Key Points
Question
Is the use of spinal manipulative therapy in the management of acute (≤6 weeks) low back pain associated with improvements in pain or function?
Findings
In this systematic review and meta-analysis of 26 randomized clinical trials, spinal manipulative therapy was associated with statistically significant benefits in both pain and function, of on average modest magnitude, at up to 6 weeks. Minor transient adverse events such as increased pain, muscle stiffness, and headache were reported in more than half of patients in the large case series.
Meaning
Among patients with acute low back pain, spinal manipulative therapy was associated with modest improvements in pain and function and with transient minor musculoskeletal harms.
Abstract
Importance
Acute low back pain is common and spinal manipulative therapy (SMT) is a treatment option. Randomized clinical trials (RCTs) and meta-analyses have reported different conclusions about the effectiveness of SMT.
Objective
To systematically review studies of the effectiveness and harms of SMT for acute (≤6 weeks) low back pain.
Data Sources
Search of MEDLINE, Cochrane Database of Systematic Reviews, EMBASE, and Current Nursing and Allied Health Literature from January 1, 2011, through February 6, 2017, as well as identified systematic reviews and RCTs, for RCTs of adults with low back pain treated in ambulatory settings with SMT compared with sham or alternative treatments, and that measured pain or function outcomes for up to 6 weeks. Observational studies were included to assess harms.
Data Extraction and Synthesis
Data extraction was done in duplicate. Study quality was assessed using the Cochrane Back and Neck (CBN) Risk of Bias tool. This tool has 11 items in the following domains: randomization, concealment, baseline differences, blinding (patient), blinding (care provider [care provider is a specific quality metric used by the CBN Risk of Bias tool]), blinding (outcome), co-interventions, compliance, dropouts, timing, and intention to treat. Prior research has shown the CBN Risk of Bias tool identifies studies at an increased risk of bias using a threshold of 5 or 6 as a summary score. The evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria.
Main Outcomes and Measures
Pain (measured by either the 100-mm visual analog scale, 11-point numeric rating scale, or other numeric pain scale), function (measured by the 24-point Roland Morris Disability Questionnaire or Oswestry Disability Index [range, 0-100]), or any harms measured within 6 weeks.
Findings
Of 26 eligible RCTs identified, 15 RCTs (1699 patients) provided moderate-quality evidence that SMT has a statistically significant association with improvements in pain (pooled mean improvement in the 100-mm visual analog pain scale, −9.95 [95% CI, −15.6 to −4.3]). Twelve RCTs (1381 patients) produced moderate-quality evidence that SMT has a statistically significant association with improvements in function (pooled mean effect size, −0.39 [95% CI, −0.71 to −0.07]). Heterogeneity was not explained by type of clinician performing SMT, type of manipulation, study quality, or whether SMT was given alone or as part of a package of therapies. No RCT reported any serious adverse event. Minor transient adverse events such as increased pain, muscle stiffness, and headache were reported 50% to 67% of the time in large case series of patients treated with SMT.
Conclusions and Relevance
Among patients with acute low back pain, spinal manipulative therapy was associated with modest improvements in pain and function at up to 6 weeks, with transient minor musculoskeletal harms. However, heterogeneity in study results was large.
This meta-analysis summarizes the effectiveness and harms of spinal manipulative therapy for acute (≤6 weeks) low back pain.
Introduction
Back pain is among the most common symptoms prompting patients to seek care. Lifetime prevalence estimates of low back pain exceed 50%.1
Many treatments are used for acute back pain. None of the therapies for acute back pain has been established as superior to others. Treatments include analgesics, muscle relaxants, exercises, physical therapy modalities, heat, spinal manipulative therapy (SMT), and others.2
There have been multiple systematic reviews on spinal manipulation. A 2003 review concluded SMT was associated with statistically significant benefits compared with a sham manipulation, but not compared with other effective treatments for acute low back pain.3 Since then, the most recent Cochrane review on the subject concluded that SMT was not associated with statistically significant benefits compared with other interventions or sham SMT,4 but another Cochrane review of “combined chiropractic interventions” (which included SMT as part of the intervention) concluded the opposite.5 A third review assessed SMT for patients with back pain of less than 3 months duration and concluded it was associated with benefits compared with placebo treatment, no treatments, or massage,6 and a fourth review concluded “the efficacy of manipulation for patients with acute or chronic low back pain remains unconvincing.”7
As new trials continue to be published,8,9,10,11,12,13 and given these differences in conclusions among studies, this review was conducted to provide updated estimates of the effectiveness and harms associated with spinal manipulation compared with other nonmanipulative therapies for adults with acute low back pain.
Methods
This systematic review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. A formal protocol was developed and submitted to PROSPERO (CRD42015017916). This review is part of a larger review commissioned by the Department of Veterans Affairs.14
Data Sources and Searches
MEDLINE, the Cochrane Database of Systematic Reviews, EMBASE, and Cumulative Index of Nursing and Allied Health Literature were searched (for the full search strategy, see the eAppendix in the Supplement). The initial search was for existing systematic reviews (from January 1, 2011, through May 7, 2015). References were retrieved from these. An updated search was performed (through February 6, 2017) to identify recently published studies, both systematic reviews and randomized clinical trials (RCTs). Experts were consulted for additional studies.
Study Selection
Titles, abstracts, and full-text articles were screened independently by 2 reviewers (N.M.P. and P.G.S.), with discrepancies discussed with the research group. We used the following inclusion criteria. Participants were adults with acute (defined as ≤6 weeks) lower-back pain. Studies that included a subset of patients with sciatica or leg pain were eligible, but studies exclusively about patients with sciatica were excluded. Studies of patients with chronic back pain were excluded, as were studies in which we could not determine the duration of pain. If studies included patients with longer durations of pain, we included them if they presented stratified results or if the majority of patients had pain for up to 6 weeks duration. The intervention was spinal manipulation by any type of clinician. Studies in which SMT was given alone or as part of a “package” of therapies were included. Chiropractic care was considered as including SMT.15 The comparator included other forms of management for acute pain, such as analgesics, exercises, physical therapy. Sham-controlled studies were included. The primary outcomes were pain and functional status. Studies had to report at least 1 outcome within 6 weeks to be eligible. Only studies in ambulatory or outpatient settings were included; studies in hospital settings were excluded. Only RCTs were eligible for assessing benefits. Both RCTs plus observational studies were used for assessing harms.
