Key Points
Question
Does a single dose of dexamethasone in the absence of antibiotics provide symptom relief for acute sore throat in adults presenting to primary care?
Findings
In this randomized clinical trial including 565 adults, the proportion of participants in the dexamethasone group achieving complete symptom resolution at 24 hours was not significantly different from those taking placebo. But at 48 hours significantly more in the dexamethasone group experienced complete resolution than did those in the placebo group.
Meaning
Among adults presenting to primary care practices with acute sore throat, a single dose of oral dexamethasone did not increase the likelihood of symptom resolution at 24 hours but did increase the likelihood at 48 hours.
This randomized controlled trial compared the effects of a single dose of dexamethasone vs placebo on sore throat symptoms among adults with acute sore throat not requiring immediate antibiotics.
Abstract
Importance
Acute sore throat poses a significant burden on primary care and is a source of inappropriate antibiotic prescribing. Corticosteroids could be an alternative symptomatic treatment.
Objective
To assess the clinical effectiveness of oral corticosteroids for acute sore throat in the absence of antibiotics.
Design, Setting, and Participants
Double-blind, placebo-controlled randomized trial (April 2013-February 2015; 28-day follow-up completed April 2015) conducted in 42 family practices in South and West England, enrolled 576 adults recruited on the day of presentation to primary care with acute sore throat not requiring immediate antibiotic therapy.
Interventions
Single oral dose of 10 mg of dexamethasone (n = 293) or identical placebo (n = 283).
Main Outcomes and Measures
Primary: proportion of participants experiencing complete resolution of symptoms at 24 hours. Secondary: complete resolution at 48 hours, duration of moderately bad symptoms (based on a Likert scale, 0, normal; 6, as bad as it could be), visual analog symptom scales (0-100 mm; 0, no symptom to 100, worst imaginable), health care attendance, days missed from work or education, consumption of delayed antibiotics or other medications, adverse events.
Results
Among 565 eligible participants who were randomized (median age, 34 years [interquartile range, 26.0-45.5 year]; 75.2% women; 100% completed the intervention), 288 received dexamethasone; 277, placebo. At 24 hours, 65 participants (22.6%) in the dexamethasone group and 49 (17.7%) in the placebo group achieved complete resolution of symptoms, for a risk difference of 4.7% (95% CI, −1.8% to 11.2%) and a relative risk of 1.28 (95% CI; 0.92 to 1.78; P = .14). At 24 hours, participants receiving dexamethasone were not more likely than those receiving placebo to have complete symptom resolution. At 48 hours, 102 participants (35.4%) in the dexamethasone group vs 75 (27.1%) in the placebo group achieved complete resolution of symptoms, for a risk difference of 8.7% (95% CI, 1.2% to 16.2%) and a relative risk of 1.31 (95% CI, 1.02 to 1.68; P = .03). This difference also was observed in participants not offered delayed antibiotic prescription, for a risk difference of 10.3% (95% CI, 0.6% to 20.1%) and a relative risk of 1.37 (95% CI, 1.01 to 1.87; P = .046). There were no significant differences in any other secondary outcomes.
Results
Of 565 eligible randomized participants (median age, 34 years [interquartile range, 26.0-45.5 years]; 75.2% women; 100% completed the intervention), 288 received dexamethasone and 277, placebo. At 24 hours, participants receiving dexamethasone were not more likely than those receiving placebo to have complete symptom resolution. Results were similar among those who were not offered an antibiotic prescription and those who were offered a delayed antibiotic prescription. At 48 hours, more participants receiving dexamethasone than placebo had complete symptom resolution. This difference also was observed in patients not offered delayed antibiotics. There were no significant differences in any other secondary outcomes.
| Total/No. (%) | Risk Difference, % (95% CI) | RR (95% CI) | P Value | ||
|---|---|---|---|---|---|
| Dexamethasone (n = 288) |
Placebo (n = 277) |
||||
| Resolution of Symptoms at 24 h (Primary Outcome) | |||||
| Overall | 65 (22.6) | 49 (17.7) | 4.7 (−1.8 to 11.2) | 1.28 (0.92-1.78) | .14 |
| Resolution of Symptoms at 48 h (Secondary Outcomes) | |||||
| Overall | 102 (35.4) | 75 (27.1) | 8.7 (1.2 to 16.2) | 1.31 (1.02-1.68) | .03 |
| No antibiotic prescription | 65/173 (37.6) | 46/169 (27.2) | 10.3 (0.6 to 20.1) | 1.37 (1.01-1.87) | .046 |
Conclusions and Relevance
Among adults presenting to primary care with acute sore throat, a single dose of oral dexamethasone compared with placebo did not increase the proportion of patients with resolution of symptoms at 24 hours. However, there was a significant difference at 48 hours.
Trial Registration
isrctn.org Identifier: ISRCTN17435450
Introduction
Acute sore throat is one of the most common symptoms among patients presenting to primary care. Sore throats resulted in 92 million estimated visits by adults to primary care practices and emergency departments in the United States between 1997 and 2010, averaging 6.6 million annually; with unnecessary antibiotic prescribing costs of at least $500 million. Antibiotics are prescribed at 60% of UK primary care sore throat consultations, and the trend is not decreasing despite the low risks of suppurative complications, limited symptomatic benefit, and national guidelines advising against prescriptions. There is a need to find alternative strategies that reduce symptoms, reduce the burden of acute illness, and reduce antibiotic consumption.
Corticosteroids inhibit transcription of proinflammatory mediators in airway endothelial cells, responsible for pharyngeal inflammation and symptoms of pain, and are beneficial in other upper respiratory tract infections such as acute sinusitis and croup. Short courses of oral steroids have been shown to be safe, in the absence of contraindications. A systematic review reported that participants with a sore throat, taking a single dose of steroid, were 3 times more likely to experience complete resolution within 24 hours. However, antibiotics were prescribed to participants in both steroid and placebo groups in all trials and only 1 trial recruited participants from primary care. Therefore, evidence for corticosteroids for sore throat in primary care, in the absence of antibiotics, is still lacking.
