Abstract
Background/Aims:
Acute liver failure (ALF) is a rare but severe medical emergency. To date, there is no established treatment for non-acetaminophen-induced acute liver failure (NAI-ALF) other than liver transplantation, and little is known about the use of N-acetylcysteine (NAC) in NAI-ALF. A randomized case control study was conducted with the aim to determine the effect of NAC on the mortality of NAI-ALF patients, as well as to evaluate the safety and efficacy of NAC use.
Patients and Methods:
A total of 80 patients diagnosed with NAI-ALF were included in the study. Forty patients received NAC infusion for 72 h whereas the control group received placebo. The variables evaluated were demographic characteristics, signs and symptoms, biochemical parameters, and clinical course during hospitalization.
Results:
The two groups (NAC and control) were comparable for various baseline characteristics (such as etiology of ALF, INR, alanine aminotransferase, creatinine, albumin, and grade of encephalopathy), except for age. Although majority of patients had undetermined etiology (32.5% in NAC group and 42.5% in control group), the second main cause was acute hepatitis E and drug or toxin-induced ALF. The mortality decreased to 28% with the use of NAC versus 53% in the control group (P = 0.023). The use of NAC was associated with shorter length of hospital stay in survived patients (P = 0.002). Moreover, the survival of patients was improved by NAC (P = 0.025). Also, drug-induced ALF showed improved outcome compared to other etiologies.
Conclusion:
The findings of the study recommend the use of NAC along with conventional treatments in patients with NAI-ALF in non-transplant centers while awaiting referrals and conclude the use of NAC as safe.
Keywords: Acute liver failure, N-acetylcysteine, non-acetaminophen-induced acute liver failure
INTRODUCTION
Acute liver failure (ALF) is a relatively rare, but severe, life-endangering medical emergency, in which the rapid deterioration of liver function results in coagulopathy (INR >1.5) and encephalopathy of an individual who previously had a normal liver.[1] An incidence of 1–6 cases per million people every year has been reported from the developed parts of the world. However, the incidence rates are probably high in developing countries where infective hepatitis is common and treatment modalities that interrupt the progression of hepatic injury and other multiorgan dysfunction are not readily available.[2,3,4] Approximately, 2500 cases occur yearly in the US, yet it accounts for up to 7% of all liver-related deaths[5] and is responsible for 6% of liver transplants.[6] The majority of cases of ALF are among the young (median age 38 years) and among females (73%).[7]
The most important step in the management of ALF is to identify the cause, which helps in execution of targeted therapies and antidotes, when available. The main etiological factors of ALF are viral, drugs including herbal and traditional medications,[8,9] autoimmune, and toxins. In the Indian subcontinent, viral hepatitis is the most common cause of ALF and accounts for 90% of all the cases.[10] Kashmir, the northern-most part of India has reported a high incidence of ALF with hepatitis E virus (HEV) as the most important etiological agent.[11] A significant number of ALF patients have indeterminate cause with poor survival and usually require emergency transplantation.[12,13]
Mortality in ALF is usually caused by cerebral edema, multiorgan dysfunction syndrome, and sepsis. Multiorgan dysfunction in ALF occurs due to oxidative stress generated by reactive oxygen and nitrogen species[14] from immunological injuries mediated by cytokines[15,16,17,18] and raised circulating neurotoxins, especially ammonia.[19] The management of patients with ALF requires thorough infrastructure and understanding to deal with the complications.[20] Therapies that have been directed at reducing tissue injury, removing accumulated toxins, and promoting hepatocyte regeneration (which include interferon, insulin and glucagon,[21] prostaglandin E1,[22] charcoal hemoperfusion,[23] exchange transfusion,[24] and hyperimmunoglobulin infusion) have proven to be ineffective and are under trial. Although many people recover with supportive treatment, liver transplantation remains the only definitive therapy for patients who are unable to achieve sufficient hepatocyte regeneration on supportive treatment. Liver transplantation has made significant impact on the survival of patients with ALF,[25,26] however, the facility is largely unaffordable and only available in limited medical centers. Earlier studies have discussed the benefits of N-acetylcysteine (NAC) use in the treatment of acetaminophen-induced ALF,[27] and some recent studies have suggested the role of NAC in non-acetaminophen-induced acute liver failure (NAI-ALF)[28,29] because of its multiple mechanisms of action.
