Abstract
Background
Infections of the genital tract are considered common causes of male fertility disorders, with a prevalence of 6–10%. Most of the affected men are asymptomatic. The diagnostic evaluation is based mainly on laboratory testing. Inconsistent diagnostic criteria have been applied to date, and this may explain the controversial debate about the role of infection and inflammation in the genital tract as a cause of infertility. The risk of an irreversible fertility disorder should not be underestimated.
Methods
This review is based on pertinent publications retrieved by a selective literature search in PubMed, including guidelines from Germany and abroad and systematic review articles.
Results
The main causes of inflammatory disease of the male genital tract are ascending sexually transmitted infections (STIs) and uropathogens. Chronic prostatitis has no more than a limited influence on ejaculate variables. By contrast, approximately 10% of men who have had acute epididymitis develop persistent azoospermia thereafter, and 30% have oligozoospermia. Obstruction of the excurrent ducts can ensue, as can post-infectious disturbances of spermatogenesis. The differential diagnostic evaluation includes the determination of testicular volumes, hormone concentrations, and ejaculate variables. Epidemiological data are lacking with regard to infertility after primary orchitis of infectious origin; however, up to 25% of testicular biopsies obtained from infertile men reveal focal inflammatory reactions. Multiple studies have suggested a deleterious effect of leukocytes and inflammatory mediators on sperm parameters. On the other hand, the clinical significance of bacteriospermia remains unclear.
Conclusion
Any suspicion of an infectious or inflammatory disease in the male genital tract should prompt a systematic diagnostic evaluation and appropriate treatment. For patients with obstructive azoospermia, the etiology and site of the obstruction determine the surgical approach to be taken. In the near future, the elucidation of underlying pathophysiological mechanisms and the identification of suitable biomarkers may enable new strategies for conservative treatment.
Globally, approximately 15% of couples of reproductive age experience infertility; however, significant regional differences exist (1, 2). Male infertility accounts for at least half of these cases. With a prevalence of 6% to 10%, infections and the resulting inflammatory reactions within the male genital tract are among the main causes for male infertility (3– 5). Ascending infections caused by sexually transmitted pathogens, such as Chlamydia (C.) trachomatis or typical uropathogens, such as Escherichia (E.) coli, play a key role in the etiopathogenesis of the condition; other factors include the hematogenous spread of systemic, typically viral infections (5– 7). Nosological classification differentiates between urethritis, prostatitis/prostato-vesiculitis, epididymitis/epididymo-orchitis, and orchitis. These pathogens or components of these pathogens or inflammatory cells and their mediators may cause irreversible damage, especially to the testis and epididymis (table 1) (7– 9).
Table 1. Organ-specific exposure of germ cells/sperm to pathogens or components of pathogens as well as cells involved in inflammatory processes and their mediators.
| Function |
Percentage of total ejaculate volume (%) |
Biochemical markers in seminal plasma |
pH of ejaculate fractions |
Duration of germ cell/sperm exposure |
|
| Testis | Spermatogenesis | – | – | – | 74 days |
| Epididymis | Sperm maturation | 5 | α-glucosidase(L-carnitine) | – | 7–14 days |
| Seminal vesicles | Accessory secretion | 50–80 | Fructose | 7.2–7.5 | Seconds |
| Prostate | Accessory secretion | 15–30 | Zinc(PSA; citrate; acid ‧phosphatase) | 6.4 | Seconds |
PSA, prostate-specific antigen
The conditions resulting from acute infections can be diagnosed with great certainty and pertinent recommendations on their treatment and the management of complications are in place (e1, e2). However, the majority of infertility patients with signs of urogenital tract infections/inflammations are asymptomatic, suggesting a high rate of chronic disease (7, 8). In these cases, the diagnosis is primarily based on the detection of the pathogen, increased white blood cell counts and/or inflammatory mediators in ejaculate, prostate secretion and urine samples. Since a compartment-specific differential diagnosis is challenging, abnormal findings are typically summarized under the term “male accessory gland infection” (MAGI), according to the definition of the World Health Organization (WHO) (etable) (10). The lack of differentiation between infection and inflammation is of concern, as inflammatory reactions not primarily related to a pathogen may also occur (box) (11). Another problem is that asymptomatic testicular inflammatory reactions are not covered by the WHO criteria (7). Therefore, the potential impact of urogenital infections on male fertility and its management remain the subject of controversy (5). In the light of the above, it appears relevant to clinical practice to summarize the currently available evidence and recommendations in regard to diagnosis and treatment approach.
eTable. WHO criteria to diagnose male accessory gland infection (MAGI) (modified according to [10]).
|
Oligo- and/or astheno- and/or teratozoospermia*1 plus 2 positive findings from 3 categories (A-C) or 2 abnormal ejaculate results (C) | ||
| A | Medical history | Urinary tract infection, sexually transmitted infection (STI), epididymitis |
| Physical examination | Epididymis thickened, tender; spermatic cord thickened; digital rectal examination abnormal | |
| B | Urine after prostatic massage | Abnormal (e.g. >10 leukocytes/ high-power microscopic field*2);detection of Chlamydia trachomatis |
| C | Ejaculate | (Peroxidase-positive) leukocytes >106/mL Culture with significant growth of pathogens Detection of Chlamydia trachomatis Increased inflammatory markers, ROS; abnormal biochemical parameters in seminal plasma |
*1 Oligozoospermia: Total sperm count (or concentration) below the lower limit (15 x 106/mL and 39 x 106, respectively);
Asthenozoospermia: percentage of progressive motile spermatozoa below the lower limit (32%);
Teratozoospermia: percentage of spermatozoa with normal morphology below the lower limit (strict criteria: 4%) (22)
*2 Magnification x 400
ROS, reactive oxygen species; WHO, World Health Organization
BOX. Pathogenesis of male infertility due to urogenital infections.
-
Urogenital infections can affect male fertility on various levels:
Direct and/or indirect damage to sperm quality and function by pathogens or components of pathogens and/or inflammation-associated molecules, such as proinflammatory cytokines or reactive oxygen species (ROS)
Dysfunction of accessory glands
Inflammation-related obstruction of male reproductive tract
Damage to spermatogenesis by direct effects of pathogens or components of pathogens and/or by induction of cellular and humoral immune responses with (irreversible) disruption to the specific local immune regulation in the testis (so-called immune privilege to prevent auto-immune reactions to antigens of post-/meiotic germ cells)
Induction of humoral immune response to sperm, i.e. production of membrane-bound anti-sperm autoantibodies
Pathogen-induced epigenetic changes (?)
As broad as the range of mechanisms of damage is the range of causative infections.
Besides immune responses induced by pathogens or components of pathogens, primarily non-infectious inflammatory reactions may occur in testis or epididymis.
Male infertility is frequently of multifactorial etiology, making it more difficult to establish the diagnostic significance of infections/inflammatory processes in the genital tract.
