Abstract
Background/Aims:
The ideal end point of treatment for chronic hepatitis B virus (HBV) infection is sustained off-therapy hepatitis B surface antigen (HBsAg) loss with or even without seroconversion to anti-HBs. We investigated the role of adding PEGylated interferon (PEG IFN) to ongoing tenofovir treatment in chronic HBV patients for achieving HBsAg clearance.
Patients and Methods:
In this randomized controlled trial, chronic HBV patients who have been receiving tenofovir for >6 months with HBV viral load <2000 IU/ml were randomized into two groups. One group (add-on therapy) was given subcutaneous PEG IFN 180 mcg weekly for 12 months in addition to tenofovir. Patients in the other group received only tenofovir 300 mg orally on a daily basis. Patients in both groups were followed up for a total of two years, and patients in both groups were given tenofovir 300 mg daily indefinitely until they developed HBsAg clearance.
Results:
Twenty-three patients were allocated to the PEG IFN and tenofovir (add-on therapy) group, and another 25 patients were recruited to the tenofovir monotherapy group. Before randomization, patients had received tenofovir for 1135 mean days (range203 to 1542 days). One patient (4.3%) in add-on therapy lost HBsAg and seroconverted. Within two years, mean HBsAg decreased significantly with add-on therapy (from 4753 IU/ml to 2402; P = 0.03); and it decreased from 5957 IU/ml to 4198; P = 0.09 in tenofovir monotherapy group. More patients in the add-on group developed serious side effects, with treatment discontinuation, and dose reductions (P = 0.3).
Conclusion:
PEG IFN and tenofovir add-on therapy was successful in achieving HBsAg clearance and seroconversion in 4.3% of the patients. Add-on therapy patients had a significant decrease in HBsAg levels in two years; and no significant decrease in HBsAg levels with the tenofovir monotherapy. With no significant HBsAg clearance, the utility of this combination regimen is questionable.
Keywords: Chronic hepatitis B, clinical trial, hepatitis B surface antigen, clearance, hepatitis B virus, seroprevalence
INTRODUCTION
Currently, two sets of treatments are available for the treatment of chronic hepatitis B virus (HBV) infection; the nucleoside/nucleotide analogs (NAs) or PEGylated interferon (PEG IFN).[1,2] The goals of the treatment are: loss of HBV DNA; loss of HBeAg, and development of anti-HBeAg antibodies; improved histology; loss of HBsAg and the seroconversion to anti-HBS antibody formation.[3] The ideal endpoint in both HBeAg-positive and HBeAg-negative patients is sustained off-therapy HBsAg loss, with or without seroconversion to anti-HBs.[2] Due to the high relapse rate after NAs treatment discontinuation, in patients with HBeAg-negative chronic hepatitis, treatment until HBsAg loss is recommended.[4] In HBeAg-positive Chronic hepatitis B infection (CHB) patients who achieve HBeAg seroconversion with undetectable HBV DNA, the relapse rates depend on the duration of consolidation therapy. Consolidation therapy of at least three years decreased the rate of relapse and increased the rate of HBsAg loss significantly.[5]
With currently available treatment, HBsAg loss is uncommon; approximately 3–5% of the patients treated with PEG IFN, and 0 to 3% of patients treated with NAs, lose HBsAg.[3]
NAs have no effect on clearing the covalently closed circular DNA (cccDNA). In turn, interferon through its immunomodulatory action might induce cytotoxic T-cell activity and lead to immune clearance of the infected cells, and this may be useful in reducing the cccDNA level. However, a high HBV DNA load reduces the T-cell response to HBV-related antigens.[6,7] HBV DNA markedly gets suppressed with NAs treatment, and as a result, the body restores CD4 and CD8 cellular immune response against HBV. The addition of IFN at this stage enhances the immunomodulatory action and clearance of the cccDNA.[6,8]
Several clinical trials have been conducted to test the above hypothesis, but the results from these studies are inconsistent.[9,10,11,12,13] The different types of trial designs and varying objectives were the reasons for inconsistent results. Only a few of them were randomized controlled trials. Different studies have used add-on therapy in various combinations. These include the simultaneous administration of the two drugs in naïve patients, or “add-on” or “switch to” strategies in patients already on therapy. They have used these combination therapies either to improve response to nucleotide/nucleoside analogues or to improve response to IFN.[14] Only a few studies were conducted with an objective of HBsAg clearance as their primary aim.[9]
We, therefore, in this randomized trial, investigated the role of adding PEG IFN alfa 2-a to chronic HBV patients who had been treated with tenofovir and had achieved a viral response. The primary objective of our study was HBsAg clearance and HBsAg seroconversion from randomization to two years of recruitment in to the study. The secondary objective was to investigate the changes in HBsAg quantification with two treatment regimens in comparison to their values before randomization, during treatment and at two years. We also examined the safety of add-on therapy in comparison to tenofovir monotherapy.
