Key Points
Question
Is patent ductus arteriosus ligation associated with adverse neonatal outcomes and neurodevelopmental impairment among extremely preterm infants?
Findings
In this cohort study of 754 extremely preterm infants, when postnatal preligation morbidities were properly accounted for, there was no difference in the composite outcome of death or neurodevelopmental impairment among infants who underwent ligation compared with those who were medically treated. While mortality was lower among infants who underwent ligation, there was no difference in neurodevelopmental impairment, chronic lung disease, or severe retinopathy or prematurity.
Meaning
Patent ductus arteriosus ligation may reduce mortality and is not associated with neurodevelopmental impairment. Previously reported associations of ligation with increased morbidity may be because of bias from confounding by indication rather than a detrimental causal effect of ligation.
Abstract
Importance
Observational studies have associated patent ductus arteriosus (PDA) ligation among preterm infants with adverse neonatal outcomes and neurodevelopmental impairment in early childhood, with a resultant secular trend away from surgical treatment. However, to our knowledge, studies have inadequately addressed sources of residual bias, including survival bias and major neonatal morbidities arising before exposure to ligation.
Objective
Evaluate the association between PDA ligation vs medical management and neonatal and neurodevelopmental outcomes.
Design, Setting, and Participants
This retrospective cohort study of preterm infants younger than 28 weeks gestational age born between January 1, 2006, and December 31, 2012, with clinical and echocardiography diagnoses of hemodynamically significant PDA was conducted at 3 tertiary neonatal intensive care units and affiliated follow-up programs.
Exposure
Surgical ligation vs medical management.
Main Outcomes and Measures
The primary outcome was a composite of death or neurodevelopmental impairment (NDI) at 18 to 24 months corrected age. Secondary outcomes included death before discharge, NDI, moderate-severe chronic lung disease, and severe retinopathy of prematurity. Multivariable logistic regression analysis was used to adjust for perinatal and postnatal confounders.
Results
Of 754 infants with hemodynamically significant PDA (mean [standard deviation] gestational age 25.7 [1.2] weeks and birth weight 813 [183] grams), 184 (24%) underwent ligation. Infants who underwent ligation had a higher frequency of morbidities before PDA closure, including sepsis, necrotizing enterocolitis, and a dependence on mechanical ventilation. After adjusting for perinatal characteristics and preligation morbidities, there was no difference in the odds of death or NDI (adjusted odds ratio (aOR), 0.83; 95% CI, 0.52-1.32), NDI (aOR, 1.27; 95% CI, 0.78-2.06), chronic lung disease (aOR, 1.36; 95% CI, 0.78-2.39) or severe retinopathy of prematurity (aOR, 1.61; 95% CI, 0.85-3.06). Ligation was associated with lower odds of mortality (aOR, 0.09; 95% CI, 0.04-0.21).
Conclusions and Relevance
Patent ductus arteriosus ligation among preterm neonates younger than 28 weeks gestational age was not associated with the composite outcome of death or NDI, and there were no differences in chronic lung disease, retinopathy of prematurity, or NDI among survivors. Mortality was lower among infants who underwent ligation, though residual survival bias could not be excluded. Previously reported associations of ligation with increased morbidity may be because of bias from confounding by indication.
This cohort study examines whether surgical ligation vs medical management among extremely preterm infants with patent ductus arteriosus is associated with higher odds of neurodevelopmental impairment.
Introduction
Over the past decade, retrospective studies have associated patent ductus arteriosus (PDA) ligation with increased neonatal and neurodevelopmental morbidity, including chronic lung disease (CLD), retinopathy of prematurity (ROP), cerebral palsy, and cognitive, hearing, and visual impairments. These results have been associated with a secular trend toward a reduction in surgical ligation for persistent symptomatic PDA. However, ligation has also been associated with lower mortality compared with medical management.
While the collective effect of previous studies has raised concern about surgical ligation, significant methodological shortcomings exist. To our knowledge, no contemporary randomized clinical trial has examined the effectiveness of PDA ligation. Importantly, previous observational studies investigating the association of PDA ligation and outcomes have only adjusted for antenatal and perinatal covariates in multivariable analyses. While this approach may be sufficient to balance confounders for interventions occurring shortly after birth, ligation often occurs weeks after birth. During the postnatal preligation period, infants may acquire multiple morbidities of prematurity (eg, sepsis and a dependence on invasive mechanical ventilation) that influence the decision to treat infants with ligation and outcomes. Failing to adjust for such confounders results in a high risk of residual bias against infants who underwent ligation because of confounding by indication and increased preligation illness severity. On the other hand, using ligation as a rescue treatment after the failure or contraindication of medical therapy confers a risk of survival bias in favor of infants who underwent ligation which has not, to our knowledge, been addressed in previous studies.