Methods
This review is based on a selective search of the PubMed database using the MeSH terms “male infertility”, ”bacteriospermia”, ”leukocytospermia”, ”urogenital infection”, “male accessory gland infection + MAGI”, ”inflammation”, ”chronic urethritis”, ”chronic prostatitis + CP/CPPS”, ”epididymitis, orchitis”. In addition, we included current guidelines in our review.
Disease presentations
Chronic prostatitis
Approximately 10% of all men experience symptoms of prostatitis syndrome, such as pelvic pain and urinary discomfort (12). However, only 5% to 10% of these patients are diagnosed with chronic bacterial prostatitis. The classification of the National Institute of Health (NIH) differentiates between chronic bacterial prostatitis (CBP, NIH category II) and inflammatory (NIH category IIIA) or non-inflammatory chronic pelvic pain syndrome (CPPS) (NIH category IIIB) (e3).
In chronic bacterial prostatitis, the bacterial count in urine after prostatic massage is, by definition, at least 10-times higher in the 2-glass test compared with the baseline urine; in 50% of cases, a significant bacterial count of the same pathogen is found in the ejaculate (bacteriospermia) as well (figure 1) (12). Besides the bacterial spectrum of complicated urinary tract infections, atypical pathogens and pathogens causing sexually transmitted infections (STIs) may play a role (6, e4, e5). Furthermore, CBP (NIH category II) and inflammatory CPPS (NIH category III A) are associated with increased white blood cell counts in prostate secretions/urine after prostatic massage and in ejaculate (figure 1) (12). Among the inflammatory markers, interleukin 8 (IL-8) has attracted special attention; in contrast, traditional biochemical ejaculate parameters are of little diagnostic value (e6, e7) (table 2).
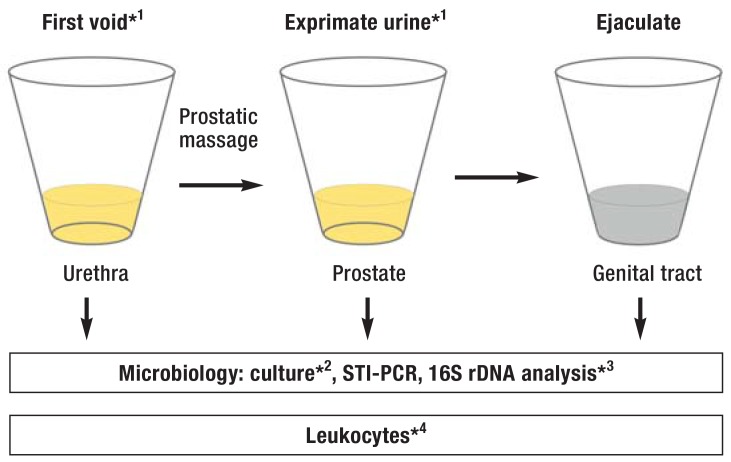
Figure 1.
Fractionated sampling and analysis of urogenital secretions: Combination of 2-glass test and subsequent semen analysis (modified according to [e8])
*1 approx. 5 mL; *2 10 x higher bacterial count (CFU/mL) in post-massage (exprimate) urine versus first void urine; bacteriospermia
*3facultative; *4 = 15 granulocytes/ high-power microscopic field in sediment (magnification x 400) → urethritis; post-massage (exprimate) urine =10/µL → prostatitis; ejaculate: >106/mL (peroxidase-positive leukocytes; no reference ranges available for macrophages, lymphocytes)
CFU, colony-forming units; PCR, polymerase chain reaction; STI, sexually transmitted infection
Table 2. Ejaculate changes with chronic inflammatory diseases of the male genital tract.
|
Ejaculate parameters (reference values)*1 |
Asymptomatic genital tract inflammation*2 |
Chronic prostatitis (NIH II, III) |
|
| Basic semen analysis | Volume (1.5 mL) | → | (↓) |
| pH (≥ 7.2) | → | (↑) | |
| Sperm concentration (15 x 106 /mL)/total (39 x 106) |
(↓) | → | |
| Motility (progressive: 32%) | ↓ | (↓) | |
| Morphology (strict criteria: 4%) | Integrity of flagellar membrane (↓) | Head segment hyper-elongation (↑) | |
|
Sperm function/ quality |
Acrosome reaction | abnormal | abnormal |
| DNA fragmentation | ↑ | ? | |
| Apoptosis | ↑ | ? | |
|
Inflammatory markers in the ejaculate |
Leukocytes (<1 x 106/ml) (neutrophilic granulocytes, macrophages) |
↑ | ↑ |
| Granulocyte elastase (<280 ng/ml) | ↑ | ↑ | |
| Proinflammatory cytokines (e.g. IL-6 [30 pg/mL], IL-8 [7000 pg/mL]) |
↑ | ↑ | |
| Accessory secretion | Zinc (≥ 2.4 µmol/ejaculate) | → | ↓ |
| Fructose (≥ 13 µmol/ejaculate) | → | → | |
| α-Glucosidase (≥ 20 mU/ejaculate) | (↓) | → |
*1 see WHO laboratory manual (22); for granulocyte elastase or cytokines (5, 12, e6, e34)
*2 infectious, post-infectious or primary non-infectious cause; especially likely with chronic inflammatory disorders affecting the epididymis, see Table 1
?, no study data available; ↑, increased; ↓, reduced; →, unchanged
NIH, National Institute of Health
In view of the prevalence of the disease among younger men, the question of the potential impact of chronic prostatitis on male fertility arises. Here, the available studies report conflicting results. Recently published meta-analyses indicate a negative effect on sperm motility and morphology (13, e8– e10) (table 2). However, the diagnostic value of basic semen analysis is limited, as demonstrated by changes in sperm function and epigenetic markers in patients with chronic prostatitis (14, e11).
Epididymitis, epididymo-orchitis and orchitis
The annual incidence of acute epididymitis is approximately 400/100 000 men (15). Typically, the cardinal symptoms of pain and swelling of the epididymis manifest on one side. Since in up to 60% of patients testicular involvement is present, many authors primarily use the term epididymo-orchitis (7). The ascent of pathogenic bacteria from the urethra into the epididymis plays a key role in the etiology of the disease. The most commonly detected microorganisms include typical uropathogens, such as E. coli, and STI pathogens (16).
From the perspective of etiopathogenesis, the notion that there is a connection between male infertility and epididymitis/epididymo-orchitis resulting from STI-related urethritis seems plausible (e12, e13). The deterioration of sperm concentration observed in the ejaculate during the acute phase of the disease is usually reversible within 3 to 6 months; however, an analysis of pooled data revealed persistent azoospermia in approximately 10% of patients and oligozoospermia in another 30% (13). Proteome analyses suggest that, despite appropriate antimicrobial treatment, qualitative sperm changes may occur in addition to reduction in sperm concentration (e14). Bacterial virulence factors, such as hemolysins, may also have an impact on the course of the disease (e15).