PATIENTS AND METHODS
Settings and location
We conducted an open-label, single center, randomized controlled trial in a tertiary teaching hospital in Saudi Arabia (King Faisal Specialist Hospital and Research Centre. Riyadh). All screened patients were getting medical care from the team members of the gastroenterology section. The screening for the study started from March 18, 2013.
Inclusion and exclusion criteria
All patients had chronic hepatitis B and had been receiving tenofovir 300 mg daily for a minimum of six months or more. Chronic HBV infection is defined if the patient has positive HBsAg or positive nucleic acid test for HBV DNA in the serum (any time in the past is acceptable) or HBeAg positive two times at least 6 months apart (any combination of these tests performed 6 months apart is acceptable).
The HBV viral load should be <2000 IU/ml. HBsAg should be positive for more than six months and HBsAg level in the serum should be quantifiable. Both HBeAg reactive and non-reactive patients were accepted. All patients were above 18 years of age.
Patients were excluded if they had contraindications for receiving PEG IFN, decompensated liver cirrhosis, post renal transplant, post liver transplant, pregnancy or lactation, advanced medical illnesses, combined HBV and HCV, HBV and HIV infection, pre-existing neutropenia (total WBC <2000 mm3); anemia (hemoglobin <12 gm/l); thrombocytopenia (<75000/mm3); renal failure with glomerular filtration rate 30 mls/mt.
Randomization
The patients were eligible for randomization if they agreed to sign the informed consent. The patients were randomized into two groups. A computer generated randomization sequence was created in blocks of 10 patients, and these blocks were used consecutively; once one block had been completed then the next block was opened. A research coordinator handled the randomization sequence, and the investigators were blinded. The study was conducted as per our institute's research guidelines, and we also followed the Declaration of Helsinki and good clinical practice guidelines.
Objectives
The primary end-point was HBsAg clearance as defined by the loss of HBsAg. HBsAg seroconversion was defined by the development of anti-HBS antibodies along with HBsAg clearance. The time frame for HBsAg clearance was any time from the randomization to two years from the day of recruitment. The secondary end point was to investigate the difference in HBsAg levels between the two treatment groups. Any patient who was randomized in the trial was assessed for efficacy by intention to treat analysis. Per-protocol analysis was not carried out.
Safety analysis
The safety analysis included any adverse event that occurred during the treatment. It also included hematology, chemistry, new symptoms, and radiological findings. All the patients who signed consent forms were accounted for adverse events.
Enrolment and follow-up
From March 18, 2013, onwards patients were screened and a total of 204 patients were screened for eligibility; most patients were excluded either because they did not meet the inclusion criteria or they declined to consent to get enrolled. The patients were recruited from April 2013 to February 2014. The last patient's visit to the research clinic was in March 2016.
Patients receiving PEG IFN were seen after four weeks from the first dose, then every three months in the first year; and then every six months in the second year (total of two years). Those receiving tenofovir monotherapy were seen every three months in the first year and then every six months for one more year (total two years). During each routine visit, hematological tests and biochemical tests were done. HBV viral load and HBsAg quantification were done every three months in the first year and every six months in the second year.
Intervention
Patients in one group (add-on therapy) were given subcutaneous PEG IFN alfa 2-a, 180 mcg weekly for 12 months in addition to tenofovir. Patients in the other group (tenofovir monotherapy) received only tenofovir 300 mg orally on a daily basis. Patients in both groups were followed up for a total of two years, and the patients in both groups were given tenofovir 300 mg daily indefinitely until they developed HBsAg clearance.
HBV detection
Hepatitis B DNA detection and quantification was performed utilizing ABBOTT real-time HBV assay, which uses polymerase chain reaction technology with homogenous real-time fluorescent detection. HBV assay provides detection limit (Analytical Measurement Range) from 15 to 1,000,000,000 IU/ml. Results were reported in international unit per milliliter (IU/mL).
HBsAg quantification
The Architect HBsAg assay was used for measuring the HBsAg level in the serum. This test is a chemiluminescent micro-particle immunoassay. The manufacturer's recommendation was clearly followed. The concentration of HBsAg in the serum was determined using a previously generated ARCHITECT HBsAg calibration curves. Calibration range: 0–250 IU/mL. The values above this were measured using the dilution technique. If the concentration of the specimen was greater than or equal to 0.05 IU/mL, the specimen was considered HBsAg reactive. All reactive samples were confirmed for HBsAg with the positive neutralizing confirmatory test. Two positive controls and negative controls were run to verify the calibration.
Stopping rules
The treatment was discontinued or doses were adjusted if any serious adverse events occurred. Granulocyte colony-stimulating factors were allowed to treat neutropenia with the absolute neutrophil count decreasing below 750 mm3. In the case of HBsAg seroconversion, the treatment was continued for another six months, and all anti-viral treatment was allowed to stop.
Sample size
From previous studies, 0–3% HBsAg clearance rate was expected from tenofovir monotherapy. Assumed HBsAg clearance response with add-on PEG IFN alfa-2a to tenofovir treatment was 30%; this estimate was made based on the literatures at the time of proposal writing.[15,16] To detect a difference of this level, with 80% power (β = 0.2) and 5% level of statistical significance (α = 0.05), the estimated number of patients in each arm were 30. The agreed recruitment time was 10 months. After 10 months, recruitment was terminated due to difficulty in enrolment.
Ethics statement
The trial design was accepted by the King Faisal Specialist Hospital and research Centre's ethics committee board and they allotted a unique RAC number 2131012. It was also approved by the research promotion group under the department of medicine. During the study, we followed guidelines set by the research committee and declaration of Helsinki for medical research involving human subjects. A written informed consent was signed by all the recruited patients. The trial was registered with International Standard Randomized Controlled Trial Number (ISRCTN) registration with a reference number 12811193.
Statistics
The baseline characteristics were compared using a Chi-square test for categorical variables and the Student's t-test for continuous variables. The HBsAg levels between the groups were compared using the paired t-test. Kaplan-Meier-test was used to compare the HBsAg loss between the groups. A two-tailed, P value of <0.05 was considered as significant. All the statistical analysis were done using SPSS version 21 (SPSS Inc. Chicago, IL, USA).
RESULTS
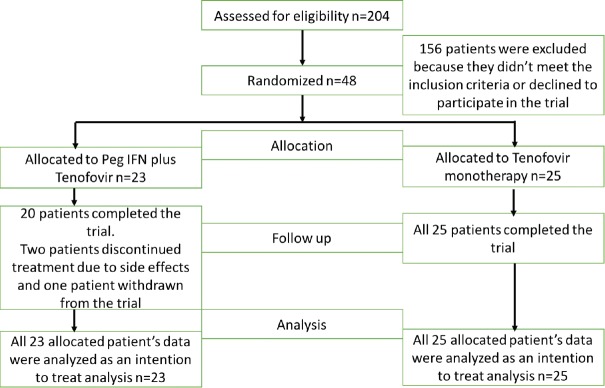
Of the patients with chronic HBV, who were screened, 48 met our inclusion criteria. Figure 1 shows the flow diagram of enrolment of patients and Figure 2 shows the trial design. All screened patients had chronic HBV and were on tenofovir for more than six months. From this cohort, 48 patients were eligible for randomization. Twenty-three were randomized into the add-on treatment; these patients were given PEG IFN 180 mg weekly and tenofovir 300 mg daily orally. Twenty-five patients were randomized into tenofovir monotherapy.
Figure 1.
Flow diagram showing the enrolment of patients
Figure 2.