The primary objective of the TOAST (Treatment Options without Antibiotics for Sore Throat) trial was to investigate, among adults 18 years or older with acute sore throat not requiring immediate antibiotic therapy, whether a single dose of oral dexamethasone compared with placebo, increased resolution of symptoms 24 hours after consultation.
Methods
Study Design and Participants
This multicenter, individually randomized, double-blind, placebo-controlled, parallel group trial involved 42 general practice (GP) clinics in South and West England. Recruitment started on April 12, 2013, and completed on February 27, 2015. Participants were followed up for 28 days after randomization (follow-up completed on April 16, 2015). The research protocol (Supplement 1) was approved by the National Research Ethics Committee South Central. Written informed consent was obtained for all participants.
Full details of the trial design, inclusion and exclusion criteria are available (Supplement 2). Briefly, included participants were aged 18 years or older, presenting to a primary care clinician (general practitioner or practice nurse) with acute symptoms (onset within the last 7 days) of sore throat and odynophagia (pain on swallowing) that the clinician judged to be caused by an infection but that did not need immediate antibiotics, and who had the capacity and willingness to give consent and complete the trial paperwork. Exclusion criteria included recent (<1 month) use of inhaled or oral corticosteroids or adenotonsillectomy, recent use (<14 days) of antibiotics, and a clear alternative diagnosis (eg, pneumonia).
Randomization, Concealment, and Masking
Randomization (1:1) was stratified by study center and by receipt of a delayed antibiotic prescription. A randomization list using block randomization with variable blocks of size 2, 4, or 6 was computer generated by an independent statistician for manufacturing. Each site was allocated to hold 2 sets (for those who were offered antibiotic prescription and those not) of packs of 2 to 3 blocks of blinded prerandomized medication. Participants in the intervention group received a single dose of 10 mg of oral dexamethasone as 5 × 2 mg of dexamethasone tablets overencapsulated by a single-size, 1-gelatin capsule. The control group received lactose overencapsulated with an identical capsule. Overencapsulation for both intervention and placebo was performed by Nottingham University Hospital NHS Trust Pharmacy. Participants, clinicians, and researchers were unaware of the allocation of the patient and remained unaware until trial completion.
Procedures
Participants were recruited on the day of presentation to their GP practice with symptoms of sore throat and pain on swallowing. Baseline clinical features and a throat swab were obtained. Rapid streptococcal antigen tests were not available to clinicians. The clinician was free to decide to offer no antibiotic prescription or a delayed antibiotic prescription with their usual instructions; typically to fill the prescription and take the antibiotics if symptoms had not improved in 48 hours. Participants were randomized and observed to take the trial medication. Primary outcome data were collected at 24 and 48 hours via text message or telephone, from days 0 through 7 using a patient symptom diary, and a review of the patient’s electronic medical records at 1 month.
Outcome Measures
The primary outcome was the complete resolution of sore throat at 24 hours as reported by the patient by either text message or telephone contact regardless of whether they were offered a prescription for delayed antibiotics. Secondary outcomes were (1) complete resolution of sore throat at 48 hours; (2) duration of moderately bad symptoms recorded by validated 7-day symptom diary, based on a Likert scale for which 0 indicates normal; 1, very little problem; 2, slight problem; 3, moderately bad; 4, bad; 5, very bad; 6, as bad as it could be; (3) patient-reported time to onset of pain relief and time to complete symptom resolution; (4) change in ratings of sore throat pain, pain on swallowing, and difficulty swallowing on a 0- to 100-mm visual analog scale, for which 0 mm indicates no pain and 100 mm, the worst pain imaginable; (5) consumption of delayed antibiotic prescription for sore throat; (6) time missed from work or education; (7) attendance or telephone contact at any health care facility (including GP clinic, urgent care clinic, emergency department, or hospital admission) within 28 days with symptoms or complications associated with sore throat (defined as direct suppurative complications or presentation with sore throat symptoms); and (8) use of over-the-counter medications and prescription medications in the first 7 days. The eMethods in Supplement 2 detail deviations from or amendments to our protocol since the inception of the trial and the reasons for this. Data were collected concurrently during the trial to inform a cost-effectiveness analysis that will be reported in a future article.
Statistical Analysis
Sample Size
Based on a previous systematic review, the minimum absolute increase in resolution of pain at 24 hours with corticosteroids was 18% (11% vs 29%) and the average was 27%. To achieve an 18% increase in resolution with 90% power and α level of 5% , required 226 participants.
Based on previous data, antibiotic prescriptions are offered to an estimated 50% of participants presenting with sore throat; therefore, in order to recruit at least 226 participants who were not offered antibiotics, 566 participants were recruited, allowing for a 20% loss to follow-up.
Statistical Methods
The primary outcome was analyzed using a log-binomial regression model adjusted for center and receipt of a delayed prescription for antibiotics. Complete resolution of sore throat by 48 hours was analyzed in the same way.
Time to onset of pain relief and to complete pain resolution was analyzed using a Cox regression model adjusting for receipt of a delayed antibiotic prescription and for health center. Participants who did not provide a valid time in their symptom diary—a time without am or pm specified and outside the feasible range of times for the study—were excluded from this analysis. The duration of moderately bad or worse symptoms was analyzed using a negative binomial model adjusting for center and delayed prescription at baseline and including completed diary days as an offset. Time missed from work or education was analyzed using linear regression adjusted for center and delayed antibiotic prescription. Reconsultation—by visiting or calling—at a health care facility, including an emergency department, after-hours primary care facility, or in-hours primary care facility, with symptoms or complications of sore throat; use of over-the-counter and prescription medications; and uptake of delayed antibiotic prescriptions were analyzed using the same methods as the primary outcome. Sore throat complications were defined a priori as direct suppurative complications such as quinsy and paratonsillar abscess; a post hoc definition that included otitis media, sinusitis, or cellulitis was also analyzed.