The objective of this study was to evaluate the association of PDA ligation with neonatal and neurodevelopmental outcomes after accounting for antenatal, perinatal, and postnatal confounders.
Methods
We conducted a retrospective cohort study of extremely preterm infants born at 27 weeks plus 6 days gestational age or younger and treated at 3 tertiary neonatal units in Toronto, Canada (Mount Sinai Hospital, Sunnybrook Health Sciences Centre, and the Hospital for Sick Children) from January 1, 2006, to December 31, 2012. Infants were included if they had a clinically significant PDA and had undergone at least 1 echocardiogram demonstrating a hemodynamically significant PDA, defined as a ductal diameter of 1.5 mm or larger. Infants with major congenital anomalies were excluded. This study was approved by the respective research ethics boards from all 3 neonatal intensive care unit institutions and the University of Toronto, who also approved a waiver of informed consent.
Management of PDA
Treatment for PDA was at the discretion of the attending neonatologist and typically occurred for a clinically and echocardiographically significant PDA. Medical treatment aimed toward facilitating ductal closure (with indomethacin or ibuprofen) was used as first-line therapy. Pharmacotherapeutic treatment could be repeated at the discretion of the attending team. Infants with hemodynamically significant PDA (HSPDA) were considered for surgical ligation after the failure of, or contraindication to, medical therapy.
The decision to refer an infant for PDA ligation was made by the attending neonatologist at each site in collaboration with a neonatologist with expertise in echocardiography. Before ligation, infants at the 2 perinatal centers were transported to The Hospital for Sick Children for surgical ligation. The surgery was performed via a left lateral thoracotomy, an intra- or extrapleural approach, and the PDA was closed using either a clip or ligature at the discretion of the attending surgeon. Postoperative intensive care was supported by targeted neonatal echocardiography including the targeted administration of intravenous milrinone for infants with critically low cardiac output in the immediate postoperative period.
Outcomes and Assessment
Surviving infants underwent neurodevelopmental assessments at 18 to 24 months corrected age, which consisted of a clinical examination, visual and hearing assessment, and cognitive evaluation using the Bayley Scales of Infant Development, Third Edition (BSID III), and/or the Ages and Stages Questionnaire (ASQ). Clinical examinations and standardized motor assessments identified the presence of cerebral palsy, which was classified according to the Gross Motor Functional Classification System. Cognition and language abilities were assessed predominantly using the BSID III, with a small number of infants assessed exclusively using the ASQ.
The primary outcome was a composite of death or moderate-severe neurodevelopmental impairment (NDI), evaluated at 18 to 24 months corrected age. Moderate-severe NDI was defined as a composite of neuromotor, neurocognitive, and/or neurosensory impairment (eTable 1 in the Supplement). Secondary outcomes included death before discharge, moderate-severe NDI, CLD (defined as treatment with supplemental oxygen or positive pressure support at 36 weeks corrected gestational age) and severe ROP (defined as treatment with laser surgery or intravitreal vascular endothelial growth factor inhibitor).
Data Sources and Collection
The hospital records of all eligible infants were reviewed to abstract antenatal, neonatal, and outcome data. The echocardiography reports of each infant were reviewed to identify the infants with an echocardiographically significant PDA, defined as at least 1 echocardiogram with PDA diameter 1.5 mm or larger.
The date and hemodynamic significance of the PDA in all echocardiograms were recorded from study reports to define the onset and longitudinal course (duration and severity) of each infant’s exposure to a ductal shunt (eTable 2 in the Supplement). The dates of echocardiography and/or clinical PDA closure were recorded. For infants who underwent ligation, the date of PDA closure was recorded as the date of surgical ligation. For medically-treated infants, echocardiography closure was recorded as the earliest date of the echocardiogram demonstrating ductal closure without a subsequent reopening. Clinical closure was determined as the earliest date of the disappearance of established clinical signs of PDA (murmur, active precordium, and bounding pulses) in an infant in whom these signs were definitively present. The PDA of an infant was considered no longer hemodynamically significant if it underwent clinical or echo closure or was “small” or “small-moderate” in size on echocardiography.
