Key Points
Question
Is light therapy effective in improving excessive daytime sleepiness and sleep quality in Parkinson disease (PD)?
Findings
In this randomized clinical trial of 31 participants with PD and coexistent excessive daytime sleepiness, light therapy administered twice daily for 2 weeks was well tolerated and resulted in significant improvements of excessive daytime sleepiness and several other metrics of sleep and PD severity.
Meaning
Light therapy may be a feasible intervention for improving sleep and alertness in patients with PD.
Abstract
Importance
Impaired sleep and alertness are some of the most common nonmotor manifestations of Parkinson disease (PD) and currently have only limited treatment options. Light therapy (LT), a widely available treatment modality in sleep medicine, has not been systematically studied in the PD population.
Objective
To determine the safety and efficacy of LT on excessive daytime sleepiness (EDS) associated with PD.
Design, Settings, and Participants
This randomized, placebo-controlled, clinical intervention study was set in PD centers at Northwestern University and Rush University. Participants were 31 patients with PD receiving stable dopaminergic therapy with coexistent EDS, as assessed by an Epworth Sleepiness Scale score of 12 or greater, and without cognitive impairment or primary sleep disorder. Participants were randomized 1:1 to receive bright LT or dim-red LT (controlled condition) twice daily in 1-hour intervals for 14 days. This trial was conducted between March 1, 2007, and October 31, 2012. Data analysis of the intention-to-treat population was conducted from November 1, 2012, through April 30, 2016.
Main Outcomes and Measures
The primary outcome measure was the change in the Epworth Sleepiness Scale score comparing the bright LT with the dim-red LT. Secondary outcome measures included the Pittsburgh Sleep Quality Index score, the Parkinson’s Disease Sleep Scale score, the visual analog scale score for daytime sleepiness, and sleep log–derived and actigraphy-derived metrics.
Results
Among the 31 patients (13 males and 18 females; mean [SD] disease duration, 5.9 [3.6] years), bright LT resulted in significant improvements in EDS, as assessed by the Epworth Sleepiness Scale score (mean [SD], 15.81 [3.10] at baseline vs 11.19 [3.31] after the intervention). Both bright LT and dim-red LT were associated with improvements in sleep quality as captured by mean (SD) scores on the Pittsburg Sleep Quality Index (7.88 [4.11] at baseline vs 6.25 [4.27] after bright LT, and 8.87 [2.83] at baseline vs 7.33 [3.52] after dim-red LT) and the Parkinson’s Disease Sleep Scale (97.24 [22.49] at baseline vs 106.98 [19.37] after bright LT, and 95.11 [19.86] at baseline vs 99.28 [16.94] after dim-red LT). Bright LT improved several self-reported mean (SD) sleep metrics, including sleep fragmentation (number of overnight awakenings, 1.51 [1.03] at baseline vs 0.92 [0.97] after the intervention), sleep quality (sleep diary score, 3.03 [1.01] at baseline vs 3.53 [0.91] after the intervention), and ease of falling asleep (sleep diary score, 2.32 [0.89] at baseline vs 1.83 [0.88] after the intervention). Light therapy was associated with increased daily physical activity as assessed by actigraphy (average activity [SD] counts, 165.01 [66.87] at baseline vs 194.59 [87.81] after the intervention).
Conclusions and Relevance
Light therapy was well tolerated and may be a feasible intervention for improving the sleep-wake cycles in patients with PD. Further studies are required to determine optimal parameters of LT for PD.
Trial Registration
clinicaltrials.gov Identifier: NCT01338649
This randomized clinical trial reports the improvements in sleep-wake cycles, alertness, and motor and nonmotor functions achieved with the use of light therapy by participants with Parkinson disease.
Introduction
Parkinson disease (PD) is the second most common neurodegenerative disorder, affecting more than 1 million people in the United States alone. Sleep disturbances are among its most common manifestations: Excessive daytime sleepiness (EDS) and nocturnal sleep fragmentation affect up to 90% of patients with PD. Sleep disturbances reduce quality of life, impair daytime functioning, and are associated with motor vehicle crashes. Chronic sleep disturbances, coupled with the adverse effects of medications prescribed for PD and sleep, limit the usefulness of available treatment strategies. Therefore, there is a great need to develop nonpharmacological approaches to preventing and managing sleep disorders in patients with PD.
In PD, the cause of sleep disturbances has largely been attributed to the symptoms, adverse effects of medications, and primary neurodegeneration of central sleep regulatory areas. Disruption of circadian rhythms, which can also result in sleep fragmentation and daytime somnolence, has not been well studied in PD, but a growing body of evidence suggests significant alterations of the circadian system. Circadian rhythms are endogenous physiologic cycles that occur on approximately a 24-hour cycle and are generated by a circadian pacemaker located in the suprachiasmatic nucleus of the hypothalamus. Circadian rhythms are synchronized to the environmental light or dark and to social activity cycles by zeitgebers (time givers). Light represents the most effective zeitgeber of the circadian timing system.
Supplementary exposure to bright light has beneficial effects on sleep quality and daytime vigilance in healthy older people and patients with dementia, and it has been increasingly applied in a variety of sleep and neuropsychiatric conditions. A few exploratory studies have examined the effects of supplemental light exposure in PD and documented significant improvements in depression, bradykinesia, rigidity, dyskinesias, and insomnia symptoms. The purpose of this study was to assess the safety and efficacy of light therapy (LT) as a novel treatment approach to excessive daytime sleepiness (EDS) associated with PD.