For visual analog scale data, the area under the curve (AUC) was calculated using the trapezoidal rule using estimates from a mixed-effects repeated-measures model adjusting for symptom at baseline, health care center, and delayed antibiotic prescription with a treatment and time interaction. The AUCs between the 2 groups were then compared using a t test. A linear regression model was fitted to the change from baseline to day 1 and to day 2 of the symptom diary.
All available data from participants who withdrew from the study were included. For the analysis of resolution of symptoms at 24 and 48 hours, participants were classified as having no resolution of symptoms if data were missing. Two-sided P values <.05 were considered significant. There was no adjustment to P values to account for multiple comparisons. All secondary outcomes are considered exploratory. Analyses were conducted using Stata software version 13 (Stata Corp).
Methods for Subgroup Analyses
The analyses of the primary and secondary outcomes were repeated in the delayed-antibiotic and no-antibiotic prescription subgroups because the study was powered for analysis within these subgroups separately. Additional subgroup analyses were prespecified in the statistical analysis plan prior to unblinding of the study data and used interaction tests. The effect of sore throat severity at baseline (defined using Centor score with a cutoff of <3 and ≥3) was assessed by including an interaction term in the log binominal regression model between treatment group and patient severity. The effect of rescue medication use (any patient-reported analgesic medication) in the first 48 hours and presence of streptococcus on throat swabs were analyzed in the same way.
Methods for Sensitivity Analyses
For the analysis of resolution of symptoms at 24 and 48 hours, it was assumed that participants who did not provide a response did not have resolution of their sore throat symptoms. Sensitivity analyses, for which missing data were assumed resolved, replaced by multiple imputation and excluded (a complete case analysis) were performed.
Many participants experienced onset of pain relief or complete resolution of pain but did not report the time to onset of pain relief or time to complete resolution of pain. A sensitivity analysis that substituted the missing time with the time in the day that the diary was completed on the day of recruitment was performed. Thus, time was computed in whole days for these participants.
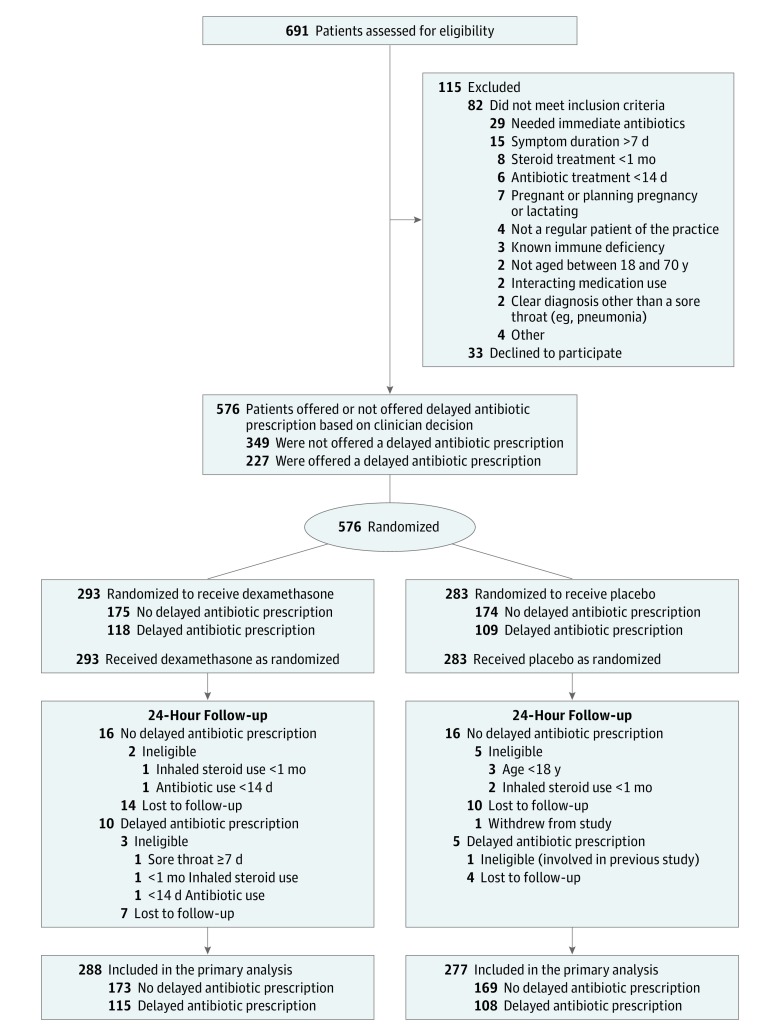
Results
Of the 691 patients treated at 42 clinics assessed for eligibility, 115 were excluded; 82 did not meet the inclusion criteria and 33 declined (Figure 1). Two hundred twenty-seven recruited participants (39.5%) were offered a delayed antibiotic prescription. Of the 576 participants (75.2% women) recruited, 11 (1.9%) were excluded after they had fully participated because they were found to be ineligible based on a review of their clinical notes, so they were not included in the primary analysis. The 36 participants (6%) who had no information for the primary outcome were included in the analysis as having no resolution of symptoms. The median age of eligible participants was 33.7 years (interquartile range [IQR], 26.3-45.8 years) in the dexamethasone group and 34.3 years (IQR, 26.0-45.0 years) in the placebo group, 76.8% were employed or in education. Baseline characteristics of eligible participants were similar between groups (Table 1). eTable 1 in Supplement 2 details clinician scores of patient symptoms at baseline, which were also similar between groups.