Despite the lack of epidemiological data on the chronification of epididymo-orchitis, testicular atrophy with permanent loss of spermatogenesis is a much-dreaded complication (7, e16). However, a recent ultrasonography study involving 80 patients with unilateral epididymitis/epididymo-orchitis showed that testicular volume returned to normal after an initial increase compared with the unaffected contralateral side, with no resulting testicular atrophy (17). These data support the view that male reproductive tract obstructions play an important role as the cause of persistently reduced semen quality.
Primary orchitis is typically observed along with the hematogenous spread of systemic, viral infections (e.g. mumps); chronic granulomatous orchitis can occur with tuberculosis, lepromatous leprosy, syphilis or brucellosis (7, 18). Epidemiological data on the prevalence of azoospermia/infertility after infection-related orchitis are almost entirely lacking. A Mongolian study confirmed the increased risk of reduced ejaculate quality after mumps orchitis or orchitis of other etiology (Odds Ratios [OR]: 3.4 and 2.3, respectively) (19).
Remarkably, asymptomatic testicular inflammatory reactions were observed in about 25% of male patients undergoing testicular biopsy as part of their infertility work-up. These primarily focal lymphocytic infiltrates correlate with the degree of damage to spermatogenesis and clinical endocrinological parameters of testicular function (7). However, only about 2% of these patients report a previous episode of (epididymo-)orchitis.
Obstruction of the male reproductive tract due to infection/inflammation
Studies following up patients after acute epididymitis found an increased risk for infection/inflammation-related azoospermia (13). Compared with obstructions of the ductus epididymidis or ductus deferens, obstructions of the ejaculatory ducts are rare (20, e17). With an overall prevalence of azoospermia in andrology centers of 5% to 15%, so-called obstructive azoospermia is present in about 40% of cases; the proportion of obstructions due to infection/inflammation among these cases ranges from 18% to 47% (21, e18, e19).
Besides normal testicular volumes and serum follicle-stimulating hormone (FSH) levels, indicating intact spermatogenesis, the differential diagnosis of obstructive azoospermia is based on volume, pH and biochemical parameters of the ejaculate (table 1) (22, e1). In addition to the site of obstruction, the differentiation between acquired infectious-inflammatory forms and congenital forms of obstructive azoospermia is a key prerequisite for the potential use of surgical treatment options, such as vasoepididymostomy (20, e18, e20).
Asymptomatic patients desiring to have children
Prevalence data on male subfertility or infertility caused by genital tract infections and inflammations are typically based on information about male accessory gland infection (MAGI), as defined by the WHO classification (etable) (10). However, due to the lack of noninvasive diagnostic markers, asymptomatic testicular inflammatory reactions are not taken into account (7). While rates of approximately 9% have been reported from andrology centers in Germany (4, 5), this percentage can rise to over 30% in countries where healthcare is inadequate (23). These regional differences are suggestive of a direct connection between STI and secondary infertility (2, e21).
In the light of the potential contamination of ejaculate samples with urethral commensals, bacteriospermia does not necessarily indicate genital infection (6, 24). For uropathogenic bacteria, a threshold of 10³ colony-forming units (CFU)/mL has been suggested to define ”significant” bacteriospermia (5, e22). In a Canadian study with almost 5000 subfertile men, however, an association between bacteriospermia, which was detected in 15% of the examined subjects, and semen parameters was only observed in patients with concomitant increase in white blood cell counts in the ejaculate (25). Studies evaluating the connection between ejaculate quality and the presence of C. trachomatis and Mycoplasma spp. reported conflicting results, despite the high prevalence of positive polymerase chain reaction (PCR) testing for these pathogens; however, significant effects on sperm function and quality were demonstrated (26, e23– e26). The available data on human papilloma viruses, for which prevalences of 10% to 36% in ejaculate have been reported (27), does not allow to draw final conclusions on their role in male infertility. Current data suggest that Trichomonas vaginalis infections do not have a negative effect on male fertility (5).
The pathological relevance of leukocytospermia, determined using the WHO threshold of 106 peroxidase-positive cells per mL of ejaculate, remains the subject of controversy (9, 22, e27, e28). In subfertile men, however, a negative correlation between white blood cell count levels and sperm concentration, motility and morphology was demonstrated (e27, e29, e30). Likewise, a negative association was found between the number of neutrophilic granulocytes in smears on the one hand and basic semen parameters as well as sperm DNA integrity on the other hand (25). Yet, the prognostic value of leukocytospermia with regard to the detection of relevant bacteria or viruses is low (25, 28).
Apart from reduced sperm motility, infection and inflammation of the male genital tract can have other negative effects on critical sperm functions (table 2) (14, e31). Oxidative damage to sperm, including DNA fragmentation caused by increased levels of reactive oxygen species (ROS) in the ejaculate, may already occur below the WHO’s 106 leukocytes/mL threshold (e32, e33). Likewise, an association between granulocyte elastase and cytokines in the ejaculate and sperm DNA fragmentation was found (e34). Macrophages play a major role as a source of proinflammatory cytokines (29, 30, e35, e36).
The prevalence of so-called immune-mediated infertility—the detection of sperm-immobilizing or sperm-agglutinating auto-antibodies in the ejaculate—is reported to be 4% to 6% (4, e37, e38). While after vasectomy sperm autoantibodies are detected in over 50% of patients, evidence in support of the induction of autoantibodies after genital tract infection/inflammation is inconclusive (31, 32). Cross-reactivity of pathogen-specific antibodies with sperm antigens is currently discussed as the cause of an association between Helicobacter pylori infection and male infertility (e39).
Diagnosis of urogenital infections and inflammations
Clinical diagnosis
Apart from fertility-relevant aspects, patient history should pay special attention to previous diseases such as STIs and other urogenital or systemic infections. Validated questionnaires to assess sexual dysfunction and prostatitis-associated symptoms are available (e1, e40).
The clinical examination of the genital organs should always be complemented by ultrasonography of the scrotal content (33). Using color-coded duplex ultrasonography, organ vascularization and perfusion can be visualized (17). Transrectal ultrasonography is indispensable in patients with suspected abscess formation due to prostatitis; yet, the relevance of the common finding of prostate calcifications remains unclear (e8).
In cases of acute urogenital infection/inflammation, laboratory testing, including blood count as well as CRP and serum PSA (indicative of concomitant prostatitis), is useful (CRP, C-reactive protein; PSA, prostate-specific antigen). Hormone status should be ordered to assess endocrine testicular function (basic: follicle-stimulating hormone [FSH], luteinizing hormone [LH], total testosterone; extended: estradiol, sexual hormone–binding globulin [SHBG], inhibin B).
Fractionated sampling and analysis of urogenital secretions
In combination with semen analysis, the 4-glass test was developed to detect the localization of inflammation/infection within the male genital tract, in particular for the diagnosis of prostatitis (12). The test involves comparative quantifying testing of urine portions before and after prostatic massage and prostatic exprimate. A simplified version of the test, the 2-glass test, has proven clinical value (figure 1) (12).