Data on postnatal morbidities was collected for each day of life for all infants from birth until death or discharge from the neonatal intensive care unit. Specifically, the date of onset of all morbidities was abstracted to include them as time-dependent covariates and characterize the timing of these morbidities in relation to ductal closure. These included intraventricular hemorrhage and periventricular echogenicity, necrotizing enterocolitis stage 2 or higher, systemic dexamethasone for the prevention or treatment of CLD (defined as a minimum 5-day course), seizures, systemic hypotension treated with inotropes, culture positive sepsis (defined as clinical sepsis accompanied by the growth of a pathogenic organism from a sterile site), culture negative sepsis (defined as clinical sepsis without a positive culture that was treated with at least 5 days of systemic antimicrobials), administration of inhaled nitric oxide, pneumothorax, and spontaneous intestinal perforation. Daily information on respiratory support (invasive positive pressure, noninvasive positive pressure, low flow oxygen, or none) and the average mean airway pressure was abstracted. Data were abstracted by study investigators at each site who collected the complete data from each infant's neonatal intensive care unit course before reviewing neurodevelopmental evaluations.
For analyses, postnatal factors were considered time-dependent and were aggregated to represent cumulative illness severity that was estimated for each day of life when infants were considered “at risk” for ligation. For infants who underwent ligation, the “at risk” period consisted of the days before surgical ligation. Medically-treated infants were considered “at risk” for ligation during the period that the PDA hemodynamic significance was at least ”moderate.” This period for medically-treated infants with a persistent PDA was truncated at 60 days of age, as this was the latest postnatal day of surgery among the group who underwent ligation.
Statistical Analysis
Preliminary estimates indicated approximately 200 extremely preterm infants were treated with ligation, and more than 600 infants received medical treatment for a clinically and echocardiographically significant PDA. Assuming 10% of infants would be lost to follow-up, we estimated that 180 infants who underwent ligation would have had the primary outcome assessed. Presuming a 60% event rate among the group who underwent ligation, a 2-sample, 2-sided test of proportions with 80% power and 5% type I error, using 180 infants who underwent ligation and 540 medically treated infants, was estimated to detect an 11% absolute difference in the primary outcome.
Study infants were categorized according to whether they underwent surgical ligation (with or without prior pharmacotherapy) or were not treated with ligation (“medically treated,” composed of cyclooxygenase inhibitor and/or conservative therapy). The distribution of perinatal and postnatal characteristics between groups who underwent ligation vs medical treatment was compared using the χ2 test for categorical variables and the t test or Wilcoxon Rank-Sum test for continuous variables. The time to PDA closure for infants who underwent ligation vs medically treated infants was compared using the Kaplan-Meier analysis.
The associations between PDA ligation and adverse outcomes were estimated using logistic regression analyses. Initially, unadjusted analyses estimated crude odds ratios and 95% confidence intervals (CI) (model 1). Multivariable logistic regression analysis was used to adjust for possible confounding. A multivariable model was constructed using only antenatal and perinatal covariates (model 2) to provide a comparison with the results of previous studies. The final model (model 3) included postnatal covariates representing morbidities that occurred during the period an infant was at risk of ligation. Variable selection for the final model was determined by backward elimination.
Subcohort Analyses
To explore the effect of survival bias (in which medically-treated infants had to survive to be eligible for ligation), subcohort analyses were conducted including only infants who survived with a HSPDA beyond specific time periods (day of life 3, 7, 14, 19, and 28). The longest periods of exclusion (19 and 28 days) were selected as they represented the earliest date of death of an infant in the group who underwent ligation (day of life 20) and the time period of major early morbidities of prematurity such as sepsis (day of life 28). An additional analysis was performed to explore the effect of possible information bias because of using the ASQ rather than the BSID among a small minority of infants. Statistical analyses were performed using SAS version 9.4 (SAS). All statistical tests were 2-sided with significance evaluated at the 5% level.
Results
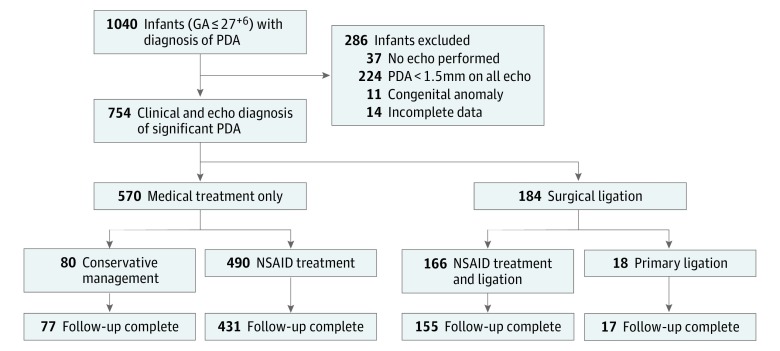
During the study period, 1040 preterm infants born at 27+6 gestational weeks or younger had a clinical diagnosis of PDA. Of these, 286 infants were excluded from the study (Figure 1). A total of 754 preterm infants had a clinical and echocardiography diagnosis (≥1.5mm) of PDA, of whom 570 received medical treatment only and 184 were treated with surgical ligation. The primary outcome of death or NDI at 18 to 24 months corrected age was known for 680 infants (90.2%). Compared with the infants who survived to discharge with known neurodevelopmental outcomes (n= 542), the surviving infants who were lost to follow-up (n = 74) were more likely to have been singleton and less likely to have been treated at Sunnybrook Health Sciences Centre. However, there were no differences in other perinatal characteristics, postnatal morbidities, or neonatal outcomes of CLD and severe ROP (eTable 3 in the Supplement).
