This randomized clinical trial reports the effects of medication reminder devices on patients’ adherence to their drug therapy.
Key Points
Question
To what extent can 3 low-cost reminder devices improve medication adherence among individuals who are receiving therapy but are poorly adherent?
Findings
In this randomized clinical trial of 53 480 enrollees of a pharmacy benefit manager, no statistically significant difference in adherence was found between those in the control group and those who received a reminder device (pill bottle strip with toggles, digital timer cap, or standard pillbox).
Meaning
Future research should focus on effective targeting of interventions and strategies that ensure sustained medication use.
Abstract
Importance
Forgetfulness is a major contributor to nonadherence to chronic disease medications and could be addressed with medication reminder devices.
Objective
To compare the effect of 3 low-cost reminder devices on medication adherence.
Design, Setting, and Participants
This 4-arm, block-randomized clinical trial involved 53 480 enrollees of CVS Caremark, a pharmacy benefit manager, across the United States. Eligible participants were aged 18 to 64 years and taking 1 to 3 oral medications for long-term use. Participants had to be suboptimally adherent to all of their prescribed therapies (with a medication possession ratio of 30% to 80%) in the 12 months before randomization. Participants were stratified on the basis of the medications they were using at randomization: medications for cardiovascular or other nondepression chronic conditions (the chronic disease stratum) and antidepressants (the antidepressant stratum). In each stratum, randomization occurred within blocks defined by whether all of the patient’s targeted medications were dosed once daily. Patients were randomized to receive in the mail a pill bottle strip with toggles, digital timer cap, or standard pillbox. The control group received neither notification nor a device. Data were collected from February 12, 2013, through March 21, 2015, and data analyses were on the intention-to-treat population.
Main Outcomes and Measures
The primary outcome was optimal adherence (medication possession ratio ≥80%) to all eligible medications among patients in the chronic disease stratum during 12 months of follow-up, ascertained using pharmacy claims data. Secondary outcomes included optimal adherence to cardiovascular medications among patients in the chronic disease stratum as well as optimal adherence to antidepressants.
Results
Of the 53 480 participants, mean (SD) age was 45 (12) years and 56% were female. In the primary analysis, 15.5% of patients in the chronic disease stratum assigned to the standard pillbox, 15.1% assigned to the digital timer cap, 16.3% assigned to the pill bottle strip with toggles, and 15.1% assigned to the control arm were optimally adherent to their prescribed treatments during follow-up. There was no statistically significant difference in the odds of optimal adherence between the control and any of the devices (standard pillbox: odds ratio [OR], 1.03 [95% CI, 0.95-1.13]; digital timer cap: OR, 1.00 [95% CI, 0.92-1.09]; and pill bottle strip with toggles: OR, 0.94 [95% CI, 0.85-1.04]). In direct comparisons, the odds of optimal adherence were higher with a standard pillbox than with the pill bottle strip (OR, 1.10 [95% CI, 1.00-1.21]). Secondary analyses yielded similar results.
Conclusions and Relevance
Low-cost reminder devices did not improve adherence among nonadherent patients who were taking up to 3 medications to treat common chronic conditions. The devices may have been more effective if coupled with interventions to ensure consistent use or if targeted to individuals with an even higher risk of nonadherence.
Trial Registration
clinicaltrials.gov Identifier: NCT02015806
Introduction
Suboptimal adherence to medications for chronic conditions, such as hypertension and diabetes, results in potentially avoidable morbidity, mortality, and health care spending. A variety of factors are responsible for patients not taking their medications as prescribed, but up to 60% of individuals identify forgetfulness as their primary explanation. Therefore, tools that remind patients to take their prescribed medications may help improve adherence by providing visual or auditory cues and by creating good habits around routine medication taking. Particular attention has been focused on electronic medication-packaging devices that provide alerts when medications are to be taken as well as feedback to patients and their caregivers about adherence. Unfortunately, there are limited and inconsistent data supporting the effectiveness of these devices; in many cases, their cost may be prohibitive for wide-scale application.
Simple and low-cost reminder devices, such as pill bottle caps with a digital timer or nonelectronic pillboxes, could be a cost-efficient alternative to overcoming forgetfulness. Small studies have found that such low-cost devices improve adherence to antiretroviral medications, but there are limited data about their ability to improve the quality of medication taking in other therapeutic areas, especially in real-world naturalistic settings. Accordingly, we conducted the Randomized Evaluation to Measure Improvements in Nonadherence from Low-Cost Devices (REMIND) trial to evaluate the effect on adherence to medications for chronic conditions commonly managed in primary care settings of 3 low-cost devices: a pill bottle strip with toggles for each day of the week, a pill bottle cap with a digital timer displaying the time elapsed since the medication was last taken, and a plastic organization box with 1 compartment for every day of the week.