Semen analysis
The examination of the ejaculate according to WHO recommendations (22) plays a key role in the diagnosis of infectious and inflammatory processes in the male genital tract. However, sperm concentration, total sperm count, and sperm motility and morphology represent only surrogate parameters in the evaluation of fertility. In addition, these are not specific for abnormalities resulting from infection/inflammation. Ejaculate volume, pH and biochemical parameters reflect the secretory function of the accessory glands (table 1). Sperm agglutination may indicate the presence of membrane-bound auto-antibodies (IgG, IgA; detection by mixed antiglobulin reaction test) (22).
Quantification of leukocytes as markers of inflammation in the ejaculate is based on the detection of peroxidase-positive granulocytes (5, 22). Further analyses extending beyond the scope of the above are currently still considered facultative/experimental by the WHO. Compared with the peroxidase method, immunocytochemical detection or flow cytometry of leukocytes have a much higher sensitivity and allow for the differentiation of leukocyte subpopulations in the ejaculate (22, 29). Other indicators of inflammation include granulocyte elastase and proinflammatory cytokines (8, e8, e34, e41).
Microbiological testing
In patients with suspected urogenital infections, urine samples and secretions/swaps are obtained for microbiological testing (figure 1). However, urogenital samples can get contaminated by the normal microbiota of the urethra, including bacteria such as S. epidermidis and viridans group streptococci. These bacteria are generally classed as non-pathogenic commensals. In healthy male subjects, only about 10% of urogenital samples are free of microorganisms (5, 24). In contrast, facultative pathogenic bacteria, such as enterobacteria (e.g. E. coli, Klebsiella spp., Proteus spp.), enterococci, ureaplasms, mycoplasms, and Staphylococcus (S.) saprophyticus as well as obligatory pathogenic bacteria, such as Neisseria (N.) gonorrhoeae and C. trachomatis, can trigger an infection (6).
According to microbiological/infectiological quality standards (MiQ), it is thus required to determine the type of the pathogen and the bacterial count when urogenital samples are tested to evaluate their etiological relevance (e42). Furthermore, proper sampling, sample transport and sample processing within 2 to 4 hours after sampling is critical (e43). Pre-analytical mistakes include lack of cleaning of the urethral orifice and its surrounding; microbiological analysis of the ejaculate should be performed only after prior urination (22, e42).
The detection of microorganisms in urogenital secretions is best achieved with a combination of culture and nucleic acid amplification techniques (NAT) (34– 36). Fast-growing bacteria are detected using culture techniques (e42), while difficult to cultivate and very sensitive bacteria are detected using culture-independent NAT (for example STI PCR) (e44). In case of negative culture and negative STI PCR, an additional universal bacterial PCR can be performed, using the 16S rDNA gene as target sequence which is highly conserved in all bacteria. The highly sensitive universal bacterial PCR with subsequent sequencing detects 99% of human pathogenic bacteria (16, 36, e45). This approach detects bacteria missed with cultural methods and STI PCR.
Treatment options
Treatment aims at the reduction or eradication of pathogenic bacteria in the ejaculate or prostate secretions, the normalization of inflammatory parameters, and the improvement of abnormal sperm parameters. Antibiotic treatment of asymptomatic genital tract infections in sub-/infertile patients follows the recommendations primarily made for acute/symptomatic conditions (16, e1, e2) (figure 2). In case an STI is detected, antimicrobial therapy is always required and should be administered as recommended in available guidelines and include the partner of the patient (37, 38, e46). The treatment for uropathogens is guided by bacterial counts and resistance testing; in the absence of chronic bacterial prostatitis or symptomatic epididymitis, the microbiological results should first be confirmed at a follow-up examination. In symptomatic patients requiring ad-hoc treatment, fluoroquinolones with activity against C. trachomatis are considered the first-line treatment because of their good local tissue penetration (e2, 12, 16).
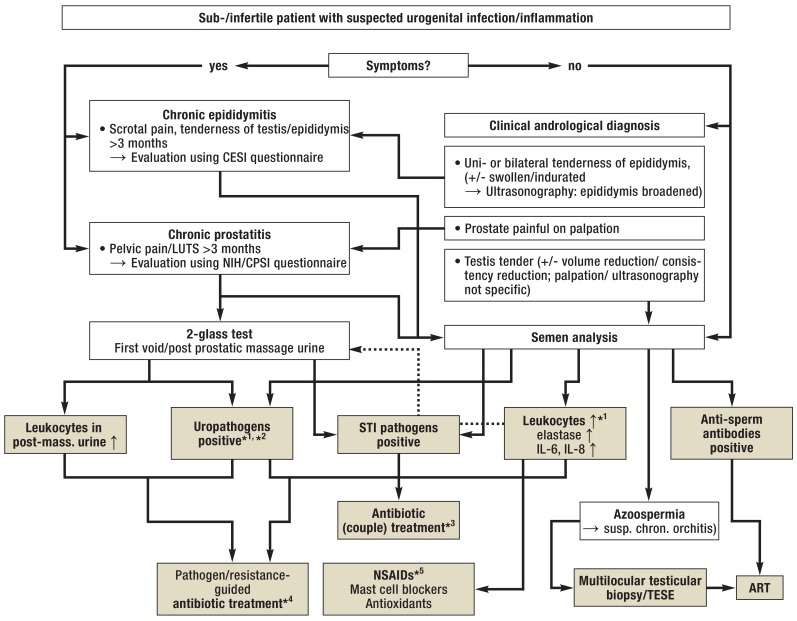
Figure 2.
Algorithm for the management of urogenital infections/inflammation in men with infertility
*1 In case of isolated findings: Confirmation by follow-up examinations; in case of increased levels of inflammatory markers in the ejaculate: advanced diagnostic assessment (2-glass test)
*2 Bacterial count in the ejaculate >103 CFU/mL, for urine samples see Figure 1
*3 Always antimicrobial therapy with co-treatment of partner according to guideline recommendations; cycle-adapted administration/contraception to prevent negative effects on early pregnancy (37, 38, e46)
*4 In case of symptom-free patients with positive inflammatory markers in the ejaculate (see Table 2): Treatment only in case of significant bacterial count and positive confirmatory test (see above; [e1, e2]); treatment duration 2 to 4 weeks, subject to pathogen/indication and medication (with the exemption of azithromycin; WARNING: current warnings for fluoroquinolones [e57]; tentative antibiotic treatment, e.g. in patients with non-significant bacterial count/isolated signs of inflammation, is not indicated). Because of the risk of negative effects on spermatogenesis/sperm function: Follow-up ejaculate testing or use of ART only after a treatment-free interval (several weeks) (cf. [22]).
*5 In case of sign of inflammation in the ejaculate (see Table 2), primarily without pathogen detection/after antibiotic treatment: Administration of nonsteroidal anti-inflammatory drugs (NSAIDs; e.g. diclofenac [100–150 mg/d] or equivalent medications, including cyclooxygenase-2 inhibitors; treatment duration 3–6 weeks); mast cell blocker, if needed; subsequent adjuvant treatment with antioxidants (e.g. vitamin E) optional.