Figure 1. Flow Diagram of Infants Included in the Study.
GA indicates gestational age; NSA, nonsteroidal anti-inflammatory drug; and PDA, patent ductus arteriosus.
Patient Characteristics
The antenatal and perinatal characteristics of medically and surgically treated infants are presented in Table 1. Infants treated with ligation had lower gestational age and birth weight and were more likely to be female, require intensive delivery room resuscitation, and receive indomethacin prophylaxis. Infants who underwent ligation had a longer exposure to an HSPDA, with most medically-treated infants experiencing ductal closure prior to the timing of ligation in surgically treated infants (eFigure 1 in the Supplement).
Table 1. Antenatal and Perinatal Characteristics of the Cohort of Extremely Preterm Infants With Clinically and Echocardiographically Significant Patent Ductus Arteriosus.
| Characteristic | No. (%) | Difference (95% CI) | P Valuea | |
|---|---|---|---|---|
| Surgical Ligation (n = 184) |
Medical Treatment Only (n = 570) |
|||
| GA, mean (SD), wk | 25.2 (1.1) | 25.9 (1.2) | −0.7 (−0.87 to −0.48) | <.001 |
| BW, mean (SD), g | 742 (163) | 835 (183) | −93 (−125 to −66) | <.001 |
| SGA, <10th percentile | 29 (15.8) | 63 (11.0) | 4.8% (−1.1 to 10.6) | .09 |
| Initial level 3 hospital | NA | .39 | ||
| SB | 74 (25.5) | 216 (74.5) | ||
| MSH | 79 (22.4) | 273 (77.6) | ||
| HSC | 31 (27.7) | 81 (72.3) | ||
| Multiple gestation | 54 (29.3) | 200 (35.1) | −6.8% (−13.4 to 1.9) | .19 |
| Male | 86 (46.7) | 322 (56.5) | −9.8% (−18.0 to −1.4) | .02 |
| Antenatal corticosteroids, full | 95 (51.6) | 317 (55.6) | −4.0% (−12.3 to 4.3) | .38 |
| Vaginal delivery | 94 (51.1) | 286 (50.2) | 0.9% (−7.4 to 9.2) | .87 |
| Not born at institution | 56 (30.4) | 161 (28.2) | 2.2% (−5.4 to 9.8) | .58 |
| Intensive delivery room resuscitationb | 181 (98.4) | 531 (93.2) | 5.2% (2.4-8.0) | .007 |
| 5-min Apgar <7 | 78 (42.4) | 217 (38.1) | 4.3% (−3.0 to 12.5) | .30 |
| Indomethacin prophylaxis | 48 (26.1) | 101 (17.7) | 8.4% (1.3-15.4) | .02 |
| SNAPII score ≥20 | 73 (39.7) | 187 (32.8) | 6.9% (−1.2 to 14.9) | .09 |
Abbreviations: BW, birth weight; GA, gestational age; HSC, Hospital for Sick Children; MSH, Mount Sinai Hospital; NA, not applicable; SB, Sunnybrook Health Sciences Centre; SGA, small for gestational age; SNAPII, score for neonatal acute physiology II.
t-test or Wilcoxon test for continuous data, or χ2 test for categorical data.
Defined as intubation, chest compressions, or epinephrine administration.