Methods
Study Design
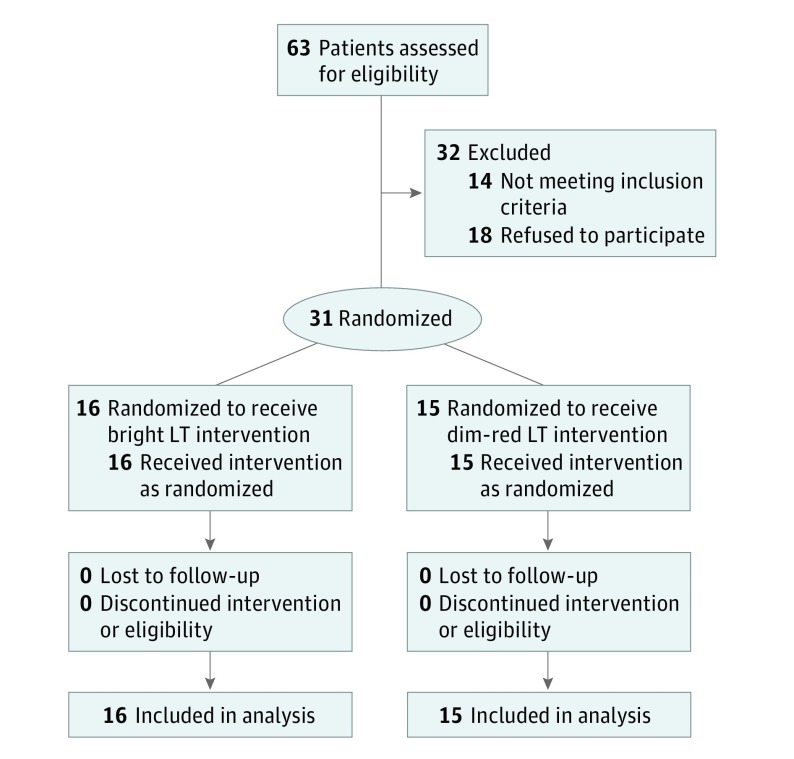
This trial was a randomized, placebo-controlled, clinical intervention of LT in patients with PD and coexistent EDS. Participants with PD who had an Epworth Sleepiness Scale (ESS) score of 12 or greater were randomized to receive a 2-week LT intervention using either bright light exposure of 10 000 lux (active group) or dim-red light exposure of less than <300 lux (controlled group) (Figure). The study protocol (available in the Supplement) was approved by the institutional review boards of Northwestern University, Chicago, Illinois, and Rush University, Chicago. All study participants provided written informed consent.
Figure. CONSORT Flow Diagram.
LT indicates light therapy.
Study Population
Thirty-one patients were recruited from the Parkinson’s Disease and Movement Disorders Centers at Northwestern University and the Parkinson’s Disease and Movement Disorders Program at Rush University. Patients were enrolled in the study if they (1) had a diagnosis of idiopathic PD, as defined by the UK Parkinson’s Disease Society Brain Bank Criteria; (2) were classified as Hoehn and Yahr stages 2 to 4; (3) had EDS, as defined by an ESS score of 12 or greater; (4) had a stable PD medication regimen for at least 4 weeks before study screening; and (5) were willing and able to give written informed consent.
Patients were excluded from participation if they (1) had atypical parkinsonian syndrome; (2) had significant sleep-disordered breathing, defined as an apnea-hypopnea index of more than 15 events per hour of sleep on screening polysomnography; (3) had significant periodic limb-movement disorder, defined as a periodic limb-movement arousal index of more than 10 events per hour of sleep on screening polysomnography; (4) had REM (rapid eye movement) sleep behavior disorder based on the presence of both clinical symptomatology and intermittent loss of REM atonia on screening polysomnography; (5) had cognitive impairment, as indicated by a Mini-Mental State Examination score less than 24; (6) had untreated hallucinations or psychosis; (7) used hypnosedative or stimulant drugs; (8) used antidepressants, unless the patient had been receiving a stable dose for at least 3 months; (9) had visual abnormalities that may interfere with LT, such as significant cataracts, narrow-angle glaucoma, or blindness; or (10) traveled across 2 or more time zones within 90 days before study screening.
Study Protocol
At the screening visit, potential study participants underwent standard neurological and ophthalmologic examinations, Hoehn and Yahr staging, the Unified Parkinson’s Disease Rating Scale (UPDRS), and the Mini-Mental State Examination. Participants also completed the ESS, the Parkinson’s Disease Sleep Scale, the Pittsburgh Sleep Quality Index, the Fatigue Severity Scale, the Beck Depression Inventory, and the Parkinson’s Disease Questionnaire-39 (39-item quality-of-life scale). Subsequently, participants underwent polysomnography to rule out sleep-disordered breathing and periodic limb-movement disorder at the General Clinical Research Center at Northwestern Memorial Hospital.
After the screening, eligible participants entered a 2-week baseline phase. They wore an actigraphy monitor (Actiwatch-2; Philips Respironics), on the nondominant wrist for 24 hours a day, 7 days a week. The monitor recorded activity in epochs of 1 minute, and a photosensor integrated into the monitor recorded light exposure. Participants completed a daily sleep log, in which they noted bedtime, wake-up time, estimated total sleep time, sleep latency, wake after sleep onset, naps, and sleep quality. A visual analog scale (VAS) score for daytime sleepiness was completed every 2 hours on Mondays, Wednesdays, and Fridays, starting at 8 am until the habitual bedtime.