NAC is a thiol-containing agent that scavenges free oxygen radicals and replenishes cellular, mitochondrial, and cytosolic glutathione stores by serving as a source of a glutathione surrogate that combines directly with reactive metabolites or serves as a source of sulfate, thus preventing hepatic damage.[30,31,32] Moreover, various trials have proved the anti-inflammatory, antioxidant, inotropic, and vasodilating effects of NAC.[33,34] NAC benefits NAI-ALF patients either by improving systemic hemodynamics and tissue oxygen delivery[35,36,37,38,39] or via other mechanisms.[40] Despite established roles of NAC in acetaminophen-induced ALF, its role in NAI-ALF remains controversial.[28,29] Owing to contradictory results, further studies are warranted to evaluate the safety and efficacy of NAC in patients with NAI-ALF. Thus, we carried out a randomized trial in Sher-i-Kashmir Institute of Medical Science (SKIMS), a center without transplantation facility to (1) assess the effect of NAC on ALF-induced mortality in patients with NAI-ALF and (2) evaluate the safety and efficacy of NAC in NAI-ALF patients and its impact on the duration of hospital stay.
PATIENTS AND METHODS
The study was a hospital-based prospective randomized case control trial of adult patients with NAI-ALF. The study was carried out in the Department of Gastroenterology of SKIMS, Soura, Jammu & Kashmir. The study was approved by the institutional ethical committee of SKIMS.
Study participants
This study was conducted over a period of 2 years from September 2011 to September 2013. A total of 80 patients confirmed with NAI-ALF and who were above the age of 18 years were invited to participate in this study. ALF was defined as biochemical evidence of ALF with INR of ≥1.5 and any degree of encephalopathy caused by illness of duration <8 weeks (fulminant hepatic failure). After informed consent which was obtained from every participant, information regarding various demographic characteristics was taken through well-structured questionnaires. In addition to a detailed history, physical examination, and biochemical work-up which included baseline investigations, liver function test (LFT) and coagulogram were carried out. The exclusion criteria of the study were (1) acetamionophen-induced ALF (on the basis of detailed history), (2) ALF during pregnancy, (3) acute or chronic liver failure, (4) prior exposure to NAC, and (5) hepatic ischemia (shock liver).
Detailed study design
After ALF was diagnosed, blood samples of all the patients were taken for etiological diagnosis of ALF, which included hepatitis B surface antigen (HBsAg), hepatitis B core IgM (HBc-IgM), hepatitis A virus IgM (HAV-IgM), and hepatitis E virus IgM (HEV-IgM), anti HCV (hepatitis C virus), anti-nuclear antibody (ANA), anti-smooth muscle antibody (ASMA), Wilson profile (serum ceruloplasmin, serum copper), and iron profile. Herpes simplex virus (HSV), cytomegalovirus (CMV), and Epstein barr virus (EBV) serology were done if non-hepatotropic viruses were suspected as a cause of ALF. Detailed history was obtained for any hepatotoxic drug intake such as homeopathic and herbal medications. Participants were then randomized by simple random method into two groups.
NAC Group: Forty NAI-ALF patients who fulfilled the eligibility criteria were treated with intravenous NAC for duration of 72 hours.
Control Group: Forty NAI-ALF patients who received 5% dextrose (placebo) infusion for 72 hours.
Study medication: The patients in NAC group were administered intravenous NAC with initial loading dose of 150 mg/kg over 1 hour, followed by 12.5 mg/kg/h for 4 hours and continuous infusion of 6.25 mg/kg/h for remaining 67 hours. Patients in the control group were given 5% dextrose infusion (placebo) for 72 hours. All the ethical considerations were taken care of during the study. Patients were given the option of liver transplant (to be done at the hospital with transplantation facility) at various stages of study when indicated.
