Key Points
Question
Are intracranial collateral blood vessels, identified using arterial spin labeling magnetic resonance imaging of the brain, associated with neurologic outcome after acute ischemic stroke?
Findings
In a cohort study of 38 patients with acute ischemic stroke, collaterals on arterial spin labeling were associated with better neurologic outcome at hospital discharge, both in unadjusted and adjusted analyses.
Meaning
This novel association between arterial spin labeling MRI collaterals and improved neurologic outcome may help guide prognosis and management, particularly in patients who are unable to undergo contrast-based radiological studies.
This cohort study assesses the association between the presence of collateral vessels identified using arterial spin labeling magnetic resonance imaging and neurologic outcome in patients after acute ischemic stroke.
Abstract
Importance
Robust collateral blood vessels have been associated with better neurologic outcome following acute ischemic stroke (AIS). The most commonly used methods for identifying collaterals are contrast-based angiographic imaging techniques, which are not possible in all patients after AIS.
Objective
To assess the association between the presence of collateral vessels identified using arterial spin labeling (ASL) magnetic resonance imaging, a technique that does not require exogenous administration of contrast, and neurologic outcome in patients after AIS.
Design, Setting, and Participants
This retrospective cohort study examined 38 patients after AIS admitted to a tertiary academic medical center between 2012 and 2014 who underwent MRI with ASL.
Main Outcomes and Measures
According to a prespecified hypothesis, ASL images were graded for the presence of collaterals by 2 neuroradiologists. Modified Rankin Scale (mRS) scores at discharge and other composite data were abstracted from the medical record by a neurologist blinded to radiologic data.
Results
Of the 38 patients, 19 (50.0%) were male, and the mean (SD) age was 61 (20) years. In 25 of 38 patients (65.8%), collaterals were detected using ASL, which were significantly associated with both a good outcome (mRS score of 0-2 at discharge; P = .02) and a 1-point decrease in mRS score at discharge (odds ratio, 6.4; 95% CI, 1.7-23.4; P = .005). In a multivariable ordinal logistic regression model, controlling for admission National Institutes of Health Stroke Scale score, history of atrial fibrillation, premorbid mRS score, and stroke parent artery status, there was a strong association between the presence of ASL collaterals and a 1-point decrease in the mRS score at discharge (odds ratio, 5.1; 95% CI, 1.2-22.1; P = .03).
Conclusions and Relevance
Following AIS, the presence of ASL collaterals is strongly associated with better neurological outcome at hospital discharge. This novel association between ASL collaterals and improved neurologic outcome may help guide prognosis and management, particularly in patients who are unable to undergo contrast-based radiological studies.
Introduction
After adjusting for stroke severity, patients after acute ischemic stroke (AIS) with robust collaterals have smaller final infarct volume and better clinical outcomes. Acute ischemic stroke is characterized by an ischemic penumbra, a region of salvageable brain tissue, that surrounds a core of irreversible ischemic infarct. The penumbra is tenuously perfused by collateral blood vessels, which, if extensive enough, can maintain penumbral perfusion, improving the odds for a larger volume of surviving brain tissue and a smaller final infarct volume. Standard methods for evaluating collaterals in the acute setting include computed tomography angiography, magnetic resonance angiography, and digital subtraction angiography.
Arterial spin labeling (ASL) is a quantitative magnetic resonance imaging (MRI) technique that uses blood as an endogenous contrast agent to assess cerebral perfusion. Arterial spin labeling has been used in patients after AIS to quantify ischemic penumbra. Its advantages include a relatively short scan time (4-6 minutes), a lack of ionizing radiation, and independence from an exogenous contrast agent (contraindicated in patients with impaired renal function or documented sensitivity). Collaterals can be identified within ASL images as foci of curvilinear hyperintensity bordering regions of hypoperfusion. Arterial spin labeling has been used to identify collaterals in patients with Moyamoya disease and ischemic stroke. We sought to explore a novel association between the presence of ASL collaterals (ASLcs) and neurological outcome in patients after AIS.