ART, assisted reproductive technology (with TESE: in-vitro fertilization with intracytoplasmic sperm injection; IVF/ICSI); CESI, chronic epididymitis symptom index (e58); CPSI, chronic prostatitis symptom index (e40); CFU, colony-forming units; LUTS, lower urinary tract symptoms; NIH, National Institute of Health; STI, sexually transmitted infections; TESE, testicular sperm extraction; ↑, increased
The example of epididymitis/epididymo-orchitis shows that the development of persistent subfertility or infertility remains unpredictable even if acute antibiotic treatment achieves the eradication of the pathogen (13, 16, e47). Likewise, successful antibiotic therapy of MAGI does not necessarily result in an improved conception rate (e48, e49). Despite reports of negative effects of antibiotics, such as sulfonamides or tetracyclines, on spermatogenesis or sperm function, irreversible treatment-related damages appear unlikely (e50), but the induction of cellular and humoral immune responses in the genital tract seems to play an important role (box) (39). Thus, despite diagnostic limitations, treatment with nonsteroidal anti-inflammatory drugs should be considered in the presence of positive inflammatory parameters in the ejaculate (without pathogen or after antibiotic therapy), since several open studies reported improvements of ejaculate quality along with a decrease in white blood cell counts (e51, e52) (figure 2). Similar results were observed with mast cell blockers (e53, e54). Weak evidence supports the efficacy of adjuvant medications, such as antioxidants (e55). In patients with anti-sperm auto-antibodies, assisted reproductive techniques are preferred over immunosuppression with glucocorticosteroids (e8, e56).
(Re-)fertilizing surgical procedures are indicated with obstructions of the epididymis and along the course of the ductus deferens (microsurgical vasoepididymo- or vasovasostomy) as well as in central parts of the prostate (transurethral resection of the ejaculatory ducts) (20). The only option for patients with non-obstructive azoospermia as the result of epididymo-orchitis or orchitis is multilocular testicular biopsy for testicular sperm extraction (20, 40).
Conclusion
Adequate pathogen-specific antimicrobial therapy does not necessarily achieve restitution of sperm parameters or fertility. New diagnostic technologies, such as transcriptome and proteome analysis, are likely to open new perspectives which allow us to differentiate between inflammatory reaction due to persistent infection and post-infectious, primary non-infectious and autoimmune processes and to identify compartment-specific markers. These methods and the research into the underlying pathomechanisms are the foundation on which new treatment strategies can be established.
Key Messages.
Given the high prevalence of infectious-inflammatory conditions of the male genital tract and the associated risk of irreversible infertility, adequate testing for infection and inflammation should be part of the basic andrological examination in patients desiring to have children.
Both STI and typical uropathogens are of etiological relevance.
Cases with asymptomatic, primarily chronic disease make diagnosis difficult. Besides pathogen detection, diagnosis is based on increased white blood cell counts and/or elevated levels of inflammatory mediators in ejaculate, prostate secretions and urine samples.
With noninvasive, organ-specific diagnostic markers not yet available, diagnosing asymptomatic inflammatory reactions, especially in epididymis and testis, is challenging; testicular biopsy is the only reliable way to prove testicular inflammation.
The detection of pathogenic bacteria is an indication for antibiotic therapy aimed at eradicating the pathogen; however, this treatment cannot prevent permanent sperm parameter abnormalities/infertility in all cases, presumably due to the induction of persistent immunopathological processes in the genital tract.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Funding
The authors were funded as members of the focus ”Male Infertility along with Infection and Inflammation (MIBIE)” of the Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz (LOEWE) by the state of Hesse.
Footnotes
Conflict of interest statement
Prof. Schuppe has received lecture fees from Merck-Serono and Ferring.
PD Dr. Diemer holds shares in Lilly Deutschland GmbH. He received consultancy fees from AMS, Jenapharm, Bayer Vital, Cheplapharm, and Pfizer.
Prof. Wagenlehner received consultancy fees and study support (third-party funding) from Achaogen, Astellas, AstraZeneca, Bionorica, Calixa Pharmaceuticals, Cerexa Pharmaceuticals, Cubist Pharmaceuticals, LEO, MSD, Pfizer, Rempex Pharmaceuticals, Rosen Pharma, Shionogi, and Vifor Pharma.
The remaining authors declare that no conflict of interest exists.
References
- 1.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13 doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001356. e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Towards more objectivity in diagnosis and management of male fertility. Results of a World Health Organization multicenter study. Int J Androl. 1987;(7):1–53. [Google Scholar]
- 4.Tüttelmann F, Nieschlag E. Nosologie andrologischer Krankheitsbilder Andrologie - Grundlagen und Klinik der reproduktiven Gesundheit des Mannes. In: Nieschlag E, Behre HM, Nieschlag S, editors. Springer. 3. Heidelberg: 2009. pp. 90–96. [Google Scholar]
- 5.Weidner W, Pilatz A, Diemer T, Schuppe HC, Rusz A, Wagenlehner F. Male urogenital infections: impact of infection and inflammation on ejaculate parameters. World J Urol. 2013;31:717–723. doi: 10.1007/s00345-013-1082-7. [DOI] [PubMed] [Google Scholar]
- 6.Schiefer HG. Microbiology of male urethroadnexitis: diagnostic procedures and criteria for aetiologic classification. Andrologia. 1998;30(1):7–13. doi: 10.1111/j.1439-0272.1998.tb02820.x. [DOI] [PubMed] [Google Scholar]
- 7.Schuppe HC, Meinhardt A, Allam JP, et al. Chronic orchitis—a neglected cause of male infertility? Andrologia. 2008;40:84–91. doi: 10.1111/j.1439-0272.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 8.Haidl G, Allam JP, Schuppe HC. Chronic epididymitis—impact on semen parameters and therapeutic options. Andrologia. 2008;40:92–96. doi: 10.1111/j.1439-0272.2007.00819.x. [DOI] [PubMed] [Google Scholar]
- 9.Wolff H. The biologic significance of white blood cells in semen. Fertil Steril. 1995;63:1143–1157. doi: 10.1016/s0015-0282(16)57588-8. [DOI] [PubMed] [Google Scholar]
