for this malignancy. To address this challenge, early detection and systemic therapy might be the solution to alter the mortality trend and gain our knowledge in lung cancer progression. Recent omics researches in lung cancer have been focused on classification of lung cancer, correlation of gene and protein expression, and identification of novel molecular targets [5].
Proteins are involved in all biological processes which can be regarded as the final stage of biological information from genome. Proteomics is extremely dynamic and complex due to the continuous response to the change of environment, drug treatment, and post-translational modification [6]. Large-scale and systematic analysis of proteins is a complete and unique profile for characterization and biological activity. Quantitative proteomics provides the relative different protein abundance in normal and disease samples which offers ultimate information for molecular interactions, signaling pathways, and biomarker identification in human disease research [7]. In addition, the integration of biomarker discovery from different pulmonary diseases and multiple sample types may serve as a valuable resource for future clinical validation studies [8, 9]. To interpret the data generated from high-throughput technologies, a combination of computational and experimental approach is required for analyzing complex interaction of many levels of biological information which may benefit our understanding in biochemical pathways, regulatory networks, and disease therapies in lung cancer [10, 11].
Development and techniques of quantitative proteomics
Proteomics is an analysis of dynamic systems in biology which consists a range of diversity that are insufficient to analyze with any single method. Quantitative proteomics not only provides a list of identified proteins, it also quantifies the changes between normal and disease sample profiles in order to generate classification models. Here, we review quantitative proteomics into four major approaches: gel-based, stable isotope labeling, label free, and targeted proteomics for lung cancer studies (Fig. 1).
Fig. 1.
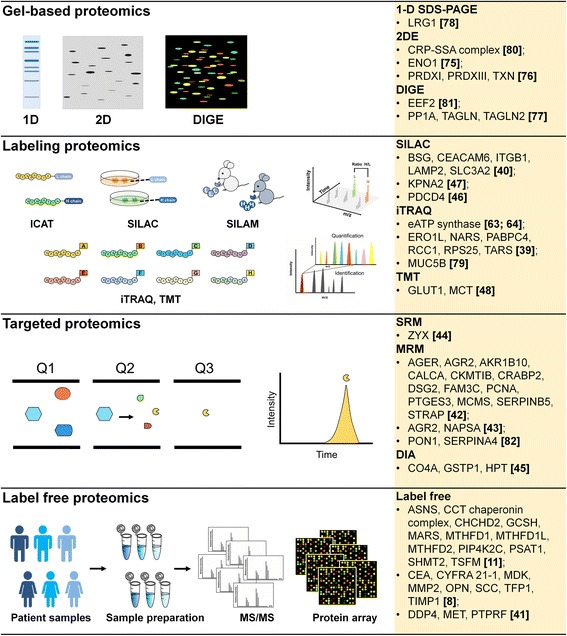
The applications of quantitative proteomics for discovery of biomarkers in lung cancer study. Quantitative proteomics not only provides a list of identified proteins, it also quantifies the changes between normal and disease sample profiles which enables to generate classification models or biomarkers. Biomarkers are measurable biological indicators found in tissue, cells, blood or other body fluids that may be used for detection, diagnosis treatment and monitoring in cancer research by the means of advanced quantitative proteomic approaches: gel-based, stable isotope labeling, targeted proteomics, and label free. In gel-based proteomics, one-dimensional (1D) gel electrophoresis, two-dimensional (2D) polyacrylamide gel electrophoresis, and difference gel electrophoresis (DIGE) approaches have been developed and utilized to separate protein from protein mixtures and identification. In vitro labeling, the peptides are modified by stable isotope labeling (ICAT, iTRAQ, TMT) prior to MS analysis. In vivo labeling, isotope labeling (SILAC and SILAM), specific supplements containing distinct forms of amino acid are given to living cells or mammals prior to MS analysis. The resulting spectrum is able to generate peptide intensity for both identification and quantitation. Targeted proteomics (SRM, MRM, and DIA) using triple quadrupole mass spectrometers systems where the mass of the intact targeted analyte is selected in the first quadrupole (Q1), and then the fragmentation of the Q1 mass-selected precursor ion by collision-induced dissociation in the second quadrupole (Q2), finally a desired product ion is selected in the third quadrupole (Q3), which is then transmitted to the detector. This method of absolute quantitation in targeted proteomics analyses is suitable for identification and quantitation of target peptides within complex mixtures independent on peptide-specific manner. Label-free quantification is an alternative method for samples that cannot directly label and enables the comparison of protein expression across different samples or treatment regardless the number of samples. Protein microarray is another label-free method which is a high-density and high-throughput microarray containing thousands of unique proteins to identify the interactions on a large scale
Gel-based and gel-free proteomics
The first and most important step in quantitative proteomic analysis is the separation of a complex protein mixture [12]. Gel-based proteomics including one-dimensional (1D) gel electrophoresis, two-dimensional (2D) polyacrylamide gel electrophoresis, and difference gel electrophoresis (DIGE) approaches have been developed and utilized to separate protein from protein mixtures and identification [13]. 1D- and 2D-PAGE are simple and straight forward in a principle of molecular weight (M r) and isoelectric point (pI)-based separation. DIGE has been developed as a multicolor detection for comparing protein abundance of samples within a single gel, where each protein sample is prelabeled with spectrally distinct fluorescent dye [14]. To reduce inter-gel variation, internal standard is applied for normalization across different gels. However, membrane proteins, low abundance proteins, alkaline proteins, and high molecular weight proteins remain an area of considerable concern in gel-based proteomics [15]. Gel-free proteomic techniques have been developed to fulfill the shortage of gel-base proteomics [13]. Both gel-based and gel-free proteomics are well-established quantitative proteomics which compare the proteome of normal and disease samples in a global aspect which led to magnify the identification of novel protein candidates associated with lung cancer [16].
Stable isotope labeling proteomics
For quantitative analysis, stable isotope labeling coupled with mass spectrometry (MS)-based techniques is often performed in cancer research [7]. Mass spectrometer is composed of two major compartments: (1) an ionization source that generates ions of target molecules and (2) a mass analyzer that sorts molecules by mass-to-charge ratio (m/z). Stable isotopic labeling methods have been developed and applied in quantitative proteomics as a routine means to analyze protein expression patterns for multiple samples as comparison (Table 1). In vitro labeling, the peptides are modified by stable isotope labeling (ICAT, iTRAQ, TMT, diLeu, and DiART) prior to MS analysis [17–21]. The resulting spectrum is able to generate peptide intensity for both identification and quantitation. In vivo approaches are based on incorporation of isotope label such as SILAC into proteins presenting in living cells that specific media containing distinct forms of amino acid are given [22]. Stable isotope labeling in mammals (SILAM) has been developed by combining 15N spirulina with a protein-free chow to overcome the limitation of SILAC to cell culture [23].