Methods
A retrospective search of an inpatient ischemic stroke database identified 38 patients admitted due to AIS between 2012 and 2014 who had undergone MRI examinations containing an acute diffusion-weighted imaging lesion and a diagnostic-quality ASL sequence. Arterial spin labeling images were obtained on a 3-Tesla MAGNETOM Tim Trio System (Siemens Healthcare), incorporating quantitative imaging of perfusion using a single subtraction, second version (QUIPSS II) with thin-slice inversion time (TI), periodic saturation (Q2TIPS) and proximal inversion with control for off-resonance effects with the following parameters: 52 label and control image pairs with slice spacing (0-mm section gap); slice thickness = 5 mm for 21 slices or 7.5 mm for 17 slices (dependent on protocol); echo time = 12 milliseconds; time to inversion 1 (bolus duration) = 800 milliseconds; time to inversion (time from application of labeling pulse to image acquisition) = 1800 milliseconds; repetition time = 3400 milliseconds; receiver bandwidth = 2367 Hz/pixel; flip angle = 90°; and field of view = 192; 64 × 64 matrix. Arterial spin labeling images underwent inline motion correction and were acquired without vascular crusher gradients. Arterial spin labeling perfusion maps were obtained from the console without further processing. This study was approved by the University of Washington Institutional Review Board and is compliant with the Health Insurance and Portability Accountability Act. Patient consent was waived owing to the retrospective nature of this study.
An experienced neuroradiologist graded ASL images for the presence of collaterals, which were defined dichotomously as the presence or absence of curvilinear areas of hyperintensity, known as arterial transit artifact (ATA), along 10% or more of the border of an ASL hypoperfusion abnormality that corresponded to a diffusion-weighted imaging lesion (Figure 1). A second neuroradiologist without experience in ASL interpretation read our description of ASLcs and then graded the images for ASLcs. The results were compared with the findings from the experienced neuroradiologist using an unweighted Cohen κ. Magnetic resonance images for individual patients were evaluated in random order at a dedicated picture archiving and communication system station. Patient medical record review was performed by a blinded vascular neurologist, who abstracted a discharge and follow-up modified Rankin Scale (mRS) score and other relevant covariates, such as admission National Institutes of Health Stroke Scale (NIHSS) score. While nearly all patients underwent vascular imaging, this included a mix of computed tomography, MRI, and catheter angiograms, precluding comparison based on a single scoring system.
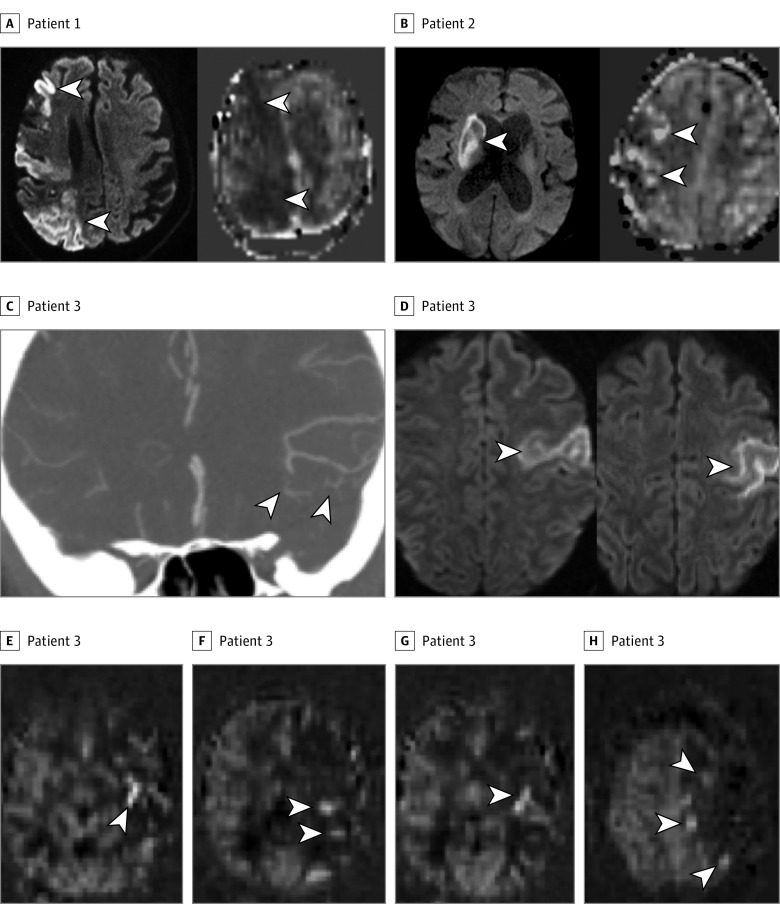
Figure 1. Three Patients With Acute Ischemic Stroke and a Range of Collateral Arterial Transit Artifact (ATA) Signal on Arterial Spin Labeling (ASL).