Data Extraction and Quality Assessment
Data were extracted by 2 reviewers (N.M.P. and P.G.S.), and discrepancies were reconciled after discussion. Data abstracted included the authors’ description of the SMT, type of professional performing the treatment, co-interventions, whether SMT was provided alone or as part of a package, whether patients were selected as more likely to respond to SMT or unselected, data on the outcomes listed above, and data needed to complete the Cochrane Back and Neck (CBN; formerly the Cochrane Back Review Group) Risk of Bias assessment.
Based on the authors’ description of the SMT provided, studies were categorized as using a thrust or nonthrust technique. Thrust was defined as high-velocity, low-amplitude technique, such as “a short-lever, high-velocity thrust.”16 Nonthrust was defined as other manual therapies that were self-described as SMT but did not meet the definition of thrust, such as a study where “most participants had several low-velocity mobilization techniques.”12 In 1 case, an original author was contacted to clarify whether the intervention was thrust or nonthrust.
Outcome data were extracted by the project statistician from results identified by the research team clinicians and checked by a reviewer (P.G.S.). We assessed outcomes at 2 time points. Based on a prior review on use of epidural steroids, outcomes at 2 weeks or less were defined as immediate-term and outcomes from 3 to 6 weeks were defined as short-term.17
For continuous outcomes, the sample size, mean, and SD were extracted for each SMT group and comparator group within each trial. For count data, the number and percentage of patients with an event were extracted.
Study quality was assessed using the CBN Risk of Bias tool. This tool has 11 items in the following domains: randomization, concealment, baseline differences, blinding (patient), blinding (care provider [blinding of the care provider is a specific quality metric used by the CBN Risk of Bias tool]), blinding (outcome), co-interventions, compliance, dropouts, timing, and intention to treat. Prior research has shown the CBN Risk of Bias tool to identify studies at an increased risk of bias using a threshold of 5 or 6 as a summary score.18
Main Outcome Measures
The a priori primary outcomes were pain, function, quality of life, and harms. Secondary outcomes included opiate use, disability claims, return to work, and health care utilization. Data were sparse for quality of life and all secondary outcomes and are not reported here. These data are included in our Evidence Report.14 Outcomes had to be measured within 6 weeks.
Data Synthesis and Analysis
Studies were pooled within outcome measures and 95% CIs were constructed: studies using a 100-mm visual analog scale (VAS), 11-point numeric rating scale (NRS), or other numeric pain scale were pooled by converting all outcomes to a 0-to-100 measure (using the appropriate multiplier); studies reporting the Roland-Morris Low Back Pain and Disability Questionnaire (RMDQ; range, 0-24) and studies reporting the Oswestry Disability Index (ODI; range, 0-100) were pooled as a functional outcome using an effect-size approach. Studies reporting none of these were not pooled, but discussed narratively.
Random-effects meta-analyses were conducted using the Hartung-Knapp-Sidik-Jonkman method.19,20 Tests of heterogeneity were performed using the I2 statistic.21 All meta-analyses were conducted with Stata statistical software (StataCorp), version 12.0,22 and R (R Foundation), version 3.2.2. The Begg rank correlation23 and Egger regression asymmetry test24 were used to examine publication bias. To further explore possible sources of heterogeneity (ie, timing, outcome, type of practitioner, and type of manipulation), bivariate meta-regressions were conducted.
The meta-analyses were organized based on 2 follow-up times and the 2 outcomes. Outcomes for 2 studies25,26 were in the period between immediate-term and short-term outcomes; they were closest to the definition of immediate-term, so they were classified in the immediate-term group. Within these 4 groupings the intervention was assessed in comparison with control interventions classified as either sham SMT or all other therapies.4 This classification was justified because many of the comparison interventions were intended to be inactive (ie, detuned diathermy, light massage) or of uncertain effectiveness (usual medical care); and for those comparisons for which the other treatment was expected to be effective, the existing RCTs and systematic reviews indicate the benefit was small, at best.27,28,29 Studies comparing SMT with sham SMT were not pooled with studies comparing SMT with other therapies. Studies were included in each pooled analysis only once.
An a priori analysis considered 3 potential sources of heterogeneity: the comparison group, the outcome, and the timing of the outcome. In addition, 3 post hoc hypotheses were developed to test possible explanations for observed heterogeneity: by type of manipulation, comparing thrust techniques with nonthrust techniques; by the types of patients enrolled (selected or not selected); and by study quality, comparing higher-quality trials with lower-quality trials.
The Intervention
Spinal manipulative therapy is a term that encompasses a large variation in the type of manual therapy. Direct evidence that different kinds of manipulation have different efficacy is lacking. However, among patients meeting a clinical prediction rule for SMT, thrust-type manipulation may be more effective than nonthrust-type manipulation.30 Therefore the intervention used in each study was classified as either thrust-type SMT or nonthrust-type SMT. Seven studies were not included because either the SMT could not be classified8,11,31 or the studies could not be included in the pooled analyses.9,32,33,34
The Patients
Each study was examined to see if the authors reported having selected patients based on certain a priori criteria they believed made patients more likely to benefit from SMT.
Study Quality
Using the CBN Risk of Bias tool,18 studies were classified as higher quality (6-11 points) or lower quality (0-5 points), and results were compared between the 2 quality categories.
Rating the Body of Evidence
The evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria, which uses the domains of study design limitations, inconsistency, indirectness, and imprecision in results.35
Results
Description of the Evidence
From the searches for systematic reviews and new trials, 40 articles were identified relevant to effectiveness, and 8 additional articles relevant to adverse events (Figure 1). Twenty-six RCTs were included in the data synthesis for effectiveness (for details, see the evidence table in eTable 1 in the Supplement).8,9,10,11,12,13,16,25,26,31,32,33,34,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51 Of the 14 articles not included in the analyses, 3 focused on the subpopulation of patients with sciatica,52,53,54 2 were only relevant to clinical prediction rule evaluations,30,55 2 did not have the necessary outcome data,56,57 and 1 had a unique patient population (pelvic joint dysfunction) that we judged as clinically dissimilar to the other studies.58 The findings of the final 6 publications have been reported in another included publication.11,36,38,59,60,61
Figure 1. Literature Search Flow Chart.
CINAHL indicates Cumulative Index of Nursing and Allied Health Literature.
SMT was provided by physical therapists in 13 studies, chiropractors in 7 studies, medical doctors in 5 studies, and osteopathic physicians in 3 studies. These were not mutually exclusive, because some studies employed multiple disciplines.
The most commonly met quality criteria were the timing criterion (25 studies) and the appropriate randomization criterion (17 studies). None of the studies met the criterion for blinding of providers. Only 4 studies met the criterion for blinding of patients. Twelve studies were classified as high quality and 14 studies were classified as low quality (eTable 2 in the Supplement).