Supportive treatment: All patients were managed with the standard supportive care treatment, which were similar throughout the study period in both the groups. The patients received treatment for the complications of ALF.[41] The treatment mainly involved continuous intravenous dextrose to prevent hypoglycemia, broad-spectrum prophylactic antimicrobials,[41,42,43,44] proton pump inhibitors for stress-related ulcers, fluid and electrolyte balance, and lactulose enema. In patients with advanced hepatic encephalopathy, intensive care management, midazolam sedation, and mannitol infusion in case of raised intracranial pressure were given. Intracranial hypertension was diagnosed by the presence of clinical signs such as abnormal pupillary reflexes, hypertonia, or decerebrate posturing. Fresh frozen plasma was given in only those patients who had spontaneous bleeding. Blood and urine cultures were obtained in suspected cases of sepsis, which were than treated according to sensitivity. Renal impairment was defined as serum creatinine level of more than 2.0 mg/dl.
Monitoring: Response to treatment was monitored clinically (Grade of encephalopathy) and biochemically (bilirubin, PT, INR, etc.). In addition, morbidity and mortality was also assessed. Patient were followed till discharge or death in hospital.
Statistical analyses: In univariate analysis, the categorical variables were compared in the two groups by using χ2 test or Fisher exact test as appropriate. For continuous variables, the independent sample t-test was used. P values <0.05 were considered statistically significant. All the analyses were performed by the Statistical Package for Social Sciences (SPSS Inc. Released 2004. SPSS for Windows, Version 13.0. Chicago, SPSS Inc.).
RESULTS
The distribution of baseline characteristics (both categorical and continuous) of the two groups (NAC vs Control) are presented in Table 1. The mean age of NAC and control group was 30.60 ± 11.64 years and 38.48 ± 20.11 years, respectively (P = 0.035). All the patients were of Kashmiri ethnicity. Coma grade at the time of admission showed that the majority of patients (38.75%) had grade I encephalopathy. The patients in both the groups were comparable for the various grades of encephalopathy (P = 0.054). Further, the two groups did not differ significantly with respect to vomiting, MELD score, interval between jaundice and encephalopathy, and biochemical measures of liver injury (INR, bilirubin, AST, ALT, and albumin).
Table 1.
Baseline characteristics of study subjects in the two treatment groups
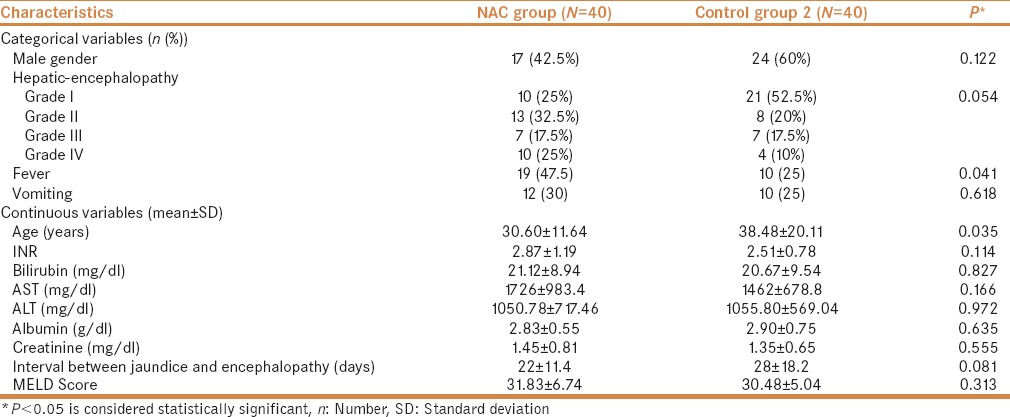
The etiology of the NAC and control group at the time of the admission are presented in Table 2. Majority of the patients, 32.5% and 42.5% in the NAC and control group, respectively, had undetermined etiology. There were 7 (17.5%) acute HEV-induced ALF cases in each group. Moreover, 10 (25.0%) patients in the NAC group and 5 (12.5%) in the control group (P = 0.399) had ALF induced by drugs and toxins (8 patients had anti-tuberculosis therapy (ATT) induced ALF and 2 patients had ayurvedic-induced ALF in NAC group and in the control group 4 and 1 patients had ATT and ayurvedic-induced ALF, respectively). Other etiology includes ALF due to Wilson, autoimmune, CMV, and HSV. There was one patient with Wilson-induced ALF in each group. Control group comprised one patient each of autoimmune, CMV, and HSV.
Table 2.