Methods
Study Design
The REMIND trial was a pragmatic, block-randomized, controlled, 4-arm comparative effectiveness study. Details of the study design and protocol have been published previously. The academic authors (N.K.C., N.F.K., A.A.K., A.Y.T., and J.M.F.) analyzed the data using an independent copy of the study database and attested to the analytic accuracy and completeness as well as the fidelity of the report to the trial protocol (available in Supplement 1).
The trial was approved by the institutional review board of Brigham and Women’s Hospital, Boston, Massachusetts, and by the Chesapeake Institutional Review Board. Because the study devices are currently available for commercial use and because participants received the devices by mail and could choose not to use them, patient-level consent was waived by Chesapeake Institutional Review Board.
Study Devices
The REMIND trial evaluated 3 low-cost adherence devices: (1) a pill bottle with an affixed strip with toggles that can be slid after each day’s dose has been taken (Take-n-Slide; IC Innovations), (2) a pill bottle cap with a digital timer displaying the time elapsed since the medication was last taken (Rx TimerCap; TimerCap LLC), and (3) a standard plastic pillbox with 1 compartment for each day of the week (Appendix eFigure 1 in Supplement 2).
Study Population
In our study, conducted from February 12, 2013, through March 21, 2015, we enrolled individuals aged 18 to 64 years whose prescription drug benefits were administered by CVS Caremark, a large pharmacy benefits manager that provides coverage to more than 65 million individual members in the United States. We included only commercially insured individuals whose plan sponsor had provided permission to contact their members for this study and who were continuously eligible for pharmacy benefits in the 12 months before the start of the study. Thus, individuals aged 65 years or older, for whom Medicare is typically the primary payer and for whom accurate outcome data would not be available, were excluded.
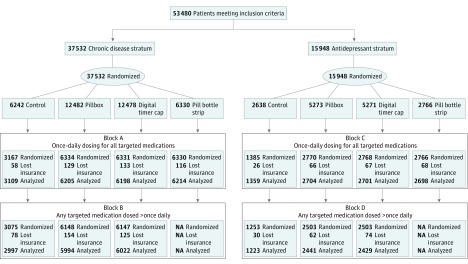
Eligible patients were identified using prescription claims data. To be included, patients must have filled between 1 and 3 oral maintenance medications for the treatment of cardiovascular disease (ie, hypertension, hyperlipidemia, coronary artery disease, congestive heart failure, or diabetes), another nondepression chronic condition (ie, breast cancer; benign prostatic hypertrophy; schizophrenia, bipolar disorder, and anxiety; arrhythmia; Parkinson disease; or seizure and epilepsy); or depression in the 12 months before February 2014, when eligibility for the study was evaluated (see eTable 1 in Supplement 2 for the study medications and eFigure 2 in Supplement 2 for the study timeline). We restricted potentially eligible individuals to those taking 3 or fewer medications to limit the number of devices that patients with more complex treatment regimens may be required to use at any point (Figure 1).
Figure 1. CONSORT Flow Diagram of Study Blocks and Sample Sizes.
In addition, to target individuals who may have been most likely to benefit from efforts to improve adherence, we required participants to have been suboptimally adherent to all of their qualifying oral maintenance medications during the 12 months prior to randomization. Adherence was assessed using prescription drug claims, and suboptimal adherence was defined as a medication possession ratio (MPR) between 30% and 80%. The 30% threshold was selected so that those patients in the cohort who normally filled more medications with every dispensation (eg, 90-day supply) would have filled at least twice in the year before randomization and thus would have had some consistency in their medication use. The MPR is a highly valid and widely used measure of long-term adherence.
Randomization and Study Procedures
Because antidepressants, unlike the other medication classes in this study, are generally not intended for lifelong use, we stratified participants prior to randomization on the basis of antidepressants being their only eligible medication (Figure 1). As a result, stratum 1 (the chronic disease stratum) consisted of patients who took and were suboptimally adherent to up to 3 medications for cardiovascular or other nondepression chronic conditions. Stratum 2 (the antidepressant stratum) consisted of patients whose only qualifying medication was an antidepressant.