- 10.Rowe PJ, Comhaire FH, Hargreave TB, Mahmoud AMA. WHO manual for the standardized investigation, diagnosis and management of the infertile male. Cambridge: Cambridge University Press. 2000 [Google Scholar]
- 11.Chan PTK, Schlegel PN. Inflammatory conditions of the male excurrent ductal system. Part I. J Androl. 2002;23:453–460. [PubMed] [Google Scholar]
- 12.Wagenlehner FME, Naber KG, Bschleipfer T, Brähler E, Weidner W. Prostatitis and male pelvic pain syndrome: diagnosis and treatment. Dtsch Arztebl Int. 2009;106:175–183. doi: 10.3238/arztebl.2009.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusz A, Pilatz A, Wagenlehner F, et al. Influence of urogenital infections and inflammation on semen quality and male fertility. World J Urol. 2012;30:23–30. doi: 10.1007/s00345-011-0726-8. [DOI] [PubMed] [Google Scholar]
- 14.Henkel R, Ludwig M, Schuppe HC, Diemer T, Schill WB, Weidner W. Chronic pelvic pain syndrome/chronic prostatitis affect the acrosome reaction in human spermatozoa. World J Urol. 2006,;24:39–44. doi: 10.1007/s00345-005-0038-y. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson A, Rait G, Murray-Thomas T, Hughes G, Mercer CH, Cassell J. Management of epididymo-orchitis in primary care: results from a large UK primary care database. Br J Gen Pract. 2010;60:e407–e422. doi: 10.3399/bjgp10X532413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilatz A, Hossain H, Kaiser R, et al. Acute epididymitis revisited: impact of molecular diagnostics on etiology and contemporary guideline recommendations. European Urology. 2015;68:428–435. doi: 10.1016/j.eururo.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Pilatz A, Wagenlehner F, Bschleipfer T, et al. Acute epididymitis in ultrasound: results of a prospective study with baseline and follow-up investigations in 134 patients. Eur J Radiol. 2013;82:e762–e768. doi: 10.1016/j.ejrad.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 18.Schuppe HC, Bergmann M. Inflammatory conditions of the testis Atlas of the human testis. In: Jezek D, editor. Springer. London: 2013. [Google Scholar]
- 19.Bayasgalan G, Naranbat D, Radnaabazar J, Lhagvasuren T, Rowe PJ. Male infertility: risk factors in Mongolian men. Asian J Androl. 2004;6:305–311. [PubMed] [Google Scholar]
- 20.Diemer T, Hauptmann A, Weidner W. Treatment of azoospermia: surgical sperm retrieval (MESA, TESE, micro-TESE) Urologe A. 2011;50:38–46. doi: 10.1007/s00120-010-2442-1. [DOI] [PubMed] [Google Scholar]
- 21.Dohle GR. Inflammatory-associated obstructions of the male reproductive tract. Andrologia. 2003;35:321–324. [PubMed] [Google Scholar]
- 22.World Health Organization. WHO Press. 5th. Geneva: 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 23.Ahmed A, Bello A, Mbibu NH, et al. Epidemiological and aetiological factors of male infertility in northern Nigeria. Niger J Clin Pract. 2010;13:205–209. [PubMed] [Google Scholar]
- 24.Cottell E, Harrison RF, McCaffrey M, et al. Are seminal fluid microorganisms of significance or merely contaminants? Fertil Steril. 2000;74:465–470. doi: 10.1016/s0015-0282(00)00709-3. [DOI] [PubMed] [Google Scholar]
- 25.Domes T, Lo KC, Grober ED, Mullen JB, Mazzulli T, Jarvi K. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil Steril. 2012;97:1050–1055. doi: 10.1016/j.fertnstert.2012.01.124. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham KA, Beagley KW. Male genital tract chlamydial infection: implications for pathology and infertility. Biol Reprod. 2008;79:180–189. doi: 10.1095/biolreprod.108.067835. [DOI] [PubMed] [Google Scholar]
- 27.Foresta C, Noventa M, De Toni L, Gizzo S, Garolla A. HPV-DNA sperm infection and infertility: from a systematic literature review to a possible clinical management proposal. Andrology. 2015;3:163–173. doi: 10.1111/andr.284. [DOI] [PubMed] [Google Scholar]
- 28.Bezold G, Politch JA, Kiviat NB, et al. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. 2007;87:1087–1097. doi: 10.1016/j.fertnstert.2006.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fathy A, Chen SJ, Novak N, Schuppe HC, Haidl G, Allam JP. Differential leucocyte detection by flow cytometry improves the diagnosis of genital tract inflammation and identifies macrophages as pro-inflammatory cytokine-producing cells in human semen. Andrologia. 2014;46:1004–1012. doi: 10.1111/and.12188. [DOI] [PubMed] [Google Scholar]
- 30.Tremellen K, Tunc O. Macrophage activity in semen is significantly correlated with sperm quality in infertile men. Int J Androl. 2010;33:823–831. doi: 10.1111/j.1365-2605.2009.01037.x. [DOI] [PubMed] [Google Scholar]
- 31.Bohring C, Krause W. Immune infertility: towards a better understanding of sperm (auto)-immunity. The value of proteomic analysis. Hum Reprod. 2003;18:915–924. doi: 10.1093/humrep/deg207. [DOI] [PubMed] [Google Scholar]
- 32.Marconi M, Pilatz A, Wagenlehner FM, et al. Are antisperm antibodies really associated with proven chronic inflammatory and infectious diseases of the male reproductive tract? Eur Urol. 2009;56:708–715. doi: 10.1016/j.eururo.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21:56–83. doi: 10.1093/humupd/dmu042. [DOI] [PubMed] [Google Scholar]
- 34.Hochreiter WW, Duncan JL, Schaeffer AJ. Evaluation of the bacterial flora of the prostate using a 16S rRNA gene based polymerase chain reaction. J Urol. 2000;163:127–130. [PubMed] [Google Scholar]
- 35.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 36.Domann E, Hong G, Imirzalioglu C, et al. Culture-independent identification of pathogenic bacteria and polymicrobial infections in the genitourinary tract of renal transplant recipients. J Clin Microbiol. 2003;41:5500–5510. doi: 10.1128/JCM.41.12.5500-5510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. (RR-03) [PMC free article] [PubMed] [Google Scholar]
- 38.Wagenlehner FM, Brockmeyer NH, Discher T, Friese K, Wichelhaus TA. The presentation, diagnosis and treatment of sexually transmitted infections. Dtsch Arztebl Int. 2016;113:11–22. doi: 10.3238/arztebl.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedger M. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl. 2011;32:625–640. doi: 10.2164/jandrol.111.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marconi M, Keudel A, Diemer T, et al. Combined trifocal- and micro-TESE is the best technique for testicular sperm retrieval in “low chance” non-obstructive azoospermia. Eur Urol. 2012;62:713–719. doi: 10.1016/j.eururo.2012.03.004. [DOI] [PubMed] [Google Scholar]