After the baseline phase, participants were randomized to bright LT or dim-red LT (control condition). Participants received 1 hour of bright LT or dim-red LT in gaze direction in the morning (9-11 am) and in the afternoon (5-7 pm) daily for 2 weeks. A light box (SunRay; The SunBox Co) was used for LT administration. The box was placed 86.4 cm away from the subject, and an 86.4-cm string attached from its side, along with detailed instructions provided to the participants, assisted with the proper exposure distance. Participants were instructed to sit quietly during LT and not to nap, and they were allowed to listen to music or audiobooks. They also recorded light exposure duration and timing daily in a light log. During the intervention phase, participants continued to wear the actigraphy monitor, maintained sleep logs, and completed the VAS for daytime sleepiness.
To examine potential carryover effects of the initial responses to LT, we had all participants continue to wear the actigraphy monitor, maintain sleep logs, and complete the VAS for daytime sleepiness for an additional 2 weeks after the conclusion of the intervention phase. The final study visit was performed after this 2-week follow-up period. One study rater (A.V.) remained blinded to the participants’ LT assignment throughout the study. Data were collected from March 1, 2007, through October 31, 2012.
Statistical Analysis
The primary outcome measure was the change in the ESS when comparing the bright light exposure with dim-red light exposure. Secondary outcome measures included the global Pittsburgh Sleep Quality Index score, Parkinson’s Disease Sleep Scale score, VAS scores for daytime sleepiness, and sleep log–derived and actigraphy-derived metrics. A variety of exploratory analyses examined the effects of bright light exposure on the Fatigue Severity Scale, the Beck Depression Inventory, quality of life, cognition, mood, and motor disability.
Efficacy analyses were performed from November 1, 2012, through April 30, 2016, using intention-to-treat analysis. The distribution of the change scores of the treatment groups was compared using a 2-sample t test. Actigraphy measures were calculated using Actiware software, version 5.71 (Mini-Mitter Co), and checked against the daily sleep logs maintained by the participants. Two-factor, repeated-measures, one-way analysis of variance, with day of study as the within-subject factor and group membership as the between-subject factor, was conducted for actigraphy output measures. Adverse events were described.
With 12 participants per treatment group completing the study protocol, there was 80% power against an effect size of 1.1 (α = .05, 2-sided, 2-sample t test). This corresponds to a mean difference in change scores of 4.6 on the ESS, with a projected within-group SD of 4 points. The projection is conservatively based on a study of modafinil for the treatment of EDS in patients with PD.
Results
Patient Characteristics
Fourteen participants failed the screening because they had an ESS score of more than 12 (n = 5), presence of obstructive sleep apnea (n = 4), a Mini-Mental State Examination score lower than 24 (n = 3), and medication changes (n = 2). Thirty-one participants (13 males and 18 females) with a mean (SD) disease duration of 5.9 (3.6) years (range, 32-77 years) were enrolled. All participants completed the study. There were no significant differences in demographic characteristics, disease duration and severity, metrics of sleep and alertness, and other disease characteristics between the active treatment group and control group (Table 1). No medication changes occurred throughout the study.
Table 1. Demographic Characteristics and Disease Metrics of the Study Cohort.
| Characteristic or Disease Metrica | Light Therapy Group, Mean (SD) | P Value | |
|---|---|---|---|
| Bright | Dim-Red | ||
| Age, y | 62.31 (10.83) | 64.07 (8.89) | .63 |
| Sex, male/female | 8/8 | 5/10 | .47 |
| Disease duration | 5.94/3.57 | 8.38/3.71 | .08 |
| Hoehn and Yahr stage, No. (%) | |||
| 2 | 14 (87.5) | 10 (66.67) | .53 |
| 2.5 | 1 (6.25) | 3 (20.00) | |
| 3 | 1 (6.25) | 1 (6.67) | |
| 4 | 1 (6.67) | ||
| UPDRS | 39.69 (15.85) | 45.07 (20.15) | .63 |
| I | 1.75 (0.86) | 2.27 (1.49) | .36 |
| II | 10.44 (5.23) | 10.40 (7.13) | .68 |
| III | 24.75 (11.26) | 29.13 (12.43) | .33 |
| IV | 2.75 (2.24) | 3.27 (2.12) | .36 |
| LED | 492.13 (362.08) | 540.27 (233.41) | .67 |
| Mini-Mental State Examination score | 29.13 (1.19) | 29.13 (1.06) | .86 |
| Beck Depression Inventory score | 8.31 (3.63) | 8.27 (4.71) | .98 |
| Epworth Sleepiness Scale score | 15.81 (3.10) | 15.47 (2.59) | .81 |
| Pittsburgh Sleep Quality Index | 7.88 (4.11) | 8.87 (2.83) | .44 |
| Parkinson’s Disease Sleep Scale score | 97.24 (22.49) | 95.11 (19.86) | .78 |
| Fatigue Severity Scale score | 41.62 (12.62) | 37.00 (9.10) | .34 |
| Parkinson’s Disease Questionnaire-39 score | 41.46 (19.30) | 36.80 (19.72) | .58 |
| Central nervous system–active medications | Sertraline, 50 mg (n = 1) Escitalopram, 10-30 mg (n = 2) |
Bupropion, 300 mg (n = 1) Escitalopram, 30 mg (n = 1) |
|
Abbreviations: LED, levodopa equivalent dose; UPDRS, Unified Parkinson’s Disease Rating Scale.