Table 1.
Common isotopic labeling methods in quantitative proteomics
| Types of Label | Principles | Comparison | Methods | Year | Ref. |
|---|---|---|---|---|---|
| Isotope-Coded Affinity Tags (ICAT) | Thiol group | Pairwise: duplexed | In-vitro | 1999 | [17] |
| Stable isotopic labeling with amino acids in cell culture (SILAC) | Metabolic incorporation of lysine or arginine | Pairwise: non-labeled (light); Lys4 and Arg6 (middle); Lys8 and Arg10 (heavy) | In-vivo | 2002 | [22] |
| Tandem Mass Tag (TMT) | Free amino group | Multiplex: 2-plex, 6-plex, and 10-plex | In-vitro | 2003 | [19] |
| Isobaric Tags for Relative and Absolute Quantification (iTRAQ) | N-terminus and lysine side chains of peptide | Multiplex: 4-plex and 8-plex | In-vitro | 2004 | [18] |
| Stable isotope labeling in mammals (SILAM) | combining 15N spirulina with a protein-free chow | Pairwise: non-labeled (light); and nitrogen 15N (heavy) | In-vivo | 2004 | [23] |
| Deuterium isobaric Amine Reactive Tag (DiART) | N-terminus and lysine side chains of peptide | Multiplex: 6-plex | In-vitro | 2010 | [21] |
| N,N-Dimethyl leucine (DiLeu) | N-terminus and ε-amino group of the lysine side chain | Multiplex: 4-plex | In-vitro | 2010 | [20] |
| Terminal amine isotopic labeling of substrates (TAILS) | neo-N-terminal peptides | Multiplex: iTRAQ-based labeling | In-vitro | 2011 | [27] |
| iTRAQ hydrazide (iTRAQH) | carbonylated peptide | Multiplex: 4-plex | In-vitro | 2012 | [24] |
| Stable isotope labeled carbonyl-reactive tandem mass tags (Glyco-TMT) | N-linked glycans | heavy/light-TMT labeled glycans | In-vitro | 2012 | [26] |
| Irreversible isobaric iodoacetyl Cys-reactive tandem mass tag (iodoTMT) | Cys-redox modifications | Multiplex: iTRAQ-based labeling | In-vitro | 2014 | [25] |
To characterize the post-transcriptional modifications in proteomics, several isobaric reagents were developed for selective labeling such as carbonylated residues and cysteine residues which might expand our knowledge of dynamic system in cancer progression [24–26]. Moreover, a novel iTRAQ-based labeling has been used in distinguishing protease-generated neo-N termini from N-termini of mature protein. This approach can be applied in characterization of post-translational modification [27].
Label free proteomics
Label-free quantification is an alternative method for samples that cannot directly label, and enables the comparison of protein expression across different samples or treatment regardless the number of samples [28]. Label-free quantification can be divided into two categories: peptide peak intensity based quantification and spectral counting quantification that depends on the number of peptides identified from a protein of interest [29]. Label-free proteome quantification encounters many limitation, several published algorithms are available for additional calculations to compute the predicted abundance of proteins in the sample. Protein microarray is another label-free method which is a high-density and high-throughput microarray containing thousands of unique proteins to identify the interactions on a large scale [30]. Protein microarray has the same concept as DNA microarray that is rapid and automated, moreover, protein microarray also solved the limitation of gene expression levels for proteomics [31]. The probe molecules labeled with fluorophores, chromogen and radioiostopes aim to compare protein expression in different samples [32].
Targeted proteomics
Selected reaction monitoring (SRM), multiple reaction monitoring (MRM), and data-independent acquisition (DIA) are widely used MS-based proteomics which have been considered as true quantification techniques for targeted quantification of protein [16, 33, 34]. Targeted quantitation using triple quadrupole mass spectrometers systems where the mass of the intact targeted analyte is selected in the first quadrupole (Q1), and then the fragmentation of the Q1 mass-selected precursor ion by collision-induced dissociation in the second quadrupole (Q2), finally a desired product ion is selected in the third quadrupole (Q3), which is then transmitted to the detector [35]. This method of absolute quantitation in targeted proteomics analyses is suitable for identification and quantitation of target peptides within complex mixtures independent on peptide-specific manner [36]. DIA requires no prior knowledge of target peptides and obtains much larger numbers of peptide than SRM or MRM. DIA analysis is a method that all peptides within a given window are subjected to fragmentation, then it is repeated until the mass spectrometer marches up the full m/z range. This powerful targeted proteomics provides accurate peptide quantification without being limited to predefined peptides of interest.
Applications of quantitative proteomics in lung cancer
Quantitative proteomics allows the discovery of molecular markers and new therapeutic targets for lung cancer. Predictive markers of drug resistance, lung cancer diagnosis, and prognostic markers in lung cancer have also been discovered by quantitative proteomics analysis [37].
Biomarkers in lung cancer
Biomarker is a measurable biological indicator found in tissue, cells, blood or other body fluids that may be used for detection, diagnosis treatment and monitoring in cancer research [38]. The characterization of specific protein patterns associated with lung cancer as a discovery strategy for biomarker identification in clinical research. Quantitative proteomics reveals several biomarker candidates in lung cancer through comparing differentially expression proteins of lung cancer and normal individual [8]. Biomarkers identified by quantitative proteomics provide valuable information for the researchers to develop better personalized medicine and early and precise diagnostic markers for the lung cancer patients (Table 2).
Table 2.