Within each of the 2 strata, randomization occurred within blocks defined by whether all of the patient’s targeted medications were dosed once daily. Patients with at least 1 medication dosed more than once daily (blocks B and D) were not eligible for the pill bottle strip with toggles, which can be used only for medications dosed once per day, and were randomized to 1 of 3 intervention arms. Within each block, randomization was carried out in a 2:1 ratio between each of the device and control arms. Patients who were randomized to one of the intervention arms received a free device in the mail along with an information card explaining how to use the device as well as whom and what telephone number to call to obtain additional information during the trial. Patients who were randomized to the pill bottle strip with toggles arm or the digital timer cap arm received 1 device for each targeted medication. Patients who were randomized to the standard pillbox arm received 1 device to use for all of their medications.
Study devices were mailed across 4 days in late March 2014 by US Postal Service first-class mail. Individuals randomized to the control arm were not contacted and did not receive any of the devices. Because the receipt dates for each patient are not precisely known, the last day of the mailing period was used as the start of follow-up for all participants. During the 6-week period between targeting and deploying the study devices, a small proportion of enrolled patients lost insurance eligibility and thus received the devices but could not be included in the analysis (Figure 1).
Study Outcomes
The study’s primary outcome was a binary measure of optimal adherence during the 12-month follow-up period to all cardiovascular or nondepression chronic disease medications for participants in the chronic disease stratum. For each eligible medication, an MPR was first calculated using administrative pharmacy claims. Patients were defined as being optimally adherent if their MPR was equal to or greater than 80% for each and all of their eligible medications. Patients who lost insurance eligibility during follow-up were censored at their first eligibility gap of greater than 7 days, and outcomes were calculated over this truncated period.
Secondary outcomes were calculated in an analogous manner and included optimal adherence to cardiovascular medications among participants targeted for these medications in the chronic disease stratum and optimal adherence to antidepressants in the antidepressant stratum.
Statistical Analysis
We randomized 37 532 patients in the chronic disease stratum to achieve more than 80% power to detect a 1% difference in the percentage of patients who were optimally adherent between each of the individual intervention arms and controls as well as between each 2-way comparison of active arms, assuming that 2% of patients in the control group were optimally adherent, and an α of 5%. The randomization sequence was generated using PROC SURVEYSELECT with a fixed seed in SAS Enterprise Guide 5.1 (SAS Institute Inc).
All analyses were performed on the basis of the intention-to-treat principle. We calculated means and frequencies of prerandomization variables separately by study arm. We also compared the average length of follow-up by study arm.
In the primary analyses, all outcomes were compared between study arms using standard logistic regression. We accounted for differences in level of optimal adherence for patients in each block by adjusting for block in the regression model. In a subsequent analysis, we compared outcomes between study arms using a generalized estimating equation with a logit link to account for clustering of participants within participating employers. We also evaluated adherence as a continuous measure, calculated as the average MPR across targeted medications, using linear regression. Finally, we conducted subgroup analyses of the primary outcome, stratifying our population by sex, age (<50 years vs ≥50 years), adherence level prior to randomization (MPR <0.55 vs ≥0.55), and number of targeted maintenance medications (between 1 and 3).
Results
Of the 53 480 potentially eligible participants, 1.5% to 2% of patients in each study arm lost insurance eligibility between targeting and deploying the devices. The final sample size for analysis was 36 739 in the chronic disease stratum and 15 555 in the antidepressant stratum. See Figure 1 for more information.
The baseline characteristics of the study participants are presented in Table 1. In the entire cohort, mean (SD) age was 45 (12) years and 56% were females. Within study blocks, patients randomized to each of the treatment arms were well balanced and had similar lengths of follow-up. Compared with patients in the chronic disease stratum, patients in the antidepressant stratum were younger, more likely to be female, and less likely to live in neighborhoods with higher incomes and a greater proportion of individuals with white race/ethnicity.
Table 1. Baseline Characteristics by Study Block.