A, Patient 1. Left, Diffusion-weighted imaging (DWI) showing multifocal infarcts (arrowheads) throughout the right middle cerebral artery (MCA) territory. Right, ASL showing a large area of decreased cerebral blood flow (CBF) without ASL collaterals (ASLcs). Arrowheads indicate the absence of ATA hyperintensity, which was present on all ASL images. B, Patient 2. Left, DWI showing a right basal ganglia infarct (arrowhead). Right, ASL showing a larger area of decreased CBF with moderate ASLcs (arrowheads). C-H, Patient 3. Computed tomographic (CT) angiogram of the neck (not shown) showed occluded left internal carotid artery. C, CT angiogram of the brain showing excellent leptomeningeal collateral vessels (arrowheads) distal to the occlusion. D, DWI showing the cortical area of infarct in the left MCA territory. E-H, ASL showing decreased CBF in the left MCA territory, with a pattern of subtle ASLcs seen on multiple slices (arrowheads).
Statistical analyses were performed using Stata version 14.1 (StataCorp). Descriptive statistics were reported using the mean and SD for variables with normal distribution and median and interquartile ranges for variables with skewed distribution. Dichotomous variables were compared with the Fisher exact test and continuous variables with the Spearman and Mann-Whitney U test. In ordinal logistic regression, the primary predictor variable was ASLc, and mRS score at hospital discharge was the primary outcome. Modified Rankin Scale scores at follow-up (90 days or more after discharge) were available in 19 patients, including only 3 without ASLc, making statistical analysis based on that outcome impossible in this small cohort.
Covariates were tested for an association with mRS score at discharge and were included in the model if their univariate association by ordinal logistic regression had a P < .20. Evaluated covariates included patient sex, age, admission NIHSS score, hypertension, hyperlipidemia, atrial fibrillation, diabetes, body mass index, cigarette smoking, anterior vs posterior circulation, less than 50% stenosis vs greater than 50% stenosis vs occluded stroke parent artery, mean systolic blood pressure, and mean diastolic blood pressure. An ordinal logistic regression model was fitted to the outcome of the mRS score at hospital discharge, although the scores were inverted to provide odds ratio (OR) estimates for a 1-point decrease in the mRS score. An additional analysis examined whether ASLcs were associated with a good outcome at discharge (ie, a mRS score of 0-2) using the Fisher exact test. Statistical significance was set at P < .05.
Results
Thirty-eight patients, including 19 (50.0%) males and females, met inclusion criteria, with a mean (SD) age of 61 (20) years, a mean (SD) NIHSS score of 10.6 (10.5), and a median (interquartile range) discharge mRS score of 3 (1-4) (Table 1). Arterial spin labeling collaterals were present in 25 patients (65.8%). The interrater agreement for ASLcs between the experienced neuroradiologist and the neuroradiologist without ASL experience was moderate (κ, 0.55; agreement, 82%). Anterior circulation stroke was present in 32 patients (84.2%). The stroke parent artery had occlusion in 11 patients (28.9%), and greater than 50% stenosis was present in 17 patients (44.7%) vs less than 50% stenosis in 10 patients (26.3%) (Table 1). The median (interquartile range) number of days from stroke onset to MRI was 1 (0-3), and MRI was performed within 24 hours of symptom onset in 25 patients (65.8%). Premorbid mRS score was 0 in 27 patients (71.1%), 1 in 9 patients (23.7%), 2 in 1 patient (2.6%), and 3 in 1 patient (2.6%).
Table 1. Demographic Information and Outcome Data.
| Variable | Patients With AIS, No. (%) | P Value | ||
|---|---|---|---|---|
| With ASL (n = 38) |
With ASL Collaterals (n = 25) |
Without ASL Collaterals (n = 13) |
||
| Male | 19 (50.0) | 12 (48.0) | 7 (53.8) | .73 |
| Age, mean (SD), y | 61 (20) | 59 (22) | 64 (15) | .45 |
| Admission NIHSS score, mean (SD) | 11 (11) | 8 (8) | 16 (13) | .03 |
| History of atrial fibrillation | 12 (31.6) | 6 (24.0) | 6 (46.2) | .15 |
| Premorbid mRS score, median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-1) | .34 |
| mRS score at discharge, median (IQR) | 3 (1-4) | 2 (1-3) | 4 (3-4) | .005 |
| Patients with good outcome at discharge (ie, mRS score, 0-2) | 17 (44.7) | 15 (60.0) | 2 (15.4) | .02 |
| Days from stroke onset to ASL MRI, mean (SD) | 1.9 (2.5) | 2.1 (2.6) | 1.6 (2.3) | .56 |
| Collaterals on ASL | 25 (65.8) | NA | NA | NA |
| Stenosis in stroke parent arterya | .44 | |||
| <50 | 11 (28.9) | 5 (20.0) | 6 (46.2) | |
| >50 | 17 (44.7) | 14 (56.0) | 3 (23.1) | |
| Occlusion of stroke parent arterya | 10 (26.3) | 6 (24.0) | 4 (30.8) | .44 |
Abbreviations: AIS, acute ischemic stroke; ASL, arterial spin labeling; IQR, interquartile range; mRS, Modified Rankin Scale; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale.