Quantitative proteomic studies in lung cancer
| Biomarker | Types of Sample | Results | Year | Ref. |
|---|---|---|---|---|
| ENO1 | Lung cancer tissue | ENO1 was consistently up-regulated in all 14 cases of lung cancer, and suggested that basaloid carcinoma is a unique subtype of NSCLC | 2004 | [75] |
| PRDXI, PRDXIII, TXN | Lung cancer tissue | Enhanced lung cancer cell survival and proliferation | 2006 | [76] |
| PPIA, TAGLN, TAGLN2 | Lung cancer tissue | Early diagnostic markers for lung cancer | 2009 | [77] |
| AGR2, NAPSA | Lung cancer tissue | Stage-related protein candidates for stage IA and IIIA lung adenocarcinoma | 2010 | [43] |
| LRG1 | Urinary exosome and lung tissue of NSCLC patient | Non-invasive diagnosis of NSCLC in urine | 2011 | [78] |
| AGER, AGR2, AKR1B10, CALCA, CKMTIB, CRABP2, DSG2, FAM3C, PCNA, PTGES3, MCMS, SERPINB5, STRAP | Lung cancer tissue | 84–88% of the protein expression differences of SCC and 44 ADC proteins measured by shotgun analyses of the SCC, ADC and normal pools were confirmed in an independent set of specimens | 2012 | [42] |
| Ectopic ATP synthase | Lung tumor xenograft and lung cancer cell | Citreoviridin revealed antitumorigenic effects in lung cancer |
2012 2013 |
[63, 64] |
| MUC5B | Adenocarcinoma tissue | Aberrant expression of MUC5B was identified in 71% of lung adenocarcinomas in the tumor tissue microarray | 2013 | [79] |
| ASNS, CCT chaperonin complex, CHCHD2, GCSH, MARS, MTHFD1, MTHFD1L, MTHFD2, PIP4K2C, PSAT1, SHMT2, TSFM | Lung cancer tissue and xenograft | Integrating the omic data from DNA, RNA, and proteins data sets to reveal new anticancer therapeutic targets for lung cancer | 2014 | [11] |
| CEA, CYFRA 21–1, MDK, MMP2, OPN, SCC, TFP1, TIMP1 | Lung cancer tissue, cell-line, and conditioned medium | Biomarker model was developed which accurately distinguished subjects with lung cancer from high risk smokers | 2015 | [8] |
| CRP-SAA complex | Serum | Higher expression of CRP-SAA level was associated with severe clinical features of lung cancer | 2015 | [80] |
| DPP4, MET, PTPRF | Pleural effusion (PE) | Diagnostic biomarkers of NSCLC from PE proteome | 2015 | [41] |
| GLUT1, MCT | Lung cancer cell line | Quantitative proteomics of TMT labeled SCC and ADC suggested that MCT1 and GLUT1 are the promising drug targets or histological markers | 2015 | [48] |
| KPNA2 | lung adenocarcinoma cell line | KPNA2-mediated modulation of cell migration in lung cancer | 2015 | [47] |
| PDCD4 | NSCLC cell | Longer overall survival of lung cancer patients with PTX treatment (personalized medicine) | 2015 | [46] |
| ZYX | Plasma | Early diagnostic marker for NSCLC | 2015 | [44] |
| BSG, CEACAM6, ITGB1, LAMP2, SLC3A2 | Lung cancer-derived exosome | NSCLC-related proteins identified from the study of exosomal proteome as promising candidates | 2016 | [40] |
| CO4A, GSTP1, HPT | Bronchoalveolar lavage fluid | More sensitive biomarkers were identified by a DIA-based quantitative proteomic approach from bronchoalveolar lavage fluid | 2016 | [45] |
| EEF2 | Lung cancer tissue | Clinical tissue studies showed that EF2 protein was significantly overexpressed in LSCC tissues, compared with the adjacent normal lung tissues | 2016 | [81] |
| ERO1L, NARS, PABPC4, RCC1, RPS25, TARS | Lung cancer tissue | ERO1L and NARS were positively associated with lymph node metastasis, in which ERO1L overexpression in patient with early stage of adenocarcinoma was associated with poor overall survival | 2016 | [39] |
| PON1, SERPINA4 | Serum | Meta-markers might have better specificity and sensitivity than a single biomarker and thus improved the differential diagnosis of lung cancer and lung disease patients | 2016 | [82] |
The survival rate of lung cancer patients is highly correlated to the stage of lung cancer; therefore, improve the diagnostic strategies for early lung cancer detection may increase patient survival. Hsu et al. identified 133 protein candidates from paired adenocarcinoma (ADC) tissues with different extents of lymph node involvement by iTRAQ-labeling technology coupled with 2D-LC-MS/MS [39]. They further validated six potential biomarkers (ERO1L, NARS, PABPC4, RCC1, RPS25, and TARS) which were highly expressed in ADC tissues compared to the adjacent normal tissues. In addition, they found ERO1L and NARS are positively associated with lymph node metastasis, in which ERO1L overexpression in patients with early stage of ADC was associated with poor overall survival. Another recent study of triple SILAC quantitative proteomics identified several biomarkers by comparing the protein abundance of immortalized normal epithelial cell derived exosomes and NSCLC exosomes [40]. Integrin beta-1 (ITGB1), Basigin (BSG), 4 F2 cell-surface antigen heavy chain (SLC3A2), lysosome-associated membrane glycoprotein 2 (LAMP2), and carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) are the NSCLC-related proteins identified from their study of exosomal proteome as promising candidates.
Label-free quantitative proteomic analysis was performed and a significant higher protein levels of hepatocyte growth factor (MET), dipeptidyl peptidase IV (DDP4) and Receptor-type tyrosine-protein phosphatase F (PTPRF) in malignant pleural effusion (PE) samples were found comparing to benign and paramalignant PE samples [41]. Proteomic profiling of body fluids presents a sensitive diagnostic tool for early cancer diagnosis and establishes a new database of differential lung tumor-proximal body fluid (PE) proteomes to facilitate the identification of biomarkers for discriminating NSCLC from nonmalignant pulmonary diseases. In a study of shotgun proteomics, Kikuchi et al. identified 3621 proteins from the analysis of pooled human samples of 20 squamous cell carcinoma (SCC), 20 adenocarcinoma (ADC), and 22 control specimens. To further assess the concordance between shotgun proteomics and targeted proteomics on the differentially expressed proteins, they analyzed 20 SCC, 20 ADC and 21 normal tissues by MRM analysis. 84–88% of the protein expression differences (42 SCC and 44 ADC proteins) measured by shotgun analyses of the SCC, ADC and normal pools were confirmed in an independent set of specimens [42]. Moreover, Kawamura et al. identified 81 proteins were associated with stage IA and stage IIIA lung adenocarcinoma by shotgun proteomics using formalin-fixed paraffin-embedded materials, then MRM targeted proteomic quantification was applied to verify for those protein candidates and found that Napsin-A (NAPSA) and anterior gradient protein 2 homolog (AGR2) might be the stage-related protein candidates for stage IA and IIIA lung adenocarcinoma [43].
A recent study applied highly multiplexed liquid chromatography-selected reaction monitoring (LC-SRM) assay to verify the biomarker candidates in plasma samples for lung cancer. A total of 17 proteins were verified as potent tumor markers, especially, a novel plasma-based biomarker, zyxin (ZYX) was identified as a potential early diagnostic marker for NSCLC [44]. Overall, targeted proteomics is able to yield high probability biomarkers for clinical validation in large patient cohorts and represents a strategy to identify and verify novel different types of diseases [36]. Moreover, integrated biomarker discovery from multiple sample types including lung cancer tissues, cell lines and conditioned medium has established to construct a biomarker model (TFPI, MDK, OPN, MMP2, TIMP1, CEA, CYFRA 21–1, SCC) which enables to classify lung cancer patients from high risk smokers [8]. A recent clinical research of the bronchoalveolar lavage fluid (BALF) proteomic analysis by combining a simple pre-treatment and a sequential windowed acquisition of all theoretical fragment ion mass spectra (SWATH) DIA MS approach provided useful resources for the discovery of potential biomarkers for lung disease [45]. BALF is usually discarded after using a portion of the fluid for standard pathological procedure, but Ortea et al. used BALF as source for proteomic analysis and identified sensitive biomarkers by targeted proteomics DIA. They found forty-four proteins with a fold-change higher than 3.75 among ADC patients compared with controls where CO4A, GTSP1, and HPT are consistent with previous studies.
