Abstract
The ocular surface is continuously exposed to potential pathogens, including free-living amoebae. Acanthamoeba species are among the most ubiquitous amoebae, yet Acanthamoeba keratitis is remarkably rare. The pathogenesis of Acanthamoeba keratitis is a complex, sequential process. Here we show that Acanthamoeba keratitis is profoundly affected by mannosylated proteins on the ocular surface, which stimulate the amoebae to elaborate a 133-kDa pathogenic protease. The mannose-induced protease (MIP133) mediates apoptosis of the corneal epithelium, facilitates corneal invasion, and degrades the corneal stroma. We show that contact lens wear upregulates mannosylated proteins on the corneal epithelium, stimulates MIP133 secretion, and exacerbates corneal disease. Corynebacterium xerosis, a constituent of the ocular flora, contains large amounts of mannose and is associated with Acanthamoeba keratitis. The present results show that amoebae exposed to C. xerosis produce increased amounts of MIP133 and more severe corneal disease. Oral immunization with MIP133 mitigates Acanthamoeba keratitis and demonstrates the feasibility of antidisease vaccines for pathogens that resist immune elimination.
Acanthamoeba keratitis is a rare but potentially blinding infection of the cornea that is caused by free-living amoebae belonging to the genus Acanthamoeba. Acanthamoeba spp. are ubiquitous organisms that can be isolated from a wide variety of environments, including public water supplies, swimming pools, freshwater reservoirs, salt water, hot tubs, ventilation ducts, soil, bottled water, and even eyewash stations (2, 18). The disease is closely associated with contact lens wear, which appears to be an important risk factor in infection. Previous studies showed that more than 80% of the cases of Acanthamoeba keratitis occurred in contact lens wearers (11, 22). Acanthamoeba spp. have been isolated from the contact lens cases of Acanthamoeba keratitis patients, and it is widely believed that contact lenses serve as vectors for transmitting infectious Acanthamoeba trophozoites to the eye. However, contact lenses can stimulate the expression of glycoproteins on the corneal epithelium (12). This in turn might exacerbate the infectious process, as mannosylated proteins promote the binding of Acanthamoeba trophozoites to the corneal epithelium via a mannose-binding protein (mannose receptor) that is expressed on the Acanthamoeba cell membrane. However, adhesion can be inhibited by the addition of free methyl-α-d-mannopyranoside (4, 25). Although engagement of the mannose receptor blocks adhesion, it does not prevent Acanthamoeba-mediated cytolysis of corneal cells (8, 9, 14). We have recently shown that methyl-α-d-mannopyranoside stimulates Acanthamoeba trophozoites to elaborate a 133-kDa protease, designated MIP133, which is produced by pathogenic strains of Acanthamoeba spp. (5) and mediates cell contact-independent apoptosis of corneal epithelial cells and degradation of the collagenous matrix that forms the corneal stroma (8, 9).
In addition to contact lens wear, the bacterial flora of the ocular surface has been implicated as a risk factor for the development of Acanthamoeba keratitis. In particular, Corynebacterium xerosis has been associated with human cases of Acanthamoeba keratitis and is an obligatory cofactor for the development of Acanthamoeba keratitis in a rat model of this disease (3). It has been suggested that C. xerosis serves as an important food source for sustaining the amoebae during the initial stages of corneal infection (3). However, the presence of other commensal bacterial species at the ocular surface is common and casts doubt on the notion that C. xerosis serves merely as a food source for the omnivorous Acanthamoeba trophozoites. C. xerosis does, however, have the highest mannose content of any constituent of the normal ocular bacterial flora (20). With this in mind, we evaluated the effect of contact lens wear and exposure to C. xerosis on the generation of the pathogenic MIP133 proteases and the pathogenicity of Acanthamoeba trophozoites. The pivotal role of MIP133 in the pathogenesis of Acanthamoeba keratitis also prompted us to test this molecule as a potential vaccinogen.
MATERIALS AND METHODS
Amoebic keratitis.
A Chinese hamster model of Acanthamoeba keratitis has been described previously (24) and was used in accordance with approval granted by our institutional animal care and use committee. Acanthamoeba castellanii ATCC 30868 was obtained from the American Type Culture Collection, Rockville, Md. Trophozoites were maintained in axenic culture at 35°C in peptone-yeast extract-glucose (PYG) medium in test tubes, as described previously (24). Miniature contact lenses were prepared from Spectra/Por dialysis membrane tubing (Medical Industries, Los Angeles, Calif.). Disks that were 3.0 mm in diameter were cut from tubing by using a sterile trephine prior to heat sterilization. Lenses were placed in sterile 96-well microtiter plates (Costar, Cambridge, Mass.) and incubated with 3 × 104 A. castellanii trophozoites at 35°C for 24 h. Attachment of amoebae to the lenses was verified microscopically before the lenses were applied to the Chinese hamster corneal surface. Acanthamoeba-laden miniature contact lenses were applied to one eye of each Chinese hamster. The contact lenses were removed 3 to 4 days postinfection, and the corneas were visually inspected by microscopy to determine the severity of disease. Visual inspection results were recorded daily during the times indicated below, and infections were scored based on the degree of corneal infiltration, corneal neovascularization, and corneal ulceration (7). The infections were scored on a scale of 0 to 5 based on the following parameters: corneal infiltration, corneal neovascularization, and corneal ulceration. The pathology was recorded as follows, as described previously (7): 0, no pathology; 1, <10% of the cornea involved; 2, 10 to 25% of the cornea involved; 3, 25 to 50% of the cornea involved; 4, 50 to 75% of the cornea involved; and 5, 75 to 100% of the cornea involved. The results were expressed as percentages of the severity of keratitis. In Chinese hamsters, Acanthamoeba keratitis resolves at approximately 3 weeks.