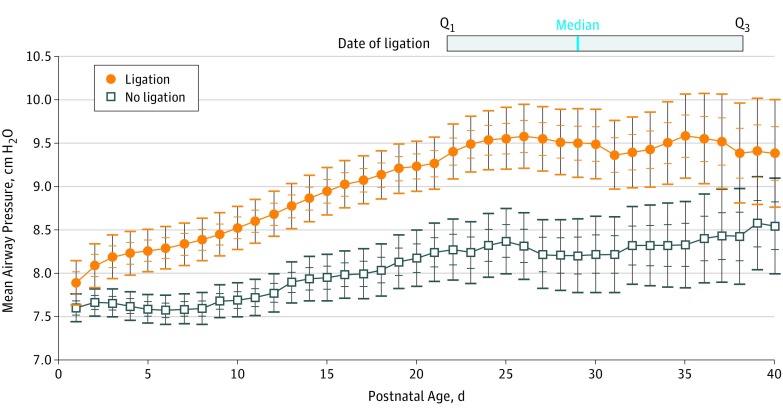
Infants who underwent ligation had higher rates of morbidity during the postnatal period of ductal patency compared with medically treated infants. (Table 2) The burden of invasive mechanical ventilation was higher among infants who underwent ligation, who required a significantly higher average daily mean airway pressure (9.6 cm H2O [±1.9] vs 7.9 cm H2O [±2.3]; 95% CI, 3.8-4.6), mean difference (1.7 cm H2O; 95% CI, 1.3-2.1), and more days of invasive mechanical ventilation (26.9 [ ± 11.0] vs 14.1 [± 13.1]; 95% CI, 22.0-28.2), mean difference (12.7 days; 95% CI, 10.7-14.8) in the postnatal period before ductal closure (Figure 2).
Table 2. Morbidity Arising During the NICU Course, Period of Ductal Patency (Before Surgical Ligation or Medical Closure), and At-Risk Period for Surgical Ligation Among the Infant Cohort (N = 754).
| Morbidity | No. (%) | P Valuea | ||||||
|---|---|---|---|---|---|---|---|---|
| Surgical Ligation (n = 184) |
Medical Treatment (n = 570) |
|||||||
| Incidence of Morbidity Before Discharge (1) | Incidence of Morbidity Before Ligation, (2) | Incidence of Morbidity Before Discharge (3) | Incidence of Morbidity Before Medical PDA Closure (4) | Incidence of Morbidity Before Medical Closure or DOL 60 (5) | (1) vs (3) | (2) vs (4) | (2) vs (5) | |
| Culture positive sepsis | 131 (71.2) | 91 (49.5) | 270 (47.4) | 172 (30.2) | 168 (29.5) | <.001 | <.001 | <.001 |
| Culture negative sepsis | 107 (58.1) | 92 (50.0) | 255 (44.7) | 225 (39.5) | 225 (39.5) | .001 | .01 | .01 |
| Grade 3/4 IVH | 31 (16.8) | 31 (16.8) | 128 (22.5) | 128 (22.5) | 111 (19.5) | .10 | .10 | .43 |
| Hypotension inotropes | 59 (32.1) | 54 (29.3) | 150 (26.3) | 128 (22.4) | 128 (22.4) | .13 | .06 | .06 |
| NEC, Stage ≥2 | 22 (12.0) | 17 (9.2) | 51 (8.9) | 29 (5.1) | 28 (4.9) | .23 | .04 | .03 |
| Pneumothoraxb | 11 (6.0) | 7 (3.8) | 23 (4.0) | 22 (3.9) | 22 (3.9) | .27 | .97 | .97 |
| Nitric oxide | 9 (4.9) | 6 (3.3) | 32 (5.6) | 27 (4.7) | 27 (4.7) | .71 | .39 | .39 |
| Seizure | 23 (12.5) | 17 (9.2) | 51 (8.9) | 41 (7.2) | 41 (7.2) | .14 | .37 | .37 |
| SIP | 9 (4.9) | 9 (4.9) | 10 (1.7) | 9 (1.6) | 9 (1.6) | .02 | .01 | .01 |
| Dexamethasonec | 31 (16.8) | 8 (4.3) | 37 (6.5) | 12 (2.1) | 12 (2.1) | <.001 | .11 | .11 |
Abbreviations: DOL, day of life; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; PDA, patent ductus arteriosus; SIP, spontaneous intestinal perforation.
χ2 test or Fisher exact test.
Pneumothorax requiring thoracostomy tube insertion.
Systemic dexamethasone administered for the prevention or treatment of chronic lung disease, minimum 5 d course.
Figure 2. Average Daily Mean Airway Pressure Over the First 40 Days of Life.
Average daily mean airway pressure (cm H2O) with 68% and 95% CIs over the first 40 days of life for medically-treated infants (black line) vs infants who underwent ligation (orange line) before ductal closure. Infants no longer contributed data after the date of ductal closure, leading to the widening of CIs over time as the number of infants with persistent hemodynamically significant patent ductus arteriosus diminished with time. The median date of ligation was day of life 29, with the interquartile range (day of life 22 to day of life 38) (solid gray box). The earliest date of ligation was on day of life 7.
Main Outcomes
On univariable analysis (model 1), ligation was associated with lower mortality but increased CLD, ROP and NDI, and there was no difference in the composite outcome of death or NDI (Table 3). After adjusting for antenatal and perinatal confounders only (model 2), the associations between ligation and NDI, CLD and ROP were attenuated but remained significant (Table 3). Ligation remained associated with lower mortality and there was no difference in the composite outcome of death or NDI.
Table 3. Neonatal and Neurodevelopmental Outcomes of Infants Who Underwent Ligation vs Medically Treated Infantsa.
| Outcome | No. (%) | Model 1: Crude OR (95% CI) | Model 2: AOR (95% CI)b Antenatal/Perinatal Covariates Only | Model 3: AOR (95% CI)c Model 2 and Postnatal, Preductal Closure Covariates | |
|---|---|---|---|---|---|
| Ligation (n = 184) |
Medical Treatment (n = 570) |
||||
| Death or moderate-severe neurodevelopmental impairmentd | 110 (59.8) | 299 (52.5) | 1.24 (0.87-1.77) | 0.97 (0.65-1.44) | 0.83 (0.52-1.32) |
| Death before dischargee | 17 (9.2) | 121 (21.2) | 0.38 (0.22-0.65) | 0.17 (0.09-0.31) | 0.09 (0.04-0.21) |
| Moderate-severe neurodevelopmental impairment | 92 (50.0) | 174 (30.5) | 1.79 (1.22-2.62) | 1.64 (1.08-2.51) | 1.27 (0.78-2.06) |
| Chronic lung disease | 141 (76.6) | 237 (41.6) | 3.67 (2.44-5.52) | 3.13 (1.96-5.00) | 1.36 (0.78-2.39) |
| Severe retinopathy of prematurity | 42 (22.8) | 30 (5.3) | 4.48 (2.70-7.45) | 2.67 (1.52-4.68) | 1.61 (0.85-3.06) |
Abbreviations: AOR, adjusted odds ratio; GA, gestational age; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; OR, odds ratio; SGA, small for gestational age; SNAP, score for neonatal acute physiology.
Reference is medically treated infants.
Adjusted for GA, SGA, antenatal corticosteroids, sex, multiple gestation, a SNAP 2 score of 20 or higher, and treatment center.
Adjusted for covariates in model 2 plus the following morbidities if they occurred before PDA closure: culture positive sepsis, severe intraventricular hemorrhage, inotrope use, NEC stage 2 or greater, average daily mean airway pressure, proportion of days of invasive mechanical ventilation, total dose of indomethacin, and systemic dexamethasone.
Primary composite outcome includes death before 18 to 24 mo neurodevelopmental follow-up.
Death before discharge from the NICU.
However, after further adjustment for postnatal, preductal closure confounders (model 3), the associations between surgical ligation and NDI (adjusted odds ratio [aOR] 1.27; 95% CI, 0.78-2.06), CLD (aOR 1.36; 95% CI, 0.78-2.39), and ROP (aOR 1.61; 95% CI, 0.85-3.06) were no longer significant (Table 3). Ligation remained associated with lower mortality (aOR 0.09; 95% CI, 0.04-0.21). and there was no significant association with the composite outcome of death or NDI (aOR 0.83; 95% CI, 0.52-1.32).
The causes and timing of death (n = 17 in the ligation group; n = 121 in the medically-treated group) are reported in eTable 4 in the Supplement. Among those who died, medically-treated infants had higher rates of sepsis and early respiratory failure, and mortality occurred earlier in the postnatal period.
Subcohort Analyses
In the subcohort analyses (to address survival bias) that included infants who survived beyond a specific age, ligation was associated with lower mortality among all subcohorts and reduced odds of the composite outcome of death/NDI in the subcohorts of infants with persistent HSPDA beyond the 20th day of life (eTable 5 in the Supplement).
The association of PDA ligation and death/NDI (aOR 0.86; 95% CI, 0.51-1.44) or NDI alone (aOR 1.42; 95% CI, 0.83-2.43) in the subcohort of infants evaluated at neurodevelopmental follow-up using the BSID only (n = 665, 158 underwent ligation) was similar to results from the primary analysis (which included infants evaluated using the BSID or ASQ).
Discussion
In this retrospective cohort study of extremely preterm infants with HSPDA, surgical ligation was not associated with higher odds of the composite outcome of death or NDI, compared with medical management alone. Ligation was also not associated with NDI, CLD, or severe ROP; however, PDA surgery was associated with reduced odds of mortality. These findings contrast with multiple large cohort studies over the past decade that have strongly associated surgical ligation with NDI, CLD, and ROP.
Methodological differences likely account for the divergence in results. Previous studies did not adjust for postnatal, preligation confounders, which represent increased illness severity. We observed similar findings as previous studies when we only included antenatal and perinatal covariates (model 2) which changed with the inclusion of postnatal covariates measured before ductal closure (model 3). The attenuation of the associations with the inclusion of the postnatal covariates suggests that bias because of confounding by indication (as sicker infants are more likely to be referred for ligation), rather than a detrimental causal effect of the surgery itself, may explain the previously reported associations of ligation and adverse outcomes.
Local clinical practices may also have contributed to the absence of an association between ligation and adverse outcomes. Targeted neonatal echocardiography was used to guide management aimed at ductal closure, including the use of a delayed, selective approach in referring infants for surgical ligation, a clinical practice which has been associated with improved neonatal and neurodevelopmental outcomes.
In this study, mortality was significantly lower for infants who underwent ligation compared with medically-treated infants, which corroborates previous studies. Although this finding is compelling, the association between ligation and lower mortality is, like prior studies, possibly influenced by survival bias or confounding by contraindication (in which infants with the highest illness severity are considered unsuitable for surgery and do not survive). For example, medically-treated infants were more likely to have a cerebral injury (eTable 4 in the Supplement), from which several died early in life after the withdrawal of life-sustaining medical therapy. In addition, medically-treated infants were more likely to die from sepsis, suggesting that increased illness severity may have rendered them too unstable for PDA surgery.
To reduce survival bias, we conducted subcohort analyses excluding infants who died or experienced PDA closure within the first weeks of life, though infants who underwent ligation continued to have significantly lower mortality compared with medically-treated infants. However, these findings should be interpreted with caution because of the possibility of residual survival bias. For example, it is unknown for an infant with a large PDA who died of sepsis on the28th day of life whether prior PDA ligation may have reduced the mortality risk. The potential for residual survival bias means that the association of ligation with outcomes that include mortality requires further evaluation before clinicians can be confident that ligation improves survival. Ultimately, the effect of survival bias and an estimate of any mortality benefit of ligation may only be reliably evaluated in a randomized clinical trial.
The strengths of this study include the in-depth abstraction of postnatal morbidities and indices of illness severity, such as daily respiratory support, in a large cohort of extremely preterm infants. This data collection included key covariates in the multivariable analyses to minimize confounding by indication. In addition, the comprehensive collection of all echocardiography data permitted identification of the “at-risk” period for ligation for each infant based on an objective estimate rather than clinician preference or perception. Finally, the proportion of infants lost to neurodevelopmental follow-up was low (9.8%) and these infants had similar characteristics as those for whom follow-up was complete, increasing the confidence in our estimates (eTable 3 in the Supplement).
Limitations
This study is limited by possible ascertainment bias, as medically-treated infants were considered eligible for ligation until the date of confirmed PDA closure, which may have been delayed relative to the true date of ductal closure. This may have led to overestimation of the “at-risk of ligation” period for some medically-treated infants and introduced bias in favor of the medically-treated group, as all morbidities that occurred before this date were adjusted for in the multivariable analyses. In addition, a small minority of infants were evaluated using the ASQ rather than the BSID for the cognition and language assessments. However, results from a subcohort analysis including only infants evaluated using the BSID were similar to those from the full cohort, suggesting minimal ascertainment bias owing to variable neurodevelopmental testing methods. Finally, there remains the potential for confounding by contraindication, residual survival bias, and residual confounding because of unmeasured covariates in this analysis.
Conclusions
Compared with medical management, surgical ligation of HSPDA is associated with lower mortality without increased CLD, ROP, NDI, or the composite of death or NDI. The previously reported associations of ligation with adverse neonatal outcomes and neurodevelopmental impairment may be because of confounding by indication rather than a detrimental causal effect of PDA surgery. However, well-designed randomized clinical trials are needed to evaluate the relative effects of medical and surgical treatment of HSPDA on neonatal and early childhood outcomes.
eFigure 1. Kaplan-Meier Analysis of Time to PDA Closure of Medically (Red) Versus Surgically (Blue) Treated Infants Over the First 10 Weeks of Life.
eTable 1. Classification of the Severity of Neurodevelopmental Impairment in Infants.
eTable 2. Echocardiographic Classification of PDA Hemodynamic Significance.
eTable 3. Antenatal and Perinatal Characteristics, Postnatal Morbidity, Ductal Characteristics and Neonatal Outcomes of Surviving Infants Based on Completion of Neurodevelopmental Evaluation at Follow-Up.
eTable 4. Causes and Timing of Death in Ligated and Nonligated Infants.
eTable 5. Associations of Ligation vs. Medical Treatment and the Primary Composite Outcome of Death or Moderate-Severe Neurodevelopmental Impairment, and the Secondary Outcome of Death before Discharge from NICU Among Cohorts Restricted to Survivors by Postnatal Age with Persistent Hemodynamically Significant PDA.