Etiology of ALF
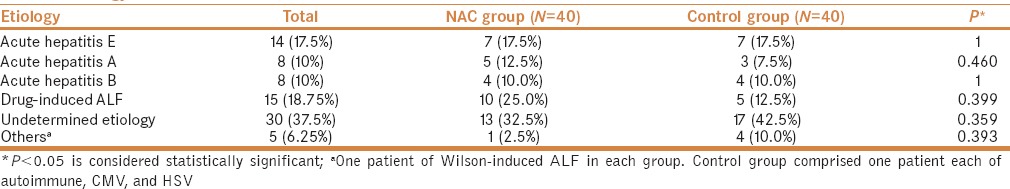
During the hospital course, a total of 15% patients developed renal failure in NAC group versus 22.5% in control group (P = 0.393). Mannitol was used for raised intracranial pressure (ICP) more frequently in the control (92.5%) than in the NAC group (75%) (P = 0.037). Complications such as infection, seizures, hypotension, development of ascites, and UGI bleed were similar between the two groups (P = ns) [Table 3].
Table 3.
Hospital course of non-acetaminophen-induced ALF in the two groups
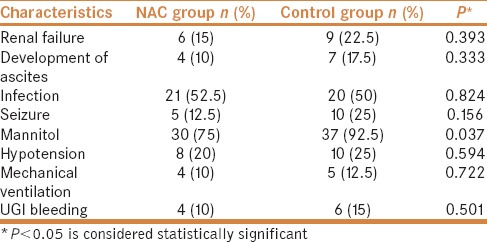
The mean number of days of hospital stay of survived patients in NAC group was 8.241 ± 2.115 versus 10.737 ± 3.106 in the control group, and the difference was statistically significant (P = 0.002) [Table 4]. A total of 32 of 80 (40%) patients died with ALF complications; 11 (27.5%) patients belonged to the NAC group and 21 (52.5%) patients to the control group (chi-square = 5.208; P = 0.023) and the mean time to death from diagnosis was 9.3 days.
Table 4.
Length of hospital stay in NAC group and controls
More patients (72.5%) survived in the NAC group than in the control group (47.5%) and the difference was statistically significant [Table 5]. When survival was stratified with various etiologies, patients with drug-induced ALF showed improved outcome as compared to other etiologies [Table 6].
Table 5.
Survival for study subjects

Table 6.
Survival for study participants stratified by etiology
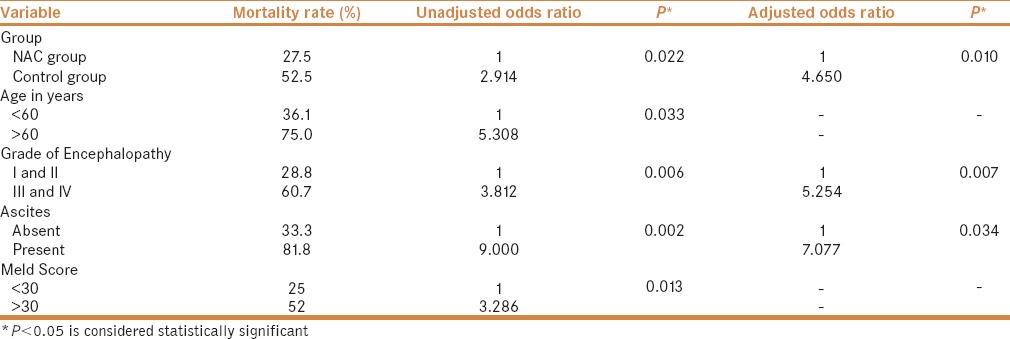
Logistic regression analysis was performed to study the role of independent risk factors on the mortality in NAI-ALF patients. In this study, not using NAC, age >60 years, III–IV grade of encephalopathy, presence of ascites, and MELD score >30 were the independent prognostic factors determining mortality. However, in adjusted models (adjusted with age and fever) not using NAC, III–IV grade of encephalopathy and presence of ascites were significant predictive markers of mortality [Table 7]. No adverse effect was noted in patients in the NAC group, which could have been attributed to NAC administration.
Table 7.