Isolation and cytotoxicity assays with mannose-induced cytolytic protein MIP133.
MIP133 was purified and characterized as previously described (7). Briefly, MIP133 was isolated and purified by size exclusion fast protein liquid chromatography and size exclusion chromatography (8). The purity of the concentrated MIP133 was confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. The protein concentration of the purified MIP133 was determined by a bicinchoninic acid protein assay with bovine serum albumin as a standard (21).
MIP133 levels were quantified by an enzyme-linked immunosorbent assay (ELISA), and MIP133-induced cytotoxicity for corneal cells was measured spectrophotometrically. Briefly, bacteria (5 × 106 cells/ml) were either cultured alone or cultured with Acanthamoeba trophozoites (1 × 106 cells/ml) in PYG medium for 3 days. The supernatants were collected, and the MIP133 production was measured by the ELISA. The MIP133 cytolytic protein (15.6 μg of protein in 25 μl of phosphate-buffered saline [PBS]) was added to 96-well plates with confluent monolayers of human corneal epithelium cells and incubated for 18 h at 37°C (13). Each well contained 200 μl of the growth medium (minimal essential medium). Additional control wells contained untreated confluent cells or cells treated with purified MIP133. Following incubation, all wells were washed three times with medium and stained with Giemsa stain (Shandon, Inc., Pittsburgh, Pa.). After staining, the wells were washed three times with PBS (pH 7.2), the contents were solubilized in 0.1 ml of 5% SDS in PBS, and the optical density at 590 nm was determined with a Molecular Devices microplate reader. The results were expressed as percentages of live cells by using the following formula: percentage of live cells = 100 − (optical density of experimental wells − optical density of supernatant alone/optical density of control cells alone).
ELISA.
Ninety-six-well assay plates were coated with 50 μg of MIP133 in carbonate buffer overnight. The plates were washed four times with PBS containing 0.05% Tween 20 (wash buffer; Sigma) and then blocked with 0.5% bovine serum albumin (BSA) in PBS (blocking buffer) for 1 h at room temperature. All antibodies were diluted with blocking buffer and incubated at room temperature. Chicken anti-MIP133 antiserum (Aveslabs, Tigard, Oreg.) was added at a 1:50, 1:75, or 1:100 dilution for 1 h and washed. Horseradish peroxidase-conjugated goat anti-chicken antiserum (Aveslabs) was added at a concentration of 1:10,000 and incubated for 1 h at 37°C. The plates were developed by adding 1.0 mM 2,2′-azinobis(3-ethyl-benzthiazoline-6-sulfonic acid) (Sigma) containing 0.003% H2O2 and incubated for 30 min at room temperature. After development, 100 μl of 10% SDS (Sigma) per well was added prior to reading with a microplate reader at 405 nm (1).
Oral immunization.
Animals received 1 ml of 0.1 M sodium carbonate (pH 9.6; Sigma) by gavage prior to administration of 500 μg of MIP133 plus 50 μg of cholera toxin. Immunizations were administered once a week for 4 weeks prior to infection with A. castellanii. The control groups included animals that were immunized with equivalent doses of cholera toxin alone or irrelevant antigen (lysozyme) and animals that were not treated prior to infection (7, 15).
Organ cultures and amoeba adhesion assays.
Pig eyes were obtained from Owen's Country Sausage, Inc. (Richardson, Tex.), and were rinsed once with a 1% iodine solution and then twice with sterile PBS. The eyes were placed on gauze with the cornea facing up, and either sterile hard contact lenses (100% polymer and 0% water; Vision Direct Inc., Austin, Tex.) or sterile hydrophilic soft contact lenses (57.5% polymer and 42.5% water; Copper Vision, Inc., Scottsville, N.Y.) were placed on the corneal surface and secured to prevent flotation by placing two sterile metal rings on the surface of each contact lens. The eyes were incubated in McCarey-Kaufman medium for 24 h. In other experiments the corneal epithelium was abraded with a sterile cotton swab. Untreated eyes were used as controls. Eye cup assays were used to determine the trophozoite binding on the surface of the cornea. Briefly, treated and untreated eyes were placed in 20-ml beakers with the epithelial surface up. The eyes were secured by placing sterile sponges around each globe. The contact lenses were removed, and sterile hollow cylinders (diameter, 0.8 cm; length, 1 cm) open at both ends were prepared by cutting the middle parts of 3-ml syringes. Cyanoacrylate glue was applied to the rim of each cylinder, and the cylinder was placed on the surface of a cornea (limbus area). Acanthamoeba trophozoites were labeled with [35S]methionine-cysteine (New England Nuclear, Boston, Mass.) as described previously (13). The corneas were washed with PBS and were incubated with 1 ml of radiolabeled trophozoites (1 × 106 cells/ml) in PYG medium. In other experiments the corneas were treated with 10 μg of succinylated concanavalin A per ml for 30 min and washed three times with PBS, and then the radiolabeled parasites were added. After incubation at 37°C for 24 h, the medium was removed, and each eye was washed three times with fresh PYG medium. The corneas were excised and transferred to scintillation vials containing 2 ml of scintillation fluid (Budget-Solve; Research Products, Mount Prospect, Ill.), and counts were determined with a liquid scintillation counter (LS 3801; Beckman, Irvine, Calif.). The number of trophozoites that adhered to the cornea was expressed in counts per minute. Eye cup assays were used to determine if exposure to the corneal epithelium induced MIP133 production. Trophozoites (1 × 106 cells/ml) were cultured in the eye cup for 72 h as described above. The supernatants were collected, and the MIP133 production was determined by the ELISA.