Association With Pain
Twenty studies reported pain outcomes for comparisons of SMT with other treatments, 17 immediate-term and 18 short-term outcomes. Fifteen studies reported outcomes using a 100-mm VAS, or 11-point NRS, or other numeric pain scale and were included in pooled analyses (1699 patients). As differences in relative effectiveness between immediate-term and short-term outcomes were small, only the pooled result with the largest number of patients is presented in Figure 2 (short-term pain, with 12 RCTs and 1421 patients). The overall random-effects pooled estimate for short-term pain was a mean effect of −9.95 mm (95% CI, −15.6 to −4.3), favoring treatments with SMT compared with other treatments. (Figure 2).8,10,12,16,31,36,37,39,46,48,50,51,59,60,61 There was heterogeneity in the results (I2 = 67%). For immediate-term pain, the overall random-effects pooled estimate was −9.76 mm (95% CI, −17.0 to −2.5) compared with other treatments. A sensitivity analysis substituting the alternative comparison group (back school instead of diathermy) in the 3-group study by Bergquist-Ullman and Larsson39 yielded a result similar to the main analysis (−8.22 mm [95% CI, −14.7 to −1.7]). Two studies of SMT vs sham SMT reported nonstatistically significant results. There was no evidence of publication bias in the overall pooled result, with a Begg rank correlation of 0.92 and an Eggar test P value of .58.
Figure 2. Short-term Pain Outcomes in Randomized Clinical Trials of Effectiveness of Spinal Manipulative Therapy for Acute Low Back Pain (N = 1421).
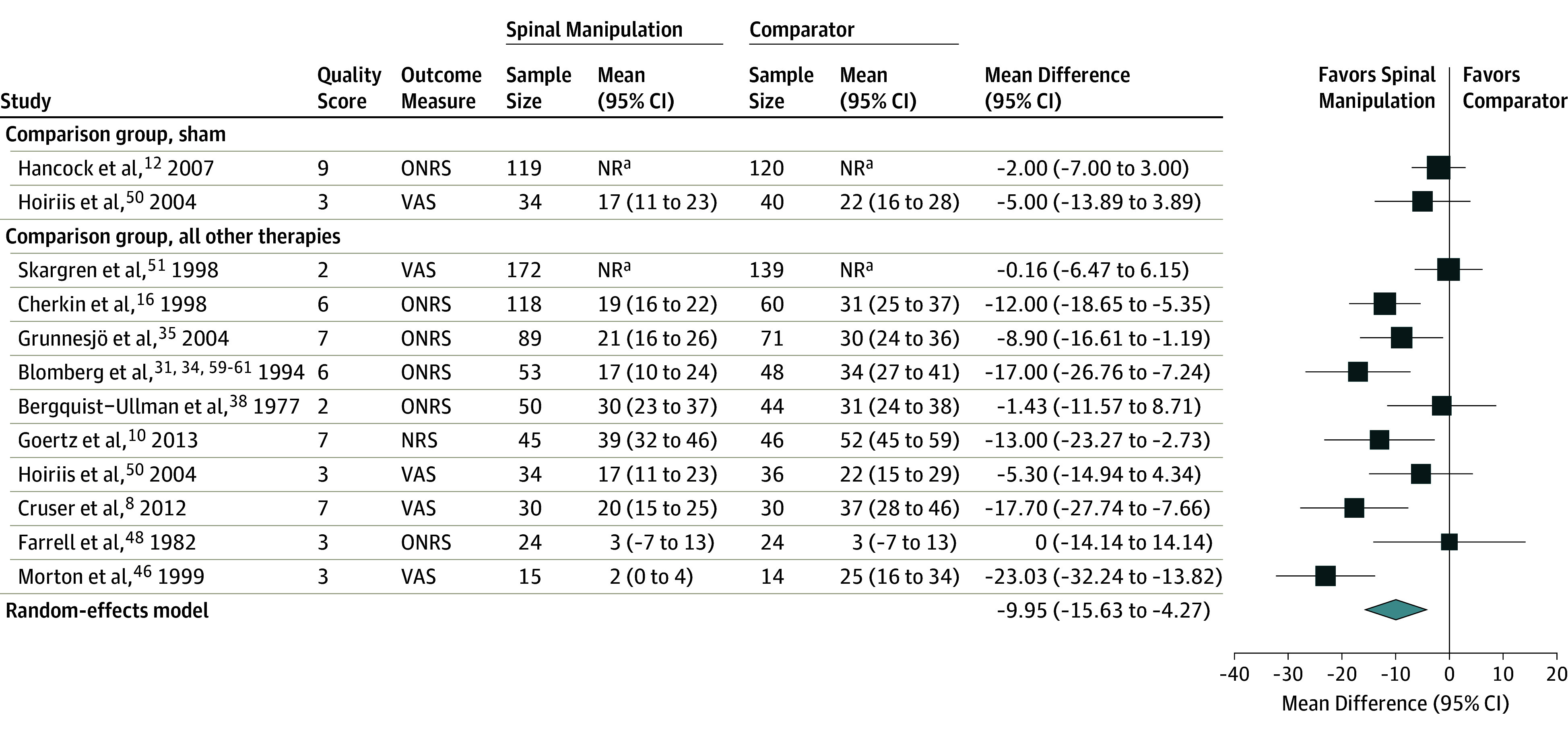
NR indicates not reported; NRS, numeric rating scale (range, 0-10; converted to 0-100); ONRS, other numeric rating scale (including ranges of 0-10, 0-70, and 0-100; all converted to 0 to 100); VAS, visual analog scale (ranges of, 0-100 mm or 1-10, converted to 0-100). Size of the data markers represent weight based on the randomized meta-analysis. A high score indicates worse pain. Quality score uses the Cochrane Back and Neck Risk of Bias tool (range, 0-11).
aOutcome data not reported by group, only between-group data reported.
Studies Not Included in the Pooled Analysis
Five studies reported outcomes that were not measured with a 100-mm VAS, NRS, or other numeric pain scale.25,40,41,42,43 All were old studies (30-40 years ago), and all but 1 were judged as low quality. Two of the 5 studies concluded SMT had an effect41,42 and 3 studies concluded it did not.25,40,43
Association With Function Outcomes
A total of 17 studies reported functional outcomes for comparisons of SMT with other therapies, 15 immediate-term and 11 short-term outcomes. Eight studies measured function using the RMDQ, and 4 studies used the ODI (1381 patients). As differences in relative effectiveness between immediate-term function and short-term function were small, only the pooled result with the largest number of patients (short-term function, with 8 RCTs and 1049 patients) is presented in the Figure 3. The overall random-effects pooled estimate for short-term function was an effect size of −0.39 (95% CI, −0.71 to −0.07) favoring treatment with SMT (Figure 3)8,10,12,16,46,50,51. There was heterogeneity in the results (I2 = 72%). For immediate-term function, the overall random-effects pooled estimate was an effect size of −0.24 (95% CI, −0.55 to 0.08). A sensitivity analysis substituting the alternative comparison group (physical therapy instead of booklet) in the 3-group study by Cherkin and colleagues16 yielded a result similar to the main analysis (−0.32 [95% CI, −0.65 to 0.02]). Two small studies of SMT vs sham SMT reported small- to medium-sized effects but neither was statistically significant. There was no evidence of publication bias, with a Begg rank correlation of 0.85 and an Eggar test P value of .10.