Mortality among two groups and impact of various factors on mortality using logistic regression analysis
DISCUSSION
In this prospective randomized control trial, we found significant improvement in the survival of patients who were treated with NAC; mortality decreased to 28% with the use of NAC versus 53% in the control group. On stratification of patient's survival on the basis of various etiological groups, patients with drug-induced ALF showed improved outcome compared to other etiologies. Furthermore, the use of NAC was safe and was associated with a shorter length of hospital stay in survived patients (P = 0.002).
Similarly, a prospective study with historical controls carried out by Mumtaz et al.[45] revealed administration of NAC causes reduction in NAI-ALF mortality and its use was safe. Furthermore, some earlier studies in adults and children[34,46,47,48,49,50,51,52] argued the safe use of NAC with minor side effects, with most of them self-limited or resolved with either the use of antihistamine drugs or by lowering the infusion rate. Retrospective studies of pediatric patients with NAI-ALF showed improved transplant-free survival and shorter hospital stays with NAC use.[45,53] Moreover, patients with drug-induced ALF (ATT induced) in this study showed improved outcome as compared to other etiologies. Baniasadi et al. also showed protective effect of NAC on antituberculosis drug-induced hepatotoxicity by monitoring LFT.[54]
The etiology of ALF has a wide geographic variation.[55] In the West, acetaminophen overdose and idiosyncratic drug reaction constitute most of the ALF cases,[20,56,57,58,59,60] whereas in the Indian subcontinent it is HEV.[10] In this study, although the majority of patients had undetermined etiology (37.5%), the main cause was drug and acute hepatitis E-induced ALF. HEV was followed by HAV and HBV.[10,61] The previous study from Kashmir (J & K) also revealed HEV as the most common cause of ALF.[11] The increase in the number of patients with undetermined etiology from previous study of 31%[11] could be because of unexpected acetaminophen toxicity,[62] a novel or unrecognized virus, metabolic, or xenobiotic injury and undiagnosed immune dysregulation.[63]
Occurrence of various complications of ALF during hospital course, such as sepsis, renal impairment, hypotension, and development of ascites were similar between the two groups. However, during hospital stay more patients with raised ICP required mannitol in the control group than in NAC group, possibly because NAC increases cerebral perfusion pressure (CPP) and decreases inflammation.
The patients in the NAC group had worse prognostic factors at their baseline, yet their survival was better as evidenced by the fact that there were more patients with fever, grade IV encephalopathy and with higher bilirubin, AST, creatinine, and INR levels in the NAC group. No adverse effects were noted in patients that could have been attributed to NAC administration.
To the best of our knowledge, only four prospective studies have reported some benefits of NAC use in NAI-ALF patients[64] and most of them have used retrospective controls. The major strengths of this study include relatively modest sample size, prospective cases and controls, and adjustments of the results for multiple potential confounding factors. Some of the limitations of our study include the two groups differed with respect to age, fever, and grade of encephalopathy; exclusion of acetaminophen-induced ALF was on the basis of history so retrospective exposure assessments recall bias rather than biochemical confirmation which was not available in the hospital; and the duration of follow up was short (hospital stay till discharge or death in the hospital).