Bacterial cultures.
The EGD strain of Listeria monocytogenes was kindly provided by Christopher Lu (University of Texas Southwestern Medical Center, Dallas) and was grown axenically in brain heart infusion medium (BBL Microbiology Systems, Becton Dickinson and Co., Cockeysville, Md.) at 37°C for 18 h (16). C. xerosis ATCC 373 was obtained from the American Type Culture Collection and was cultured in brain heart infusion medium. The concentration of bacteria was estimated by using McFarland standards.
Induction and detection of MIP133.
To determine if C. xerosis and L. monocytogenes induced MIP133 production, bacteria (5 × 106 cells/ml) were cultured with Acanthamoeba trophozoites (1 × 106 cells/ml) in PYG medium for 3 days, and the MIP133 production was measured as described above. Acanthamoeba trophozoites were cocultured with bacteria for 3 days, and then the cultures were treated with antibiotics (0.7 mg of penicillin/ml and 1.04 mg of streptomycin/ml). The trophozoites exposed to bacteria were cultured with contact lenses and used for in vivo infection as described above. No bacteria were cultured on chocolate and blood agar plates from the coculture of trophozoites and bacteria after antibiotic treatment. This indicated that the trophozoite culture was axenic. Production of MIP133 was also determined by coculture of trophozoites (1 × 106 cells/ml) with C. xerosis (5 × 106 cells/ml) that was exposed to heat (56°C), ethanol (70%), formalin (4%), or UV radiation (800 mJ/cm2).
To determine if mannosylated contact lenses or latex beads stimulated MIP133 production, trophozoites (1 × 106 cells/ml) were cultured with either 25-μm-diameter latex beads covalently conjugated to mannose or 2.0-mm-diameter contact lenses coated with mannosylated bovine serum albumin (Mann-BSA). Preparations exposed to other sugars, such as lactose and fucose, served as controls. Trophozoites cultured with either mannose-BSA- or galactose-BSA-coated contact lenses were also used for in vivo infection.
Statistics.
Statistical analyses of all data except clinical scores were performed by using unpaired Student t tests. Clinical severity scores were analyzed by the Mann-Whitney test. Differences between means were considered significant at a P value of <0.05.
RESULTS
Kinetics of MIP133 induction.
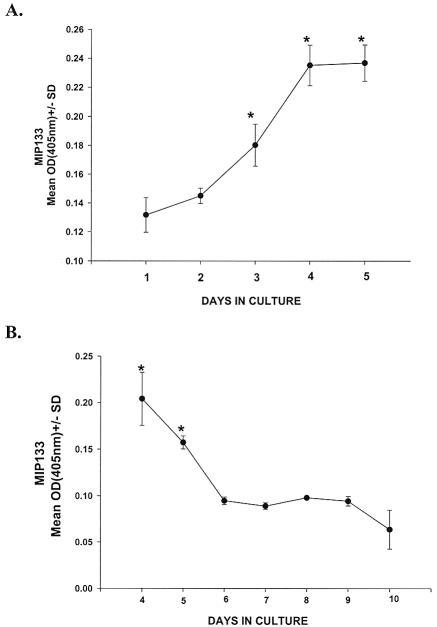
We have recently shown that methyl-α-d-mannopyranoside stimulates Acanthamoeba trophozoites to release a 133-kDa serine protease (mannose-induced protein 133 [MIP133]) that mediates cell contact-independent apoptosis of corneal epithelial cells and degradation of the collagenous matrix that forms the corneal stroma (8, 9). An ELISA was used to determine the kinetics of MIP133 production and its persistence after the removal of mannose. To do this, A. castellanii trophozoites were incubated with 100 mM mannose, and the supernatants were assessed for MIP133 by the ELISA. The results indicate that MIP133 production rose steadily and peaked at 4 days (Fig. 1A). In additional experiments we examined the persistence of MIP133 production following trophozoite removal from mannose. Trophozoites were incubated in 100 mM mannose for 3 days, washed in PBS, and returned to culture. Supernatants were examined for MIP133 at 24-h intervals. Trophozoites continued to produce significant quantities of MIP133 for 48 h after they were removed from mannose (Fig. 1B).
FIG. 1.
Effect of mannose on MIP133 production by Acanthamoeba trophozoites. (A) A. castellanii trophozoites (1 × 106 cells/ml) were incubated with 100 mM mannose, and MIP133 was measured by the ELISA. (B) Trophozoites were incubated in 100 mM mannose for 3 days and washed in PBS, and MIP133 production was measured by the ELISA at 24-h intervals after the trophozoites were removed from mannose. An asterisk indicates that the P value is <0.05. The ELISA was performed by coating the plates with 50 μg of MIP133 protein in carbonate buffer. The plates were washed with PBS and then blocked with PBS containing 0.5% BSA. Chicken anti-MIP133 was added at dilution of 1:100 for 1 h and washed. Horseradish peroxidase-conjugated goat anti-chicken IgY was added at a 1:10,000 dilution. The plates were developed, and the optical density at 405 nm [OD(405nm)] was determined.