Figure 3. Short-term Function Outcomes in Randomized Clinical Trials of Effectiveness of Spinal Manipulative Therapy for Acute Low Back Pain (N = 1049).
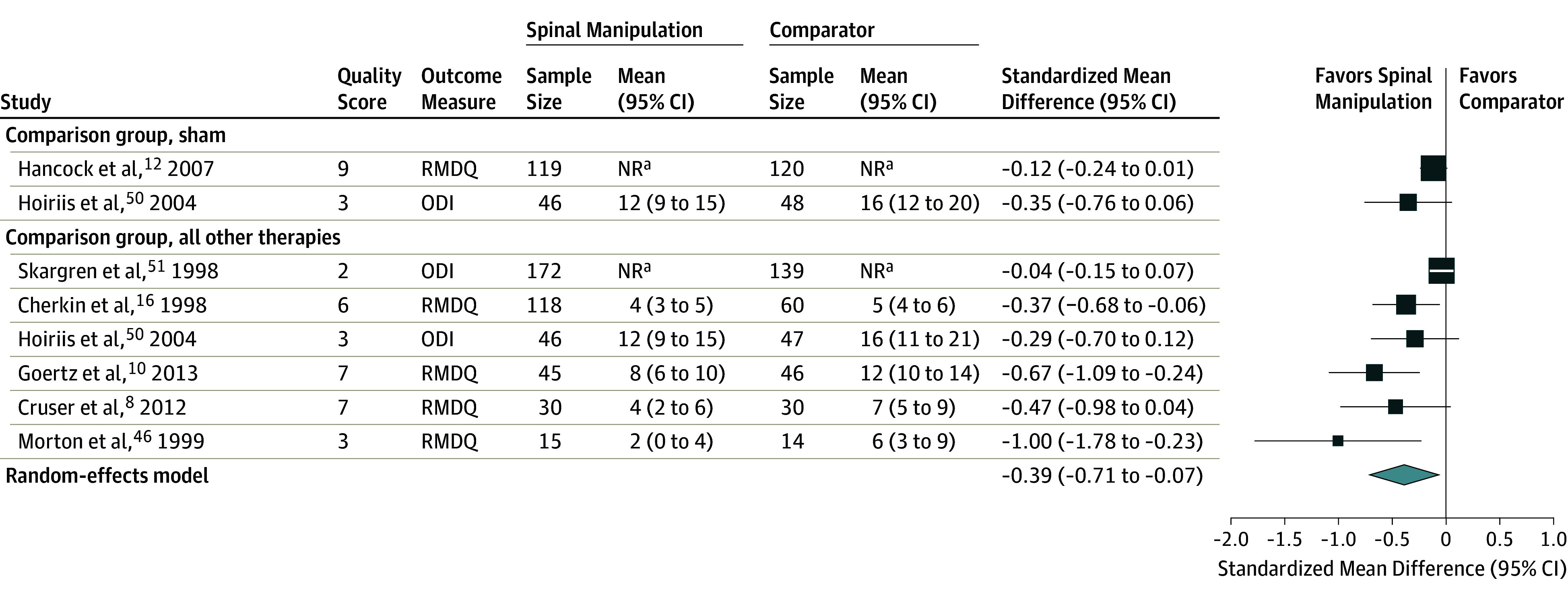
NR indicates not reported; ODI, Oswestry Disability Index (range, 0-100); RMDQ, Roland Morris Disability Questionnaire (range, 0-24). Size of the data markers represent weight based on the randomized meta-analysis. Quality score uses the Cochrane Back and Neck Risk of Bias tool (range, 0-11).
aOutcome data not reported by group, only between-group data reported.
Studies Not Included in the Pooled Analysis
Five studies did not report function outcomes using the RMDQ or ODI.11,25,36,43,44 With 1 exception, all the studies were performed more than 20 years ago. Three studies were judged as high quality and 2 studies were low quality. Three studies concluded SMT had an effect compared with usual medical care, advice to stay active, or advice on posture, exercises, and avoidance of occupational stress,11,36,44 and 2 studies concluded it did not.25,43
Exploring Sources of Heterogeneity
Meta-regression did not show any statistically significant differences in association by timing, outcome, type of manipulating clinician, or whether SMT was delivered alone or with other interventions. Differences in pooled effects between patients receiving thrust compared with nonthrust SMT were not statistically significant. However, in 3 of the 4 comparisons the pooled effect size for thrust-type manipulation was about twice as large as the pooled effect size for nonthrust manipulation, or the effect size of individual RCTs of nonthrust therapy. Five studies reported having selected patients based on an increased probability of response to SMT, but 4 were a set of similar studies (discussed below) and no conclusions were drawn from the 1 remaining study. Both meta-regression and stratified analysis showed no statistically significant differences between groups based on study quality.
Studies Considered Separately Because of Shared Characteristics
Four studies meeting all eligibility criteria were not included in the pooled analysis because they all shared some common characteristics: (1) all used a similar method to select patients considered more likely to benefit from a specific kind of manual therapy; (2) all used the same SMT technique; (3) all studies were authored by professionally related physical therapists; (4) three of these studies reported the largest effect sizes for their primary outcome, short-term function (more than 3 times greater than the average for other SMT studies). Because all of these studies shared some common characteristics and because including them in the pooled analysis greatly increased both heterogeneity and the size of the effect, they were most appropriately discussed as their own group.
The first 2 studies were authored by the same group of researchers, were small (24 patients in each), were classified as low quality, and reported large benefits in favor of the patients receiving the SMT.32,33 The third study was a randomized trial of a clinical prediction rule to identify patients most likely to benefit from SMT, and classified as high quality. Based on prior work that used a prospective cohort to identify variables,62 the authors proposed 5 criteria—any 4 of which identified a patient as more likely to benefit from SMT: duration of episode less than 16 days, no symptoms radiating below the knee, less than 19 points on the Fear-Avoidance Beliefs Questionnaire work subscale, and 2 physical findings. Among patients who met criteria for likely to respond to SMT, those patients treated with SMT had a large benefit in function at 1 week compared with those patients not treated with SMT.34
A fourth RCT reported results from participants selected using a similar clinical prediction rule and treated with the same type of thrust manipulation. Although this study found statistically significant benefits in both pain and function in patients treated with SMT, the size of the benefit was smaller than in the prior 3 studies.9 This discrepancy was attributable to better outcomes in the patients not treated with SMT in this study compared with the prior 3 studies.