| Characteristic | Block A: Medications Dosed 1 × Daily | Block B: ≥1 Medication Dosed >1× Daily | Block C: Medications Dosed 1× Daily | Block D: ≥1 Medication Dosed >1 × Daily | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Standard Pillbox | Digital Timer Cap | Pill Bottle Strip With Toggles | Control | Standard Pillbox | Digital Timer Cap | Control | Standard Pillbox | Digital Timer Cap | Pill Bottle Strip With Toggles | Control | Standard Pillbox | Digital Timer Cap | |
| No. | 3109 | 6205 | 6198 | 6214 | 2997 | 5994 | 6022 | 1359 | 2704 | 2701 | 2698 | 1223 | 2441 | 2429 |
| Age, mean, y | 48.7 | 48.7 | 48.7 | 48.6 | 45.6 | 45.5 | 45.6 | 39.5 | 39.2 | 39.4 | 39.2 | 39.6 | 39.8 | 40.5 |
| Female, % | 46.3 | 44.5 | 46.5 | 45.1 | 56.3 | 54.9 | 56.4 | 68.7 | 69.5 | 73.5 | 70.8 | 71.0 | 71.5 | 72.0 |
| Median income, mean in zip code, $ | 59 543 | 59 751 | 59 729 | 59 804 | 57 359 | 58 441 | 58 631 | 62 108 | 61 734 | 62 342 | 61 835 | 61 207 | 61 501 | 61 435 |
| Black race, mean % in zip code, % | 10.8 | 10.6 | 10.4 | 10.6 | 10.7 | 10.4 | 10.6 | 7.3 | 7.2 | 6.9 | 6.9 | 7.5 | 7.4 | 7.4 |
| Region, % | ||||||||||||||
| Midwest | 16.2 | 17.8 | 16.6 | 17.3 | 17.6 | 18.1 | 17.9 | 21.0 | 22.3 | 22.0 | 23.0 | 21.1 | 19.4 | 20.1 |
| Northeast | 51.5 | 49.6 | 50.3 | 51.2 | 48.4 | 48.4 | 49.0 | 51.9 | 50.2 | 50.6 | 51.1 | 51.6 | 52.1 | 52.8 |
| South | 26.6 | 27.1 | 27.5 | 26.0 | 28.0 | 27.2 | 27.3 | 22.4 | 21.1 | 21.5 | 20.7 | 19.8 | 21.5 | 20.2 |
| West | 5.6 | 5.4 | 5.6 | 5.4 | 6.0 | 6.2 | 5.8 | 4.7 | 6.3 | 5.8 | 5.2 | 7.5 | 6.9 | 6.9 |
| No. of targeted medications, mean | 1.4 | 1.4 | 1.4 | 1.4 | 1.5 | 1.5 | 1.5 | 1.1 | 1.1 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Baseline adherence, MPR | 44.3 | 44.1 | 43.9 | 43.9 | 41.3 | 40.8 | 40.9 | 40.5 | 40.6 | 40.9 | 40.4 | 40.2 | 40.1 | 39.7 |
| Follow-up, mean, d | 338.3 | 336.7 | 336.5 | 336.2 | 333.5 | 332.6 | 329.7 | 325.2 | 329.9 | 330.2 | 328.1 | 329.8 | 327.4 | 330.6 |
Abbreviation: MPR, medication possession ratio.
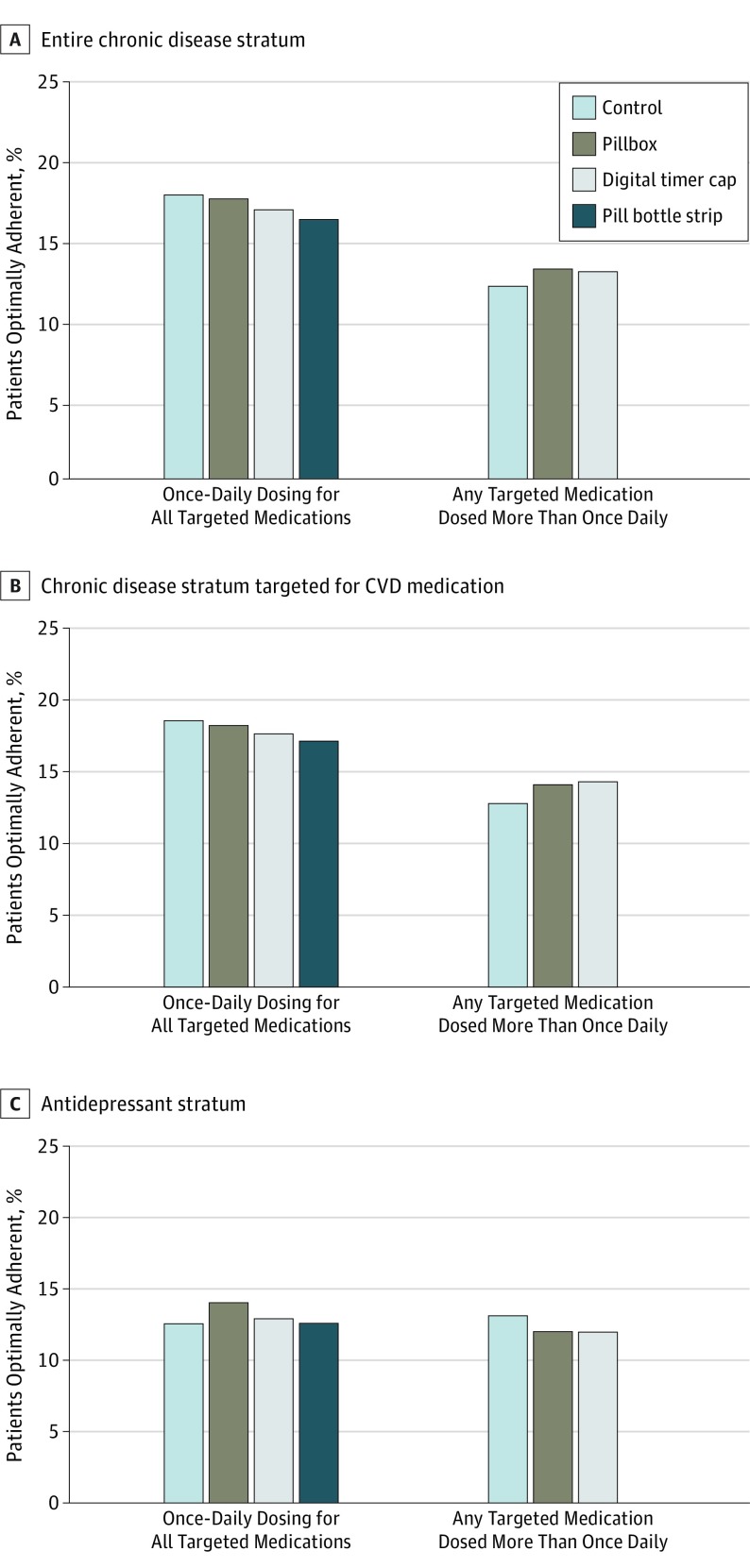