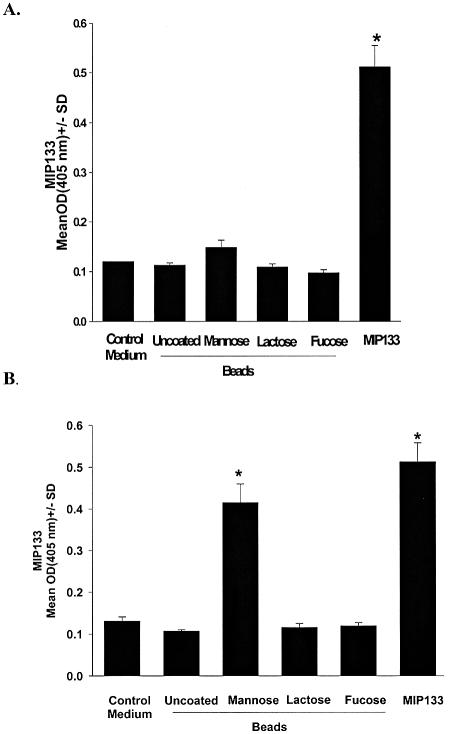
Experiments were performed to determine if mannose simply served as a nutrient that was necessary for the generation of MIP133 or if engagement of mannose with the mannose receptor on A. castellanii was a cue for the production of MIP133. To determine if ingestion of mannose was necessary for the production of MIP133, trophozoites were exposed to 25-μm-diameter latex beads, which were slightly larger than Acanthamoeba trophozoites and thus could not be phagocytosed. Trophozoites were also exposed to latex beads that were covalently conjugated with mannose, lactose, or fucose. Even though mannsose-conjugated latex beads were not phagocytosed (data not shown), significant MIP133 production was detected at 48 h (Fig. 2). By contrast, uncoated latex beads or beads coated with either lactose or fucose did not stimulate MIP133 production.
FIG. 2.
Specificity of mannose-induced MIP133 production. A. castellanii trophozoites (1 × 106 cells/ml) were incubated with 25-μm-diameter sterile latex beads that were either not coated or covalently bound with mannose. Preparations exposed to other sugars, such as lactose and fucose, served as controls. MIP133 was quantified by the ELISA after 48 h (A) and 72 h (B) of incubation. Purified MIP133 served as the positive control. An asterisk indicates that the P value is <0.05. Abbreviation: OD(405nm), optical density at 405 nm.
Contact lens-induced production of MIP133.
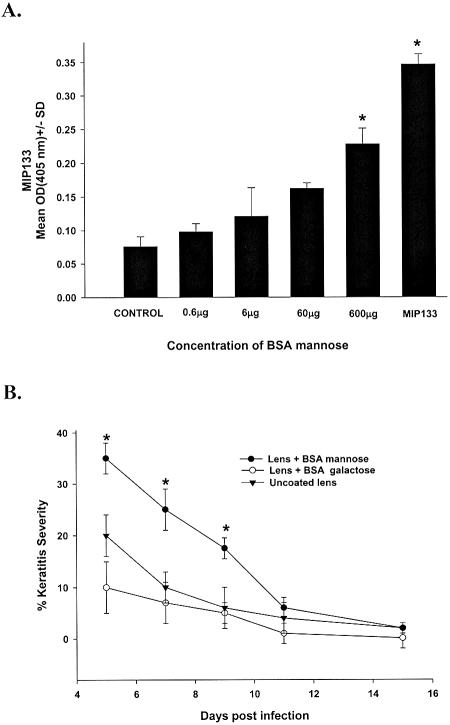
Biofilms and proteinaceous deposits on contact lenses are known to be risk factors for corneal infections. Accordingly, 2.0-mm-diameter sterile contact lenses were coated with mannose-BSA and incubated overnight with A. castellanii trophozoites to determine if a mannosylated protein film stimulated MIP133 production and increased the pathogenicity of A. castellanii trophozoites. Mannose-coated contact lenses induced trophozoites to produce MIP133 in a dose-dependent manner (Fig. 3A). In vivo experiments were performed to determine if the mannose-BSA-coated contact lenses made A. castellanii trophozoites more pathogenic. Chinese hamsters were infected by using either uncoated contact lenses or lenses that were coated with mannose-BSA or galactose-BSA prior to overnight incubation with trophozoites. Chinese hamsters which received uncoated contact lenses or lenses pretreated with galactose-BSA developed typical, self-limiting Acanthamoeba keratitis, which resolved by day 15. By contrast, animals fitted with mannose-BSA-coated, parasite-laden contact lenses developed Acanthamoeba keratitis that was significantly more severe than the Acanthamoeba keratitis in either the galactose-BSA-coated contact lens group or the untreated contact lens group (Fig. 3B).
FIG. 3.
Induction of MIP133 by mannose-coated contact lenses. (A) Contact lenses were incubated overnight in various concentrations of Mann-BSA. The lenses were washed in PBS and incubated for 3 days with A. castellanii trophozoites (1 × 106 cells/ml), and the supernatants were tested for MIP133 by the ELISA. Preparations exposed to other sugars, such as lactose and fucose, served as controls. (B) Uncoated contact lenses or contact lenses coated with either mannose-BSA or galactose-BSA were incubated overnight with trophozoites, and the Acanthamoeba-laden contact lenses were applied to the eyes of Chinese hamsters. The contact lenses were removed 5 days later, and the severity of keratitis was scored as described in Materials and Methods. An asterisk indicates that the P value is <0.05 (n = 8 for each group). Abbreviation: OD(405nm), optical density at 405 nm.
Induction of MIP133 synthesis by constituents of the ocular bacterial flora.