The scales are explained in the Study Population subsection of the Methods section.
Adherence Metrics
The total LT exposure for this experimental protocol is 28 hours divided in 2 sessions daily for 14 days. The mean (SD) self-reported use of LT in participants treated with bright LT was 20.8 (5.3) hours (range, 10.3-27.4 hours) and 21.8 (6.0) hours (range, 7-28 hours) in participants treated with dim-red LT.
Effects of LT as Self-reported or Assessed in Questionnaires or Scales
Bright LT resulted in significant improvements in EDS, assessed by the ESS score (Table 2). Both bright LT and dim-red LT were associated with improvements in sleep quality as assessed by the Pittsburgh Sleep Quality Index. The Parkinson’s Disease Sleep Scale captured improvements in sleep after LT in both study groups (Table 2). These beneficial effects of LT on 3 self-reported markers of sleep and alertness were not present 2 weeks after cessation of LT. Light therapy improved self-reported alertness (VAS measures) in the bright LT group.
Table 2. Effects of LT on Parkinson Disease Severity, Sleep, Alertness, Depression, Fatigue, and Quality of Life.
| Outcome Measurea | Mean (SD) |
P Value, Group/ Condition/ Interaction |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Postintervention (Week 4) |
P Value, Group/ Condition/ Interaction |
Postwashout (Week 6) | |||||
| Bright LT | Dim-Red LT | Bright LT | Dim-Red LT | Bright LT | Dim-Red LT | |||
| UPDRS | 39.69 (15.85) | 45.07 (20.15) | 35.47 (17.67) | 40.02 (19.48) | .39/.001 | 35.94 (12.29) | 40.20 (18.68) | .43/.78 |
| I | 1.75 (0.86) | 2.27 (1.49) | 1.40 (1.06) | 1.33 (1.18) | .49/.008 | 1.56 (1.41) | 1.87 (1.36) | .74/.07 |
| II | 10.44 (5.23) | 10.40 (7.13) | 8.87 (4.87) | 9.53 (6.39) | .84/.03 | 10.13 (4.30) | 10.40 (6.66) | .79/.02 |
| III | 24.75 (11.26) | 29.13 (12.43) | 22.67 (13.12) | 25.87 (11.87) | .35/.01 | 21.94 (8.84) | 24.60 (11.79) | .44/.52 |
| IV | 2.75 (2.24) | 3.27 (2.12) | 2.53 (1.85) | 3.47 (2.67) | .33/.80 | 2.31 (1.85) | 3.33 (2.64) | .22/.47 |
| BDI | 8.31 (3.63) | 8.27 (4.71) | 7.88 (4.53) | 9.33 (10.57) | .84/.26 | 7.06 (5.56) | 10.40 (10.49) | .94/.73 |
| ESS | 15.81 (3.10) | 15.47 (2.59) | 11.19 (3.31) | 13.67 (4.78) | .37/<.001/.005 | 11.38 (4.15) | 13.13 (3.56) | .10/.81 |
| PSQI | 7.88 (4.11) | 8.87 (2.83) | 6.25 (4.27) | 7.33 (3.52) | .40/.006 | 5.56 (3.60) | 6.00 (2.39) | .51/.08 |
| PDSS | 97.24 (22.49) | 95.11 (19.86) | 106.98 (19.37) | 99.28 (16.94) | .4792/.001 | 103.23 (21.76) | 100.49 (17.87) | .43/.53 |
| FSS | 41.62 (12.62) | 37.00 (9.10) | 37.92 (13.65) | 36.53 (11.54) | .57/.48 | 37.07 (13.01) | 39.21 (10.39) | .94/.63 |
| PDQ-39 | 41.46 (19.30) | 36.80 (19.72) | 43.00 (14.84) | 39.40 (26.17) | .49/.41 | 41.00 (18.68) | 37.20 (27.38) | .64/.23 |
Abbreviations: BDI, Beck Depression Inventory; ESS, Epworth Sleepiness Scale; FSS, Fatigue Severity Scale; LT, light therapy; PDQ-39, Parkinson’s Disease Questionnaire (39-item); PDSS, Parkinson’s Disease Sleep Scale; PSQI, Pittsburgh Sleep Quality Index; UPDRS, Unified Parkinson’s Disease Rating Scale.
For the BDI, a score of 0 to 13 indicates minimal depression; 14 to 19, mild depression; 20 to 28, moderate depression; and 29 to 63, severe depression. For the ESS, a score equal to or greater than 10 is considered indicative of excessive daytime sleepiness. For the PSQI, a global PSQI score equal to or greater than 5 indicates poor sleep quality. For the PDSS, a higher score indicates better sleep. For the UPDRS, a higher score indicates more severe disease. For the FSS, a total score greater than 36 is indicative of fatigue.
Effects of LT as Self-reported in Sleep Diaries
Light therapy improved multiple sleep metrics as assessed by participant-reported sleep diaries: Overnight awakenings, sleep quality, and ease of falling asleep were all significantly improved in participants treated with bright LT (Table 3). Sleep latency, total sleep time, and wake time after sleep onset became shorter with the administration of either bright LT or dim-red LT. All participants reported being more refreshed in the morning during LT, compared with the baseline status. Actual light brightness (95th percentile) was positively related to the measure wake time during night and negatively related to the measure dreaming.