Harms
SMT for Acute Low Back Pain
In the 26 RCTs of SMT for acute low back pain included in the pooled analyses, 18 publications did not describe assessment of harms, 3 publications made nonspecific comments about harms (ie, no adverse effects were documented), and 5 publications reported on specific harms (Table 1), none of which were considered related to the treatment except that “the treatment hurts” was statistically more common in the group of patients receiving SMT (along with other interventions) compared with those receiving conventional medical care.61
Table 1. Adverse Events Reported in Randomized Clinical Trials of Effectiveness of Spinal Manipulative Therapy for Acute Low Back Pain.
| Source | Sample Size | Method for Assessing Adverse Events | Adverse Events |
|---|---|---|---|
| Blomberg et al,61 1993 | 149 | Closed-end questionnaires at 1, 2, and 4 mo | Has a table of adverse effects by group; “The treatment hurts” was statistically significantly more likely in the group treated with SMT than continued medical care |
| Fritz et al,9 2015 | 220 | Open-end and closed-end questionnaire at 4 wk | 12.0% of patients reported a total of 20 adverse effects from treatment including increased pain, stiffness, spasm, shooting pain, and fatigue |
| Goertz et al,10 2013 | 91 | Not specified | No serious adverse events (2 mild adverse events were reported in SMT group, both were pain that resolved in 24-48 h) |
| Hancock,12 2007 | 240 | Spontaneous reporting and open-ended questions | No serious adverse reactions associated with SMT |
| Heymann et al,45 2013 | 100 | Not specified | Safety analysis showed no unexpected untoward events in either group |
| Juni et al,13 2009 | 104 | Not specified | Two serious adverse events occurred in the experimental group (4%) and 2 in the control group (4%); in the experimental group there was 1 patient with acute pancreatitis and 1 patient with an acute loss of motor and sensory function due to a herniated disk after randomization, but before any SMT treatment was initiated; in the control group, there was 1 patient with symptomatic cholelithiasis and 1 patient with a femoroacetabular impingement syndrome |
| Morton et al,46 1999 | 29 | Not specified | No adverse effects for either group |
| Waterworth et al,43 1985 | 108 | Not specified | Adverse experiences with therapy were not specifically itemized, but their seriousness and drug relationship were recorded; patients receiving SMT experienced less adverse reactions to treatments on the second assessment (at 10-12 days of therapy) than patients receiving nonsteroidal anti-inflammatory drugs. |
Abbreviation: SMT, spinal manipulative therapy.
SMT in General
Eight studies prospectively assessed harms in patients receiving SMT. Harms were typically assessed by asking consecutive patients receiving SMT from a sample of manual therapy clinicians to complete a questionnaire. Results of these studies, which ranged from 68 to 1058 patients, were generally consistent. Mild, transient harms were reported by 50% to 67% of patients. The most commonly reported adverse effects were local discomfort or increased pain (Table 2). In 1 randomized trial focused on SMT harms, although approximately 50% of patients receiving SMT reported harms, this was not statistically different than the proportion reporting harms in patients randomized to receive manual therapy without SMT or manual therapy without stretching exercises.69 No serious harms were reported in any of these studies.
Table 2. Results From Cohort Studies and Randomized Clinical Trials Focused on Adverse Events of Spinal Manipulative Therapy.
| Source | Sample Size | Method for Assessing Adverse Events | Interventions | Findings |
|---|---|---|---|---|
| Prospective Cohort Studies | ||||
| Barrett et al,63 2000 | 68 Patients; 11 chiropractors | Questionnaires given to 12 consecutive new patients | All received SMT | 53% reported an adverse event, mostly increased or radiating pain |
| Cagnie et al,64 2004 | 465 Patients; 51 manipulating clinicians | Questionnaires given to 15 consecutive new patients | All received SMT | 283 patients (61%) reported at least 1 reaction; headache, stiffness, aggravation of complaints, and radiating discomfort accounted for two-thirds of reactions |
| Leboeuf-Yde et al,65 1997 | 625 Patients; 66 chiropractors | Questionnaires given to 10 consecutive patients | All received SMT | Treatment reactions were common, but benign and short lasting |
| Rubinstein et al,66 2008 | 529 Patients with neck pain; 79 chiropractors | Questionnaires completed at regularly scheduled visits | All received SMT | All patients were treated for neck pain; 56% of patients reported at least 1 adverse event; more than 70% of reported adverse events were musculoskeletal or pain |
| Senstad et al,67 1997 | 1050 Patients; 102 chiropractors | Chiropractor asked 12 consecutive patients a set of standardized questions | All received SMT | At least 1 reaction was reported by 580 patients (55%), 53% reported reactions were local discomfort |
| Randomized Clinical Trials | ||||
| Maiers et al,68 2014 | 194 Elderly patients with neck pain | Standardized solicitation by clinicians, unsolicited reporting of patients, and qualitative interviews with patients | SMT, home exercise, or supervised rehabilitation exercise | 130 patients (67%) reported at least 1 adverse event; SMT patients reported about twice as many adverse events as patients randomized to home exercise (74 for SMT vs 40 for home exercise) |
| Paanalahti et al,69 2014 | 767 Patients | Questionnaires at each return visit | SMT, manual therapy without SMT, and manual therapy without stretching | About 50% of patients reported an adverse event; the most common adverse event was soreness in muscles, followed by increased pain, stiffness, and tiredness; there were no differences between patients receiving SMT, manual therapy without SMT, or manual therapy without stretching |
| Walker et al,70 2013 | 198 Patients; 12 chiropractors | Questionnaires completed within 48 h of treatment | Usual chiropractic care (96% received SMT) or a sham | 42% of usual care patients and 33% of sham care patients reported an adverse event; the most common adverse events were increased pain, muscle stiffness, headache, and radiating discomfort |
Abbreviation: SMT, spinal manipulative therapy.
Serious Harms
Numerous case reports, collections of case reports, and systematic and nonsystematic reviews have included discussion of serious harms of SMT in general and of SMT for low back pain.71,72 However, these case reports could not assess causality or calculate incidence and results of these case reports were not included in this review.
Grading the Quality of Evidence
The quality of evidence was judged as moderate that treatment with SMT was associated with improved pain and function in patients with acute low back pain, which was downgraded from high due to inconsistency of results.
The quality of evidence was judged as high that SMT is commonly associated with transient minor musculoskeletal harms, although they may be equally common following non-SMT manual therapy.
Discussion
The principal conclusion of this review was that SMT treatments for acute low back pain were associated with statistically significant benefit in pain and function at up to 6 weeks, that was, on average, clinically modest. The size of the benefit for pain (−9.95 mm) is about the same as the benefit for nonsteroidal anti-inflammatory drugs in acute low back pain (−8.39 mm) according to the Cochrane review on this topic.27 For function, the effect size of −0.39 is approximately equivalent to an improvement in the RMDQ score of between 1 and 2.5 points, using the range of SDs for the RMDQ in the included studies. However, heterogeneity was high, and could not be explained by differences in patients, clinicians, type of manipulation, study quality, or timing of the outcome. Evaluation of these differences was limited by the quality of reporting in the primary studies.
This review adds to the existing literature by including a greater number of eligible RCTs in the pooled analysis than prior reviews, and also providing a higher level of precision to the pooled analysis. For example, 2 prior reviews included 37 and 45 RCTs and did not perform a pooled analysis. Another review included 27 studies,6 but patients could have had pain for up to 3 months’ duration, and it is unclear how many RCTs were included in their pooled analysis and whether or not they pooled sham-controlled studies with active therapy comparisons. The most recent Cochrane review on SMT for acute low back pain reports pooled results for pain and function at 4-week follow-up that included only 3 studies for each outcome.4 In the current review, 10 studies for pain and 6 studies for function were included in pooled analyses for short-term outcomes.
The studies reporting the largest benefits were 3 studies that used clinical criteria to select patients as more likely to benefit.32,33,34 In a recent RCT, the physical therapy research team reported statistically significant benefits of much smaller magnitude.9 Possible hypotheses include that the comparison group (usual care along with education and reassurance based on The Back Book) was more effective than the exercises given to the comparison groups in the prior studies or that it is due to patient selection, as the most recent study recruited patients directly from primary care and not from patients already referred to physical therapy (and therefore possibly having less successful spontaneous improvement). The recent study also selected patients using a modification of the prediction rule that is more pragmatic for clinical implementation but is known to sacrifice specificity in identifying likely SMT responders.
Limitations
This study has limitations. First, there were limitations in the quantity and quality of the original research. More studies were classified as low quality than high quality. Nevertheless, high-quality studies tended to report larger benefits. Second, some studies did not describe the manipulation in sufficient detail to allow application in practice. Third, there was significant unexplained heterogeneity. There were too few studies to use meta-regression methods to simultaneously test for variables possibly associated with heterogeneity. The most fruitful area for further research is likely to be assessing the role of patient selection and type of SMT on explaining heterogeneity in treatment effects. Fourth, the minimum clinically important difference for these outcomes has not been well established, raising questions about the size of the clinical benefit. Fifth, the possibility of publication bias exists, although no statistical evidence for it was detected.
Conclusions
Among patients with acute low back pain, spinal manipulation therapy was associated with modest improvements in pain and function at up to 6 weeks, with transient minor musculoskeletal harms. However, heterogeneity in study results was large.
eAppendix. Systematic review search strategies
eTable 1. Evidence table of 26 randomized clinical trials of spinal manipulative therapy for acute low back pain
eTable 2. Quality scores of 26 randomized clinical trials of spinal manipulation therapy for acute low back pain
eReferences
References
- 1.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363-370. [DOI] [PubMed] [Google Scholar]
- 2.Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976). 2009;34(10):1078-1093. [DOI] [PubMed] [Google Scholar]
- 3.Assendelft WJ, Morton SC, Yu EI, Suttorp MJ, Shekelle PG. Spinal manipulative therapy for low back pain: a meta-analysis of effectiveness relative to other therapies. Ann Intern Med. 2003;138(11):871-881. [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein SM, Terwee CB, Assendelft WJ, de Boer MR, van Tulder MW. Spinal manipulative therapy for acute low-back pain. Cochrane Database Syst Rev. 2012;(9):CD008880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker BF, French SD, Grant W, Green S. Combined chiropractic interventions for low-back pain. Cochrane Database Syst Rev. 2010;(4):CD005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira ML, Ferreira PH, Latimer J, Herbert R, Maher CG. Efficacy of spinal manipulative therapy for low back pain of less than three months’ duration. J Manipulative Physiol Ther. 2003;26(9):593-601. [DOI] [PubMed] [Google Scholar]
- 7.Avery S, O’Driscoll ML. Randomised controlled trials on the efficacy of spinal manipulation therapy in the treatment of low back pain (structured abstract). Phys Ther Rev. 2004;(3):146-152. http://onlinelibrary.wiley.com/o/cochrane/cldare/articles/DARE-12004008835/frame.html. Accessed March 24, 2017. [Google Scholar]
- 8.Cruser A, Maurer D, Hensel K, Brown SK, White K, Stoll ST. A randomized, controlled trial of osteopathic manipulative treatment for acute low back pain in active duty military personnel. J Man Manip Ther. 2012;20(1):5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz JM, Magel JS, McFadden M, et al. Early physical therapy vs usual care in patients with recent-onset low back pain: a randomized clinical trial. JAMA. 2015;314(14):1459-1467. [DOI] [PubMed] [Google Scholar]
- 10.Goertz CM, Long CR, Hondras MA, et al. Adding chiropractic manipulative therapy to standard medical care for patients with acute low back pain: results of a pragmatic randomized comparative effectiveness study. Spine (Phila Pa 1976). 2013;38(8):627-634. [DOI] [PubMed] [Google Scholar]
- 11.Grunnesjö MI, Bogefeldt JP, Blomberg SI, Strender LE, Svärdsudd KF. A randomized controlled trial of the effects of muscle stretching, manual therapy and steroid injections in addition to “stay active” care on health-related quality of life in acute or subacute low back pain. Clin Rehabil. 2011;25(11):999-1010. [DOI] [PubMed] [Google Scholar]
- 12.Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomised controlled trial. Lancet. 2007;370(9599):1638-1643. [DOI] [PubMed] [Google Scholar]
- 13.Jüni P, Battaglia M, Nüesch E, et al. A randomised controlled trial of spinal manipulative therapy in acute low back pain. Ann Rheum Dis. 2009;68(9):1420-1427. [DOI] [PubMed] [Google Scholar]
- 14.Shekelle PG, Paige NM, Miake-Lye IM, Beroes JM, Booth MS, Shanman R. Effectiveness and harms of spinal manipulative therapy for the treatment of acute neck and lower back pain [intranet only]. US Dept of Veterans Affairs. http://www.hsrd.research.va.gov/publications/esp/reports.cfm. Accessed March 14, 2017. [PubMed]
- 15.Hurwitz EL, Coulter ID, Adams AH, Genovese BJ, Shekelle PG. Use of chiropractic services from 1985 through 1991 in the United States and Canada. Am J Public Health. 1998;88(5):771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherkin DC, Deyo RA, Battié M, Street J, Barlow W. A comparison of physical therapy, chiropractic manipulation, and provision of an educational booklet for the treatment of patients with low back pain. N Engl J Med. 1998;339(15):1021-1029. [DOI] [PubMed] [Google Scholar]
- 17.Chou R, Hashimoto R, Friedly J, et al. Epidural corticosteroid injections for radiculopathy and spinal stenosis: a systematic review and meta-analysis. Ann Intern Med. 2015;163(5):373-381. [DOI] [PubMed] [Google Scholar]
- 18.van Tulder MW, Suttorp M, Morton S, Bouter LM, Shekelle P. Empirical evidence of an association between internal validity and effect size in randomized controlled trials of low-back pain. Spine (Phila Pa 1976). 2009;34(16):1685-1692. [DOI] [PubMed] [Google Scholar]
- 19.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Meca J, Marín-Martínez F. Confidence intervals for the overall effect size in random-effects meta-analysis. Psychol Methods. 2008;13(1):31-48. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.StataCorp . Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godfrey CM, Morgan PP, Schatzker J. A randomized trial of manipulation for low-back pain in a medical setting. Spine (Phila Pa 1976). 1984;9(3):301-304. [DOI] [PubMed] [Google Scholar]
- 26.Hallegraeff JM, de Greef M, Winters JC, Lucas C. Manipulative therapy and clinical prediction criteria in treatment of acute nonspecific low back pain [correction appears in Percept Mot Skills. 2009;108(3):981]. Percept Mot Skills. 2009;108(1):196-208. [DOI] [PubMed] [Google Scholar]
- 27.Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33(16):1766-1774. [DOI] [PubMed] [Google Scholar]
- 28.Cherkin DC, Deyo RA, Street JH, Hunt M, Barlow W. Pitfalls of patient education. Limited success of a program for back pain in primary care. Spine (Phila Pa 1976). 1996;21(3):345-355. [DOI] [PubMed] [Google Scholar]
- 29.Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleland JA, Fritz JM, Kulig K, et al. Comparison of the effectiveness of three manual physical therapy techniques in a subgroup of patients with low back pain who satisfy a clinical prediction rule: a randomized clinical trial. Spine (Phila Pa 1976). 2009;34(25):2720-2729. [DOI] [PubMed] [Google Scholar]
- 31.Blomberg S, Svärdsudd K, Tibblin G. A randomized study of manual therapy with steroid injections in low-back pain: telephone interview follow-up of pain, disability, recovery, and drug consumption. Eur Spine J. 1994;3(5):246-254. [DOI] [PubMed] [Google Scholar]
- 32.Erhard RE, Delitto A, Cibulka MT. Relative effectiveness of an extension program and a combined program of manipulation and flexion and extension exercises in patients with acute low back syndrome. Phys Ther. 1994;74(12):1093-1100. [DOI] [PubMed] [Google Scholar]
- 33.Delitto A, Cibulka MT, Erhard RE, Bowling RW, Tenhula JA. Evidence for use of an extension-mobilization category in acute low back syndrome: a prescriptive validation pilot study. Phys Ther. 1993;73(4):216-222; discussion 223-228. [DOI] [PubMed] [Google Scholar]
- 34.Childs JD, Fritz JM, Flynn TW, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004;141(12):920-928. [DOI] [PubMed] [Google Scholar]
- 35.GRADE working group website. http://www.gradeworkinggroup.org/. Accessed March 14, 2017.
- 36.Blomberg S, Hallin G, Grann K, Berg E, Sennerby U. Manual therapy with steroid injections—a new approach to treatment of low back pain: a controlled multicenter trial with an evaluation by orthopedic surgeons. Spine (Phila Pa 1976). 1994;19(5):569-577. [DOI] [PubMed] [Google Scholar]
- 37.Grunnesjö MI, Bogefeldt JP, Svärdsudd KF, Blomberg SI. A randomized controlled clinical trial of stay-active care vs manual therapy in addition to stay-active care: functional variables and pain. J Manipulative Physiol Ther. 2004;27(7):431-441. [DOI] [PubMed] [Google Scholar]
- 38.Bogefeldt J, Grunnesjö MI, Svärdsudd K, Blomberg S. Sick leave reductions from a comprehensive manual therapy programme for low back pain: the Gotland Low Back Pain Study. Clin Rehabil. 2008;22(6):529-541. [DOI] [PubMed] [Google Scholar]
- 39.Bergquist-Ullman M, Larsson U. Acute low back pain in industry. A controlled prospective study with special reference to therapy and confounding factors. Acta Orthop Scand. 1977;(170):1-117. [DOI] [PubMed] [Google Scholar]
- 40.Glover JR, Morris JG, Khosla T. Back pain: a randomized clinical trial of rotational manipulation of the trunk. Br J Ind Med. 1974;31(1):59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmussen G. Manipulation in treatment of low back pain: a randomized clinical trial. Man Med. 1979;1:8-10. [Google Scholar]
- 42.Postacchini F, Facchini M, Palieri P. Efficacy of various forms of conservative treatment in low back pain: a comparative study. Neuro-orthopedics. 1988;6(1):28-35. [Google Scholar]
- 43.Waterworth RF, Hunter IA. An open study of diflunisal, conservative and manipulative therapy in the management of acute mechanical low back pain. N Z Med J. 1985;98(779):372-375. [PubMed] [Google Scholar]
- 44.MacDonald RS, Bell CM. An open controlled assessment of osteopathic manipulation in nonspecific low-back pain. Spine (Phila Pa 1976). 1990;15(5):364-370. [DOI] [PubMed] [Google Scholar]
- 45.von Heymann WJ, Schloemer P, Timm J, Muehlbauer B. Spinal high-velocity low amplitude manipulation in acute nonspecific low back pain: a double-blinded randomized controlled trial in comparison with diclofenac and placebo. Spine (Phila Pa 1976). 2013;38(7):540-548. [DOI] [PubMed] [Google Scholar]
- 46.Morton JE. Manipulation in the treatment of acute low back pain. J Manual Manip Ther. 1999;7(4):182-189. [Google Scholar]
- 47.Cramer GD, Humphreys CR, Hondras MA, McGregor M, Triano JJ. The Hmax/Mmax ratio as an outcome measure for acute low back pain. J Manipulative Physiol Ther. 1993;16(1):7-13. [PubMed] [Google Scholar]
- 48.Farrell JP, Twomey LT. Acute low back pain: comparison of two conservative treatment approaches. Med J Aust. 1982;1(4):160-164. [PubMed] [Google Scholar]
- 49.Hadler NM, Curtis P, Gillings DB, Stinnett S. A benefit of spinal manipulation as adjunctive therapy for acute low-back pain: a stratified controlled trial. Spine (Phila Pa 1976). 1987;12(7):702-706. [PubMed] [Google Scholar]
- 50.Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398. [DOI] [PubMed] [Google Scholar]
- 51.Skargren EI, Carlsson PG, Oberg BE. One-year follow-up comparison of the cost and effectiveness of chiropractic and physiotherapy as primary management for back pain: subgroup analysis, recurrence, and additional health care utilization. Spine (Phila Pa 1976). 1998;23(17):1875-1883. [DOI] [PubMed] [Google Scholar]
- 52.Santilli V, Beghi E, Finucci S. Chiropractic manipulation in the treatment of acute back pain and sciatica with disc protrusion: a randomized double-blind clinical trial of active and simulated spinal manipulations. Spine J. 2006;6(2):131-137. [DOI] [PubMed] [Google Scholar]
- 53.Mathews W, Morkel M, Mathews J. Manipulation and traction for lumbago and sciatica: physiotherapeutic techniques used in two controlled trials. Physiother Theory Pract. 1988;4(4):201-206. [Google Scholar]
- 54.Mathews JA, Mills SB, Jenkins VM, et al. Back pain and sciatica: controlled trials of manipulation, traction, sclerosant and epidural injections. Br J Rheumatol. 1987;26(6):416-423. [DOI] [PubMed] [Google Scholar]
- 55.Brennan GP, Fritz JM, Hunter SJ, Thackeray A, Delitto A, Erhard RE. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain: results of a randomized clinical trial. Spine (Phila Pa 1976). 2006;31(6):623-631. [DOI] [PubMed] [Google Scholar]
- 56.Hussain I, Hussain Shah SI, Amjad I. Efficacy of spinal manual therapy in non-specific acute low back pain. Rawal Med J. 2013;38(4):358-360. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/341/CN-00915341/frame.html. Accessed March 24, 2017. [Google Scholar]
- 57.Seferlis T, Németh G, Carlsson AM, Gillström P. Conservative treatment in patients sick-listed for acute low-back pain: a prospective randomised study with 12 months’ follow-up. Eur Spine J. 1998;7(6):461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wreje U, Nordgren B, Aberg H. Treatment of pelvic joint dysfunction in primary care—a controlled study. Scand J Prim Health Care. 1992;10(4):310-315. [DOI] [PubMed] [Google Scholar]
- 59.Blomberg S, Svärdsudd K, Mildenberger F. A controlled, multicentre trial of manual therapy in low-back pain: initial status, sick-leave and pain score during follow-up. Scand J Prim Health Care. 1992;10(3):170-178. [DOI] [PubMed] [Google Scholar]
- 60.Blomberg S, Svärdsudd K, Tibblin G. Manual therapy with steroid injections in low-back pain. Improvement of quality of life in a controlled trial with four months’ follow-up. Scand J Prim Health Care. 1993;11(2):83-90. [DOI] [PubMed] [Google Scholar]
- 61.Blomberg S, Tibblin G. A controlled, multicentre trial of manual therapy with steroid injections in low-back pain: functional variables, side effects, and complications during four months follow-up. Clin Rehabil. 1993;7(1):49-62. [Google Scholar]
- 62.Flynn T, Fritz J, Whitman J, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine (Phila Pa 1976). 2002;27(24):2835-2843. [DOI] [PubMed] [Google Scholar]
- 63.Barrett AJ, Breen AC. Adverse effects of spinal manipulation. J R Soc Med. 2000;93(5):258-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cagnie B, Vinck E, Beernaert A, Cambier D. How common are side effects of spinal manipulation and can these side effects be predicted? Man Ther. 2004;9(3):151-156. [DOI] [PubMed] [Google Scholar]
- 65.Leboeuf-Yde C, Hennius B, Rudberg E, Leufvenmark P, Thunman M. Side effects of chiropractic treatment: a prospective study. J Manipulative Physiol Ther. 1997;20(8):511-515. [PubMed] [Google Scholar]
- 66.Rubinstein SM, Leboeuf-Yde C, Knol DL, de Koekkoek TE, Pfeifle CE, van Tulder MW. Predictors of adverse events following chiropractic care for patients with neck pain. J Manipulative Physiol Ther. 2008;31(2):94-103. [DOI] [PubMed] [Google Scholar]
- 67.Senstad O, Leboeuf-Yde C, Borchgrevink C. Frequency and characteristics of side effects of spinal manipulative therapy. Spine (Phila Pa 1976). 1997;22(4):435-440. [DOI] [PubMed] [Google Scholar]
- 68.Maiers M, Evans R, Hartvigsen J, Schulz C, Bronfort G. Adverse events among seniors receiving spinal manipulation and exercise in a randomized clinical trial. Man Ther. 2015;20(2):335-341. [DOI] [PubMed] [Google Scholar]
- 69.Paanalahti K, Holm LW, Nordin M, Asker M, Lyander J, Skillgate E. Adverse events after manual therapy among patients seeking care for neck and/or back pain: a randomized controlled trial. BMC Musculoskelet Disord. 2014;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker BF, Hebert JJ, Stomski NJ, et al. Outcomes of usual chiropractic: the OUCH randomized controlled trial of adverse events. Spine (Phila Pa 1976). 2013;38(20):1723-1729. [DOI] [PubMed] [Google Scholar]
- 71.Hebert JJ, Stomski NJ, French SD, Rubinstein SM. Serious adverse events and spinal manipulative therapy of the low back region: a systematic review of cases. J Manipulative Physiol Ther. 2015;38(9):677-691. [DOI] [PubMed] [Google Scholar]
- 72.Oliphant D. Safety of spinal manipulation in the treatment of lumbar disk herniations: a systematic review and risk assessment. J Manipulative Physiol Ther. 2004;27(3):197-210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Systematic review search strategies
eTable 1. Evidence table of 26 randomized clinical trials of spinal manipulative therapy for acute low back pain
eTable 2. Quality scores of 26 randomized clinical trials of spinal manipulation therapy for acute low back pain
eReferences