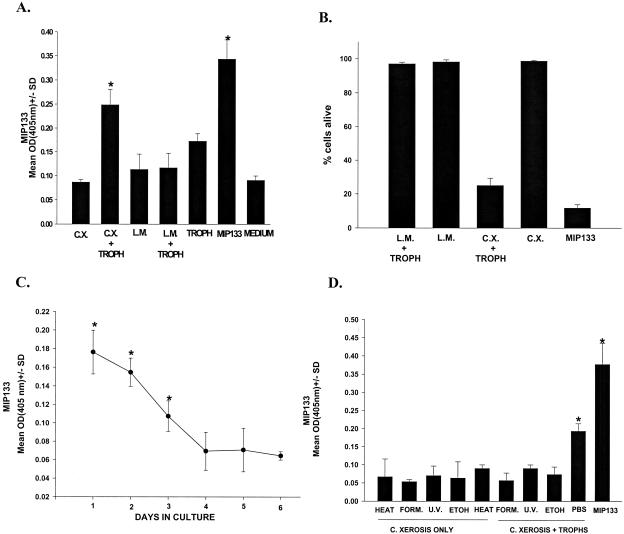
In addition to contact lens wear, the bacterial flora of the ocular surface has been implicated as a risk factor for the development of Acanthamoeba keratitis. In particular, C. xerosis has been associated with human cases of Acanthamoeba keratitis and is an obligatory cofactor for the development of Acanthamoeba keratitis in a rat model of this disease (3). C. xerosis has the highest mannose content of any constituent of the normal ocular bacterial flora (20). This prompted us to test the capacity of C. xerosis cells to stimulate the release of MIP133. A. castellanii trophozoites were incubated with either C. xerosis (20 to 35% mannose in the cell wall) (20) or L. monocytogenes (2% mannose in the cell wall) (6) to determine if either bacterium induced the production of MIP133. Acanthamoeba trophozoites were cocultured with bacteria for 72 h, and then the cultures were treated with antibiotics to kill extracellular bacteria. Exposure to L. monocytogenes did not induce MIP133 production, but incubation with C. xerosis elicited a 40% increase in the amount of MIP133 elaborated by Acanthamoeba trophozoites (Fig. 4A). Moreover, the MIP133 induced by C. xerosis was cytopathic for human corneal epithelial cells (Fig. 4B). MIP133 production persisted for at least 3 days after removal of viable C. xerosis (Fig. 4C). Although production of MIP133 continued after removal of C. xerosis, the induction of MIP133 synthesis required viable C. xerosis, as treatment with formalin, ethanol, or UV irradiation prevented C. xerosis cells from inducing trophozoites to produce MIP133 (Fig. 4D). In additional experiments we examined the effects of a bacterium containing a high level of mannose (C. xerosis) and a bacterium containing a low level of mannose (L. monocytogenes) on the pathogenicity of A. castellanii trophozoites. Trophozoites incubated with L. monocytogenes produced corneal infections that were no more severe than those produced by trophozoites incubated in control culture medium (Fig. 5). By contrast, trophozoites incubated with C. xerosis produced more severe corneal infections at all times examined.
FIG. 4.
Induction of MIP133 synthesis by C. xerosis. (A) MIP133 production was induced by incubating A. castellanii trophozoites (1 × 106 cells/ml) with C. xerosis or L. monocytogenes at a concentration of 5 × 106 cells/ml for 72 h in PYG medium in vitro. Trophozoites without bacteria or bacteria alone (medium) served as controls. The supernatants were collected, and the MIP133 production was measured by the ELISA. (B) Supernatants were also tested for the capacity to produce cytopathic effects on human corneal epithelial cell monolayers. Bacteria (5 × 106 cells/ml) were either cultured alone or cultured with Acanthamoeba trophozoites (1 × 106 cells/ml) in PYG medium for 3 days, the supernatants were collected, and the MIP133 production was measured by the ELISA. The MIP133 cytolytic protein (15.6 μg of protein in 25 μl of PBS) was added to 96-well plates with confluent monolayers of human corneal epithelial cells. The cytopathic effects of the MIP133 protein were assessed by spectrophotometric analysis of Giemsa-stained corneal epithelial cell monolayers after 24 h of exposure to the MIP133 protein as described in Materials and Methods. (C) Kinetics of MIP133 production. Acanthamoeba trophozoites were cocultured with bacteria for 72 h, and then the cultures were treated with antibiotics (0.7 mg of penicillin/ml and 1.04 mg of streptomycin/ml) to kill extracellular bacteria. Axenic cultures of trophozoites were washed extensively and cultured without mannose, and MIP133 was measured by the ELISA for 5 days. (D) Production of MIP133 in the presence of viable and nonviable C. xerosis was assessed by culturing amoebae with viable C. xerosis or C. xerosis treated with 70% ethanol, heat (56°C), 4% formalin, or 800 mJ of UV irradiation/cm2. Supernatants were collected 3 days later, and MIP133 was measured by the ELISA. Purified MIP133 (15.6 μg/200 μl) was used as a positive control. An asterisk indicates that a result is significantly different from the result for the PBS control (P < 0.05). Abbreviations: OD(405nm), optical density at 405 nm; C.X., C. xerosis; L.M. L. monocytogenes; TROPH, trophozoites; FORM., formalin; U.V., UV irradiation; ETOH, ethanol.
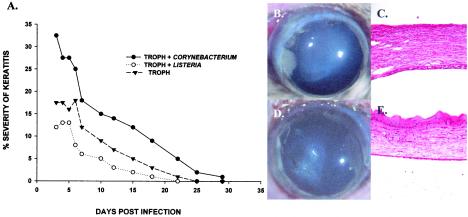
FIG. 5.