Table 3. Effects of LT on Self-reported Sleep Diaries.
| Sleep Diary Measurea | Mean (SD) |
P Value, Group/ Condition/ Interaction |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Postintervention (Week 4) |
P Value, Group/ Condition/ Interaction |
Postwashout (Week 6) | |||||
| Bright LT | Dim-Red LT | Bright LT | Dim-Red LT | Bright LT | Dim-Red LT | |||
| Sleep | ||||||||
| Duration, h | 7.42 (0.99) | 7.43 (1.31) | 6.95 (0.95) | 7.25 (1.29) | .71/.05 | 7.03 (0.90) | 7.35 (1.29) | .43/.59 |
| Latency, min | 37 (45) | 24 (16) | 23 (21) | 22 (10) | .42/.02 | 20 (16) | 24 (12) | .65/.64 |
| No. of awakenings | 1.51 (1.03) | 2.18 (1.09) | 0.92 (0.97) | 1.77 (1.08) | .02/<.001/.006 | 1.28 (1.13) | 1.76 (1.02) | .03/.01/.004 |
| Wake during night, min | 45.18 (50.63) | 38.56 (21.69) | 39.74 (40.93) | 31.77 (23.28) | .54/.05 | 40.19 (48.46) | 29.53 (19.62) | .44/.89 |
| Sleep quality | 3.03 (1.01) | 3.58 (0.53) | 3.53 (0.91) | 3.61 (0.75) | .38/<.001/.002 | 3.47 (0.82) | 3.79 (0.43) | .54/.73 |
| Feeling refreshed | 2.93 (0.84) | 3.39 (0.77) | 3.03 (1.15) | 3.44 (0.95) | .24/.053 | 3.14 (1.00) | 3.65 (0.70) | .22/.08 |
| Easily waking up | 2.42 (0.65) | 2.33 (1.28) | 2.35 (0.62) | 2.32 (1.17) | .74/.59 | 2.45 (0.88) | 2.26 (1.14) | .69/.56 |
| Easily falling asleep | 2.32 (0.89) | 2.65 (1.11) | 1.83 (0.88) | 2.49 (1.10) | .18/<.001/<.001 | 1.85 (0.82) | 2.58 (1.10) | .05/.33 |
| Dreaming | 1.79 (0.78) | 1.83 (0.74) | 1.71 (0.70) | 1.74 (0.65) | .89/.24 | 1.81 (0.80) | 1.63 (0.62) | .91/.66 |
Abbreviation: LT, light therapy.
For the 5 sleep measures, scores range from 1 to 5. For the sleep quality and feeling refreshed measures, higher scores are better; for the easily waking up and easily falling asleep measures, lower scores are better; and for the dreaming measure, lower scores indicate less dreaming.
Effects of LT on Actigraphy-Derived Measures of Sleep-Wake Cycles
During actigraphy-defined sleep, sleep-onset latency diminished after the completion of bright LT and remained improved at the final study visit following the completion of the washout period (Table 4). Although actigraphy-derived sleep efficiency did not change after LT, total physical activity counts and the average physical activity counts became higher in both lighting conditions.
Table 4. Effects of LT on Actigraphy-Derived Sleep and Wake Metrics.
| Actigraphy Marker | Mean (SD) |
P Value, Group/ Condition/ Interaction |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Postintervention (Week 4) |
P Value, Group/ Condition/ Interaction |
Postwashout (Week 6) | |||||
| Bright LT | Dim-Red LT | Bright LT | Dim-Red LT | Bright LT | Dim-Red LT | |||
| Sleep Period | ||||||||
| Sleep onset latency, min | 30.16 (36.05) |
23.54 (21.45) |
27.68 (22.43) |
17.06 (13.04) |
.43/.75/.03 | 20.82 (10.67) |
16.88 (9.75) |
.36/.73/.03 |
| Total activity counts | 7247.5 (7242.5) |
8538.3 (9560.7) |
8680.9 (7807.1) |
7898.9 (4750.0) |
.54/.002 | 8755.8 (7445.1) |
7012.7 (4456.9) |
.79/.61 |
| Average activity counts | 19.74 (15.21) |
22.31 (18.58) |
24.09 (20.05) |
21.17 (11.91) |
.65/.002 | 22.22 (14.86) |
18.02 (11.38) |
.93/.73 |
| Sleep efficiency | 74.85 (15.41) |
75.94 (15.26) |
74.65 (16.23) |
79.32 (9.02) |
.60/.23 | 76.64 (10.43) |
81.06 (9.61) |
.39/.06 |
| Wake after sleep onset, min | 38.53 (40.67) |
43.21 (34.64) |
44.31 (42.52) |
43.37 (22.78) |
.37/<.001 | 48.06 (43.07) |
40.58 (28.02) |
.71/.45/.003 |
| Sleep time, min | 310.05 (72.49) |
327.49 (87.11) |
310.92 (82.14) |
322.35 (66.48) |
.49/.33 | 317.78 (51.63) |
334.32 (58.79) |
.49/.05 |
| Average light/illuminance, lux | 4.13 (8.35) |
1.46 (2.40) |
10.41 (32.89) |
7.47 (25.30) |
.04/.21 | 1.43 (2.09) |
0.75 (0.94) |
.02/.03 |
| Maximum light/highest illuminance, lux | 51.30 (41.39) |
39.89 (56.95) |
87.71 (180.37) |
26.66 (27.84) |
.24/.11/.02 | 34.57 (33.53) |
16.71 (13.91) |
.008/.26 |
| Wake Period | ||||||||
| Total activity counts | 164 847 (71 139) |
180 818 (64 901) |
189 413 (88 824) |
189 676 (47 246) |
.59/<.001 | 170 951 (90 948) |
184 419 (47 372) |
.49/.01 |
| Average activity counts | 165.01 (66.87) |
185.15 (56.63) |
194.59 (87.81) |
194.09 (47.35) |
.39/<.001/.02 | 173.70 (89.08) |
183.97 (43.43) |
.51/.007 |
| Sleep time, min | 385.72 (145.76) |
290.78 (82.84) |
344.09 (103.68) |
302.19 (109.43) |
.06/.24 | 340.53 (114.14) |
345.29 (136.13) |
.45/.69 |
| % Scored total sleep time | 38.85 (14.58) |
32.18 (10.16) |
34.99 (10.61) |
30.12 (9.80) |
.12/<.001 | 37.61 (13.56) |
32.29 (9.78) |
.15/.07 |
| Average light/illuminance, lux | 507.42 (445.48) |