P value is for comparison between all 3 categories for stroke parent vessel status using the Wilcoxon rank sum test.
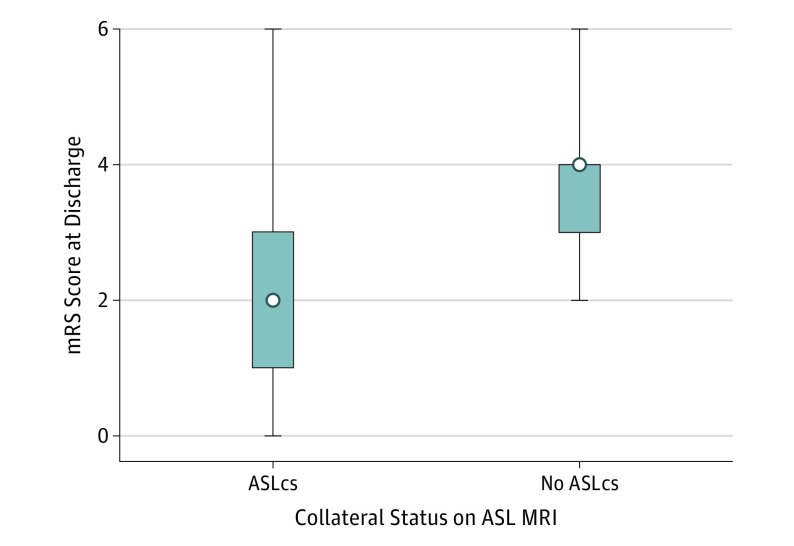
Stroke severity, as measured by admission NIHSS score, was associated with discharge mRS score (ρ, 0.69; P < .001) and also with ASLcs (ρ, −0.33; P = .04). The presence of ASLcs was associated with lower median mRS scores at discharge (2 vs 4; P = .005) (Figure 2). Arterial spin labeling collaterals were also associated with a good outcome at discharge (mRS score, 0-2), which was present in 15 of 25 patients (60.0%) with ASLcs and only 2 of 13 (15.4%) without ASLcs (P = .02). In univariate ordinal logistic regression, the presence of ASLcs was associated with an OR of 6.4 (95% CI, 1.7-23.4; P = .005) for a 1-point decrease in the mRS score at discharge (Table 2). After controlling for admission NIHSS score, history of atrial fibrillation, premorbid mRS score, and stroke parent artery status, multivariable ordinal logistic regression showed ASLcs were associated with a 1-point decrease in discharge mRS score, with an OR of 5.1 (95% CI, 1.2-22.1; P = .03).
Figure 2. Box Plot of Modified Rankin Scale (mRS) Scores in Patients With vs Without Arterial Spin Labeling Collaterals (ASLcs).
The box plots demonstrate a lower median mRS score in the group with ASLcs (z, 3.0; P = .005). The box indicates the interquartile range (25/75); error bars, range; white dot, the median. MRI indicates magnetic resonance imaging.
Table 2. One-Point Decrease in mRS Score at Dischargea.
| Variable | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Admission NIHSS score | 0.86 (0.80-0.93) | <.001 | NA | NA |
| Premorbid mRS score | 0.28 (0.11-0.72) | .008 | NA | NA |
| History of atrial fibrillation | 0.19 (0.05-0.71) | .01 | NA | NA |
| Parent vessel statusb | 0.41 (0.17-0.99) | .05 | NA | NA |
| Collaterals on ASL | 6.36 (1.73-23.4) | .005 | 5.14 (1.19-22.1) | .03 |
Abbreviations: ASL, arterial spin labeling; mRS, Modified Rankin Scale; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio.
Multivariable ordinal logistic regression model, adjusting for patient age, admission NIHSS score, atrial fibrillation, parent vessel status, and ASL collaterals.