Effect of C. xerosis on the severity of Acanthamoeba keratitis. (A) A. castellanii trophozoites were cultured with C. xerosis or L. monocytogenes for 3 days before bacteria were eliminated with antibiotics. Trophozoites were then incubated overnight with contact lenses, which were applied to eyes of Chinese hamsters. The contact lenses were removed 3 days later, and the severity of keratitis was scored as described in Materials and Methods. Keratitis was scored at least three times per week. (B to E) Typical clinical appearance and histopathological features of eyes on day 7 following infection with trophozoites pretreated with C. xerosis (B and C) or L. monocytogenes (D and E). Two separate experiments were performed, and there were eight hamsters in each group. Abbreviation: TROPH, trophozoites.
Effect of contact lens wear on MIP133 production and the pathogenicity of Acanthamoeba trophozoites.
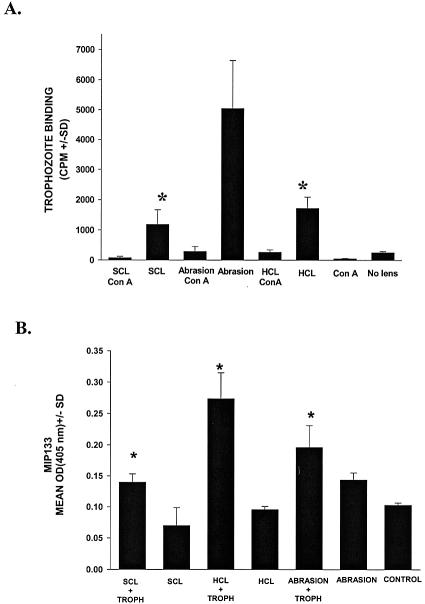
Mild trauma can induce a steep upregulation of mannosylated proteins on the corneal epithelium (10) and promote the adhesion of bacteria to the corneal surface (12). In order to determine if contact lens wear promoted the adhesion of trophozoites to the corneal surface, whole pig eyes were fitted with sterile hard contact lenses or sterile soft, high-water-content contact lenses and placed into organ culture for 24 h before radiolabeled trophozoites were added. Evaluation of the organ-cultured eyes revealed that trophozoites adhered more extensively to corneas conditioned with either soft or hard contact lenses than to unmanipulated corneas (Fig. 6A). In order to determine if the enhanced adhesion was due to upregulation of mannosylated proteins, contact lenses were removed after 24 h of incubation, and the eyes were incubated with the mannose-binding lectin succinyl concanavalin A and washed extensively prior to the addition of radiolabeled trophozoites. Sequestration of mannose moieties on the corneal epithelium with succinyl concanavalin A reversed the enhanced binding associated with contact lens wear. In addition to promoting the adhesion of trophozoites to the corneal surface, contact lens wear also stimulated the release of MIP133 (Fig. 6B).
FIG. 6.
Effect of contact lens wear on amoeba binding to pig corneas and induction of MIP133 synthesis. (A) Pig eyes were placed in eye cups with the cornea facing up, and either sterile hard contact lenses or soft contact lenses were placed on the corneal surface and maintained in organ culture for 24 h. Controls consisted of untreated eyes and eyes whose corneas were abraded with sterile cotton-tipped swabs but not fitted with contact lenses. Twenty-four hours later, the contact lenses were removed, and the corneas were washed with PBS. Eyes were treated with the mannose-binding lectin succinyl concanavalin A prior to addition of [35S]methionine-cysteine-labeled trophozoites. The corneas were excised and transferred to scintillation vials containing 2 ml of scintillation fluid (Budget-Solve; Research Products). The results were determined 24 h later by liquid scintillation counting after extensive washing of the corneal surface. The amoeba binding to corneas was expressed in counts per minute. An asterisk indicates that the P value is <0.05. (B) The effect of contact lens wear on Acanthamoeba production of MIP133 was tested by applying contact lenses to eyes in organ cultures similar to those described above. Twenty-four hours later, the contact lenses were removed, and the corneas were washed with PBS. Trophozoites were added to the corneal surface of each eye in an organ culture as described in Materials and Methods. Controls consisted of untreated eyes without contact lenses. Supernatants were collected 72 h after incubation, and MIP133 was measured by the ELISA. An asterisk indicates that the P value is <0.05. Abbreviations: HCL, hard contact lenses; SCL, soft contact lenses; ConA, succinyl concanavalin A; TROPH, trophozoites.
Efficacy of MIP133 as an antidisease vaccinogen.
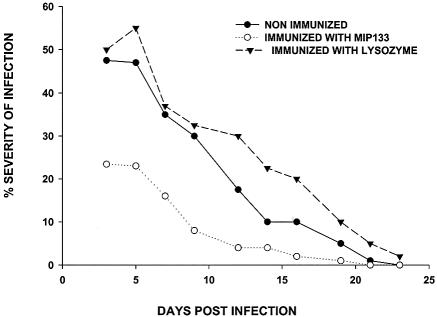
MIP133 is known to facilitate cytolysis of the corneal epithelium, penetration of the basement membrane, and dissolution of the stroma (8, 9). Therefore, neutralizing MIP133 might have a salutary effect on mitigating the pathogenesis of Acanthamoeba keratitis. This possibility was tested by immunizing Chinese hamsters with MIP133 in the presence of neutralized cholera toxin as a means of inducing the production of mucosal immunoglobulin A (IgA) antibodies that appear in the tears (8). The results of two independent experiments demonstrated that animals immunized with MIP133 developed significantly milder Acanthamoeba keratitis than untreated controls or hamsters orally immunized with an irrelevant antigen developed (Fig. 7).