We suggest that the use of NAC along with conventional treatments will benefit the patients with NAI-ALF in centers where transplantation facility is not available. Furthermore, the use of NAC is safe.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–98. [PubMed] [Google Scholar]
- 2.Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102:2459–63. doi: 10.1111/j.1572-0241.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Brandsaeter B, Hockerstedt K, Friman S. Fulminant hepatic failure: Outcome after listing for highly urgent liver transplantation-12 years experience in the Nordic countries. Liver Transpl. 2002;8:1055–62. doi: 10.1053/jlts.2002.35556. [DOI] [PubMed] [Google Scholar]
- 4.Escorsell A, Mas A. Acute liver failure in Spain: Analysis of 267 cases. Liver Transpl. 2007;13:89–95. doi: 10.1002/lt.21119. [DOI] [PubMed] [Google Scholar]
- 5.Hoofnagle JH, Carithers RL, Shapiro C. Fulminant hepatic failure: Summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 6.Rockville. Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. 2007 [Google Scholar]
- 7.Ostapowicz G, Fontana RJ, Schiodt FV. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 8.Bjornsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. doi: 10.1016/j.dld.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Wai CT, Tan BH, Chan CL, Sutedja DS, Lee YM, Khor C, et al. Drug-induced liver injury at an Asian center: A prospective study. Liver Int. 2007;27:465–74. doi: 10.1111/j.1478-3231.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 10.Acharya SK, Dasarathy S, Kumer TL, Sushma S, Prasanna KS, Tandon A, et al. Fulminant hepatitis in tropical population: Clinical course, cause, and early predictors of outcome. Hepatology. 1996;23:1448–55. doi: 10.1002/hep.510230622. [DOI] [PubMed] [Google Scholar]
- 11.Khuroo MS, Kamili S. Aetiology and prognostic factors in acute liver failure in India. J Viral Hepat. 2003;10:224–31. doi: 10.1046/j.1365-2893.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 12.Bernal W. Changing patterns of causation and the use of transplantation in the United Kingdom. Semin Liver Dis. 2003;23:227–37. doi: 10.1055/s-2003-42640. [DOI] [PubMed] [Google Scholar]
- 13.Wei G, Kalaitzakis E, Bergquist A, Bjornsson E. Long-term followup of patients with acute liver failure of indeterminate aetiology. Scand J Gastroenterol. 2008;43:984–91. doi: 10.1080/00365520801965399. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury. J Gastroenterol Hepatol. 2000;15:718–24. doi: 10.1046/j.1440-1746.2000.02207.x. [DOI] [PubMed] [Google Scholar]
- 15.Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988;2:72–4. doi: 10.1016/s0140-6736(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 16.Nagaki M, Iwai H, Naiki T, Ohnishi H, Muto Y, Moriwaki H. High levels of serum interleukin-10 and tumor necrosis factor-alpha are associated with fatality in fulminant hepatitis. J Infect Dis. 2000;182:1103–8. doi: 10.1086/315826. [DOI] [PubMed] [Google Scholar]
- 17.Iwai H, Nagaki M, Naito T, Ishiki Y, Murakami M, Sugihara J. Removal of endotoxin and cytokines by plasma exchange in patients with acute hepatic failure. Crit Care Med. 1998;26:873–6. doi: 10.1097/00003246-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Sood GK, Katz J. Acute liver Failure. Medscape. 2011 [Google Scholar]
- 19.Bjerring PN, Eefsen M, Hansen BA, Larsen FS. The brain in acute liver failure. A tortuous path from hyperammonemia to cerebral edema. Metab Brain Dis. 2009;24:5–14. doi: 10.1007/s11011-008-9116-3. [DOI] [PubMed] [Google Scholar]
- 20.Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: Definitions and causes. Semin Liver Dis. 1986;6:97–106. doi: 10.1055/s-2008-1040593. [DOI] [PubMed] [Google Scholar]
- 21.Woolf GM, Redeker AG. Treatment of fulminant hepatic failure with insulin and glucagon: A randomized, controlled trial. Dig Dis Sci. 1991;36:92–6. doi: 10.1007/BF01300094. [DOI] [PubMed] [Google Scholar]
- 22.Sinclair SB, Greig PD, Blendis LM, Abecassis M, Roberts EA, Phillips MJ, et al. Biochemical and clinical response of fulminant viral hepatitis to administration of prostaglandin E: A preliminary report. J Clin Invest. 1989;84:1063–9. doi: 10.1172/JCI114268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Grady JG, Gimson AE, O'Brien CJ, Pucknell A, Hughes RD, Williams R. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology. 1988;94:1186–92. doi: 10.1016/0016-5085(88)90011-x. [DOI] [PubMed] [Google Scholar]