In the chronic disease stratum, 15.1% of control patients became optimally adherent during follow-up compared with 15.5% of patients in the pillbox arm, 15.1% of patients in the digital timer cap arm, and 16.3% of patients in the pill bottle strip with toggles arm (Figure 2A). In logistic models comparing study arms to controls and adjusted for study block, the odds of full adherence were not substantially different among the intervention arms or controls (Table 2). In direct comparisons between devices, patients randomized to receive a pillbox had a statistically significant 10% higher odds of optimal adherence than those randomized to receive a pill bottle strip with toggles (odds ratio [OR], 1.10; 95% CI, 1.00-1.21). Analyses were similar when accounting for the clustering of patients within employers (OR, 1.10; 95% CI, 0.98-1.24; eTable 2 in Supplement 2). Across subgroups, males with the pill bottle strip with toggles had substantially higher adherence than did females with the same device, and patients with higher levels of adherence before randomization responded better to the digital timer cap (OR, 1.07; 95% CI, 1.01-1.15) than did patients with lower prerandomization levels of adherence (OR, 0.96; 95% CI, 0.90-1.03; P value for interaction = .02). Effects across other subgroups, defined by sex and number of medications taken at randomization, were consistent (Table 3).
Figure 2. Optimal Adherence by Study Arm and Outcome Definition.
Percentage of patients optimally adherent in the chronic disease stratum (A), in the chronic disease stratum and targeted for a cardiovascular medication (B), and in the antidepressant stratum (C). CVD indicates cardiovascular disease.
Table 2. Odds Ratio (OR) of Optimal Adherence.
| Outcome | vs Control, OR (95% CI) | Head-to-Head Comparison, OR (95% CI) | ||||
|---|---|---|---|---|---|---|
| Standard Pillbox | Digital Timer Cap | Pill Bottle Strip With Toggles |
Standard Pillbox vs Digital Timer Cap | Standard Pillbox vs Pill Bottle Strip With Toggles | Digital Timer Cap vs Pill Bottle Strip With Toggles | |
| Primary | ||||||
| All CVD and nondepression maintenance medications in chronic disease stratum | 1.03 (0.95-1.13) | 1.00 (0.92-1.09) | 0.94 (0.85-1.04) | 1.03 (0.96-1.11) | 1.10 (1.00-1.21) | 1.05 (0.95-1.15) |
| Secondary | ||||||
| All CVD medications | 1.03 (0.94-1.13) | 1.01 (0.92-1.11) | 0.95 (0.86-1.06) | 1.02 (0.94-1.10) | 1.08 (0.98-1.19) | 1.04 (0.94-1.43) |
| Antidepressants (antidepressant stratum) | 1.02 (0.89-1.18) | 0.97 (0.84-1.11) | 0.93 (0.79-1.11) | 1.14 (1.02-1.29) | 1.14 (0.97-1.33) | 1.03 (0.88-1.21) |
Abbreviation: CVD, cardiovascular disease.