FIG. 7.
Effect of oral immunization with MIP133 on Acanthamoeba keratitis. Hamsters were orally immunized with either 400 μg of MIP133 or an irrelevant antigen (lysozyme) along with neutralized cholera toxin once a week for 4 weeks prior to ocular infection. Trophozoites (1 × 106 cells/ml) were cocultured with C. xerosis (1 × 106 cells/ml) for 3 days before the bacteria were eliminated with antibiotics. Trophozoites were then incubated overnight with contact lenses, which were applied to the eyes of Chinese hamsters. The lenses were removed 3 days postinfection, and the severity of keratitis was scored as described in Materials and Methods. The results are representative of two separate experiments (n = 8 for each group in each experiment). The severity scores were significantly lower than the scores for nonimmunized hamsters and hamsters immunized with lysozyme for all observations made during the first 15 days of infection (P < 0.05).
DISCUSSION
The pathogenesis of Acanthamoeba keratitis is a sequential, multifaceted process that involves (i) binding of trophozoites to the corneal epithelium, (ii) amoeba-mediated apoptosis of corneal epithelial cells, (iii) penetration of the basement membrane, and (iv) degradation of the collagenous stroma that forms the middle layer of the cornea. The mannose binding protein on the surface of Acanthamoeba facilitates amoeba adhesion to the corneal epithelium and induces the release of MIP133. MIP133, in turn, affects the subsequent steps in the pathogenic cascade of Acanthamoeba keratitis, including the cytopathic effects on the corneal epithelium and keratocytes in the stroma, penetration of the basement membrane, and the dissolution of the collagenous stroma. The results reported here indicate that at least three of the important risk factors in Acanthamoeba keratitis involve the stimulation of MIP133.
Contact lens wear and trauma to the corneal epithelium are well-recognized risk factors in Acanthamoeba keratitis (2, 18). The conventional view has held that a contact lens merely serves as a passive vehicle for transmitting Acanthamoeba trophozoites from the environment to the ocular surface. Previous studies in a Chinese hamster model of Acanthamoeba keratitis confirmed the suspicion shared by many clinicians that mild trauma to the corneal surface enhances the binding of trophozoites to the cornea (24). However, the present results reveal additional mechanisms related to these risk factors that might contribute to the pathogenesis of Acanthamoeba keratitis. The results of investigations with pig eyes maintained in organ cultures demonstrated that mild trauma to the corneal surface produced by contact lens wear not only renders the cornea more susceptible to trophozoite binding but also provokes Acanthamoeba trophozoites to produce increased quantities of the pathogenic protease MIP133. The present results also suggest that in addition to serving as a mechanical vector, contact lenses can acquire biofilms and proteinaceous deposits of mannosylated proteins, which stimulate trophozoites to produce increased amounts of MIP133 and create more severe corneal infections.
C. xerosis is a constituent of the ocular bacterial flora and has been implicated as a risk factor in Acanthamoeba keratitis (3). It is noteworthy that the cell wall of C. xerosis has one of the highest mannose contents of any bacterium (20), and exposure to C. xerosis stimulates a steep increase in the elaboration of MIP133 by Acanthamoeba trophozoites and exacerbates their pathogenic behavior in vivo. By contrast, other bacteria, such as L. monocytogenes, which expresses only small quantities of mannose yet can serve as a food source for Acanthamoeba spp., do not affect the pathogenic behavior of Acanthamoeba trophozoites.
Although Acanthamoeba keratitis is relatively rare, it produces extraordinary pain and in some cases blindness. Management is complicated by the biology of the organism. Acanthamoeba is one of the few human protozoan parasites that encysts in human tissues. The cysts are remarkably resistant to a wide variety of antimicrobial agents and can remain viable for up to 24 years (1, 17). Recrudescence can occur years after clinical disease has disappeared. Attempts to produce immunity in experimental animals by conventional immunization protocols have not been successful, even though high titers of Acanthamoeba-specific IgG antibodies and delayed-type hypersensitivity responses were induced (19). Moreover, pathogenic species of Acanthamoeba express complement regulatory proteins that render the trophozoites resistant to complement-mediated lysis (23). The results reported here suggest that targeting a key protease in the pathogenic cascade of Acanthamoeba keratitis is a facile method for managing infection by an organism that is highly resistant to immune attack. In certain conditions, such antidisease vaccines might be more effective than antimicrobial vaccines for the treatment of infectious diseases caused by microorganisms that are either poorly immunogenic or have highly evolved immune escape mechanisms.
Acknowledgments
This work was supported by Public Health Service grant EY09756 from the National Institutes of Health, by NIAID molecular microbiology training grant AI07520, and by an unrestricted grant from Research to Prevent Blindness, Inc., New York, N.Y.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aksozek, A., K. McClellan, K. Howard, J. Y. Niederkorn, and H. Alizadeh. 2002. Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J. Parasitol. 88:621-623. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh, H., J. Y. Niederkorn, and J. P. McCulley. 1996. Acanthamoebic keratitis, p. 1062-1071. In J. S. Pepose, G. N. Holland, and K. R. Wilhelmus (ed.), Ocular infection and immunity. Mosby, St. Louis, Mo.
- 3.Badenoch, P. R., A. M. Johnson, P. E. Christy, and D. J. Coster. 1990. Pathogenicity of Acanthamoeba and a Corynebacterium in the rat cornea. Arch. Ophthalmol. 108:107-112. [DOI] [PubMed] [Google Scholar]
- 4.Cao, Z., D. M. Jefferson, and N. Panjwani. 1998. Role of carbohydrate-mediated adherence in cytopathogenic mechanisms of Acanthamoeba. J. Biol. Chem. 273:15838-15845. [DOI] [PubMed] [Google Scholar]
- 5.Garate, M., Z. Cao, E. Bateman, and N. Panjwani. 2004. Cloning and characterization of a novel mannose-binding protein of Acanthamoeba. J. Biol. Chem. 279:29849-29856. [DOI] [PubMed] [Google Scholar]
- 5a.Hendrickson, D. A. 1985. Reagents and stains, p. 1093-1107. In E. H. Lennette, A. Balows, W. J. Hausler, Jr., and H. J. Shadomy (ed.), Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, D. C.
- 6.Hether, N. W., P. A. Campbell, L. A. Baker, and L. L. Jackson. 1983. Chemical composition and biological functions of Listeria monocytogenes cell wall preparations. Infect. Immun. 39:1114-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurt, M., S. Apte, H. Leher, K. Howard, J. Niederkorn, and H. Alizadeh. 2001. Exacerbation of Acanthamoeba keratitis in animals treated with anti-macrophage inflammatory protein 2 or antineutrophil antibodies. Infect. Immun. 69:2988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurt, M., S. Neelam, J. Niederkorn, and H. Alizadeh. 2003. Pathogenic Acanthamoeba spp. secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease. Infect. Immun. 71:6243-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurt, M., J. Niederkorn, and H. Alizadeh. 2003. Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 44:3424-3431. [DOI] [PubMed] [Google Scholar]
- 10.Jaison, P. L., Z. Cao, and N. Panjwani. 1998. Binding of Acanthamoeba to mannose-glycoproteins of corneal epithelium: effect of injury. Curr. Eye Res. 17:770-776. [PubMed] [Google Scholar]
- 11.Jones, D. B., G. S. Visvesvara, and N. M. Robinson. 1975. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans. Ophthalmol. Soc. U. K. 95:221-232. [PubMed] [Google Scholar]
- 12.Klotz, S. A., R. P. Misra, and S. I. Butrus. 1990. Contact lens wear enhances adherence of Pseudomonas aeruginosa and binding of lectins to the cornea. Cornea 9:266-270. [PubMed] [Google Scholar]
- 13.Leher, H., K. Kinoshita, H. Alizadeh, F. L. Zaragoza, Y. He, and J. Niederkorn. 1998. Impact of oral immunization with Acanthamoeba antigens on parasite adhesion and corneal infection. Investig. Ophthalmol. Vis. Sci. 39:2337-2343. [PubMed] [Google Scholar]
- 14.Leher, H., R. Silvany, H. Alizadeh, J. Huang, and J. Y. Niederkorn. 1998. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect. Immun. 66:5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leher, H. F., H. Alizadeh, W. M. Taylor, A. S. Shea, R. S. Silvany, F. Van Klink, M. J. Jager, and J. Y. Niederkorn. 1998. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Investig. Ophthalmol. Vis. Sci. 39:2666-2673. [PubMed] [Google Scholar]
- 16.Ma, D., R. D. Gerard, X. Y. Li, H. Alizadeh, and J. Y. Niederkorn. 1997. Inhibition of metastasis of intraocular melanomas by adenovirus-mediated gene transfer of plasminogen activator inhibitor type 1 (PAI-1) in an athymic mouse model. Blood 90:2738-2746. [PubMed] [Google Scholar]
- 17.Mazur, T., E. Hadas, and I. Iwanicka. 1995. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop. Med. Parasitol. 46:106-108. [PubMed] [Google Scholar]
- 18.Niederkorn, J. Y., H. Alizadeh, H. Leher, and J. P. McCulley. 1999. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1:437-443. [DOI] [PubMed] [Google Scholar]
- 19.Niederkorn, J. Y., H. Alizadeh, H. F. Leher, and J. P. McCulley. 1999. The immunobiology of Acanthamoeba keratitis. Springer Semin. Immunopathol. 21:147-160. [DOI] [PubMed] [Google Scholar]
- 20.Puech, V., M. Chami, A. Lemassu, M. A. Laneelle, B. Schiffler, P. Gounon, N. Bayan, R. Benz, and M. Daffe. 2001. Structure of the cell envelope of corynebacteria: importance of the non-covalently bound lipids in the formation of the cell wall permeability barrier and fracture plane. Microbiology 147:1365-1382. [DOI] [PubMed] [Google Scholar]
- 21.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 22.Stehr-Green, J. K., T. M. Bailey, and G. S. Visvesvara. 1989. The epidemiology of Acanthamoeba keratitis in the United States. Am. J. Ophthalmol. 107:331-336. [DOI] [PubMed] [Google Scholar]
- 23.Toney, D. M., and F. Marciano-Cabral. 1998. Resistance of Acanthamoeba species to complement lysis. J. Parasitol. 84:338-344. [PubMed] [Google Scholar]
- 24.van Klink, F., H. Alizadeh, Y. He, J. A. Mellon, R. E. Silvany, J. P. McCulley, and J. Y. Niederkorn. 1993. The role of contact lenses, trauma, and Langerhans cells in a Chinese hamster model of Acanthamoeba keratitis. Investig. Ophthalmol. Vis. Sci. 34:1937-1944. [PubMed] [Google Scholar]
- 25.Yang, S., and C. Villemez. 1994. Cell surface control of differentiation in Acanthamoeba. J. Cell. Biochem. 56:592-596. [DOI] [PubMed] [Google Scholar]