304.74 (337.10) |
706.49 (717.92) |
314.82 (505.38) |
.01/<.001/.002 | 569.81 (511.67) |
236.20 (234.39) |
.00/<.001 |
| Maximum light/highest illuminance, lux | 23 870 (13 716) |
14 539 (13 134) |
24 509 (13 189) |
13 291 (17 333) |
.004/.001 | 23 637 (14 272) |
14 451 (12 564) |
.008/.004/.03 |
Abbreviation: LT, light therapy.
During actigraphy-defined wake, both types of LT were associated with significant increases in total physical activity counts that persisted at the end of the washout period (Table 4). Bright LT was associated with higher average physical activity count, compared with dim-red LT. The percentage of scored sleep time during the wake interval diminished in both LT groups.
Effects of LT on Disease Severity and Other Metrics
Light therapy led to improvements of disease severity as assessed by the UPDRS (Table 2); it was associated with significant improvements in the total UPDRS score and in the UPDRS parts I, II, and III scores (Table 2). No significant differences in the total and composite UPDRS scores were observed between the 2 LT arms. After the washout period, the favorable effect of LT persisted in the domain of activities of daily living as assessed by the UPDRS part II. Light therapy was not associated with significant changes in depression, anxiety, or quality of life.
Adverse Effects of LT
Light therapy was well tolerated. Within the bright LT group, 2 study participants reported 1 adverse effect each: headache and sleepiness. One participant in the dim-red LT group reported itchy eyes. These adverse effects resolved spontaneously.
Discussion
Light therapy is a noninvasive, nonpharmacological, well-tolerated intervention widely available and used as a treatment modality for several sleep and psychiatric disorders. This treatment modality is relatively easy to prescribe and incorporate into a clinical practice. Important aspects of LT are the timing of its administration and the spectral properties of the light applied in a specific LT regimen. The timing of LT is important because it may affect the circadian phase, causing either phase advance or delay. These changes are most likely to occur if LT is administered soon after the habitual wake time or in close proximity to the habitual sleep time. Bright LT remains the main treatment modality, but other wavelengths, such as blue and green lights, have been used as well.
In this study, we assessed the effects of timed LT on sleep and alertness and explored LT’s effects on motor and nonmotor manifestations and the quality of life for patients with PD. To our knowledge, this is the first study to target impaired sleep-wake cycles in PD with LT intervention. We used dim-red LT as the placebo arm because dim-red light has been accepted as an appropriate experimental control condition in studies of LT.
The LT improved several metrics of sleep and excessive sleepiness. It was the most beneficial in enhancing daytime alertness and sleep quality. Daytime sleepiness (as assessed by the ESS) and sleep quality, latency, and fragmentation (as assessed by self-reported sleep diaries) were significantly better in the bright LT group than in the dim-red LT group. Sleep latency (captured by sleep diaries) and overall sleep quality (assessed by the Parkinson’s Disease Sleep Scale and Pittsburgh Sleep Quality Index) improved in both treatment arms. After the 2-week washout period, sleep diary–assessed sleep fragmentation as well as actigraphy-assessed wake after sleep onset and sleep latency remained improved compared with the baseline status. These results are encouraging and relevant because sleep fragmentation and EDS are frequently present in patients with PD and represent a substantial portion of nonmotor PD symptoms with unmet therapeutic needs.
Of interest are the improvements in PD severity. These include improvements (which were present in both study arms after the LT intervention) in UPDRS total score and individual scores in parts I, II, and III. The UPDRS part II score remained better after the washout period than the baseline status score. This part is reflective of PD-specific effects on activities of daily living; therefore, it is encouraging to report potential benefits of LT on activities of daily living of patients with PD.
Light therapy was well tolerated, and the participants’ adherence to the study protocol was excellent. This finding is in agreement with results reported in the available literature on LT, which documented mild and transient side effects, including headache, nausea, and hypomania.