Figure 1. Flow of Patients Through the Treatment Options Without Antibiotics for Sore Throat (TOAST) in Adults Randomized Clinical Trial (N = 293).
Table 1. Baseline Characteristics of Eligible Patients.
| All Patients | Antibiotic Prescription | |||||
|---|---|---|---|---|---|---|
| Not Offered | Delayed Prescription Offered | |||||
| Dexamethasone (n = 288) |
Placebo (n = 277) |
Dexamethasone (n = 173) |
Placebo (n = 169) |
Dexamethasone (n = 115) |
Placebo (n = 108) |
|
| Age, median (IQR), y | 33.7 (26.3-45.8) | 34.3 (26.0-45.0) | 36.7 (27.0-48.3) | 37.0 (27.5-48.1) | 31.8 (24.0-43.2) | 31.9 (24.5-40.3) |
| Men, No. (%) | 67 (23.3) | 73 (26.4) | 42 (24.3) | 46 (27.2) | 25 (21.7) | 27 (25.0) |
| Working or in school, No. (%) | 217 (75.3) | 217 (78.3) | 122 (70.5) | 134 (79.3) | 95 (82.6) | 83 (76.9) |
| Smoker, No. (%) | 52 (18.1) | 51 (18.4) | 28 (16.2) | 26 (15.4) | 24 (20.9) | 25 (23.2) |
| Study center, No. (%) | ||||||
| Bristol | 79 (27.4) | 72 (26.0) | 47 (27.2) | 46 (27.2) | 32 (27.8) | 26 (24.1) |
| Oxford | 143 (49.7) | 139 (50.2) | 90 (52.0) | 89 (52.7) | 53 (46.1) | 50 (46.3) |
| Southampton | 66 (22.9) | 66 (23.8) | 36 (20.8) | 34 (20.1) | 30 (26.1) | 32 (29.6) |
| Duration of sore throat, mean (SD), d | 3.86 (1.67) | 3.91 (1.79) | 3.99 (1.68) | 4.14 (1.85) | 3.7 (1.60) | 3.5 (1.60) |
| Duration of pain on swallowing, median (IQR), d | 3 (2-4) | 3 (2-4) | 3 (2-4) | 3 (2-5) | 3 (2-4) | 3 (2-4) |
| Pharyngeal inflammation, No. (%) | 254 (88.2) | 248 (89.5) | 144 (83.2) | 148 (87.6) | 110 (95.8) | 100 (92.6) |
| Self-report of moderate or severe sore throat, No. (%) | 277 (96.2) | 268 (96.8) | 165 (95.4) | 164 (97.0) | 112 (97.4) | 104 (96.3) |
| Self-report of moderate or severe difficulty swallowing, No. (%) | 198 (68.9) | 196 (70.8) | 114 (65.9) | 113 (66.9) | 84 (73.0) | 83 (76.9) |
| Tonsils visible on examination, No. (%) | 201 (69.8) | 190 (68.6) | 113 (65.3) | 110 (65.1) | 88 (76.5) | 80 (74.1) |
| Purulent tonsils, No. (%) | 30 (10.4) | 31 (11.2) | 11 (6.4) | 5 (3.0) | 19 (16.5) | 26 (24.1) |
| Temperature, mean (SD), °C | 36.8 (0.5) | 36.8 (0.6) | 36.7 (0.5) | 36.8 (0.5) | 36.9 (0.5) | 36.8 (0.7) |
| Centor score, No. (%)a | ||||||
| ≥3 | 41 (14.2) | 40 (14.4) | 14 (8.1) | 12 (7.1) | 27 (23.5) | 28 (25.9) |
| ≥4 | 7 (2.4) | 7 (2.5) | 3 (1.7) | 0 | 4 (3.5) | 7 (6.5) |
| Throat swab culture positive for streptococcus, No. (%) | ||||||
| Group A | 30 (11.5) | 33 (13.6) | 17 (11.0) | 16 (11.0) | 13 (12.3) | 17 (17.5) |
| Group C | 7 (2.7) | 10 (4.1) | 4 (2.6) | 3 (2.1) | 3 (2.8) | 7 (7.2) |
| Group G | 1 (0.4) | 3 (1.2) | 1 (0.7) | 1 (0.7) | 0 (0) | 2 (2.1) |
| Total, No. (%)b | 38 (14.6) | 46 (19.0) | 22 (14.3) | 20 (13.8) | 16 (15.1) | 26 (26.8) |
Abbreviation: IQR, interquartile range.
The Centor score (range, 0-4) uses the following criteria: presence of tonsillar exudate, tender anterior cervical lymphadenopathy or lymphadenitis, history of fever, and absence of cough. A score of 3 or 4 indicates a higher likelihood of a bacterial sore throat.
Sixty-three swabs were lost in transit (28 dexamethasone, 35 placebo); thus, the percentage of swabs positive for streptococcus that were received is reported herein.
Complete Resolution of Sore Throat
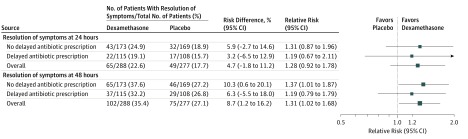
For the primary outcome, a single dose of oral dexamethasone did not significantly increase the proportion of participants reporting complete resolution of their sore throat at 24 hours. Sixty-five participants (22.6%) of 288 receiving dexamethasone and 49 (17.7%) of 277 receiving placebo reported complete resolution within 24 hours, for a relative risk (RR) of 1.28 (95% CI, 0.92 to 1.78, P = .14) and a risk difference of 4.7% (95% CI, −1.8% to 11.2%; Figure 2).
Figure 2. Effect of Dexamethasone Without Immediate Antibiotics vs Placebo on Acute Sore Throat in Adults.