- 24.Redeker AG, Yamahiro HS. Controlled trial of exchange-transfusion therapy in fulminant hepatitis. Lancet. 1973;1:3–6. doi: 10.1016/s0140-6736(73)91220-8. [DOI] [PubMed] [Google Scholar]
- 25.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–26. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 26.Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 27.Smilkstein MJ, Bronstein AC, Linden C, Augenstein WL, Kulig KW, Rumack BH. Acetaminophen overdose: A 48-hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med. 1991;20:1058–63. doi: 10.1016/s0196-0644(05)81352-6. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Ari Z, Vaknin H, Tur-Kaspa R. N-Acetylcysteine in acute hepatic failure (non-paracetamol-induced) Hepatogastroenterology. 2000;47:786–9. [PubMed] [Google Scholar]
- 29.Katoonizadeh A, Decaestecker J, Wilmer A, Aerts R, Verslype C, Vansteenbergen W. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int. 2007;27:329–34. doi: 10.1111/j.1478-3231.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 30.Halliwell B, Gutterridge JM. Free radicals in biology and medicine. Oxford: Oxford University Press; 1999. pp. 840–2. [Google Scholar]
- 31.Cotgreave IA. N-acetylcysteine pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–27. [PubMed] [Google Scholar]
- 32.Kharazmi A, Nielsen H, Schiotz PO. N-acetylcysteine inhibits human neutrophil and monocyte chemotaxis and oxidative metabolism. Int J Immunopharmacol. 1988;10:39–46. doi: 10.1016/0192-0561(88)90148-8. [DOI] [PubMed] [Google Scholar]
- 33.Harrison P, Wendon J, Williams R. Evidence of increased guanylate cyclase activation by acetylcysteine in fulminant hepatic failure. Hepatology. 1996;23:1067–72. doi: 10.1053/jhep.1996.v23.pm0008621135. [DOI] [PubMed] [Google Scholar]
- 34.Harrison PM, Wendon JA, Gimson AE, Alexander GJ, Williams R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852–7. doi: 10.1056/NEJM199106273242604. [DOI] [PubMed] [Google Scholar]
- 35.Rank N, Michel C, Haertel C. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit Care Med. 2000;28:3799–807. doi: 10.1097/00003246-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Schneider J, Lutun P, Boudjema K. In vivo evidence of enhanced guanylyl cyclase activation during the hyperdynamic circulation of acute liver failure. Hepatology. 1994;19:38–44. [PubMed] [Google Scholar]
- 37.Alonso A, Lau J, Jaber BL. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: A meta-analysis of randomized, controlled trials. Am J Kidney Dis. 2004;43:1–9. doi: 10.1053/j.ajkd.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Zwingmann C, Bilodeau M. Metabolic insights into the hepatoprotective effect of N-acetylcysteine in mouse liver. Hepatology. 2006;443:454–63. doi: 10.1002/hep.21075. [DOI] [PubMed] [Google Scholar]
- 39.Lee WM, Hynan LS, Lorenzo R, Fontana RJ, Stravitz RT. Intravenous N-Acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–64. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bémeur C, Vaquero J, Desjardins P, Butterworth RF. N-Acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: Antioxidant and anti-inflammatory mechanisms. Metab Brain Dis. 2010;25:241–9. doi: 10.1007/s11011-010-9201-2. [DOI] [PubMed] [Google Scholar]
- 41.Rolando N, Harvey F, Brahm J, Howard JP, Alexander G, Gimson A, et al. Prospective study of bacterial infections in acute liver failure: An analysis of fifty patients. Hepatology. 1990;11:49–53. doi: 10.1002/hep.1840110110. [DOI] [PubMed] [Google Scholar]
- 42.Rolando N, Wade J, Davalos M, Wendon J, Howard JP, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–9. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 43.Rolando N, Harvey F, Brahm J, Howard JP, Alexander G, Gimson A, et al. Fungal infection: A common, unrecognised complication of acute liver failure. J Hepatol. 1991;12:1–9. doi: 10.1016/0168-8278(91)90900-v. [DOI] [PubMed] [Google Scholar]