A few other studies examined the effects of supplemental light exposure on PD. In a pilot study of 36 patients with PD, Paus and colleagues administered bright light of 7500 lux and 950 lux (control condition) for 30 minutes in the morning for 14 days. These investigators observed significant improvements in tremor; UPDRS parts I, II, and IV; depression; and sleepiness. The control they used might not have been a true placebo because its intensity may have had active properties. In a case series of 12 patients with PD, white fluorescent light was administered for 1 to 1½ hours at an intensity of 100 to 1500 lux 1 hour before habitual sleep time. Within 2 weeks of that treatment, bradykinesia and rigidity were improved. With ongoing LT administration, improvements were noted in agitation, dyskinesias, and psychiatric symptoms with concomitant reduction of dopaminergic therapy. In an open-label study, 120 patients with PD were prescribed bright LT at the dose of 4000 to 6000 lux for 60 minutes before habitual bedtime; these patients were followed up from a few months to 8 years. Patients with good adherence to the therapy achieved enhancements in mood, anxiety, and motor function.
Several metrics of sleep, alertness, and motor PD severity improved in both LT arms of this study. One possible explanation for these findings is the placebo effect. Another possibility may be that a relatively short duration of light exposure in our study did not allow for differentiation of LT effects across the study groups. It has been suggested that the duration of LT exposure has to be at least 6 to 8 weeks for its beneficial effects to emerge. Another possible reason is that some individuals in the control group still had significant daily exposure to ambient bright light during the day (at times different from the LT times). Finally, anchoring the LT to a strict twice-daily regimen provided means for structuring daily activities, which itself may be an interesting possible mechanism underlying the beneficial effects of both bright LT and dim-red LT, given that a regular sleep-wake cycle may feed back to increase the amplitude of the circadian oscillator. Emerging experimental evidence suggests that behavioral manifestations of circadian disturbances may be modulated by a social context in which lifestyle factors contribute to symptom onset and manifestations.
There is no consensus on the optimal timing, dosage, and treatment duration for LT. We applied LT with 10 000 lux in the active arm. Most commonly used light intensities are between 2500 lux and 10 000 lux, administered in bursts of 1 to 2 hours. Exploratory studies in patients with PD used light intensity of 1000 to 7500 lux. Furthermore, light intensity of 1000 lux has been confirmed to synchronize rhythms in healthy people and to improve circadian rhythm abnormalities in older patients with dementia. Future studies of LT in the PD population need to address the optimal treatment parameters of LT.
The mechanisms that underlie the effects of LT on PD observed in this study are not clear. Light therapy may be exhibiting its therapeutic benefits through direct alerting effects. This mechanism could explain more the improvements in daytime alertness and less the benefits on sleep and motor symptoms. Another plausible mechanism of action is the potential influence on the circadian system. Activation of the suprachiasmatic nucleus has been hypothesized as one mechanism of bright light effects on mood, sleep, and circadian rhythm. Emerging evidence reveals dampening of the amplitude of the circadian system in PD. Parkinson disease is also associated with decreased exposure to environmental light and a decline in retinal function, which collectively reduce the input of the main alerting signal—bright light—to the suprachiasmatic nucleus. Light exposure via its effects on the circadian pathways may strengthen circadian signal and improve consolidation of sleep-wake cycles in PD.
Limitations
A major limitation of this study was that light levels were not measured at other times of the day. Therefore, some individuals in the dim-red LT goup received as much or more light exposure (from nonlightbox lighting sources) than those under the bright LT group. To adjust for this difference, we performed some analyses using the light levels recorded by the actigraphy monitors. However, this difference in exposure may have affected our ability to find differences between the groups. Future studies may be more strict in controlling such exposures.
Conclusions
Light therapy is a well-tolerated, feasible intervention for impaired sleep-wake cycles associated with PD. Bright LT was associated with improvements in EDS, sleep fragmentation, and sleep quality. Light therapy may also be beneficial for motor symptoms of PD. Based on these results, the next logical step is to optimize various parameters of LT (eg, intensity, duration, and wavelength) not only for impaired sleep and alertness but also for other motor and nonmotor manifestations of PD. Such chronobiological treatment strategies would be highly desirable because pharmacological interventions for sleep disturbances in PD have been of modest benefit and may cause unacceptable side effects.