References
- 1.Kabra NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A; Trial of Indomethacin Prophylaxis in Preterms Investigators . Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. J Pediatr. 2007;150(3):229-234, 234.e1. [DOI] [PubMed] [Google Scholar]
- 2.Madan JC, Kendrick D, Hagadorn JI, Frantz ID III; National Institute of Child Health and Human Development Neonatal Research Network . Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics. 2009;123(2):674-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirea L, Sankaran K, Seshia M, et al. ; Canadian Neonatal Network . Treatment of patent ductus arteriosus and neonatal mortality/morbidities: adjustment for treatment selection bias. J Pediatr. 2012;161(4):689-94.e1. [DOI] [PubMed] [Google Scholar]
- 4.Chorne N, Leonard C, Piecuch R, Clyman RI. Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics. 2007;119(6):1165-1174. [DOI] [PubMed] [Google Scholar]
- 5.Bourgoin L, Cipierre C, Hauet Q, et al. . Neurodevelopmental outcome at 2 years of age according to patent ductus arteriosus management in very preterm infants. Neonatology. 2016;109(2):139-146. [DOI] [PubMed] [Google Scholar]
- 6.Janz-Robinson EM, Badawi N, Walker K, Bajuk B, Abdel-Latif ME; Neonatal Intensive Care Units Network . Neurodevelopmental outcomes of premature infants treated for patent ductus arteriosus: a population-based cohort study. J Pediatr. 2015;167(5):1025-1032, e3. [DOI] [PubMed] [Google Scholar]
- 7.Clyman R, Cassady G, Kirklin JK, Collins M, Philips JB III. The role of patent ductus arteriosus ligation in bronchopulmonary dysplasia: reexamining a randomized controlled trial. J Pediatr. 2009;154(6):873-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lokku A, Mirea L, Lee SK, Shah PS; Canadian Neonatal Network . Trends and outcomes of patent ductus arteriosus treatment in very preterm infants in Canada [published online September 20, 2016]. Am J Perinatol. [DOI] [PubMed] [Google Scholar]
- 9.Hagadorn JI, Brownell EA, Trzaski JM, et al. . Trends and variation in management and outcomes of very low-birth-weight infants with patent ductus arteriosus. Pediatr Res. 2016;80(6):785-792. [DOI] [PubMed] [Google Scholar]
- 10.Weisz DE, More K, McNamara PJ, Shah PS. PDA ligation and health outcomes: a meta-analysis. Pediatrics. 2014;133(4):e1024-e1046. [DOI] [PubMed] [Google Scholar]
- 11.Jain A, Sahni M, El-Khuffash A, Khadawardi E, Sehgal A, McNamara PJ. Use of targeted neonatal echocardiography to prevent postoperative cardiorespiratory instability after patent ductus arteriosus ligation. J Pediatr. 2012;160(4):584-589.e1. [DOI] [PubMed] [Google Scholar]
- 12.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214-223. [DOI] [PubMed] [Google Scholar]
- 13.Clyman RI. Surgical ligation of the patent ductus arteriosus: treatment or morbidity? J Pediatr. 2012;161(4):583-584. [DOI] [PubMed] [Google Scholar]
- 14.Wickremasinghe AC, Rogers EE, Piecuch RE, et al. . Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arteriosus. J Pediatr. 2012;161(6):1065-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157(3):381-387, 387.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristman V, Manno M, Côté P. Loss to follow-up in cohort studies: how much is too much? Eur J Epidemiol. 2004;19(8):751-760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Kaplan-Meier Analysis of Time to PDA Closure of Medically (Red) Versus Surgically (Blue) Treated Infants Over the First 10 Weeks of Life.
eTable 1. Classification of the Severity of Neurodevelopmental Impairment in Infants.
eTable 2. Echocardiographic Classification of PDA Hemodynamic Significance.
eTable 3. Antenatal and Perinatal Characteristics, Postnatal Morbidity, Ductal Characteristics and Neonatal Outcomes of Surviving Infants Based on Completion of Neurodevelopmental Evaluation at Follow-Up.
eTable 4. Causes and Timing of Death in Ligated and Nonligated Infants.
eTable 5. Associations of Ligation vs. Medical Treatment and the Primary Composite Outcome of Death or Moderate-Severe Neurodevelopmental Impairment, and the Secondary Outcome of Death before Discharge from NICU Among Cohorts Restricted to Survivors by Postnatal Age with Persistent Hemodynamically Significant PDA.