The major challenge for lung cancer therapy is chemoradioresistance, where protein markers might serve as the potential molecular predictors of drug resistance and overcome this shortage. Recent study of a SILAC-based quantitative proteomic approach has been utilized to evaluate the cellular protein abundance changes upon paclitaxel (PTX) treatment. Tumor suppressor programmed cell death 4 (PDCD4) in lung cancer tissues were positively correlated with the longer overall survival of lung cancer patients with PTX treatment, suggesting that PDCD4 may be used as a predictive marker of resistance to PTX in lung cancer patients [46]. Furthermore, SILAC-based quantitative proteomic strategy has been applied to reveal the functional role of invasiveness-associated KPNA2 protein complex in lung adenocarcinoma cell lines [47]. Integrating the omic data from DNA, RNA, and proteins data sets might represent new anticancer therapeutic targets for lung cancer. Li et al. integrated the genomic and proteomic data sets of lung cancer to construct omic map to represent non-small cell lung carcinoma [11]. In addition, a proteogenomic study of lung adenocarcinoma identified 565 proteins and 629 genes to be differentially expressed between SCC and ADC by TMT labeled quantitative proteomics, and suggested MCT1 and GLUT1 are the promising drug targets or histological marker [48].
Discovery of therapeutic targets by quantitative proteomics
During the stages of drug development, proteomics can also take place in a high-throughput analysis for the identification and optimization of suitable lead compounds. Several tyrosine kinase inhibitors (TKIs) have been approved from the US Food and Drug Administration (FDA) for use in advanced lung cancer. Epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) are the two common biological targets for lung cancer drug development [49, 50]. Application of these targeted therapies in selected patients has shown consistent benefits with regard to clinical outcomes [51]. Quantitative proteomics is able to predict for which patient might benefit from targeted therapy by understanding the molecular mechanism underneath (Table 3).
Table 3.
Drug target and molecular mechanism in lung cancer
| Target | Mechanism | Ref. |
|---|---|---|
| ALK | Alectinib (C30H34N4O2) is an inhibitor of ALK, which binds to and inhibits not only ALK kinase but also the L1196M mutant. Ceritinib (C28H36ClN5O3S) is a ATP-competitive, tyrosine kinase inhibitor of ALK, especially for ALK-rearranged NSCLC. Crizotinib (C21H22Cl2FN5O) is a kinase inhibitor for ALK, c-Met, and ROS1, especially for ROS1-rearranged NSCLC. |
[59] [58] [60] |
| ATP synthase | Citreoviridin (C23H30O6) inhibits the mitochondrial ATP synthetase system. It has been used to target ectopic ATPase activity in lung cancer cells in order to modulate the metabolic activity associated with tumorigenesis. | [64] |
| BRAF | Vemurafenib (C23H18ClF2N3O3S) selectively binds to the ATP-binding site of BRAF (V600E) kinase, since most BARF gene mutations exist at residue 600 which has been found to be over-activates the MAPK signaling pathway. | [83] |
| EGFR | Afatinib (C24H25ClFN5O3) selectively inhibits ErbB1, ErbB2, ErbB4 and EGFR mutants (L858R and T790M). It may inhibit tumor progression and angiogenesis. Erlotinib (C22H23N3O4) is a protein kinase inhibitor which inhibits EGFR phosphorylation and blocks signal transduction. However, the FDA-approval is limited to EGFR mutations. Gefitnib (C22H24ClFN4O3) inhibits the catalytic activity of tyrosine kinase that competes with the binding affinity of ATP to the tyrosine kinase domain of EGFR. It inhibits signal transduction by inhibiting the receptor for phosphorylation. |
[55] [54] [53] |
EGFR has become an important biological target for lung cancer. Inhibitors that target EGFR and block the signaling pathway have been developed and are clinically active [52]. Three EGFR inhibitors including afatinib, erlotinib, and gefitinib are used in NSCLC with EGFR mutated patients. Gefitnib inhibits the catalytic activity of tyrosine kinase that competes with the binding affinity of ATP to the tyrosine kinase domain of EGFR [53]. It inhibits signal transduction by inhibiting the receptor for phosphorylation. Erlotinib is a protein kinase inhibitor which inhibits EGFR phosphorylation and blocks signal transduction [54]. However, the FDA-approval is limited to EGFR mutations. For lung cancer patients who has EGFR mutation, an initial treatment with EGFR TKI is preferred. Afatinib selectively inhibits ErbB1, ErbB2, ErbB4 and especially EGFR mutants (L858R and T790M) which inhibits tumor progression as well as angiogenesis [55]. Comparative proteome profiling across 23 NSCLC cell lines revealed the significant expression differences in cell lines harboring oncogenic KRAS and EGFR mutations [56]. This study provided valuable information for the identification of candidate therapeutic targets, which mediate oncogenic processes driven by K-Ras or EGFR mutant protein expression. A multicohort cross-institutional study performed by Taguchi et al., they classified NSCLC patients for clinical outcome after treatment with EGFR TKI by mass spectrometry. Serum collected from 139 NSCLC patients were analyzed by mass spectrometry which might provide valuable resources for clinical benefit from a molecularly anticancer drug [57].
Chromosomal rearrangements of ALK have been found to be associated with lung cancer and its inhibitors ceritinib are superior for patients with chemotherapy [49]. Ceritinib is a ATP-competitive, tyrosine kinase inhibitor of ALK, especially for ALK-rearranged NSCLC [58]. Alectinib is also an inhibitor of ALK, which binds to and inhibits not only ALK kinase but also the L1196M mutant [59]. Furthermore, crizotinib is a kinase inhibitor for multiple lung cancer oncogene including ALK, c-Met, and ROS1, especially for ROS1-rearranged NSCLC [60]. Current diagnostic test for ALK arrangement is based on low throughput fluorescence in situ hybridization (FISH), Hembrough et al. developed an ALK protein assay that could save time and the expense of multiple FISH testing to detect different biomarkers [61]. They used SRM approach to quantify absolute amounts of ALK in 188 formalin-fixed paraffin-embedded NSCLC tissues and the results were correlated with patients response to crizotinib.
Our recent study demonstrated ectopic ATP synthase that presents on the plasma membrane of lung cancer cells is a potential biological target for drug development [62, 63]. Citreoviridin serves as ATP synthase inhibitor which selectively suppresses the proliferation and growth of lung cancer without affecting normal cells [63]. Comprehensive proteomics were also performed using lung tumor xenografts treat with citreovirdin that reveals its antitumorigenic effects in lung cancer, which may lead to a better understanding of the links between metabolism and tumorigenesis in lung cancer drug development [62, 64].
Quantitative proteomics enables to link proteins into functional networks
Modern high-throughput technologies generate a huge amount of data, however, proper data mining tools is the key for discovery of cancer-related proteins and networks. Fully understanding of the biological significance of differential protein networks from normal to disease cells depends on the information generated from proteomic datasets. Exploration of proteomic datasets using bioinformatic analysis enables us to elucidate new molecular interactions, protein functional annotation, protein motif and complex interaction and disease pathway.
With the advent of high-throughput omics data, bioinformatics has become a viable tool to improve our knowledge of health and disease individuals and it also provides a systems-level approach to interpret organisms and functional activities of their components by studying underneath interactions. Bioinformatics can be defined as a combination of mathematical and computational strategies for interpreting biological processes from the existing raw data. Data curation, tool development, and practical applications are the three major aims for bioinformatics [65]. To date, many biological databases are standardized and annotated for researchers to access existing information and also to submit new entries. Biological databases consist the information for protein sequencing (Uniprot, Swiss-Prot, Pfam), proteomics (PRIDE, ProteomeScout, OWL), protein structure (PDB, SCOP), and protein model (Swiss-model, SIMAP).
Mathematical and statistical approaches have become essential components for bioinformatics tool development. For example, developing a tool for protein structure requires serious consideration of the primary protein sequence, differential geometry and topology of the protein folding regardless of its biological functions. It is painstaking task for experimental biologists to interpret their dataset without bioinformatics tools, therefore, bioinformatics tool development takes an important part of proteomic and biological researches.
Protein-protein interaction networks
Protein-protein interaction (PPI) networks provide an overall picture for the understanding of biological processes in cancer research. Proteins are not functioning solely, they have interactions with other proteins or molecules that mediate signaling pathways and biological processes. Hub proteins are highly connected to other proteins in a network, whereas some others have few interactions [66]. The dysfunction of protein-protein interactions is one of the causes for many diseases, including cancer [67]. Therefore, cancer can be enlightened through protein interaction networks, which in turn can appraise methods for cancer prevention, early diagnosis, and drug discovery. Many web-based resources such as STRING and Reactome are available for functional protein association network and signal transduction pathway analyses.
A dynamic PPI network of lung cancer associated with smoking was constructed by bioinformatic analysis using Human Protein Reference Database and Gene Expression Omnibus Database. Yu et al. used the support vector machine (SVM) model and found 520 dynamic proteins and 2754 static proteins and further predicted 7 dynamic PPI subnetworks for lung cancer patients with smoking history [68].
Mathematical modeling
Mathematical modeling is a time and cost-effective method that provides insight into underlying molecular reactions and biological processes as alternatives to conventional laboratory experiments [69]. Mathematical modeling empowers the researchers to examine the relationship between the biological processes in the real world and the predictions in the conceptual world (Fig. 2). It is a computational simulation tool that utilizes mathematical approaches of quantitative calculation for hundreds of components and their interactions and thus have the potential of truly explanation for complex diseases such as cancer [70]. Researchers are able to systematically investigate systems perturbations, develop hypotheses to design new experiments, and ultimately predict the reliable candidates as novel therapeutic targets [71].
Fig. 2.
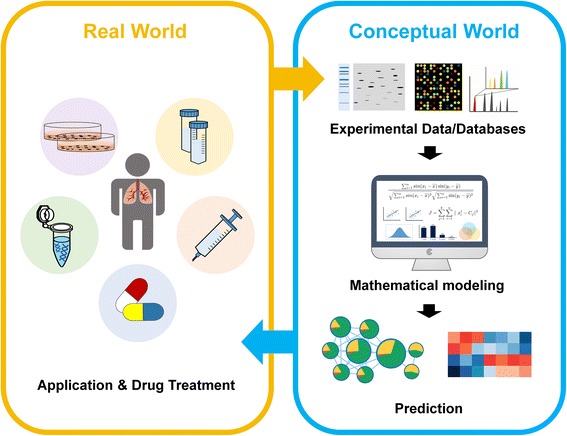
The depiction of the mathematical modeling in the conceptual world to the real world. Mathematical modeling empowers the researchers to examine the relationship between the biological processes in the real world and the predictions in the conceptual world. With the advent of high-throughput omics data, bioinformatics and mathematical modeling have become viable tools to improve our knowledge of molecular mechanism of cancer related phenomenon. It is a computational simulation that applied mathematical approaches of quantitative calculation for hundreds of components and their interactions and thus have the potential of truly explanation for complex diseases such as lung cancer. Researchers are able to systematically investigate systems perturbations, develop hypotheses to design new experiments, and ultimately predict the reliable candidates as novel therapeutic targets
Chmielecki et al. developed isogenic TKI-sensitive and TKI-resistant pairs of cell line that mimic the behavior of NSCLC with evolutionary cancer modeling [72]. They combined in vitro experiments, multiple clinical data sets, and mathematical modeling to describe NSCLC behavior. Their mathematical modeling proposed that alternative therapeutic strategies could prolong the benefit of TKI against EGFR-mutant lung cancer by delaying the development of resistance. Our recent study of a dynamic network in response to an ATP synthase inhibitor citreovirdin by mathematical modeling and bioinformatics analysis revealed that citreoviridin suppresses lung cancer cell growth via mitogen-activated protein kinase signaling by dephosphorylation of heat shock protein 90 β on Serine 255 [62]. Construction of protein networks provides an opportunity to interpret disease pathway and improve our understanding in cancer therapeutic strategies.
Conclusions
Since lung cancer is a heterogeneous disease, a comprehensive and in-depth discovery of lung cancer proteomic profiling is needed for precise target treatment. The microenvironment interface of the tumor cells and host cells directly impacts the tumor-host communication system by affecting signaling and growth factors, therefore cancer processing [73]. To understand the biological significance of differential protein networks from normal to disease cells depends on the proteomic datasets, where new molecular interactions, protein functional annotation, protein motif and complex interaction and disease pathway are able to analyze by bioinformatics analysis. Furthermore, the functional diversity of proteins is generated by post-transcriptional modifications (PTM) such as phosphorylation, acetylation, and ubiquitination. To characterize the post-transcriptional modifications might expand our knowledge of dynamic system in cancer progression. The majority of PTM research for proteomics are shotgun proteomics; however, the complexity of proteomics datasets requires standards to ensure reproducibility and unambiguous interpretation. An alternative method using targeted proteomics by SRM and MRM with sensitive detection enable us to identify specific PTM and give the absolute copy number of proteins in a single cell. Recent study of phosphorylation dynamics in non-small cell lung cancer by targeted proteomics including SRM, MRM, and DIA enabled the quantification of 42 PI3K-mTOR and MAPK phosphosites and provides valuable conclusion on each assessment [74]. Quantitative proteomics provides the information for synthetic biologists to engineer or rewire the key pathways, furthermore to offers the best therapeutic strategy for lung cancer.
Acknowledgement
We thank the Ministry of Science and Technology, Taiwan for funding supports.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 102-2628-B-002-041-MY3, MOST 104-2627-B-002-001 and MOST 105-2320-B-002-057-MY3).
Availability of data and materials
Data and materials related to this work are available upon request.
Authors’ contributions
The authors CHYC and H-FJ declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors approve the manuscript for publication.
Ethics approval and consent to participate
No applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- 1D
One-dimensional
- 2D
Two-dimensional
- ADC
Adenocarcinoma
- ALK
Anaplastic lymphoma kinase
- ATP
Adenosine triphosphate
- BALF
Bronchoalveolar lavage fluid
- BRAF
Serine/threonine-protein kinase B-raf
- DIA
Data-independent acquisition
- DIGE
Difference gel electrophoresis
- EGFR
Epidermal growth factor receptor
- FDA
The US Food and Drug Administration
- iTRAQ
Isobaric tags for relative and absolute quantification
- m/z
Mass-to-charge ratio
- MET
Hepatocyte growth factor receptor
- Mr
Molecular weight
- MRM
Multiple reaction monitoring
- MS
Mass spectrometry
- NSCLC
Non-small cell lung cancer
- PE
Pleural effusion
- pI
Isoelectric point
- PTX
Paclitaxel
- ROS1
Proto-oncogene tyrosine-protein kinase ROS
- SCC
Squamous cell carcinoma
- SCLC
Small cell lung cancer
- SILAC
Stable isotopic labeling with amino acids in cell culture
- SILAM
Stable isotope labeling in mammals
- SRM
Selected reaction monitoring
- TKI
Tyrosine kinase inhibitors
Contributor Information
Chantal Hoi Yin Cheung, Email: d02b43001@ntu.edu.tw.
Hsueh-Fen Juan, Phone: +886-2-33664536, Email: yukijuan@ntu.edu.tw.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chan BA, Coward JI. Chemotherapy advances in small-cell lung cancer. J Thorac Dis. 2013;5(Suppl 5):565–78. doi: 10.3978/j.issn.2072-1439.2013.07.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leon G, MacDonagh L, Finn SP, Cuffe S, Barr MP. Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways. Pharmacol Ther. 2016;158:71–90. doi: 10.1016/j.pharmthera.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Crino L, Weder W, van Meerbeeck J, Felip E, Group EGW. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v103–15. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 5.Granville CA, Dennis PA. An overview of lung cancer genomics and proteomics. Am J Respir Cell Mol Biol. 2005;32(3):169–76. doi: 10.1165/rcmb.F290. [DOI] [PubMed] [Google Scholar]
- 6.Pandey A, Mann M. Proteomics to study genes and genomes. Nature. 2000;405(6788):837–46. doi: 10.1038/35015709. [DOI] [PubMed] [Google Scholar]
- 7.Cifani P, Kentsis A. Towards comprehensive and quantitative proteomics for diagnosis and therapy of human disease. Proteomics. 2017; 17(1–2) [DOI] [PMC free article] [PubMed]
- 8.Birse CE, et al. Blood-based lung cancer biomarkers identified through proteomic discovery in cancer tissues, cell lines and conditioned medium. Clin Proteomics. 2015;12(1):18. doi: 10.1186/s12014-015-9090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii K, Nakamura H, Nishimura T. Recent mass spectrometry-based proteomics for biomarker discovery in lung cancer, COPD, and asthma. Expert Rev Proteomics. 2017;14(4):373–86. doi: 10.1080/14789450.2017.1304215. [DOI] [PubMed] [Google Scholar]
- 10.10. Juan HF, Huang HC. Systems Biology: applications in cancer-related research, ed., World Scientific. 2012
- 11.Li L, et al. Integrated omic analysis of lung cancer reveals metabolism proteome signatures with prognostic impact. Nat Commun. 2014;5:5469. doi: 10.1038/ncomms6469. [DOI] [PubMed] [Google Scholar]
- 12.Neverova I, Van Eyk JE. Role of chromatographic techniques in proteomic analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815(1–2):51–63. doi: 10.1016/j.jchromb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Abdallah C, Dumas-Gaudot E, Renaut J, Sergeant K. Gel-based and gel-free quantitative proteomics approaches at a glance. Int J Plant Genomics. 2012;2012:494572. doi: 10.1155/2012/494572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unlu M, Morgan ME, Minden JS. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18(11):2071–7. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier F. Highlights on the capacities of “Gel-based” proteomics. Proteome Sci. 2010;8:23. doi: 10.1186/1477-5956-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan S, Aebersold R, Chen R, Rush J, Goodlett DR, McIntosh MW, Zhang J, Brentnall TA. Mass spectrometry based targeted protein quantification: methods and applications. J Proteome Res. 2009;8(2):787–97. doi: 10.1021/pr800538n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17(10):994–9. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 18.Ross PL, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3(12):1154–69. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Thompson A, Schafer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AK, Hamon C. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem. 2003;75(8):1895–904. doi: 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]
- 20.Xiang F, Ye H, Chen R, Fu Q, Li L. N, N-dimethyl leucines as novel isobaric tandem mass tags for quantitative proteomics and peptidomics. Anal Chem. 2010;82(7):2817–25. doi: 10.1021/ac902778d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Wang Y, Li S. Deuterium isobaric amine-reactive tags for quantitative proteomics. Anal Chem. 2010;82(18):7588–95. doi: 10.1021/ac101306x. [DOI] [PubMed] [Google Scholar]
- 22.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1(5):376–86. doi: 10.1074/mcp.M200025-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Wu CC, MacCoss MJ, Howell KE, Matthews DE, Yates JR., 3rd Metabolic labeling of mammalian organisms with stable isotopes for quantitative proteomic analysis. Anal Chem. 2004;76(17):4951–9. doi: 10.1021/ac049208j. [DOI] [PubMed] [Google Scholar]
- 24.Palmese A, De Rosa C, Chiappetta G, Marino G, Amoresano A. Novel method to investigate protein carbonylation by iTRAQ strategy. Anal Bioanal Chem. 2012;404(6–7):1631–5. doi: 10.1007/s00216-012-6324-9. [DOI] [PubMed] [Google Scholar]