Table 3. Odds Ratio (OR) of Optimal Adherence, by Patient Subgroup.
| Patient Subgroup | vs Control, OR (95% CI) | |||||
|---|---|---|---|---|---|---|
| Standard Pillbox | P Value | Digital Timer Cap | P Value | Pill Bottle Strip With Toggles | P Value | |
| Age, y | ||||||
| <50 | 1.05 (0.99-1.12) | .67 | 0.99 (0.92-1.06) | .40 | 1.00 (0.91-1.09) | .12 |
| ≥50 | 1.03 (0.96-1.10) | 1.03 (0.96-1.09) | 0.91 (0.84-0.98) | |||
| Sex | ||||||
| Female | 1.04 (0.98-1.11) | .98 | 1.04 (0.98-1.11) | .15 | 0.86 (0.79-0.93) | <.001 |
| Male | 1.04 (0.99-1.09) | 1.01 (0.96-1.06) | 0.95 (0.90-1.01) | |||
| Prerandomization adherence | ||||||
| Lower adherence (MPR <0.55) | 1.05 (0.98-1.12) | .80 | 0.96 (0.90-1.03) | .02 | 0.99 (0.91-1.08) | .23 |
| Higher adherence (MPR ≥0.55) | 1.04 (0.97-1.11) | 1.07 (1.01-1.15) | 0.92 (0.85-1.00) | |||
| No. of targeted medications | ||||||
| 1 | 1.01 (0.94-1.09) | 1.01 (0.93-1.09) | 0.99 (0.91-1.09) | |||
| 2 | 1.06 (0.97-1.16) | .34a | 1.04 (0.95-1.14) | .52a | 0.91 (0.81-1.02) | .11a |
| 3 | 0.94 (0.77-1.15) | .29a | 0.99 (0.81-1.21) | .77a | 1.15 (0.90-1.46) | .09a |
Abbreviation: MPR, medication possession ratio.
Compared with 1 targeted medication.
Analyses of secondary outcomes were consistent with those of the primary outcome (Table 2 and Figure 2B and C). None of the devices differed substantially from the control arm with respect to optimal adherence to cardiovascular medications or antidepressants. In direct comparisons, patients randomized to the pillbox arm had a 14% higher odds of optimal adherence to antidepressants than patients randomized to the digital timer cap arm (OR, 1.14; 95% CI, 1.02-1.29; Table 2). Analyses considering adherence as a continuous outcome calculated as the mean MPR across eligible medications also yielded similar results (eTable 3 in Supplement 2).
Discussion
In this pragmatic, comparative-effectiveness randomized clinical trial of more than 50 000 individuals who took up to 3 long-term medications to treat chronic conditions but were nonadherent to these therapies, 3 low-cost devices—pill bottle strip with toggles, digital timer cap, and standard pillbox—did not improve medication adherence. In head-to-head comparisons of individual devices, patients who received the standard pillbox tended to have higher adherence than patients who received the digital timer cap and the pill bottle strip with toggles, although these effects were of relatively small magnitude and were of inconsistent statistical significance.
There are several potential explanations for our null findings. First, we selected for inclusion those patients who had suboptimal adherence and then we anticipated that, during our yearlong follow-up, 2% of our controls would become optimally adherent. In contrast, we observed that 12% to 18% of controls actually became optimally adherent without specific intervention. This finding is consistent with recent observations that adherence, like other health behaviors, is dynamic, with a sizable proportion of previously nonadherent patients demonstrating periods of adherence and vice versa. As such, it may have been preferable to instead target patients who were predicted to be nonadherent in the future (by using recently described methods, for example) rather than target those who were nonadherent at the point of randomization.
Second, in an intention-to-treat framework, low uptake or inconsistent use could have obscured any true effects among those who used the devices regularly. Our study was powered to detect a 1% mean improvement in the rate of optimal adherence under the assumption that approximately 20% of patients in the intervention arms would use the devices. In a survey of 618 patients randomized to one of the intervention arms conducted in the third month of follow-up, more than 68% reported using the devices. However, given the overall null results of the intervention, such use either was not sustained in the yearlong follow-up or did not lead to measurable changes in the rate of filling. In some cases, patients may not have used the devices as intended; although an information card accompanied the devices, 40% of those surveyed either did not read the card or found it unhelpful. For patients randomized to the digital timer cap arm, we used pharmacy data to predict which size cap would fit over patients’ existing pill bottles; however, size discrepancies could have prevented some patients from using the timer cap device. Those surveyed frequently reported difficulties with putting on and taking off the digital timer cap device. The fact that the standard pillbox, ubiquitous in pharmacies and other care settings, resulted in marginally greater improvements in adherence than the other two devices may suggest that familiarity and comfort with a device could have facilitated uptake and the resultant behavior change.
Third, to be pragmatic, the mailing of devices was not coordinated with actual medication refills. Thus, patients may have had difficulty transitioning to the device in the middle of a prescription fill. Or patients may have not used the devices at all if their medication refill had lapsed completely, leaving them without medication to put in the device at the time it was received.
Another possible explanation for our findings is that these devices simply do not improve adherence. These 3 devices are simple by design, intended to be intuitive to use, and minimally disruptive. However, for patients without established routines around medication taking, the additional cues from these devices may not be sufficient to overcome forgetfulness. Moreover, the devices may not have promoted periodic medication refilling, which is necessary for long-term adherence. For these devices to work, they may need to be administered with additional support mechanisms.
Alternatively, although forgetfulness is the most frequent barrier to adherence that patients report, this factor may not have been the primary driver of nonadherence in our study population. For example, a common theme in the patient survey for the trial participants was a belief that adherence was not a problem for them, suggesting that gaps in knowledge or motivation could have been the primary contributors to suboptimal medication taking. Similarly, because nonadherence is a multidimensional problem, addressing forgetfulness alone may have been insufficient to improve actual medication taking. This idea is consistent with the modest effects seen in other studies that address single barriers, such as high out-of-pocket medication costs. Conversely, multicomponent interventions, particularly those led by pharmacists, appear to be effective. Trials are now being conducted to test ways to increase the efficiency of pharmacy-patient interactions, including delivering pharmacy services by telephone and linking these services to other resources, such as text messages and performance reports drawn from routinely collected administrative claims and electronic health record data.
Limitations
This study has several limitations. Our multiarm study design necessitated making multiple comparisons and conducting several hypothesis tests. Thus, the few statistically significant findings reported here could be the result of chance. A small proportion of patients lost insurance eligibility before the devices were mailed, and another set of patients lost eligibility during follow-up. We did not observe substantial differences in the loss-to-follow-up rate between study arms because much of this loss to follow-up can be attributed to normal insurance churn. A higher-than-anticipated proportion of control patients were observed to be adherent during follow-up; however, given that almost no effect was observed across any comparisons in our study, we do not believe the interpretation of our results would change. Targeting for the trial and the outcomes were evaluated using medication dispensing data. Such data sources have been demonstrated to be valid and accurate measures of medication-taking behavior, but some misclassification of actual use could have occurred if patients stopped taking their medications midway through a fill. Similarly, claims data do not allow for the differentiation of patients who are intermittent users from patients who have discontinued therapy altogether. Such misclassification could undermine our measurement of the effectiveness of these devices in improving adherence. Given the minimal impact of these devices in improving rates of medication filling, we do not expect the devices to have differentially caused patients to stop taking their medications midway through a fill.
Included in our list of targeted medications were anxiolytics. Although these drugs are used by many patients for long periods, they are not intended for lifelong use. For very few patients, an anxiety medication was the only drug that qualified them for inclusion in the chronic disease stratum; we found this to be reassuring, and our results were virtually identical in our prespecified analysis restricted to cardiovascular medications. Our trial population consisted of patients who were continuously enrolled in a commercial pharmacy benefits manager for 12 to 24 months and who were taking between 1 and 3 maintenance medications. Some of these patients may have been simultaneously enrolled in an auto-refill program; however, we expect the distribution of enrolled patients to be balanced by design. Finally, our results may not be fully generalizable to older and/or Medicare-insured populations and to those with greater medication maintenance complexity.
Conclusions
In a large, pragmatic, comparative-effectiveness randomized clinical trial of patients across a broad range of chronic conditions, low-cost devices did not measurably improve medication adherence. Future research should focus on effective strategies to ensure uptake and sustained use of these interventions.
Trial Protocol
eFigure 1. Three Low-Cost Reminder Devices
eFigure 2. Trial Timeline
eTable 1. Oral Maintenance Medications
eTable 2. Odds Ratio of Optimal Adherence by Study Arm, Adjusted for Correlation Within Employer Using Generalized Estimating Equation Results
eTable 3. Percent Difference in Mean MPR by Study Arm
References
- 1.Gadkari AS, McHorney CA. Unintentional non-adherence to chronic prescription medications: how unintentional is it really? BMC Health Serv Res. 2012;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gazmararian JA, Kripalani S, Miller MJ, Echt KV, Ren J, Rask K. Factors associated with medication refill adherence in cardiovascular-related diseases: a focus on health literacy. J Gen Intern Med. 2006;21(12):1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egan BM, Lackland DT, Cutler NE. Awareness, knowledge, and attitudes of older Americans about high blood pressure: implications for health care policy, education, and research. Arch Intern Med. 2003;163(6):681-687. [DOI] [PubMed] [Google Scholar]
- 4.Vervloet M, Linn AJ, van Weert JCM, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahtani KR, Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev. 2011;(9):CD005025. [DOI] [PubMed] [Google Scholar]
- 6.Wroe AL. Intentional and unintentional nonadherence: a study of decision making. J Behav Med. 2002;25(4):355-372. [DOI] [PubMed] [Google Scholar]
- 7.Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. JAMA. 2014;312(12):1237-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zedler BK, Kakad P, Colilla S, Murrelle L, Shah NR. Does packaging with a calendar feature improve adherence to self-administered medication for long-term use? a systematic review. Clin Ther. 2011;33(1):62-73. [DOI] [PubMed] [Google Scholar]
- 9.Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45(7):908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhry NK, Krumme AA, Ercole PM, et al. Rationale and design of the Randomized Evaluation to Measure Improvements in Non-adherence from Low-Cost Devices (REMIND) trial. Contemp Clin Trials. 2015;43:53-59. [DOI] [PubMed] [Google Scholar]
- 11.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565-574. [DOI] [PubMed] [Google Scholar]
- 12.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7-8):1280-1288. [DOI] [PubMed] [Google Scholar]
- 13.El-Mallakh RS, Gao Y, Jeannie Roberts R. Tardive dysphoria: the role of long term antidepressant use in-inducing chronic depression. Med Hypotheses. 2011;76(6):769-773. [DOI] [PubMed] [Google Scholar]
- 14.Fava GA. Can long-term treatment with antidepressant drugs worsen the course of depression? J Clin Psychiatry. 2003;64(2):123-133. [DOI] [PubMed] [Google Scholar]
- 15.HEDIS Summary table of measures, product lines, and changes. http://www.ncqa.org/Portals/0/HEDISQM/Hedis2015/List_of_HEDIS_2015_Measures.pdf. Published 2015. Accessed April 28, 2016.
- 16.Franklin JM, Shrank WH, Pakes J, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789-796. [DOI] [PubMed] [Google Scholar]
- 17.Franklin JM, Krumme AA, Shrank WH, Matlin OS, Brennan TA, Choudhry NK. Predicting adherence trajectory using initial patterns of medication filling. Am J Manag Care. 2015;21(9):e537-e544. [PubMed] [Google Scholar]
- 18.Franklin JM, Shrank WH, Lii J, et al. Observing vs predicting: initial patterns of filling predict long-term adherence more accurately than high-dimensional modeling techniques. Health Serv Res. 2016;51(1):220-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimolzak AJ, Spettell CM, Fernandes J, et al. Early detection of poor adherers to statins: applying individualized surveillance to pay for performance. PLoS One. 2013;8(11):e79611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhry NK, Avorn J, Glynn RJ, et al. ; Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) Trial . Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365(22):2088-2097. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785-795. [DOI] [PubMed] [Google Scholar]
- 22.Choudhry NK, Isaac T, Lauffenburger JC, et al. Rationale and design of the Study of a Tele-pharmacy Intervention for Chronic diseases to Improve Treatment adherence (STIC2IT): a cluster-randomized pragmatic trial. Am Heart J. 2016;180:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44(5):471-477. [DOI] [PubMed] [Google Scholar]
- 24.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Three Low-Cost Reminder Devices
eFigure 2. Trial Timeline
eTable 1. Oral Maintenance Medications
eTable 2. Odds Ratio of Optimal Adherence by Study Arm, Adjusted for Correlation Within Employer Using Generalized Estimating Equation Results
eTable 3. Percent Difference in Mean MPR by Study Arm