Outcomes at 24 hours did not differ among the participants who were not offered delayed antibiotic prescription vs those who were offered a delayed antibiotic prescription. Among patients not offered a delayed antibiotic prescription, 43 participants (24.9%) of the 173 in the dexamethasone group and 32 (18.9%) of the 169 in the placebo group experienced complete resolution of sore throat for an RR of 1.31 (95% CI, 0.87 to 1.96; P = .19) and a risk difference of 5.9% (95% CI, −2.7% to 14.6%). Similarly among those who were offered a delayed antibiotic prescription, 22 participants (19.1%) of 115 in the dexamethasone group and 17 (15.7%) of 108 in the placebo group experienced complete resolution of sore throat for an RR of 1.19 (95% CI, 0.67 to 2.11; P = .55) and a risk difference of 3.2% (95% CI, −6.5% to 12.9%). These findings were consistent in all sensitivity analyses (eTables 2 through 4 in Supplement 2).
By 48 hours, 102 participants (35.4%) in the dexamethasone group vs 75 (27.1%) in the placebo group experienced complete resolution of symptoms, for an RR of 1.31 (95% CI, 1.02 to 1.68; P = .03) and a risk difference of 8.7% (95% CI, 1.2% to 16.2%; number needed to treat, 12; 95% CI, 7 to 146) (Figure 2). Among participants who were not offered an antibiotic prescription, 65 (37.6%) in the dexamethasone group and 46 (27.2%) in the placebo group experienced complete sore throat resolution for an RR of 1.37 (95% CI, 1.01 to 1.87; P = .046) and risk difference of 10.3% (95% CI, 0.6% to 20.1%; number needed to treat, 10; 95% CI, 6 to 234), but no significant difference was observed for participants offered a delayed prescription: 37 (32.2%) in the dexamethasone group and 29 (26.8%) in the placebo group for an RR of 1.19 (95% CI, 0.79 to 1.79; P = .41) and a risk difference of 6.3% (−5.5% to 18.0%). Findings were consistent in all sensitivity analyses (eTables 2 through 4 in Supplement 2).
Reported use of over-the-counter medications that contained analgesics (topical and oral) for symptom relief in the first 48 hours was similar for both groups (dexamethasone, 148 patients [77.1%] of 192; placebo, 153 patients [78.9%] of 194) and was not significantly different for participants reporting complete resolution of their symptoms at 48 hours (interaction effect, 1.05; 95% CI, 0.61 to 1.79; P = .86).
Neither severity of sore throat at baseline, with an interaction effect at 24 hours of 0.59 (95% CI, 0.16 to 2.14; P = .42) and at 48 hours of 0.52 (95% CI, 0.25 to 1.11; P = .09), nor the presence of streptococcus on throat swabs, with an interaction effect at 24 hours of 1.93 (95% CI, 0.37 to 5.51; P = .22) and at 48 hours of 1.13 (95% CI, 0.52 to 2.45; P = .75), were moderators of group differences.
Patient Symptom Outcome Measures
The number of days of moderately bad symptoms was not significantly different between the 2 groups either overall or within the subgroups who were offered no antibiotic prescription or a delayed antibiotic prescription (eTable 5 in Supplement 2). Ratings of sore throat pain, pain on swallowing, and difficulty swallowing by using a visual analog scale showed no significant differences in change from baseline to day 1, change from baseline to day 2, or in the AUC summary statistic, either overall or for either subgroup (eTable 6 in Supplement 2).
There were no significant differences between groups in time to onset of pain relief or time to complete resolution of pain, either overall or in the subgroups that were offered or not offered a delayed antibiotic prescription (Table 2). A sensitivity analysis that included all the participants who reported onset or complete resolution of symptoms but who did not report a valid time had similar findings (eTable 7 in Supplement 2).
Table 2. Median Time to Onset of Pain Relief and Median Time to Complete Resolution of Symptomsa.
| Dexamethasone, Median (IQR), h | No. of Patients | Placebo, Median (IQR), h | No. of Patients | Hazard Ratio (95%CI)b | P Value | |
|---|---|---|---|---|---|---|
| Time to Onset of Pain Relief | ||||||
| Full cohort | 27.5 (21.0-44.5) | 129 | 27.0 (21.4-45.8) | 102 | 1.106 (0.850-1.440) | .45 |
| No antibiotic prescription | 27.0 (19.7-37.0) | 78 | 25.8 (20.9-44.7) | 64 | 1.251 (0.887-1.763) | .20 |
| Delayed antibiotic prescription | 30.7(22.8-62.9) | 51 | 34.2 (23.3-54.1) | 38 | 0.843 (0.543-1.307) | .45 |
| Time to Complete Symptom Resolution | ||||||
| Full cohort | 65.8 (41.0-105.9) | 101 | 60.0 (39.8-92.3) | 94 | 1.043 (0.781-1.393) | .78 |
| No antibiotic prescription | 67.0 (40.2-96.9 | 61 | 54.0 (35.8-91.8) | 60 | 0.996 (0.687-1.442) | .98 |
| Delayed antibiotic prescription | 64.4 (43.4-116.8) | 40 | 67.6 (41.5-96.4) | 34 | 1.251 (0.771-2.031) | .37 |
Abbreviation: IQR, interquartile range.
Two participants (1 from each treatment group) were excluded from the time-to-event analysis for having time-to-event values outside the feasible range, ie, <0 or >200 h. Participants who did not complete any days of their symptom diary were excluded from the analysis (n = 174 of 565) alongside all who did not provide their time of pain relief nor whether the time was am or pm (n = 152 of 565). All who recorded “NA” in answer to “Has your sore throat become less painful in the last 24 hours?” are assumed to have missing data and excluded from the analysis (n = 6 of 565).
Hazard ratios are from proportional hazards models adjusted for center and delayed antibiotic prescription (where applicable).
Effect on Health Care Use, Medication Use, and Productivity
Of the 157 participants given a delayed prescription who reported this outcome, 37 (46.8%) of 79 in the dexamethasone group reported consuming the delayed prescription within 7 days vs 44 (56.4%) of 78 in the placebo group (RR, 0.83; 95% CI, 0.61 to 1.13; P = .23; risk difference, −9.4%; 95% CI, −25.0% to 6.1%). Post hoc analysis showed that 3 participants (2.5%) in the dexamethasone group and 5 (4.0%) in the placebo group who were not offered a delayed prescription reported taking antibiotics in the first 48 hours. There were no significant differences between groups in the use of pain relief medications (topical and oral), antibiotics for sore throat, or antibiotics for other conditions and no differences in the proportion of participants missing any time away from work or education (Table 3).
Table 3. Time Away From Work or School, Medication Use, and Health Contactsa.
| Total No. of Patients | No. (%) of Patients | Risk Difference, % (95% CI) | Relative Risk (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| Dexamethasone | Placebo | |||||
| Reconsultation at Health Care Facility | ||||||
| Overall | 565 | 31/288 (10.8) | 20/277 (7.2) | 3.3 (−0.6 to 7.3) | 1.48 (0.87 to 2.53) | .15 |
| No antibiotic prescription | 342 | 23/173 (13.3) | 16/169 (9.5) | 3.5 (−3.1 to 10.0) | 1.40 (0.77 to 2.56) | .27 |
| Delayed antibiotic prescription | 223 | 8/115 (7.0) | 4/108 (3.7) | 3.2 (−1.8 to 8.2) | 1.73 (0.54 to 5.50) | .36 |
| Patients Missing Work or Education | ||||||
| Overall | 411 | 61/202 (30.2) | 73/209 (34.9) | −4.2 (−13.0 to 4.7) | 0.86 (0.65 to 1.13) | .26 |
| No antibiotic prescription | 254 | 32/123 (26.0) | 39/131 (29.8) | −3.1 (−14.0 to 7.8) | 0.87 (0.59 to 1.30) | .50 |
| Delayed antibiotic prescription | 157 | 29/79 (36.7) | 34/78 (45.6) | −6.2 (−21.3 to 8.9) | 0.84 (0.58 to 1.23) | .37 |
| Antibiotics Consumed for Sore Throat | ||||||
| Overall | 417 | 50/206 (24.3) | 59/211 (28.0) | −2.4 (−9.6 to 4.7) | 0.83 (0.62 to 1.11) | .22 |
| No antibiotic prescription | 260 | 16/127 (12.6) | 17/133 (12.8) | −0.2 (−8.3 to 7.9) | 0.99 (0.52 to 1.87) | .97 |
| Delayed antibiotic prescription | 157 | 34/79 (43.0) | 42/78 (53.8) | −10.8 (−26.4 to 4.7) | 0.80 (0.58 to 1.11) | .18 |
| Antibiotics for Other Reasons | ||||||
| Overall | 417 | 5/206 (2.4) | 9/211 (4.3) | −1.7 (−5.8 to 2.3) | 0.58 (0.20 to 1.70) | .32 |
| No antibiotic prescription | 260 | 5/127 (3.9) | 6/133 (4.5) | 0.1 (−4.5 to 4.8) | 0.86 (0.27 to 2.73) | .79 |
| Delayed antibiotic prescription | 157 | 0/79 | 3/78 (3.8) | |||
| Pain Relief Preparationsb | ||||||
| Overall | 417 | 147/206 (71.4) | 154/211 (73.0) | −1.3 (−9.8 to 7.1) | 0.98 (0.87 to 1.10) | .77 |
| No antibiotic prescription | 260 | 88/127 (69.3) | 98/133 (73.7) | −4.4 (−15.3 to 6.6) | 0.94 (0.81 to 1.09) | .42 |
| Delayed antibiotic prescription | 157 | 59/79 (74.7) | 56/78 (71.8) | 3.2 (−10.1 to 16.6) | 1.05 (0.88 to 1.25) | .62 |
Log-binomial model adjusted for center and delayed antibiotic prescription.
Analysis includes only participants who provided follow-up information on the name of antibiotics used, including lozenges, linctus, and sprays that contain a local anesthetic or anti-inflammatory.
Dexamethasone also did not result in a significant difference in the mean number of hours missed from work or education (mean difference, 0.24 hours longer in dexamethasone group; 95% CI, −2.14 to 2.61; P = .85). There were no significant differences in the number of participants reconsulting at emergency departments, urgent care, or their usual GP clinic for symptoms or suppurative complications of sore throat (Table 3). In a post hoc analysis, the addition of otitis media, sinusitis, or cellulitis to the complications of the sore throat definition did not alter outcomes by treatment group among all patients (RR, 1.39; 95% CI, 0.86 to 2.24; P = .18; risk difference, 3.0%; 95% CI, −1.5% to 7.5%; among those who were not offered an antibiotic prescription (RR, 1.32; 95% CI, 0.77 to 2.26; P = .31; risk difference, 3.2%; 95% CI, −3.9% to 10.4%); or among those who were offered a delayed antibiotic prescription (RR, 1.60; 95% CI, 0.56 to 4.60; P = .38, risk difference, 2.9%; 95% CI, −2.9% to 8.6%).
Serious Adverse Events
Of the 5 serious adverse events, 2 occurred among participants in the dexamethasone group, 1 of which was considered related to the trial (hospital admission with parapharyngeal abscess), and 3 serious adverse events occurred in the placebo group (hospital admission with peritonsillar abscess, hospital admission with severe tonsillitis, and hospital admission with pneumonia, with subsequent death after hospital discharge).
Discussion
For patients seeking primary care for a sore throat that is not judged to require immediate antibiotics, no significant benefit of dexamethasone on complete resolution of symptoms at 24 hours was found. However, at 48 hours dexamethasone resulted in a significant increase in the proportion of participants reporting complete resolution, requiring an average of 12 patients to be treated for 1 additional patient to experience symptom resolution. A similar effect was evident in the subgroup of participants not offered a delayed antibiotic prescription. No significant differences were observed in participant-reported duration of moderately bad symptoms, visual analog scale ratings, time to onset and time to complete resolution of symptoms, or time away from school or work when comparing dexamethasone with placebo groups. Three study participants, 2 receiving placebo and 1 dexamethasone, required hospitalization related to sore throat, which is a complication rate similar to previous observational studies of sore throat treated in primary care. Reattendance with symptoms or complications of sore throat was similar in both groups.
This trial found an RR of 1.28 (95% CI, 0.92 to 1.78) for complete resolution at 24 hours, which was lower than effect sizes reported in previous studies of use of oral corticosteroids in sore throat, which reported RRs ranging from 1.67 to 4.41. However, all previous trials gave antibiotics to both corticosteroid and placebo treatment groups. It is possible that there is a synergistic effect of corticosteroids and antibiotics when treating sore throat, such as that suggested for acute sinusitis, for which oral corticosteroid monotherapy was ineffective for symptom relief in a primary care–based trial, but corticosteroids in addition to antibiotics offer evidence of benefit.
A second explanation for the smaller effect size in this study is that corticosteroids are most beneficial for severe sore throat. Two previous randomized trials have reported outcomes following a single dose of oral dexamethasone on a subgroup of children (n = 154) who tested negative for the rapid group A streptococcus antigen, to whom antibiotics were offered only after throat swab results confirmed diagnosis or reassessment by a clinician. The trial, which included children with a minimum subjective pain score, demonstrated that symptom duration and pain intensity were reduced, whereas the trial in which 24% of children initially reported mild pain found no effect on symptom duration.
Because this study aimed to evaluate dexamethasone in the absence of antibiotics, participants requiring immediate antibiotics, likely to have the most severe sore throat, were excluded, resulting in a different population from trials based in emergency departments. Forty-two percent of adults involved in an emergency department trial had purulent tonsils compared with 10% of those participating in the current trial. More severe symptoms might correlate with more severe inflammation and therefore the anti-inflammatory benefits of corticosteroids would be greater in this patient group. In this trial the test of interaction between severity or streptococcal presence and outcome revealed no significant effect, but this analysis was limited by the small number of participants in these groups (14.5% and 15.2%, respectively).
To our knowledge, this is the first trial to evaluate the benefits of oral corticosteroids for acute sore throat in a primary care setting—where the majority of patients with sore throat seek treatment—and to evaluate the benefits of oral corticosteroids in the absence of antibiotics. Participant adherence with medication was 100%, and the primary outcome measure was collected from 93.6% of participants. Participant allocation by treatment group was well balanced at baseline. The characteristics of the population recruited were similar to those reported in a previous large observational cohort of participants presenting to primary care with sore throat.
This study has several limitations. This trial may have recruited a less severely ill patient group by excluding those requiring immediate antibiotics. Children were also excluded. Three trials involving children have demonstrated a significant benefit of corticosteroids when administered in combination with antibiotics. The return rate of symptom diaries or follow-up questionnaires was 75%, similar to return rates for other sore throat trials in primary care; however, poor completion rates of diaries resulted in lower rates for 2 outcome measures. The primary outcome was not validated, but it is widely used in trials and systematic reviews of acute sore throat interventions and was chosen to ensure a timely and high response rate, key in evaluating response in acute infection. Other symptom measures used in the study have been validated in primary care infectious disease research. In addition, this study was underpowered to detect a modest effect on the primary outcome or to detect a difference in adverse effect profiles. A larger study may have identified a statistically significant difference between the treatments.
Uncertainty remains about the role of oral corticosteroids for patients presenting in primary care with sore throat. Corticosteroids may have clinical benefit in addition to antibiotics for severe sore throat, for example, to reduce hospital admissions of those patients who are unable to swallow fluids or medications. There have been no trials of corticosteroid use involving these patient groups. A recent systematic review of 3 small trials suggests early but unsustained symptomatic benefit in peritonsillar abscess with 1 trial demonstrating a reduction in duration of hospitalization.
Adverse effects of corticosteroids may be more significant in patients with comorbidities, such as diabetes and heart failure, who were excluded from this trial. Given that patients could receive a larger cumulative dose of corticosteroids through multiple primary care visits with sore throat, the potential longer-term effects of increased steroid consumption (eg, osteoporosis, hypertension) should also be considered.
Conclusions
Among adults presenting to primary care practices with acute sore throat, a single dose of oral dexamethasone compared with placebo did not increase the proportion of patients with resolution of symptoms at 24 hours. However, there was a significant difference at 48 hours.
Trial Protocol
eMethods. Protocol and statistical analysis plan amendments
eTable 1. Baseline clinician rated symptoms
eTable 2. Sensitivity analysis for complete sore throat resolution at 24 and 48 hours: missing responses assumed to be completely resolved
eTable 3. Sensitivity analysis for complete sore throat resolution at 24 and 48 hours: multiple imputation of missing data
eTable 4. Sensitivity analysis for complete sore throat resolution at 24 and 48 hours: complete case analysis
eTable 5. Duration of moderately bad symptoms recorded by validated symptom diary over the 7 days from treatment onset present for all participants who returned a complete symptom diary
eTable 6. Change in ratings of sore throat pain, difficulty swallowing, and pain on swallowing by visual analogue scale
eTable 7. Sensitivity analysis for time to onset of pain relief and time to complete resolution of pain
References
- 1.Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulliford M, Latinovic R, Charlton J, Little P, van Staa T, Ashworth M. Selective decrease in consultations and antibiotic prescribing for acute respiratory tract infections in UK primary care up to 2006. J Public Health (Oxf). 2009;31(4):512-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997-2010. JAMA Intern Med. 2014;174(1):138-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulliford MC, Dregan A, Moore MV, et al. . Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open. 2014;4(10):e006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawker JI, Smith S, Smith GE, et al. . Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995-2011: analysis of a large database of primary care consultations. J Antimicrob Chemother. 2014;69(12):3423-3430. [DOI] [PubMed] [Google Scholar]
- 6.Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;11(11):CD000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Respiratory Tract Infections—Antibiotic Prescribing: Prescribing of Antibiotics for Self-Limiting Respiratory Tract Infections in Adults and Children in Primary Care. London, England: National Institute for Health and Clinical Excellence; 2008. [PubMed] [Google Scholar]
- 8.Pelucchi C, Grigoryan L, Galeone C, et al. . Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012;18(suppl 1):1-28. [DOI] [PubMed] [Google Scholar]
- 9.Mygind N, Nielsen LP, Hoffmann HJ, et al. . Mode of action of intranasal corticosteroids. J Allergy Clin Immunol. 2001;108(1)(suppl):S16-S25. [DOI] [PubMed] [Google Scholar]
- 10.Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013;12(12):CD005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell KF, Liang Y, O’Gorman K, Johnson DW, Klassen TP. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955. [DOI] [PubMed] [Google Scholar]
- 12.Weinberger M. Safety of oral corticosteroids. Eur J Respir Dis Suppl. 1982;122:243-251. [PubMed] [Google Scholar]
- 13.Hayward G, Thompson MJ, Perera R, Glasziou PP, Del Mar CB, Heneghan CJ. Corticosteroids as standalone or add-on treatment for sore throat. Cochrane Database Syst Rev. 2012;10:CD008268. [DOI] [PubMed] [Google Scholar]
- 14.Watson L, Little P, Moore M, Warner G, Williamson I. Validation study of a diary for use in acute lower respiratory tract infection. Fam Pract. 2001;18(5):553-554. [DOI] [PubMed] [Google Scholar]
- 15.Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239-246. [DOI] [PubMed] [Google Scholar]
- 16.Little P, Stuart B, Hobbs FD, et al. ; DESCARTE Investigators . Antibiotic prescription strategies for acute sore throat: a prospective observational cohort study. Lancet Infect Dis. 2014;14(3):213-219. [DOI] [PubMed] [Google Scholar]
- 17.Venekamp RP, Bonten MJ, Rovers MM, Verheij TJ, Sachs AP. Systemic corticosteroid monotherapy for clinically diagnosed acute rhinosinusitis: a randomized controlled trial. CMAJ. 2012;184(14):E751-E757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venekamp RP, Thompson MJ, Hayward G, et al. . Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev. 2014;3(3):CD008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olympia RP, Khine H, Avner JR. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Arch Pediatr Adolesc Med. 2005;159(3):278-282. [DOI] [PubMed] [Google Scholar]
- 20.Bulloch B, Kabani A, Tenenbein M. Oral dexamethasone for the treatment of pain in children with acute pharyngitis: a randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2003;41(5):601-608. [DOI] [PubMed] [Google Scholar]
- 21.Wei JL, Kasperbauer JL, Weaver AL, Boggust AJ. Efficacy of single-dose dexamethasone as adjuvant therapy for acute pharyngitis. Laryngoscope. 2002;112(1):87-93. [DOI] [PubMed] [Google Scholar]
- 22.Niland ML, Bonsu BK, Nuss KE, Goodman DG. A pilot study of 1 vs 3 days of dexamethasone as add-on therapy in children with streptococcal pharyngitis. Pediatr Infect Dis J. 2006;25(6):477-481. [DOI] [PubMed] [Google Scholar]
- 23.Little P, Hobbs FD, Moore M, et al. ; PRISM Investigators . Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: randomised controlled trial of PRISM (primary care streptococcal management). BMJ. 2013;347:f5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiderman A, Yaphe J, Bregman J, Zemel T, Furst AL. Adjuvant prednisone therapy in pharyngitis: a randomised controlled trial from general practice. Br J Gen Pract. 2005;55(512):218-221. [PMC free article] [PubMed] [Google Scholar]
- 25.Tasar A, Yanturali S, Topacoglu H, Ersoy G, Unverir P, Sarikaya S. Clinical efficacy of dexamethasone for acute exudative pharyngitis. J Emerg Med. 2008;35(4):363-367. [DOI] [PubMed] [Google Scholar]
- 26.Bird JH, Biggs TC, King EV. Controversies in the management of acute tonsillitis: an evidence-based review. Clin Otolaryngol. 2014;39(6):368-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YJ, Jeong YM, Lee HS, Hwang SH. The efficacy of corticosteroids in the treatment of peritonsillar abscess: a meta-analysis. Clin Exp Otorhinolaryngol. 2016;9(2):89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Protocol and statistical analysis plan amendments
eTable 1. Baseline clinician rated symptoms
eTable 2. Sensitivity analysis for complete sore throat resolution at 24 and 48 hours: missing responses assumed to be completely resolved
eTable 3. Sensitivity analysis for complete sore throat resolution at 24 and 48 hours: multiple imputation of missing data
eTable 4. Sensitivity analysis for complete sore throat resolution at 24 and 48 hours: complete case analysis
eTable 5. Duration of moderately bad symptoms recorded by validated symptom diary over the 7 days from treatment onset present for all participants who returned a complete symptom diary
eTable 6. Change in ratings of sore throat pain, difficulty swallowing, and pain on swallowing by visual analogue scale
eTable 7. Sensitivity analysis for time to onset of pain relief and time to complete resolution of pain