- E1.Jungwirth A, Diemer T, Dohle GR, Kopa Z, Krausz C, Tournaye H. European Association of Urology (EAU) guidelines on male infertility. www.uroweb.org/guideline/male-infertility (last accessed on 31 March. 2017) doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- E2.Pickard R, Bartoletti R, Bjerklund-Johansen TE, et al. European Association of Urology (EAU) guidelines on urological infections 2016. www.uroweb.org/guideline/urological-infections (last accessed on 31 March 2017) [Google Scholar]
- E3.Krieger JN, Nyberg L Jr., Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- E4.Weidner W, Schiefer HG, Krauss H, Jantos C, Friedrich HJ, Altmannsberger M. Chronic prostatitis: a thorough search for etiologically involved microorganisms in 1,461 patients. Infection. 1991;19(3):119–125. doi: 10.1007/BF01643680. [DOI] [PubMed] [Google Scholar]
- E5.Motrich RD, Cuffini C, Oberti JP, Maccioni M, Rivero VE. Chlamydia trachomatis occurrence and its impact on sperm quality in chronic prostatitis patients. J Infect. 2006;53:175–183. doi: 10.1016/j.jinf.2005.11.007. [DOI] [PubMed] [Google Scholar]
- E6.Penna G, Mondaini N, Amuchastegui S, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51:524–533. doi: 10.1016/j.eururo.2006.07.016. [DOI] [PubMed] [Google Scholar]
- E7.Marconi M, Pilatz A, Wagenlehner F, et al. Impact of infection on the secretory capacity of the male accessory glands. Int Braz J Urol. 2009;35:299–309. doi: 10.1590/s1677-55382009000300006. [DOI] [PubMed] [Google Scholar]
- E8.Benelli A, Hossain H, Pilatz A, Weidner W. Prostatitis and it’s management. Eur Urol Suppl. 2017;16:132–137. [Google Scholar]
- E9.Fu W, Zhou Z, Liu S, et al. The effect of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) on semen parameters in human males: a systematic review and meta-analysis. PLoS One. 2014,;9 doi: 10.1371/journal.pone.0094991. e94991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Shang Y, Liu C, Cui D, Han G, Yi S. The effect of chronic bacterial prostatitis on semen quality in adult men: a meta-analysis of case-control studies. Sci Rep. 2014;4 doi: 10.1038/srep07233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Schagdarsurengin U, Teuchert LM, Hagenkoetter C, et al. Chronic prostatitis affects male reproductive health and associates with systemic and local epigenetic inactivation of CXCL12 receptor CXCR4. Urologia Int. 2017;98:89–101. doi: 10.1159/000452251. [DOI] [PubMed] [Google Scholar]
- E12.Ness RB, Markovic N, Carlson CL, Coughlin MT. Do men become infertile after having sexually transmitted urethritis? An epidemiologic examination. Fertil Steril. 1997;68:205–213. doi: 10.1016/s0015-0282(97)81502-6. [DOI] [PubMed] [Google Scholar]
- E13.Ochsendorf FR. Sexually transmitted infections: impact on male fertility. Andrologia. 2008;40:72–75. doi: 10.1111/j.1439-0272.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- E14.Pilatz A, Lochnit G, Karnati S, et al. Acute epididymitis induces alterations in sperm protein composition. Fertil Steril. 2014;101:1609–1617. doi: 10.1016/j.fertnstert.2014.03.011. [DOI] [PubMed] [Google Scholar]
- E15.Lang T, Dechant M, Sanchez V, et al. Structural and functional integrity of spermatozoa is compromised as a consequence of acute uropathogenic E coli associated epididymitis. Biol Reprod. 2013;89 doi: 10.1095/biolreprod.113.110379. [DOI] [PubMed] [Google Scholar]
- E16.Osegbe DN. Testicular function after unilateral bacterial epididymo-orchitis. Eur Urol. 1991;19:204–208. doi: 10.1159/000473620. [DOI] [PubMed] [Google Scholar]
- E17.Peng J, Yuan Y, Cui W, et al. Causes of suspected epididymal obstruction in Chinese men. Urology. 2012;80:1258–1261. doi: 10.1016/j.urology.2012.08.057. [DOI] [PubMed] [Google Scholar]
- E18.Berardinucci D, Zini A, Jarvi K. Outcome of microsurgical reconstruction in men with suspected epididymal obstruction. J Urol. 1998;159:831–834. [PubMed] [Google Scholar]
- E19.Han H, Liu S, Zhou XG, et al. Aetiology of obstructive azoospermia in Chinese infertility patients. Andrologia. 20164;8:761–764. doi: 10.1111/and.12509. [DOI] [PubMed] [Google Scholar]
- E20.Chen XF, Chen B, Liu W, et al. Microsurgical vasoepididymostomy for patients with infectious obstructive azoospermia: cause, outcome, and associated factors. Asian J Androl. 2016;18:759–762. doi: 10.4103/1008-682X.175095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Lunenfeld B, van Steirteghem A. Infertility in the third millennium: implications for the individual, family and society: condensed meeting report from the Bertarelli Foundation‘s second global conference. Hum Reprod Update. 2004;10:317–326. doi: 10.1093/humupd/dmh028. [DOI] [PubMed] [Google Scholar]
- E22.Virecoulon F, Wallet F, Fruchart-Flamenbaum A, et al. Bacterial flora of the lower male genital tract in patients consulting for infertility. Andrologia. 2005;37:160–165. doi: 10.1111/j.1439-0272.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- E23.Gimenes F, Medina FS, Abreu AL, et al. Sensitive simultaneous detection of seven sexually transmitted agents in semen by multiplex-PCR and of HPV by single PCR. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098862. e98862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Gdoura R, Kchaou W, Ammar-Keskes L, et al. Assessment of chlamydia trachomatis, ureaplasma urealyticum, ureaplasma parvum, mycoplasma hominis, and mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl. 2008;29:198–206. doi: 10.2164/jandrol.107.003566. [DOI] [PubMed] [Google Scholar]
- E25.Huang C, Zhu HL, Xu KR, Wang SY, Fan LQ, Zhu WB. Mycoplasma and ureaplasma infection and male infertility: a systematic review and meta-analysis. Andrology. 2015;3:809–816. doi: 10.1111/andr.12078. [DOI] [PubMed] [Google Scholar]
- E26.Eley A, Pacey AA, Galdiero M, Galdiero M, Galdiero F. Can chlamydia trachomatis directly damage your sperm? Lancet Infect Dis. 2005;5:53–57. doi: 10.1016/S1473-3099(04)01254-X. [DOI] [PubMed] [Google Scholar]
- E27.Henkel R, Maaß G, Hajimohammad M, et al. Urogenital inflammation: changes of leucocytes and ROS. Andrologia. 2003;35:309–313. [PubMed] [Google Scholar]
- E28.Aitken RJ, Baker MA. Oxidative stress, spermatozoa and leukocytic infiltration: relationships forged by the opposing forces of microbial invasion and the search for perfection. J Reprod Immunol. 2013;100:11–19. doi: 10.1016/j.jri.2013.06.005. [DOI] [PubMed] [Google Scholar]
- E29.Yanushpolsky EH, Politch JA, Hill JA, Anderson DJ. Is leukocytospermia clinically relevant? Fertil Steril. 1996;66:822–825. [PubMed] [Google Scholar]