Parent vessel to the ischemic stroke status, defined as an ordinal variable: 0 represented <50% stenosis, 1 represented >50% stenosis, and 2 represented occlusion.
Discussion
Our data show a significant association between the presence of ASLcs and improved neurologic outcome at hospital discharge in patients after AIS. After adjusting for potential confounders, patients with ASLcs were 5-fold more likely to have a 1-point decrease in mRS score at hospital discharge compared with those without ASLcs. Patients with ASLcs were also more likely to have a good neurologic outcome (mRS score, 0-2) compared with those without ASLcs.
To our knowledge, ASL has not historically been used to visualize collaterals, although a pilot study demonstrated a strong agreement with digital subtraction angiography in predicting the presence and intensity of collateral blood flow (identified as ATA) in patients with Moyamoya disease. A subsequent study identified ATA in 12 of 17 patients after ischemic stroke but did not attempt to correlate their presence with outcome. Our results extend this research to demonstrate that collaterals on ASL are also associated with improved neurologic outcome at hospital discharge, which can provide relevant clinical information for a subset of patients unable to tolerate intravenous contrast.
In ASL, the postlabel delay (PLD), ie, the time between blood labeling and MRI image acquisition, competes with the arterial transit time (ATT), a parameter that is dependent on cerebrovascular physiology and autonomic regulation (under nonpathological conditions). The PLD is individually adjusted based on patient age (pediatric patients have shorter ATT owing to smaller body size and higher flow velocities), expected pathophysiology (ATT is extended in the setting of poor cardiac output, hypovolemia, and intracranial atherosclerosis), and vendor-dependent or technique-dependent parameters, such as labeling efficiency. Arterial spin labeling is quantitative when the intravascular label (labeled water protons in the blood pool) reaches the target tissue, crosses the blood-brain barrier, and successfully exchanges with intracellular water before the PLD has expired, marking the commencement of image acquisition. While the PLD can be varied per experiment, the ATT is a physiologically dependent variable that differs by patient or arterial vessel and is initially unknown at the time of the ASL experiment. For this reason, longer PLDs are generally preferred, allowing for slower-traveling blood to reach the target tissue in exchange for slightly lower ASL signal (and signal-to-noise ratio) within the target tissue.
Intravascular label that has not yet reached the target tissue by the time imaging commences (vessels in which ATT is greater than PLD) will manifest as bright signal within vessels that are still transiting at the time of imaging. In the context of collateral vessels, these will primarily be curvilinear in trajectory and pial in distribution and will overlie the greater hemispheric convexities. In addition, hyperintense intravascular signal may also be identified within the major branches of the circle of Willis vessels, representing slow flow owing to either more proximal or distal vascular lesions. There are 2 key features that are critical for correctly identifying high signal in collateral vessels: (1) the high signal must be curvilinear (intravascular), along the expected trajectory of the circle of Willis vessels, and/or overlying the greater convexities (representative of pial collateral vessels), and (2) the adjacent target tissue must be hypoperfused relative to nonischemic brain regions. These 2 findings are mutually dependent on each other, as ischemic tissue (in AIS) is typically ischemic as a result of delayed ATT, which permits simultaneous evaluation of cerebral hypoperfusion and collateral vessels using ASL.
Traditional collateral angiographic imaging modalities have shown that robust collaterals result in better long-term functional outcome, adjusting for stroke severity, as a result of salvaged penumbra and smaller final infarct volume. Compared with patients with poor collaterals, those with robust collaterals also derive additional benefit from acute therapies aimed at improving stroke outcome, such as intravenous tissue plasminogen activator and endovascular thrombectomy. The collateral vascularity in the circle of Willis and leptomeningeal distribution varies widely between pateints. The best-established risk factor for poor collaterals is patient age, but hypertension and metabolic syndrome may also have an important negative influence on collaterals. Without a reliable clinical indicator of collateral status, the radiologic determination of collaterals is a potentially important aspect of the acute stroke evaluation, which can help guide management decisions following AIS, including the usefulness of endovascular thrombectomy.
Arterial spin labeling has several advantages over traditional imaging techniques for detecting collaterals; it provides data for quantitative cerebral blood flow evaluation, has minimal sensitivity to blood-brain barrier disruption, requires no exogenous contrast agent or radiation exposure, lends itself to serial measurements, and poses little to no risk to the patient. Furthermore, ASL is commercially available on all major MRI vendor platforms, and its reproducibility has been demonstrated in multicenter studies. Previously described disadvantages of ASL include the relative lack of expeditious MRI scanner availability in evaluating hyperacute ischemic strokes, inferior signal-noise ratio compared with other computed tomography and MRI perfusion techniques, and the possibility of prominent artifacts created by arterial transit delays within large vessels, although the lattermost disadvantage (ie, ATA) proved to be beneficial in this analysis.