- 44.Rolando N, Howard JP, Williams R. Bacterial and fungal infection in acute liver failure. Semin Liver Dis. 1996;16:389–402. doi: 10.1055/s-2007-1007252. [DOI] [PubMed] [Google Scholar]
- 45.Mumtaz K, Azam Z, Hamid S, Abid S, Memon S, Shah HA, et al. Role of N-Acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int. 2009;3:563–70. doi: 10.1007/s12072-009-9151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison PM, Keays R, Bray GP, Alexander GJ, Williams R. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335:1572–3. doi: 10.1016/0140-6736(90)91388-q. [DOI] [PubMed] [Google Scholar]
- 47.Smilkstein MJ, Bronstein AC, Linden C, Augenstein WL, Kulig KW, Rumack BH. Acetaminophen overdose: A 48-hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med. 1991;20:1058–63. doi: 10.1016/s0196-0644(05)81352-6. [DOI] [PubMed] [Google Scholar]
- 48.Bateman DN, Woodhouse KW, Rawlins MD. Adverse reactions to N-acetylcysteine. Human Toxicol. 1984;3:393–8. doi: 10.1177/096032718400300504. [DOI] [PubMed] [Google Scholar]
- 49.Mant TG, Tempowski JH, Volans GN, Talbot JC. Adverse reactions to acetylcysteine and effects of overdose. BMJ. 1984;289:217–9. doi: 10.1136/bmj.289.6439.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenenbein M. Hypersensitivity-like reactions to N-acetylcysteine. Vet Hum Toxicol. 1984;26:3–5. [PubMed] [Google Scholar]
- 51.Dawson AH, Henry DA, McEwen J. Adverse reactions to N-acetylcysteine during treatment for paracetamol poisoning. Med J Aust. 1989;150:329–31. doi: 10.5694/j.1326-5377.1989.tb136496.x. [DOI] [PubMed] [Google Scholar]
- 52.Falk JL. Oral N-acetylcysteine given intravenously for acetaminophen overdose: We shouldn't have to but we must. Crit Care Med. 1998;26:7. doi: 10.1097/00003246-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Kortsalioudaki C, Taylor RM, Cheeseman P, Bansal S, Mieli-Vergani G, Dhawan A. Safety and efficacy of N-acetylcysteine in children with non-acetaminophen-induced acute liver failure. Liver Transpl. 2008;14:25–30. doi: 10.1002/lt.21246. [DOI] [PubMed] [Google Scholar]
- 54.Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR, et al. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur J Gastroenterol Hepatol. 2010;22:1235–8. doi: 10.1097/MEG.0b013e32833aa11b. [DOI] [PubMed] [Google Scholar]
- 55.Lee WM, Sorrell MF. Developing a world view toward acute liver failure. Hepatology. 1996;24:270–1. doi: 10.1002/hep.510240143. [DOI] [PubMed] [Google Scholar]
- 56.Liang TJ, Jeffers L, Reddy RK, Silva MO, Cheinquer H, Findor A, et al. Fulminant or subfulminant non-A, non-B viral hepatitis: The role of hepatitis C and E viruses. Gastroenterology. 1993;104:556–62. doi: 10.1016/0016-5085(93)90426-d. [DOI] [PubMed] [Google Scholar]
- 57.Vale JA, Proudfoot AT. Paracetamol (acetaminophen) poisoning. Lancet. 1995;346:547–52. doi: 10.1016/s0140-6736(95)91385-8. [DOI] [PubMed] [Google Scholar]
- 58.Schiodt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112–7. doi: 10.1056/NEJM199710163371602. [DOI] [PubMed] [Google Scholar]
- 59.Wright TL. Etiology of fulminant hepatic failure: Is another virus involved? Gastroenterology. 1993;104:640–53. doi: 10.1016/0016-5085(93)90437-h. [DOI] [PubMed] [Google Scholar]
- 60.Fagan EA. Acute liver failure of unknown pathogenesis: The hidden agenda. Hepatology. 1994;19:1307–12. [PubMed] [Google Scholar]
- 61.Nanda SK, Yalchinkaya K, Panigrahi AK, Acharya SK, Jameel S, Panda SK. Etiological role of hepatitis E virus in sporadic fulminant hepatitis. J Med Virol. 1994;42:133–7. doi: 10.1002/jmv.1890420207. [DOI] [PubMed] [Google Scholar]
- 62.James LP, Alonso EM, Hynan LS, Hinson JA, Davern TJ, Lee WM, et al. Detection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics. 2006;118:e676–81. doi: 10.1542/peds.2006-0069. [DOI] [PubMed] [Google Scholar]
- 63.Squires RH, Dhawan A, Alonso E, Narkewicz MR, Shneider BL, Baez NR, et al. Intravenous N-acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: A placebo-controlled clinical trial. Hepatology. 2013;57:1542–9. doi: 10.1002/hep.26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu J, Zhang Q, Ren X, Sun Z, Quan Q. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015;39:594–9. doi: 10.1016/j.clinre.2015.01.003. [DOI] [PubMed] [Google Scholar]