- E30.Menkveld R, Huwe P, Ludwig M, Weidner W. Morphological sperm alterations in different types of prostatitis. Andrologia. 2003;35:288–293. [PubMed] [Google Scholar]
- E31.Zalata AA, Ahmed AH, Allamaneni SS, et al. Relationship between acrosin activity of human spermatozoa and oxidative stress. Asian J Androl. 2004;6:313–318. [PubMed] [Google Scholar]
- E32.Henkel R, Kierspel E, Stalf T, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–642. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- E33.Erenpreiss J, Hlevicka S, Zalkalns J, Erenpreisa J. Effect of leukocytospermia on sperm DNA integrity: a negative effect in abnormal semen samples. J Androl. 2002;23:717–723. [PubMed] [Google Scholar]
- E34.Kopa Z, Wenzel J, Papp GK, et al. Role of granulocyte elastase and interleukin-6 in the diagnosis of male genital tract inflammation. Andrologia. 2005;37:188–194. doi: 10.1111/j.1439-0272.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- E35.Pelliccione F, D‘Angeli A, Cordeschi G, et al. Seminal macrophages in ejaculates from men with couple infertility. Int J Androl. 2009;32:623–628. doi: 10.1111/j.1365-2605.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- E36.Haidl F, Haidl G, Oltermann I, Allam JP. Seminal parameters of chronic male genital inflammation are associated with disturbed sperm DNA integrity. Andrologia. 2015;47:464–469. doi: 10.1111/and.12408. [DOI] [PubMed] [Google Scholar]
- E37.Mazumdar S, Levine AS. Antisperm antibodies: etiology, pathogenesis, diagnosis, and treatment. Fertil Steril. 1998;70:799–810. doi: 10.1016/s0015-0282(98)00302-1. [DOI] [PubMed] [Google Scholar]
- E38.Chamley LW, Clarke GN. Antisperm antibodies and conception. Semin Immunopathol. 2007;29:169–184. doi: 10.1007/s00281-007-0075-2. [DOI] [PubMed] [Google Scholar]
- E39.Moretti E, Figura N, Collodel G, Ponzetto A. Can helicobacter pylori infection influence human reproduction? World J Gastroenterol. 2014;20:5567–5574. doi: 10.3748/wjg.v20.i19.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E40.Litwin MS, McNaughton-Collins M, Fowler FJ, et al. The National Institutes of Health Chronic Prostatitis Symptom Index: development and validation of a new outcome measure Chronic Prostatitis Collaborative Research Network. J Urol. 1999;162:369–375. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- E41.Pilatz A, Hudemann C, Wagenlehner F, et al. Seminal cytokines in urogenital disorders—is quantification useful? Urologe A. 2013;52:359–366. doi: 10.1007/s00120-013-3141-5. [DOI] [PubMed] [Google Scholar]
- E42.MIQ 10. Genitalinfektionen, Teil I Infektionen des weiblichen und des männlichen Genitaltraktes Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik. In: Podbielski A, Mauch H, Kniehl E, Herrmann M, Rüssmann H, editors. Urban & Fischer. 2. München: 2011. [Google Scholar]
- E43.MIQ 11b. Genitalinfektionen, Teil II Infektionserreger: Parasiten und Viren Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik. In: Podbielski A, Mauch H, Kniehl E, Herrmann M, Rüssmann H, editors. Urban & Fischer. 2. München: 2011. [Google Scholar]
- E44.MIQ 11a. Genitalinfektionen, Teil II Infektionserreger: Bakterien Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik. In: Podbielski A, Mauch H, Kniehl E, Herrmann M, Rüssmann H, editors. Urban & Fischer. 2. München: 2011. [Google Scholar]
- E45.MiQ 01. Podbielski A, Mauch H, Kniehl E, editors. Nukleinsäure-Amplifikationstechniken Qualitätsstandards in der mikrobiologischen Diagnostik. Urban & Fischer. (3) 2011 [Google Scholar]
- E46.Bremer V, Brockmeyer N, Coenenberg J, et al. Leitlinie STI/STD - Beratung, Diagnostik und Therapie. www.awmf.org/leitlinien/detail/ll/059-006.html (last accessed on 31 March 2017) [Google Scholar]
- E47.Vicari E. Effectiveness and limits of antimicrobial treatment on seminal leukocyte concentration and related reactive oxygen species production in patients with male accessory gland infection. Hum Reprod. 2000;15:2536–2544. doi: 10.1093/humrep/15.12.2536. [DOI] [PubMed] [Google Scholar]
- E48.Comhaire FH, Rowe PJ, Farley TM. The effect of doxycycline in infertile couples with male accessory gland infection: a double blind prospective study. Int J Androl. 1986;9:91–98. doi: 10.1111/j.1365-2605.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- E49.Hamada A, Agarwal A, Sharma R, French DB, Ragheb A, Sabanegh ES Jr. Empirical treatment of low-level leukocytospermia with doxycycline in male infertility patients. Urology. 2011;78:1320–1325. doi: 10.1016/j.urology.2011.08.062. [DOI] [PubMed] [Google Scholar]
- E50.Samplaski MK, Nangia AK. Adverse effects of common medications on male fertility. Nat Rev Urol. 2015;12:401–413. doi: 10.1038/nrurol.2015.145. [DOI] [PubMed] [Google Scholar]
- E51.Lackner JE, Herwig R, Schmidbauer J, et al. Correlation of leukocytospermia with clinical infection and the positive effect of anti-inflammatory treatment on semen quality. Fertil Steril. 2006;86:601–606. doi: 10.1016/j.fertnstert.2006.01.032. [DOI] [PubMed] [Google Scholar]
- E52.Gambera L, Serafini F, Morgante G, et al. Sperm quality and pregnancy rate after COX-2 inhibitor therapy of infertile males with abacterial leukocytospermia. Hum Reprod. 2007;22:1047–1051. doi: 10.1093/humrep/del490. [DOI] [PubMed] [Google Scholar]
- E53.Oliva A, Multigner L. Ketotifen improves sperm motility and sperm morphology in male patients with leukocytospermia and unexplained infertility. Fertil Steril. 2006;85:240–243. doi: 10.1016/j.fertnstert.2005.06.047. [DOI] [PubMed] [Google Scholar]
- E54.Azadi L, Abbasi H, Deemeh MR, et al. Zaditen (ketotifen), as mast cell blocker, improves sperm quality, chromatin integrity and pregnancy rate after varicocelectomy. Int J Androl. 2011;34:446–452. doi: 10.1111/j.1365-2605.2010.01112.x. 5 Pt 1. [DOI] [PubMed] [Google Scholar]
- E55.Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014;12 doi: 10.1002/14651858.CD007411.pub3. CD007411. [DOI] [PubMed] [Google Scholar]
- E56.Zini A, Lefebvre J, Kornitzer G, et al. Anti-sperm antibody levels are not related to fertilization or pregnancy rates after IVF or IVF/ICSI. J Reprod Immunol. 2011;88:80–84. doi: 10.1016/j.jri.2010.09.002. [DOI] [PubMed] [Google Scholar]
- E57.FDA Drug Safety Communication. FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. www.fda.gov/Drugs/DrugSafety/ucm511530.htm (last accessed on 31 March 2017) [Google Scholar]
- E58.Nickel JC. Chronic epididymitis: a practical approach to understanding and managing a difficult urologic enigma. Rev Urol. 2003;5:209–215. [PMC free article] [PubMed] [Google Scholar]