Limitations
This study has notable limitations, including a small sample size and the lack of a reference standard angiographic comparison to allow for definitive identification of ATA as evidence of collaterals. Although the interrater agreement on ASLcs between the experienced and inexperienced neuoradiologist raters was moderate, this demonstrates that without formal training, a reader of this article could begin to grade for ASLcs. An additional comparison between 2 raters with ASL experience showed near-perfect agreement. Correlation of collateral status with a standardized 90-day mRS score would have been preferable but would have prohibitively decreased the sample size. This was also a retrospective study, which did not permit uniform ASL acquisition at a set time point. Finally, the possibility of a selection bias for patients in whom ASL was performed as part of their AIS work-up cannot be excluded, as the inclusion of the ASL sequence was dependent on protocol selection prior to MRI acquisition and may have introduced the preference of the protocolling neuroradiologist. These limitations can be addressed with a prospective study design.
Conclusions
The novel association between ASLcs and better neurologic outcome at hospital discharge may help guide AIS prognosis and management, particularly in patients who are unable to undergo contrast-based radiological studies. Arterial spin labeling is underused for acute stroke, largely because of a lack of familiarity with the technique, and may eventually provide a safer standard of care for acute AIS imaging. If ASL can provide useful information about penumbral and collateral status in a single acquisition, its usefulness in acute stroke would be even more apparent. In light of our results, additional prospective study is warranted to further explore the complex relationships between ASLcs, penumbra, collaterals identified with other modalities, effectiveness of AIS interventions in the setting of ASLcs, and long-term functional outcome.
References
- 1.McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012;33(3):576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher M, Bastan B. Identifying and utilizing the ischemic penumbra. Neurology. 2012;79(13, suppl 1):S79-S85. [DOI] [PubMed] [Google Scholar]
- 3.Siegler JE, Boehme AK, Kumar AD, et al. . Identification of modifiable and nonmodifiable risk factors for neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(7):e207-e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am. 2005;15(3):553-573, x. [DOI] [PubMed] [Google Scholar]
- 5.Hartkamp NS, van Osch MJP, Kappelle J, Bokkers RPH. Arterial spin labeling magnetic resonance perfusion imaging in cerebral ischemia. Curr Opin Neurol. 2014;27(1):42-53. [DOI] [PubMed] [Google Scholar]
- 6.Bivard A, Krishnamurthy V, Stanwell P, et al. . Arterial spin labeling versus bolus-tracking perfusion in hyperacute stroke. Stroke. 2014;45(1):127-133. [DOI] [PubMed] [Google Scholar]
- 7.Niibo T, Ohta H, Yonenaga K, Ikushima I, Miyata S, Takeshima H. Arterial spin-labeled perfusion imaging to predict mismatch in acute ischemic stroke. Stroke. 2013;44(9):2601-2603. [DOI] [PubMed] [Google Scholar]
- 8.Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke. 2011;42(9):2485-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutke MA, Madai VI, von Samson-Himmelstjerna FC, et al. . Clinical evaluation of an arterial-spin-labeling product sequence in steno-occlusive disease of the brain. PLoS One. 2014;9(2):e87143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gevers S, van Osch MJ, Bokkers RPH, et al. . Intra- and multicenter reproducibility of pulsed, continuous and pseudo-continuous arterial spin labeling methods for measuring cerebral perfusion. J Cereb Blood Flow Metab. 2011;31(8):1706-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsop DC, Detre JA, Golay X, et al. . Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke. 2015;46(11):3302-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons MW, Albers GWMR. MR RESCUE: is the glass half-full or half-empty? Stroke. 2013;44(7):2055-2057. [DOI] [PubMed] [Google Scholar]
- 14.Kucinski T, Koch C, Eckert B, et al. . Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology. 2003;45(1):11-18. [DOI] [PubMed] [Google Scholar]
- 15.Liebeskind DS, Jahan R, Nogueira RG, Zaidat OO, Saver JL; SWIFT Investigators . Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke. 2014;45(7):2036-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arsava EM, Vural A, Akpinar E, et al. . The detrimental effect of aging on leptomeningeal collaterals in ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23(3):421-426. [DOI] [PubMed] [Google Scholar]
- 17.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. [DOI] [PubMed] [Google Scholar]