Trial Protocol
References
- 1.Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson’s disease. J Neurol. 2008;255(suppl 5):18-32. [DOI] [PubMed] [Google Scholar]
- 2.Dorsey ER, Constantinescu R, Thompson JP, et al. . Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384-386. [DOI] [PubMed] [Google Scholar]
- 3.Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clin Neuropharmacol. 1988;11(6):512-519. [DOI] [PubMed] [Google Scholar]
- 4.Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson’s disease. Mov Disord. 1998;13(6):895-899. [DOI] [PubMed] [Google Scholar]
- 5.Karlsen KH, Tandberg E, Arsland D, Larsen JP. Health related quality of life in Parkinson’s disease: a prospective longitudinal study. J Neurol Neurosurg Psychiatry. 2000;69(5):584-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaravilli T, Gasparoli E, Rinaldi F, Polesello G, Bracco F. Health-related quality of life and sleep disorders in Parkinson’s disease. Neurol Sci. 2003;24(3):209-210. [DOI] [PubMed] [Google Scholar]
- 7.Meindorfner C, Körner Y, Möller JC, Stiasny-Kolster K, Oertel WH, Krüger HP. Driving in Parkinson’s disease: mobility, accidents, and sudden onset of sleep at the wheel. Mov Disord. 2005;20(7):832-842. [DOI] [PubMed] [Google Scholar]
- 8.Factor SA, McAlarney T, Sanchez-Ramos JR, Weiner WJ. Sleep disorders and sleep effect in Parkinson’s disease. Mov Disord. 1990;5(4):280-285. [DOI] [PubMed] [Google Scholar]
- 9.Stocchi F, Barbato L, Nordera G, Berardelli A, Ruggieri S. Sleep disorders in Parkinson’s disease. J Neurol. 1998;245(suppl 1):S15-S18. [DOI] [PubMed] [Google Scholar]
- 10.Paus S, Brecht HM, Köster J, Seeger G, Klockgether T, Wüllner U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s disease. Mov Disord. 2003;18(6):659-667. [DOI] [PubMed] [Google Scholar]
- 11.Rye DB, Bliwise DL, Dihenia B, Gurecki P. FAST TRACK: daytime sleepiness in Parkinson’s disease. J Sleep Res. 2000;9(1):63-69. [DOI] [PubMed] [Google Scholar]
- 12.Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 1997;82(11):3763-3770. [DOI] [PubMed] [Google Scholar]
- 13.Myers BL, Badia P. Changes in circadian rhythms and sleep quality with aging: mechanisms and interventions. Neurosci Biobehav Rev. 1995;19(4):553-571. [DOI] [PubMed] [Google Scholar]
- 14.Bruguerolle B, Simon N. Biologic rhythms and Parkinson’s disease: a chronopharmacologic approach to considering fluctuations in function. Clin Neuropharmacol. 2002;25(4):194-201. [DOI] [PubMed] [Google Scholar]
- 15.Willis GL. Parkinson’s disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci. 2008;19(4-5):245-316. [DOI] [PubMed] [Google Scholar]
- 16.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201-206. [DOI] [PubMed] [Google Scholar]
- 17.Dijk DJ, Lockley SW. Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol (1985). 2002;92(2):852-862. [DOI] [PubMed] [Google Scholar]
- 18.Moore-Ede MC, Czeisler CA, Richardson GS. Circadian timekeeping in health and disease. Part 2. Clinical implications of circadian rhythmicity. N Engl J Med. 1983;309(9):530-536. [DOI] [PubMed] [Google Scholar]
- 19.Czeisler CA, Allan JS, Strogatz SH, et al. . Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233(4764):667-671. [DOI] [PubMed] [Google Scholar]
- 20.Wever RA, Polásek J, Wildgruber CM. Bright light affects human circadian rhythms. Pflugers Arch. 1983;396(1):85-87. [DOI] [PubMed] [Google Scholar]
- 21.Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: effects on sleep and activity rhythms. J Sleep Res. 2000;9(4):373-379. [DOI] [PubMed] [Google Scholar]
- 22.Rios Romenets S, Creti L, Fichten C, et al. . Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson’s disease: a randomized study. Parkinsonism Relat Disord. 2013;19(7):670-675. [DOI] [PubMed] [Google Scholar]
- 23.Willis GL, Turner EJ. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int. 2007;24(3):521-537. [DOI] [PubMed] [Google Scholar]
- 24.Paus S, Schmitz-Hübsch T, Wüllner U, Vogel A, Klockgether T, Abele M. Bright light therapy in Parkinson’s disease: a pilot study. Mov Disord. 2007;22(10):1495-1498. [DOI] [PubMed] [Google Scholar]
- 25.Adler CH, Caviness JN, Hentz JG, Lind M, Tiede J. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Mov Disord. 2003;18(3):287-293. [DOI] [PubMed] [Google Scholar]
- 26.van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med Rev. 2016;29:52-62. [DOI] [PubMed] [Google Scholar]
- 27.Kogan AO, Guilford PM. Side effects of short-term 10,000-lux light therapy. Am J Psychiatry. 1998;155(2):293-294. [DOI] [PubMed] [Google Scholar]
- 28.Willis GL, Moore C, Armstrong SM. A historical justification for and retrospective analysis of the systematic application of light therapy in Parkinson’s disease. Rev Neurosci. 2012;23(2):199-226. [DOI] [PubMed] [Google Scholar]
- 29.Morton AJ, Rudiger SR, Wood NI, et al. . Early and progressive circadian abnormalities in Huntington’s disease sheep are unmasked by social environment. Hum Mol Genet. 2014;23(13):3375-3383. [DOI] [PubMed] [Google Scholar]
- 30.Reid KJ, Burgess HJ. Circadian rhythm sleep disorders. Prim Care. 2005;32(2):449-473. [DOI] [PubMed] [Google Scholar]
- 31.Middleton B, Stone BM, Arendt J. Human circadian phase in 12:12 h, 200: <8 lux and 1000: <8 lux light-dark cycles, without scheduled sleep or activity. Neurosci Lett. 2002;329(1):41-44. [DOI] [PubMed] [Google Scholar]
- 32.Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41(9):955-963. [DOI] [PubMed] [Google Scholar]
- 33.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11(6):453-464. [DOI] [PubMed] [Google Scholar]
- 34.McEnany GW, Lee KA. Effects of light therapy on sleep, mood, and temperature in women with nonseasonal major depression. Issues Ment Health Nurs. 2005;26(7):781-794. [DOI] [PubMed] [Google Scholar]
- 35.Hubbard J, Ruppert E, Gropp CM, Bourgin P. Non-circadian direct effects of light on sleep and alertness: lessons from transgenic mouse models. Sleep Med Rev. 2013;17(6):445-452. [DOI] [PubMed] [Google Scholar]
- 36.Videnovic A, Noble C, Reid KJ, et al. . Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71(4):463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinson’s disease. Brain. 2009;132(pt 5):1128-1145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol