Abstract
Background/Aims:
The aim of the study was to evaluate the safety of non-anesthesia provider (NAPP) administered propofol sedation in patients undergoing non-advanced gastrointestinal (GI) endoscopic procedures.
Materials and Methods:
Pubmed, Embase, Cochrane central register of controlled trials, Scopus, and Web of Science databases were searched for prospective observational trials involving non-advanced endoscopic procedures. From a total of 608 publications, 25 [colonoscopy (9), upper GI endoscopy (5), and combined procedures (11)] were identified to meet inclusion criteria and were analyzed. Data was analyzed for hypoxia rates, airway intervention rates, and airway complication rates.
Results:
A total of 137,087 patients were involved. A total of 2931 hypoxia episodes (defined as an oxygen saturation below 90%) were reported with a pooled hypoxia rate of 0.014 (95% CI being 0.008-0.023). Similarly, pooled airway intervention rates and pooled airway complication rates were 0.002 (95% CI being 0.006–0.001) and 0.001 (95% CI being 0.000–0.001), respectively.
Conclusions:
The rates of adverse events in patients undergoing non-advanced GI endoscopic procedures with NAPP sedation are extremely small. Similar data for anesthesia providers is not available. It is prudent for anesthesia providers to demonstrate their superiority in prospective randomized controlled trials, if they like to retain exclusive ownership over propofol sedation in patients undergoing GI endoscopy.
Keywords: Airway complication, airway intervention, colonoscopy, endoscopy, esophagogastroduodenoscopy, hypoxia, non-advanced endoscopic procedures, propofol, sedation
INTRODUCTION
Propofol is the sine qua non of sedation during gastrointestinal (GI) endoscopy. In spite of its many shortcomings such as increased incidence of desaturation, aspiration, cardiac arrests, hypotension, colonic perforation, and bleeding, its role in the field of endoscopy sedation is unchallenged.[1,2,3,4] The primary reason appears to be a very high patient and endoscopist satisfaction.[5,6,7,8,9,10,11,12,13,14] However, at least in the USA, the added costs involved with providing propofol sedation are a significant constraint. With shrinking health care budgets and escalating costs of treatment, it is essential to find cost-effective ways of providing propofol sedation. Payment to anesthesia providers involved in the administration of propofol is a case in point.[15] Although the fears associated with the use of propofol by non-anesthesia providers are based on sound theoretical principles, they are not borne out of scientific studies.
In view of this, it is important to conduct a meta-analysis of all the scientific publications that have studied the safety of non-anesthesia provider administered propofol in patients presenting for endoscopic procedures. The safety of such practice in patients undergoing advanced endoscopic procedures has been studied previously.[16] Publications studying the safety of non-advanced endoscopic procedures wherein propofol was administered by anesthesia providers (anesthesia administered propofol – AAP) are not available. As a result, the current pooled analysis deals with non-advanced endoscopic procedures where propofol was administered by the non-anesthesia providers (non-anesthesia administered propofol-NAAP). The studies that involve propofol administration by non-anesthesia providers presenting for advanced endoscopic procedures are not included in the current study.
The aim of the current meta-analysis is to study the safety of propofol sedation in patients undergoing non-advanced endoscopic procedures when administered by non-anesthesia providers. For the purpose of the study, advanced procedures included endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasound, balloon-assisted deep enteroscopy, peroral endoscopic myotomy (POEM), endoscopic mucosal resection (EMR), and HALO radiofrequency ablation (RFA).
MATERIALS AND METHODS
Literature search was conducted for terms “propofol sedation endoscopy,” “propofol sedation colonoscopy,” and nurse administered propofol sedation. The following databases were searched PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Scopus, and Web of Science. Articles published until April 2015 (both in English and non-English languages) were included in the present pooled analysis. Article screening was done independently by two authors and final inclusion after meeting the PICOS framework [Figure 1] was included after the consensus of a third author. After removing the duplicates using the computer program “Endnote” (Thomson Reuters Inc, USA), we narrowed down the list of publications meeting the search criteria to 608. Efforts were made to contact the corresponding author if the study information was incomplete or conflicting. The final selection included 25 studies (prospective observational and randomized controlled trials combined; Table 1) on which the pooled analysis was conducted.
Figure 1.
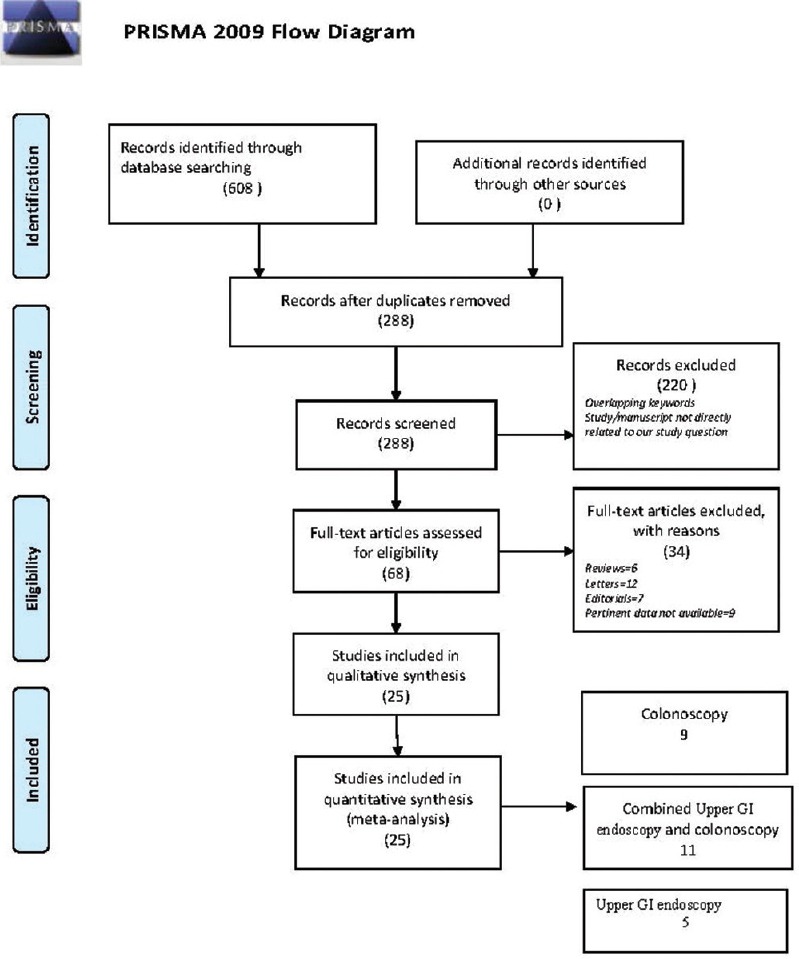
PRISMA flow diagram. Based upon the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” showing the number of studies screened, included, and excluded in the final analysis
Table 1.
Master table representing the characteristics of all the studies included in the pooled meta-analysis
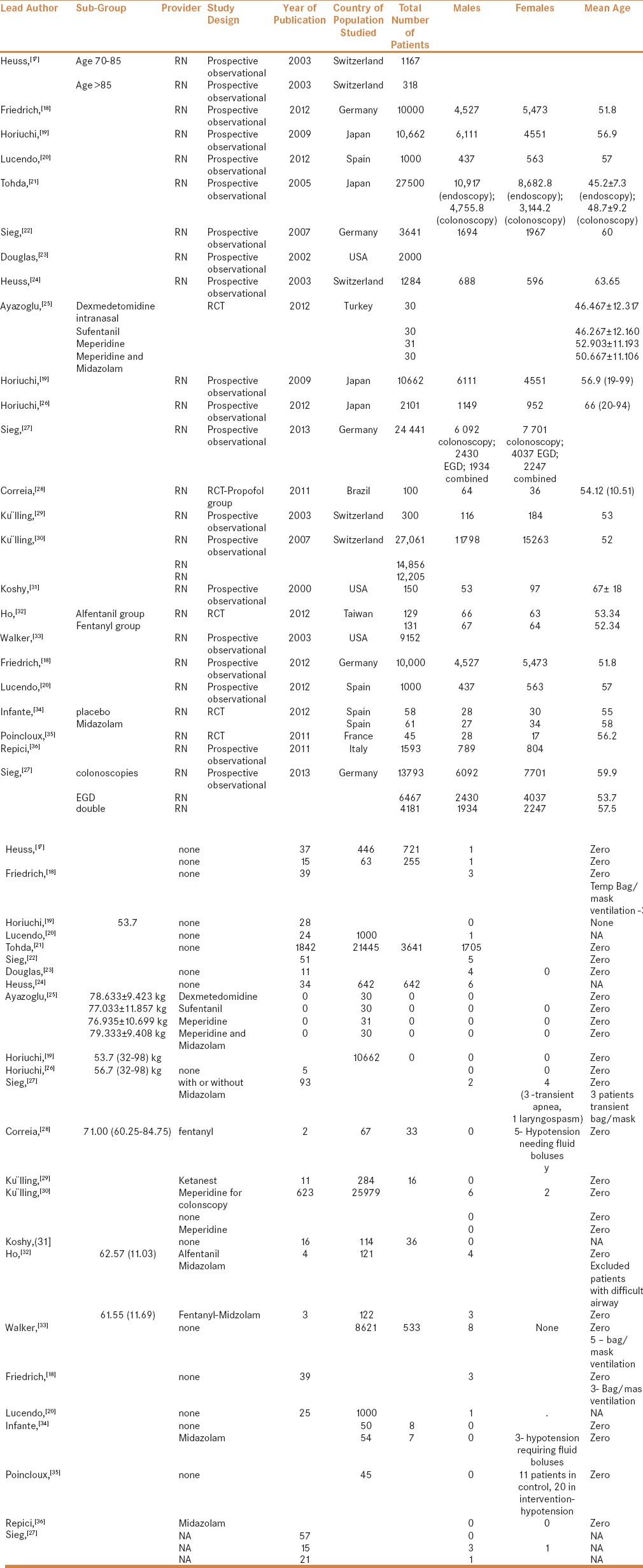
We included both prospective and retrospective studies in the analysis, provided they reported at least one of the desired variables. In all the trials included, sedation was administered by a registered nurse under the supervision of a gastroenterologist. The following parameters were included in the pooled analysis.
Hypoxia rates – Number of patients developing pulse oximeter measured saturation of 90% or below
Airway related interventions during the procedures – The interventions included in the analysis were jaw thrust, patients needing bag mask ventilation, oral/nasopharyngeal airway, and airway-related procedure interruption and intubation
Airway related complications – this group included the following events: Laryngospasm, unexpected hospital admissions, and unplanned conversion to general anesthesia
Data extraction
A standardized format was prepared for data extraction for the analysis. The following data was extracted from the relevant trials: first author of the study, characteristics of population studied, nature of procedures performed, frequency of patients' desaturation below 90%, need for intervention to maintain airway, type of intervention, total propofol dose used, complications during the procedure, any mortality, or any immediate cardiopulmonary complications. Specific, individual study related findings were also documented and are represented in Table 1.
Statistical analysis
The data for individual study was collected using a template spreadsheet in Microsoft Excel (Version 2013, Microsoft Inc, USA). Meta-analysis was performed using Comprehensive Meta-analysis version 2 (Biostat Inc, USA). Pooling for outcome rates was done initially using fixed-effects modeling and eventually with random-effects methods (after assessment of heterogeneity with fixed modeling). The extent of heterogeneity between the trials was quantified using the I2 statistic. Values of I2 <40% were considered unimportant, 40–50% were considered to represent moderate heterogeneity, and 50–90% represented high heterogeneity. Pooled values of results with associated alpha error of less than 5% were deemed statistically significant. Results of end points (hypoxia, airway intervention rate, and complication rate) were expressed as event rate (per patient) with 95% CI. Funnel plot was used for evaluation of any potential publication bias. To account for the high heterogeneity in our analysis, different methods were used. A sensitivity analysis, by removing a single study at a time, was performed. Further evaluation of heterogeneity was done by creating possible subgroups based upon upper and lower gastroenterological procedures. All values reported for analysis with I2 more than 40% are from random-effects modeling only.
RESULTS
None of the studies reported the need for endotracheal intubation, and the conversion rate to general anesthesia was zero in the included trials [Table 1]. The following is an analysis of the pooled results of various variables mentioned above.
Hypoxia rates
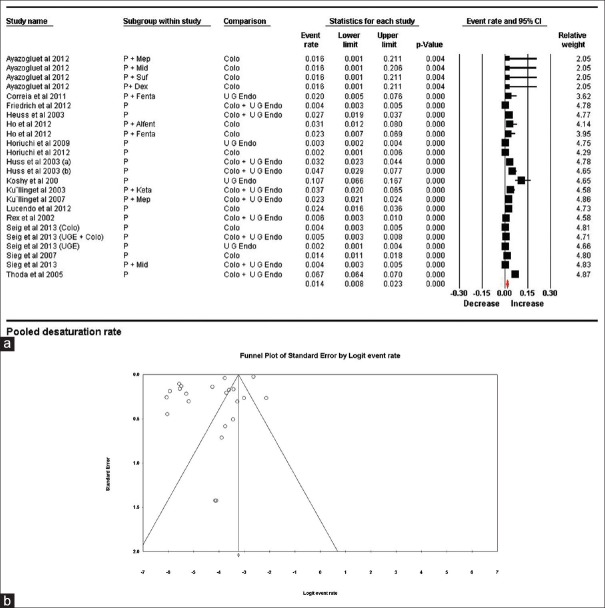
Studies reporting pulse oximeter derived saturation of 90% or lower were included in this group. None of the studies documented the duration of such desaturation or the effects of interventions; thus, time-based analysis was not possible. We analyzed the documented hypoxia variables to derive pooled hypoxia rates. Variables from 24 trials/subgroups were included in this estimate. A total of 137,087 patients were involved in these trials and 2931 desaturation episodes were reported. The pooled hypoxia rate was 0.014 (95% CI being 0.008–0.023). Because of the presence of high heterogeneity (99.03%), random effect modeling was used. To explore the heterogeneity, sensitivity analysis using “single study removal method” was used. The study by Thoda et al. contributed maximally to the heterogeneity; however, upon its removal, heterogeneity dropped only marginally to 97.15%. To further account for heterogeneity, we subdivided the studies into 3 groups. Their associated heterogeneity and pooled rates are shown in Table 2 and Figure 2a and b.
Table 2.
Desaturation (Hypoxia) rates of various subgroups by procedure

Figure 2.
(a) Pooled hypoxia (desaturation) rates. The diamond (red) at the bottom shows the final net effect with the 95% confidence interval. The line width of individual contributing study/subgroups in the forest plot is proportional to the final effect size. (b) Funnel plot for publication bias in desaturation rates showing asymmetrical distribution of published studies demonstrating a possibility of publication bias
Pooled desaturation rates were also calculated for anesthetic agents used for endoscopy
Propofol alone
Propofol was used as the sole anesthetic agent in 14 trials where 2195 patients showed desaturation among a total of 84264 patients. Pooled estimate was 0.012 (0.005–0.026) (P < 0.001) with a heterogeneity of 99.16%.
Propofol with adjuvants
Ten trial/subgroups used propofol in combination with an adjuvant. These adjutants included fentanyl, meperidine, and midazolam in two trials each, and dexmeditomedine, ketamine, sufentanyl and alfentanyl in one trial each. Desaturation was noted in 736 patients out of 52823 patients in this subgroup. The pooled estimated desaturation rate was 0.018 (95% CI being 0.008–0.041) (P < 0.001) with heterogeneity of 96.64%.
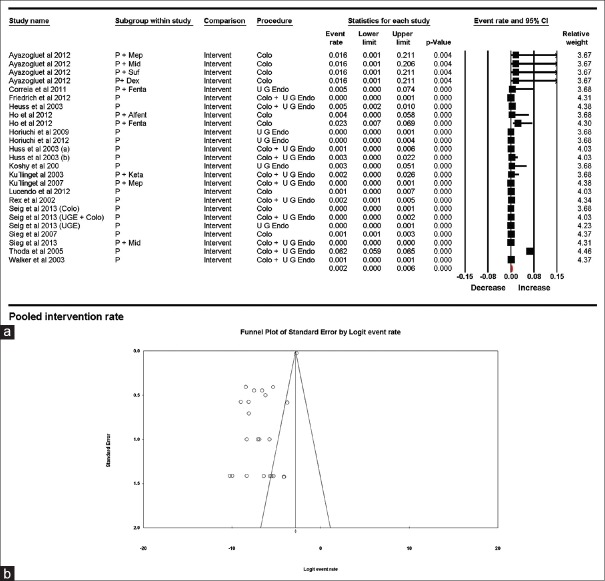
Airway intervention rate
Airway intervention for treating desaturation during the procedure was reported by 25 subgroups where 1746 patients required intervention from 146239 patients. Pooled estimate for intervention rate was 0.002 (95% CI being 0.006–0.001), with a heterogeneity of 97.31%. Once again, the study by Thoda et al. had the highest contribution to heterogeneity. On sensitivity analysis by “single study removal method,” and by dropping this study from the analysis, the heterogeneity decreased to 79.97%. Intervention rates related to anesthetic agent and type of procedure were also estimated; the results are displayed in Table 3 and Figures 3a and b.
Table 3.
Pooled airway Intervention (AI) rates based on the procedure and the anesthetic employed
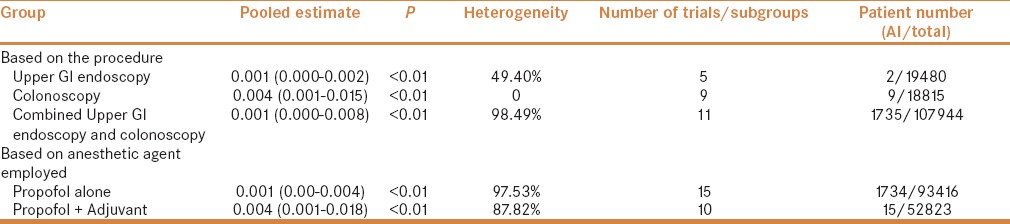
Figure 3.
(a) Pooled airway intervention rates. The diamond (red) at the bottom shows the final net effect with the 95% confidence interval. The line width of individual contributing study/subgroups in the forest plot is proportional to the final effect size. (b) Funnel plot for publication bias in intervention rates showing asymmetrical distribution of published studies demonstrating a possibility of publication bias
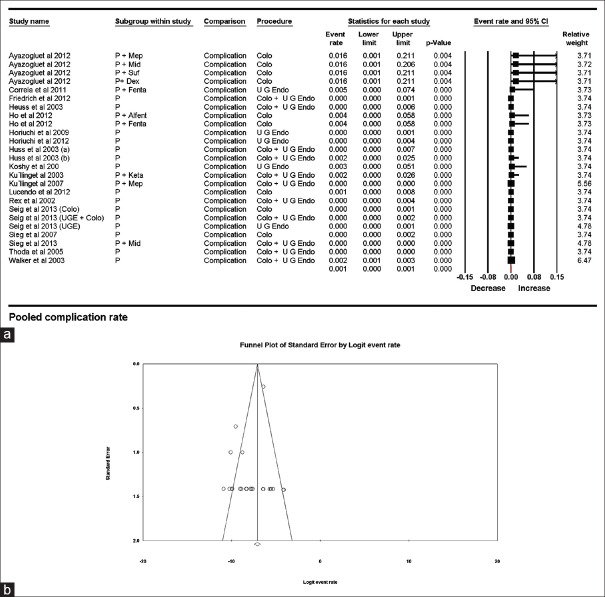
Airway-related complications
Twenty-five trials (146239 patients) reported only 19 airway-related complications. Estimated pooled complication rate was 0.001 (95% CI being 0.000–0.001) with I2 being 69.97%. The study by Walker et al. had the highest contribution to heterogeneity, and upon sensitivity analysis using “single study removal method,” the heterogeneity dropped to 64.65%. The results obtained from various subgroupings are shown in Table 4 and Figure 4a and b. It is interesting to note that no airway-related complications were reported during colonoscopy; however, the estimated pooled rate has a 95% confidence interval, which is more than upper GI endoscopy. The large sample size of patients undergoing upper GI endoscopy explains the paradox.
Table 4.
Pooled rates of airway related complications (ARC) classified by procedure and anesthetic employed
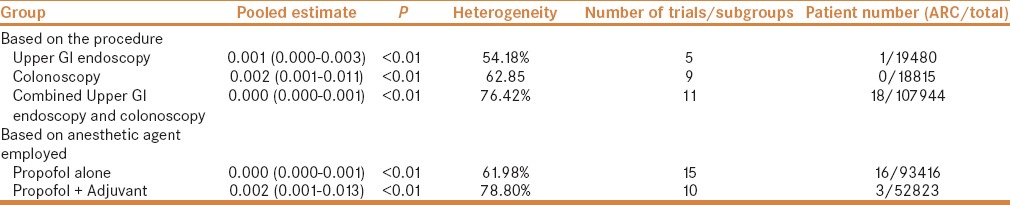
Figure 4.
(a) Pooled airway complication rates. The diamond (red) at the bottom shows the final net effect with the 95% confidence interval. The line width of individual contributing study/subgroups in the forest plot is proportional to the final effect size. (b) Funnel plot demonstrating complications rates with symmetrical distribution of published studies thus demonstrating a publication bias being unlikely
Publication bias
Funnel plot analysis was conducted for all three outcomes.
Hypoxia rates
The graphical funnel plot of the included studies is asymmetrical and deviated to the left. Egger's regression test calculated an intercept value of − 6.48, (P = <0.01), suggesting a likely publication bias. Thus, the possibility of underreporting of desaturation cannot be ruled out [Figure 2b].
Airway intervention
Funnel plot showed asymmetric distribution of studies/groups, which was further confirmed using Egger's regression test, which showed an X-axis intercept at −5.096 (P = <0.01). Once again the possibility of underreporting of airway interventions remains [Figure 3b].
Airway-related complications
A symmetrical distribution was found on funnel plot, and Egger's regression test showed intercept at −0.762, (P = 0.23). Thus, publication bias of reporting airway-related complications is unlikely [Figure 4b].
DISCUSSION
The Holy Grail for endoscopic sedation is yet to be found. Newer drugs purported as safe and effective replacements of propofol (e.g., remimazolam) are yet to prove their mettle.[37] As a result, propofol is likely to remain the mainstream sedative for endoscopic procedures in the short to intermediate term.
Hypoxemia is the most common adverse event encountered with propofol sedation during endoscopic procedures.[4,38,39] The reported incidence of this adverse event is varied depending on the place of research, method of sedation, preemptive use of airway adjuncts, and the personnel involved in the administration of propofol. It is proposed that the pharmacological variability of propofol (both pharmacokinetic and pharmacodynamic) is a major factor contributing to unpredictable hypoxemia. Both light sedation (with associated coughing, laryngospasm) and deep sedation (associated apnea) potentially cause insufficient ventilation and hypoxemia. Yet, our meta-analysis has showed an extremely low risk of hypoxemia when propofol was administered by registered nurses under the guidance of a gastroenterologist. As we do not have any reason to doubt the conduct and reporting of the data, the results of the meta-analysis must be accepted.
The results of our meta-analysis might be useful for strategic planning when delivering health care.[40,41,42] Health care spending accounts for more than 17.4% of gross domestic product in USA.[43] However, not all indicators of health are directly related to health care spending. In fact, many other countries in the Organisation for Economic Co-operation and Development (OECD) have fared better in many areas compared to USA,[44,45,46] in spite of lower health care budgets. As a result, it is important to use prudence when it comes to health care spending. In a recent study, Ladabaum et al. reported that mean anesthesia payments for diagnostic colonoscopy amounted to $494.00 per procedure.[47,48] In the absence of any proven benefits, such as a decrease in the complication rates, it is a waste of resources. However, considering patient satisfaction was better in patients sedated by anesthesia providers, it is essential to consider this aspect of patient care as well.[16]
Various societies entrusted with formulating sedation guidelines might find the results of our meta-analysis informative. In 2010, members of the European Society of Gastrointestinal Endoscopy (ESGE), the European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA), and the European Society of Anaesthesiology (ESA) published guidelines for non-anesthesiologist administration of propofol for gastrointestinal endoscopy.[49] However, 21 national societies of anesthesia felt that non-anesthesiologists should not be allowed to administer propofol.[50,51,52,53] As a result, these endorsements were later retracted by the societies that endorsed them.[54] It prompted the Dumonceau to question the wisdom and doubt if these retraction statements were all about job preservation and money.
The study has essentially answered the question posed in a recent editorial “Improving safety during sedation by nonanesthesiologists: Do we lead or follow?”[55] It is prudent on the part of anesthesia providers that some of the practices used in the included studies be adapted in their current practice to increase the safety of propofol sedation.
Limitations
It is acknowledged that the practice with regards to supplemental oxygen administration during sedation varies across the world. As a result, some of the findings may be disputable. Yet, considering that both the number of included studies and the patients is very large, the findings cannot be ignored.
The second limitation pertains to our decision to include only propofol studies. Many gastroenterologists perform non-advanced procedures utilizing intravenous conscious sedation, typically using midazolam and fentanyl. However, considering that the propofol typically produces “unconscious sedation,” such a meta-analysis is unlikely to be of significant benefit.
CONCLUSIONS
The current meta-analysis suggests that the risk of sedation related adverse events in patients administered propofol by non-anesthesiologists is extremely low. We could not compare such outcomes with anesthesia providers, as similar studies are not available. It is prudent for anesthesia providers to demonstrate their superiority in prospective randomized controlled trials, if they like to retain exclusive ownership over propofol sedation in patients undergoing GI endoscopy. However, one should also bear in mind that the patient safety demonstrated in clinical trials may not be applicable in non-trial settings unless proper training and regulatory requirements are implemented.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Goudra BGB, Singh PM. Cardiac arrests during endoscopy with anesthesia assistance. JAMA Intern Med. 2013;173:1659–60. doi: 10.1001/jamainternmed.2013.8756. [DOI] [PubMed] [Google Scholar]
- 2.Cooper GS, Kou TD, Rex DK. Complications following colonoscopy with anesthesia assistance: A population-based analysis. JAMA Intern Med. 2013;173:551–6. doi: 10.1001/jamainternmed.2013.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coté GA, Hovis RM, Ansstas MA, Waldbaum L, Azar RR, Early DS, et al. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010;8:137–42. doi: 10.1016/j.cgh.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Goudra B, Nuzat A, Singh PM, Gouda GB, Carlin A, Manjunath AK. Cardiac Arrests in Patients Undergoing Gastrointestinal Endoscopy: A Retrospective Analysis of 73,029 Procedures. Saudi J Gastroenterol. 2015;21:400–11. doi: 10.4103/1319-3767.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berzin TM, Sanaka S, Barnett SR, Sundar E, Sepe PS, Jakubowski M, et al. A prospective assessment of sedation-related adverse events and patient and endoscopist satisfaction in ERCP with anesthesiologist-administered sedation. Gastrointest Endosc. 2011;73:710–7. doi: 10.1016/j.gie.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Mazanikov M, Udd M, Kylänpää L, Mustonen H, Lindström O, Halttunen J, et al. Patient-controlled sedation for ERCP: A randomized double-blind comparison of alfentanil and remifentanil. Endoscopy. 2012;44:487–92. doi: 10.1055/s-0031-1291655. [DOI] [PubMed] [Google Scholar]
- 7.Mazanikov M, Udd M, Kylänpää L, Lindström O, Aho P, Halttunen J, et al. Patient-controlled sedation with propofol and remifentanil for ERCP: A randomized, controlled study. Gastrointest Endosc. 2011;73:260–6. doi: 10.1016/j.gie.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Mazanikov M, Udd M, Kylänpää L, Mustonen H, Lindström O, Färkkilä M, et al. A randomized comparison of target-controlled propofol infusion and patient-controlled sedation during ERCP. Endoscopy. 2013;45:915–9. doi: 10.1055/s-0033-1344712. [DOI] [PubMed] [Google Scholar]
- 9.Redondo-Cerezo E, Sánchez-Robaina A, Martínez Cara JG, Ojeda-Hinojosa M, Matas-Cobos A, Sánchez Capilla AD, et al. Gastroenterologist-guided sedation with propofol for endoscopic ultrasonography in average-risk and high-risk patients: A prospective series. Eur J Gastroenterol Hepatol. 2012;24:506–12. doi: 10.1097/MEG.0b013e328350fcbd. [DOI] [PubMed] [Google Scholar]
- 10.Dewitt J, McGreevy K, Sherman S, Imperiale TF. Nurse-administered propofol sedation compared with midazolam and meperidine for EUS: A prospective, randomized trial. Gastrointest Endosc. 2008;68:499–509. doi: 10.1016/j.gie.2008.02.092. [DOI] [PubMed] [Google Scholar]
- 11.Lee CK, Lee S-H, Chung I-K, Lee TH, Park S-H, Kim E-O, et al. Balanced propofol sedation for therapeutic GI endoscopic procedures: A prospective, randomized study. Gastrointest Endosc. 2011;73:206–14. doi: 10.1016/j.gie.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH, Lee CK, Park S-H, Lee S-H, Chung I-K, Choi HJ, et al. Balanced propofol sedation versus propofol monosedation in therapeutic pancreaticobiliary endoscopic procedures. Dig Dis Sci. 2012;57:2113–21. doi: 10.1007/s10620-012-2234-0. [DOI] [PubMed] [Google Scholar]
- 13.Angsuwatcharakon P, Rerknimitr R, Ridtitid W, Kongkam P, Poonyathawon S, Ponauthai Y, et al. Cocktail sedation containing propofol versus conventional sedation for ERCP: A prospective, randomized controlled study. BMC Anesthesiol. 2012;12:20. doi: 10.1186/1471-2253-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Delius S, Salletmaier H, Meining A, Wagenpfeil S, Saur D, Bajbouj M, et al. Bispectral index monitoring of midazolam and propofol sedation during endoscopic retrograde cholangiopancreatography: A randomized clinical trial (the EndoBIS study) Endoscopy. 2012;44:258–64. doi: 10.1055/s-0031-1291485. [DOI] [PubMed] [Google Scholar]
- 15.Dumonceau JM. Nonanesthesiologist administration of propofol: It's all about money. Endoscopy. 2012;44:453–5. doi: 10.1055/s-0031-1291658. [DOI] [PubMed] [Google Scholar]
- 16.Goudra BG, Singh PM, Gouda G, Borle A, Gouda D, Dravida A, et al. Safety of Non-anesthesia Provider-Administered Propofol (NAAP) Sedation in Advanced Gastrointestinal Endoscopic Procedures: Comparative Meta-Analysis of Pooled Results. Dig Dis Sci. 2015;60:2612–27. doi: 10.1007/s10620-015-3608-x. [DOI] [PubMed] [Google Scholar]
- 17.Heuss LT, Schnieper P, Drewe J, Pflimlin E, Beglinger C. Conscious sedation with propofol in elderly patients: A prospective evaluation. Aliment Pharmacol Ther. 2003;15(17):1493–501. doi: 10.1046/j.1365-2036.2003.01608.x. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich K, Stremmel W, Sieg A. Endoscopist-administered propofol sedation is safe-a prospective evaluation of 10,000 patients in an outpatient practice. J Gastrointest Liver Dis. 2012;21:259–63. [PubMed] [Google Scholar]
- 19.Horiuchi A, Nakayama Y, Hidaka N, Ichise Y, Kajiyama M, Tanaka N. Low-dose propofol sedation for diagnostic esophagogastroduodenoscopy: Results in 10,662 adults. Am J Gastroenterol. 2009;104:1650–5. doi: 10.1038/ajg.2009.250. [DOI] [PubMed] [Google Scholar]
- 20.Lucendo AJ, Olveira A, Friginal-Ruiz AB, Guagnozzi D, Angueira T, Fernández-Fuente M, et al. Nonanesthesiologist-administered propofol sedation for colonoscopy is safe and effective: A prospective Spanish study over 1000 consecutive exams. Eur J Gastroenterol Hepatol. 2012;24:787–92. doi: 10.1097/MEG.0b013e328353fcbc. [DOI] [PubMed] [Google Scholar]
- 21.Tohda G, Higashi S, Wakahara S, Morikawa M, Sakumoto H, Kane T. Propofol sedation during endoscopic procedures: Safe and effective administration by registered nurses supervised by endoscopists. Endoscopy. 2006;38:360–7. doi: 10.1055/s-2005-921192. [DOI] [PubMed] [Google Scholar]
- 22.Sieg A. Propofol sedation in outpatient colonoscopy by trained practice nurses supervised by the gastroenterologist: A prospective evaluation of over 3000 cases. Z Für Gastroenterol. 2007;45:697–701. doi: 10.1055/s-2007-963349. [DOI] [PubMed] [Google Scholar]
- 23.Rex DK, Overley C, Kinser K, Coates M, Lee A, Goodwine BW, et al. Safety of propofol administered by registered nurses with gastroenterologist supervision in 2000 endoscopic cases. Am J Gastroenterol. 2002;97:1159–63. doi: 10.1111/j.1572-0241.2002.05683.x. [DOI] [PubMed] [Google Scholar]
- 24.Heuss LT, Schnieper P, Drewe J, Pflimlin E, Beglinger C. Safety of propofol for conscious sedation during endoscopic procedures in high-risk patients-a prospective, controlled study. Am J Gastroenterol. 2003;98:1751–7. doi: 10.1111/j.1572-0241.2003.07596.x. [DOI] [PubMed] [Google Scholar]
- 25.Akarsu Ayazoğlu T, Polat E, Bolat C, Yasar NF, Duman U, Akbulut S, et al. Comparison of propofol-based sedation regimens administered during colonoscopy. Rev Médica Chile. 2013;141:477–85. doi: 10.4067/S0034-98872013000400009. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi A, Nakayama Y, Kajiyama M, Kato N, Kamijima T, Ichise Y, et al. Safety and effectiveness of propofol sedation during and after outpatient colonoscopy. World J Gastroenterol. 2012;18:3420–5. doi: 10.3748/wjg.v18.i26.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieg A, Beck S, Scholl SG, Heil FJ, Gotthardt DN, et al. bng-Study-Group. Safety analysis of endoscopist-directed propofol sedation: A prospective, national multicenter study of 24441 patients in German outpatient practices. J Gastroenterol Hepatol. 2014;29:517–23. doi: 10.1111/jgh.12458. [DOI] [PubMed] [Google Scholar]
- 28.Correia LM, Bonilha DQ, Gomes GF, Brito JR, Nakao FS, Lenz L, et al. Sedation during upper GI endoscopy in cirrhotic outpatients: A randomized, controlled trial comparing propofol and fentanyl with midazolam and fentanyl. Gastrointest Endosc. 2011;73:45–51. doi: 10.1016/j.gie.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Külling D, Rothenbühler R, Inauen W. Safety of nonanesthetist sedation with propofol for outpatient colonoscopy and esophagogastroduodenoscopy. Endoscopy. 2003;35:679–82. doi: 10.1055/s-2003-41518. [DOI] [PubMed] [Google Scholar]
- 30.Külling D, Orlandi M, Inauen W. Propofol sedation during endoscopic procedures: How much staff and monitoring are necessary? Gastrointest Endosc. 2007;66:443–9. doi: 10.1016/j.gie.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Koshy G, Nair S, Norkus EP, Hertan HI, Pitchumoni CS. Propofol versus midazolam and meperidine for conscious sedation in GI endoscopy. Am J Gastroenterol. 2000;95:1476–9. doi: 10.1111/j.1572-0241.2000.02080.x. [DOI] [PubMed] [Google Scholar]
- 32.Ho WM, Yen CM, Lan CH, Lin CY, Yong SB, Hwang KL, et al. Comparison between the recovery time of alfentanil and fentanyl in balanced propofol sedation for gastrointestinal and colonoscopy: A prospective, randomized study. BMC Gastroenterol. 2012;12:164. doi: 10.1186/1471-230X-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker JA, McIntyre RD, Schleinitz PF, Jacobson KN, Haulk AA, Adesman P, et al. Nurse-administered propofol sedation without anesthesia specialists in 9152 endoscopic cases in an ambulatory surgery center. Am J Gastroenterol. 2003;98:1744–50. doi: 10.1111/j.1572-0241.2003.07605.x. [DOI] [PubMed] [Google Scholar]
- 34.Molina-Infante J, Dueñas-Sadornil C, Mateos-Rodriguez JM, Perez-Gallardo B, Vinagre-Rodríguez G, Hernandez-Alonso M, et al. Nonanesthesiologist-administered propofol versus midazolam and propofol, titrated to moderate sedation, for colonoscopy: A randomized controlled trial. Dig Dis Sci. 2012;57:2385–93. doi: 10.1007/s10620-012-2222-4. [DOI] [PubMed] [Google Scholar]
- 35.Poincloux L, Laquière A, Bazin J-E, Monzy F, Artigues F, Bonny C, et al. A randomized controlled trial of endoscopist vs. anaesthetist-administered sedation for colonoscopy. Dig Liver Dis. 2011;43:553–8. doi: 10.1016/j.dld.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Repici A, Pagano N, Hassan C, Carlino A, Rando G, Strangio G, et al. Balanced propofol sedation administered by nonanesthesiologists: The first Italian experience. World J Gastroenterol. 2011;17:3818–23. doi: 10.3748/wjg.v17.i33.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goudra BG, Singh PM. Remimazolam: The future of its sedative potential. Saudi J Anaesth. 2014;8:388–91. doi: 10.4103/1658-354X.136627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goudra B, Singh PM. More questions than answers: Comparison of the risk of cardiopulmonary adverse events between propofol and traditional anesthesia for gastrointestinal endoscopy. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.09.137. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Goudra B, Nuzat A, Singh PM, Borle A, Carlin A, Gouda G. Association between Type of Sedation and the Adverse Events Associated with Gastrointestinal Endoscopy: An Analysis of 5 Years’ Data from a Tertiary Center in the USA. Clin Endosc. 2016 doi: 10.5946/ce.2016.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goudra B, Singh PM. Forward Progress of Sedation for Gastrointestinal Endoscopy Requires Taking a Step Back. Gastroenterology. 2016;151:562–3. doi: 10.1053/j.gastro.2016.02.091. [DOI] [PubMed] [Google Scholar]
- 41.Goudra B, Singh PM. Providing Deep Sedation for Advanced Endoscopic Procedures: The Esthetics of Endoscopic Anesthetics. Dig Dis Sci. 2016;61:1426–8. doi: 10.1007/s10620-016-4157-7. [DOI] [PubMed] [Google Scholar]
- 42.Dumonceau J-M, Riphaus A, Schreiber F, Vilmann P, Beilenhoff U, Aparicio JR, et al. Non-anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates Guideline-Updated June 2015. Endoscopy. 2015;47:1175–89. doi: 10.1055/s-0034-1393414. [DOI] [PubMed] [Google Scholar]
- 43.Medicare C for, Baltimore MS 7500 SB, Usa M. National Health Accounts Historical [Internet] 2014. [Last accessed on 2015 Apr 19]. Available from: http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html .
- 44.Murray CJL, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacDorman MF, Matthews TJ, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. Natl Vital Stat Rep. 2014;63:1–6. [PubMed] [Google Scholar]
- 46.Moser A, Panczak R, Zwahlen M, Clough-Gorr KM, Spoerri A, Stuck AE, et al. What does your neighbourhood say about you? A study of life expectancy in 1.3 million Swiss neighbourhoods. J Epidemiol Community Health. 2014;68:1125–32. doi: 10.1136/jech-2014-204352. [DOI] [PubMed] [Google Scholar]
- 47.Ladabaum U, Mannalithara A. Comparative Effectiveness and Cost Effectiveness of a Multitarget Stool DNA Test to Screen for Colorectal Neoplasia. Gastroenterology. 2016;151:427–39. doi: 10.1053/j.gastro.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Ladabaum U, Levin Z, Mannalithara A, Brill JV, Bundorf MK. Colorectal testing utilization and payments in a large cohort of commercially insured US adults. Am J Gastroenterol. 2014;109:1513–25. doi: 10.1038/ajg.2014.64. [DOI] [PubMed] [Google Scholar]
- 49.Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JTA, Ortmann M, et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2010;42:960–74. doi: 10.1055/s-0030-1255728. [DOI] [PubMed] [Google Scholar]
- 50.Perel A. Non-anaesthesiologists should not be allowed to administer propofol for procedural sedation: A Consensus Statement of 21 European National Societies of Anaesthesia. Eur J Anaesthesiol. 2011;28:580–4. doi: 10.1097/EJA.0b013e328348a977. [DOI] [PubMed] [Google Scholar]
- 51.Delaunay L, Gentili M, Cittanova ML, Delbos A, Lile A. Anaesthesia by non-anaesthesiologists: The Pandora Box is open! Eur J Anaesthesiol. 2012;29:50–1. doi: 10.1097/EJA.0b013e32834ad9d3. [DOI] [PubMed] [Google Scholar]
- 52.Knape JTA. Procedural sedation and analgesia and the propofol affair: A unique opportunity for anaesthesiology. Eur J Anaesthesiol. 2012;29:51–2. doi: 10.1097/EJA.0b013e32834d2102. [DOI] [PubMed] [Google Scholar]
- 53.Werner C, Smith A, Van Aken H. Guidelines on non-anaesthesiologist administration of propofol for gastrointestinal endoscopy: A double-edged sword. Eur J Anaesthesiol. 2011;28:553–5. doi: 10.1097/EJA.0b013e328348a9db. [DOI] [PubMed] [Google Scholar]
- 54.Pelosi P. Retraction of endorsement: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2012;44:302. doi: 10.1055/s-0031-1291648. [DOI] [PubMed] [Google Scholar]
- 55.Rosow C. Improving safety during sedation by nonanesthesiologists: Do we lead or follow? Anesth Analg. 2014;119:7–8. doi: 10.1213/ANE.0000000000000277. [DOI] [PubMed] [Google Scholar]