- 25.Pan KT, Chen YY, Pu TH, Chao YS, Yang CY, Bomgarden RD, Rogers JC, Meng TC, Khoo KH. Mass spectrometry-based quantitative proteomics for dissecting multiplexed redox cysteine modifications in nitric oxide-protected cardiomyocyte under hypoxia. Antioxid Redox Signal. 2014;20(9):1365–81. doi: 10.1089/ars.2013.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahne H, Neubert P, Kuhn K, Etienne C, Bomgarden R, Rogers JC, Kuster B. Carbonyl-reactive tandem mass tags for the proteome-wide quantification of N-linked glycans. Anal Chem. 2012;84(8):3716–24. doi: 10.1021/ac300197c. [DOI] [PubMed] [Google Scholar]
- 27.Kleifeld O, Doucet A, Prudova A, auf dem Keller U, Gioia M, Kizhakkedathu JN, Overall CM. Identifying and quantifying proteolytic events and the natural N terminome by terminal amine isotopic labeling of substrates. Nat Protoc. 2011;6(10):1578–611. doi: 10.1038/nprot.2011.382. [DOI] [PubMed] [Google Scholar]
- 28.Wong JW, Cagney G. An overview of label-free quantitation methods in proteomics by mass spectrometry. Methods Mol Biol. 2010;604:273–83. doi: 10.1007/978-1-60761-444-9_18. [DOI] [PubMed] [Google Scholar]
- 29.Arike L, Peil L. Spectral counting label-free proteomics. Methods Mol Biol. 2014;1156:213–22. doi: 10.1007/978-1-4939-0685-7_14. [DOI] [PubMed] [Google Scholar]
- 30.Sutandy FX, Qian J, Chen CS, Zhu H. Overview of protein microarrays. Curr Protoc Protein Sci. 2013; Chapter 27:Unit 27 21. [DOI] [PMC free article] [PubMed]
- 31.Hall DA, Ptacek J, Snyder M. Protein microarray technology. Mech Ageing Dev. 2007;128(1):161–7. doi: 10.1016/j.mad.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu X, LaBaer J. High-throughput identification of proteins with AMPylation using self-assembled human protein (NAPPA) microarrays. Nat Protoc. 2015;10(5):756–67. doi: 10.1038/nprot.2015.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics. 2006;5(4):573–88. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 34.Venable JD, Dong MQ, Wohlschlegel J, Dillin A, Yates JR. Automated approach for quantitative analysis of complex peptide mixtures from tandem mass spectra. Nat Methods. 2004;1(1):39–45. doi: 10.1038/nmeth705. [DOI] [PubMed] [Google Scholar]
- 35.Shi T, Su D, Liu T, Tang K, Camp DG, II, Qian WJ, Smith RD. Advancing the sensitivity of selected reaction monitoring-based targeted quantitative proteomics. Proteomics. 2012;12(8):1074–92. doi: 10.1002/pmic.201100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mustafa GM, Larry D, Petersen JR, Elferink CJ. Targeted proteomics for biomarker discovery and validation of hepatocellular carcinoma in hepatitis C infected patients. World J Hepatol. 2015;7(10):1312–24. doi: 10.4254/wjh.v7.i10.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho WC. Application of proteomics in non-small-cell lung cancer. Expert Rev Proteomics. 2016;13(1):1–4. doi: 10.1586/14789450.2016.1121813. [DOI] [PubMed] [Google Scholar]
- 38.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–6. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu CH, Hsu CW, Hsueh C, Wang CL, Wu YC, Wu CC, Liu CC, Yu JS, Chang YS, Yu CJ. Identification and characterization of potential biomarkers by quantitative tissue proteomics of primary lung Adenocarcinoma. Mol Cell Proteomics. 2016;15(7):2396–410. doi: 10.1074/mcp.M115.057026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark DJ, Fondrie WE, Yang A, Mao L. Triple SILAC quantitative proteomic analysis reveals differential abundance of cell signaling proteins between normal and lung cancer-derived exosomes. J Proteomics. 2016;133:161–9. doi: 10.1016/j.jprot.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Liu PJ, et al. In-depth proteomic analysis of six types of exudative pleural effusions for nonsmall cell lung cancer biomarker discovery. Mol Cell Proteomics. 2015;14(4):917–32. doi: 10.1074/mcp.M114.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kikuchi T, et al. In-depth proteomic analysis of nonsmall cell lung cancer to discover molecular targets and candidate biomarkers. Mol Cell Proteomics. 2012;11(10):916–32. doi: 10.1074/mcp.M111.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawamura T, Nomura M, Tojo H, Fujii K, Hamasaki H, Mikami S, Bando Y, Kato H, Nishimura T. Proteomic analysis of laser-microdissected paraffin-embedded tissues: (1) Stage-related protein candidates upon non-metastatic lung adenocarcinoma. J Proteomics. 2010;73(6):1089–99. doi: 10.1016/j.jprot.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Kim YJ, Sertamo K, Pierrard MA, Mesmin C, Kim SY, Schlesser M, Berchem G, Domon B. Verification of the biomarker candidates for non-small-cell lung cancer using a targeted proteomics approach. J Proteome Res. 2015;14(3):1412–9. doi: 10.1021/pr5010828. [DOI] [PubMed] [Google Scholar]
- 45.Ortea I, Rodriguez-Ariza A, Chicano-Galvez E, Arenas Vacas MS, Jurado GB. Discovery of potential protein biomarkers of lung adenocarcinoma in bronchoalveolar lavage fluid by SWATH MS data-independent acquisition and targeted data extraction. J Proteomics. 2016;138:106–14. doi: 10.1016/j.jprot.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Xu H, et al. Proteomic profiling of paclitaxel treated cells identifies a novel mechanism of drug resistance mediated by PDCD4. J Proteome Res. 2015;14(6):2480–91. doi: 10.1021/acs.jproteome.5b00004. [DOI] [PubMed] [Google Scholar]
- 47.Wang CI, Wang CL, Wu YC, Feng HP, Liu PJ, Chang YS, Yu JS, Yu CJ. Quantitative proteomics reveals a novel role of karyopherin alpha 2 in cell migration through the regulation of vimentin-pErk protein complex levels in lung cancer. J Proteome Res. 2015;14(4):1739–51. doi: 10.1021/pr501097a. [DOI] [PubMed] [Google Scholar]
- 48.Stewart PA, et al. A pilot proteogenomic study with data integration identifies MCT1 and GLUT1 as prognostic markers in lung Adenocarcinoma. PLoS One. 2015;10(11):e0142162. doi: 10.1371/journal.pone.0142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw AT, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 50.Lindeman NI, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137(6):828–60. doi: 10.5858/arpa.2012-0720-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheikine Y, Rangachari D, McDonald DC, Huberman MS, Folch ES, VanderLaan PA, Costa DB. EGFR Testing in advanced non-small-cell lung cancer, a mini-review. Clin Lung Cancer. 2016 [DOI] [PubMed]
- 52.da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- 53.Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 54.Rosell R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 55.Sequist LV, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 56.Grundner-Culemann K, Dybowski JN, Klammer M, Tebbe A, Schaab C, Daub H. Comparative proteome analysis across non-small cell lung cancer cell lines. J Proteomics. 2016;130:1–10. doi: 10.1016/j.jprot.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Taguchi F, et al. Mass spectrometry to classify non-small-cell lung cancer patients for clinical outcome after treatment with epidermal growth factor receptor tyrosine kinase inhibitors: a multicohort cross-institutional study. J Natl Cancer Inst. 2007;99(11):838–46. doi: 10.1093/jnci/djk195. [DOI] [PubMed] [Google Scholar]
- 58.Shaw AT, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw AT, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17(2):234–42. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Awad MM, Shaw AT. ALK inhibitors in non-small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol. 2014;12(7):429–39. [PMC free article] [PubMed] [Google Scholar]
- 61.Hembrough T, et al. Quantification of anaplastic lymphoma kinase protein expression in non-small cell lung cancer tissues from patients treated with crizotinib. Clin Chem. 2016;62(1):252–61. doi: 10.1373/clinchem.2015.245860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu CW, Hsu CL, Wang YC, Ishihama Y, Ku WC, Huang HC, Juan HF. Temporal phosphoproteome dynamics induced by an ATP synthase inhibitor citreoviridin. Mol Cell Proteomics. 2015;14(12):3284–98. doi: 10.1074/mcp.M115.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang HY, Huang HC, Huang TC, Yang PC, Wang YC, Juan HF. Ectopic ATP synthase blockade suppresses lung adenocarcinoma growth by activating the unfolded protein response. Cancer Res. 2012;72(18):4696–706. doi: 10.1158/0008-5472.CAN-12-0567. [DOI] [PubMed] [Google Scholar]
- 64.Wu YH, Hu CW, Chien CW, Chen YJ, Huang HC, Juan HF. Quantitative proteomic analysis of human lung tumor xenografts treated with the ectopic ATP synthase inhibitor citreoviridin. PLoS One. 2013;8(8):e70642. doi: 10.1371/journal.pone.0070642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luscombe NM, Greenbaum D, Gerstein M. What is bioinformatics? A proposed definition and overview of the field. Methods Inf Med. 2001;40(4):346–58. [PubMed] [Google Scholar]
- 66.Tsai CJ, Ma B, Nussinov R. Protein-protein interaction networks: how can a hub protein bind so many different partners? Trends Biochem Sci. 2009;34(12):594–600. doi: 10.1016/j.tibs.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kar G, Gursoy A, Keskin O. Human cancer protein-protein interaction network: a structural perspective. PLoS Comput Biol. 2009;5(12):e1000601. doi: 10.1371/journal.pcbi.1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu W, He LR, Zhao YC, Chan MH, Zhang M, He M. Dynamic protein-protein interaction subnetworks of lung cancer in cases with smoking history. Chin J Cancer. 2013;32(2):84–90. doi: 10.5732/cjc.012.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vergnaud JM, Rosca I-D. Assessing bioavailability of drug delivery systems : mathematical and numerical treatment. Boca Raton: Taylor & Francis; 2005. [Google Scholar]
- 70.Voit EO, Qi Z, Miller GW. Steps of modeling complex biological systems. Pharmacopsychiatry. 2008;41(Suppl 1):S78–84. doi: 10.1055/s-2008-1080911. [DOI] [PubMed] [Google Scholar]
- 71.Fischer HP. Mathematical modeling of complex biological systems: from parts lists to understanding systems behavior. Alcohol Res Health. 2008;31(1):49–59. [PMC free article] [PubMed] [Google Scholar]
- 72.Chmielecki J, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 74.Schmidlin T, Garrigues L, Lane CS, Mulder TC, van Doorn S, Post H, de Graaf EL, Lemeer S, Heck AJ, Altelaar AF. Assessment of SRM, MRM(3), and DIA for the targeted analysis of phosphorylation dynamics in non-small cell lung cancer. Proteomics. 2016;16(15–16):2193–205. doi: 10.1002/pmic.201500453. [DOI] [PubMed] [Google Scholar]
- 75.Li LS, Kim H, Rhee H, Kim SH, Shin DH, Chung KY, Park KS, Paik YK, Chang J, Kim H. Proteomic analysis distinguishes basaloid carcinoma as a distinct subtype of nonsmall cell lung carcinoma. Proteomics. 2004;4(11):3394–400. doi: 10.1002/pmic.200400901. [DOI] [PubMed] [Google Scholar]
- 76.Park JH, et al. Expression of peroxiredoxin and thioredoxin in human lung cancer and paired normal lung. Respirology. 2006;11(3):269–75. doi: 10.1111/j.1440-1843.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 77.Rho JH, Roehrl MH, Wang JY. Tissue proteomics reveals differential and compartment-specific expression of the homologs transgelin and transgelin-2 in lung adenocarcinoma and its stroma. J Proteome Res. 2009;8(12):5610–8. doi: 10.1021/pr900705r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011;32(15):1976–83. doi: 10.1002/elps.201000598. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Wang X, Ao M, Gabrielson E, Askin F, Zhang H, Li QK. Aberrant Mucin5B expression in lung adenocarcinomas detected by iTRAQ labeling quantitative proteomics and immunohistochemistry. Clin Proteomics. 2013;10(1):15. doi: 10.1186/1559-0275-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang XY, Zhang G, Jiang Y, Liu D, Li MZ, Zhong Q, Zeng SQ, Liu WL, Zeng MS. The prognostic value of serum C-reactive protein-bound serum amyloid A in early-stage lung cancer. Chin J Cancer. 2015;34(8):335–49. doi: 10.1186/s40880-015-0039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song Y, et al. Elevated eukaryotic elongation factor 2 expression is involved in proliferation and invasion of lung squamous cell carcinoma. Oncotarget. 2016;7(36):58470–82. doi: 10.18632/oncotarget.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim YI, Ahn JM, Sung HJ, Na SS, Hwang J, Kim Y, Cho JY. Meta-markers for the differential diagnosis of lung cancer and lung disease. J Proteomics. 2016;148:36–43. doi: 10.1016/j.jprot.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 83.Gautschi O, Pauli C, Strobel K, Hirschmann A, Printzen G, Aebi S, Diebold J. A patient with BRAF V600E lung adenocarcinoma responding to vemurafenib. J Thorac Oncol. 2012;7(10):e23–4. doi: 10.1097/JTO.0b013e3182629903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials related to this work are available upon request.