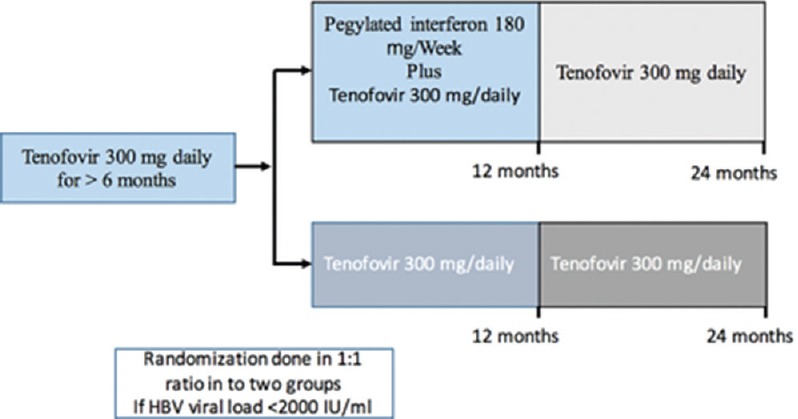
Trial design
Two patients in the add-on treatment discontinued treatment within 1-month time due to adverse events; one patient withdrew from the study before receiving the first dose of PEG IFN. Twenty patients from the add-on treatment group and 25 patients randomized to tenofovir group completed the study protocol, and data was available for analysis by the end of the two years. All 48 randomized patients' data were analyzed according to the intention to treat analysis.
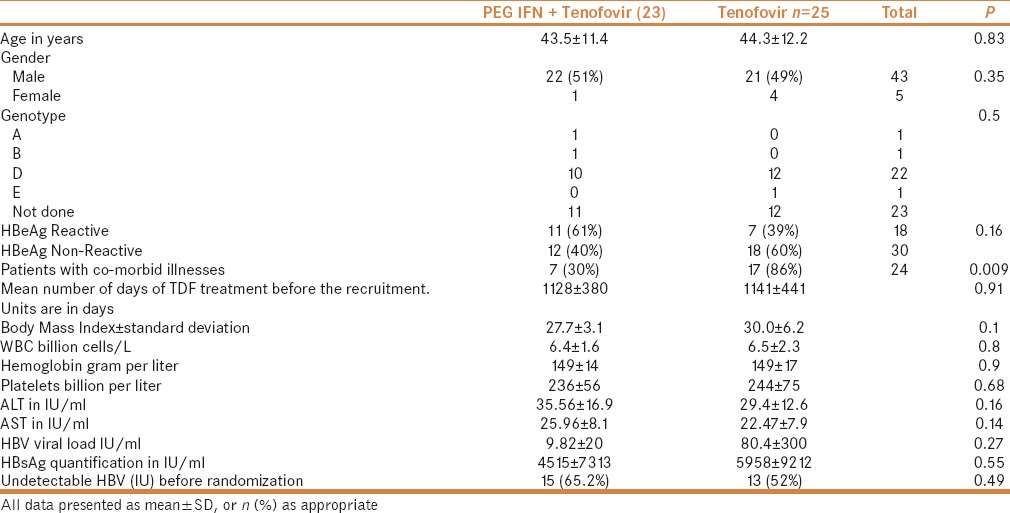
Baseline characteristics of 48 patients are given in Table 1. All baseline parameters were comparable between the two groups except for the add-on group having less comorbid illnesses. Before randomization, patients received tenofovir for an average 1135 days (ranging from 203 to 1542 days). In 25 patients, the genotype results were known; among them, 22 (88%) were genotype D.
Table 1.
Baseline characteristics of the recruited patients
One patient in the add-on treatment lost HBsAg and developed anti-HBs. This was observed after 9 months of the combination treatment. In this patient, anti-viral therapy was stopped after six months of seroconversion; anti HBS titer was 244 IU/ml after two years. The patient who seroconverted was genotype D; HBeAg non-reactive; with grade 2 liver fibrosis on biopsy (Metavir scoring system); and had earlier received Interferon, Lamivudine, PEG IFN, adefovir and then adefovir plus lamivudine; and finally tenofovir for 1325 days before getting enrolled into the study. None of the patients in tenofovir group had HBsAg seroconversion. Figure 3 shows the Kaplan Meier graph for the difference in HBsAg clearance between the two treatment groups.
Figure 3.
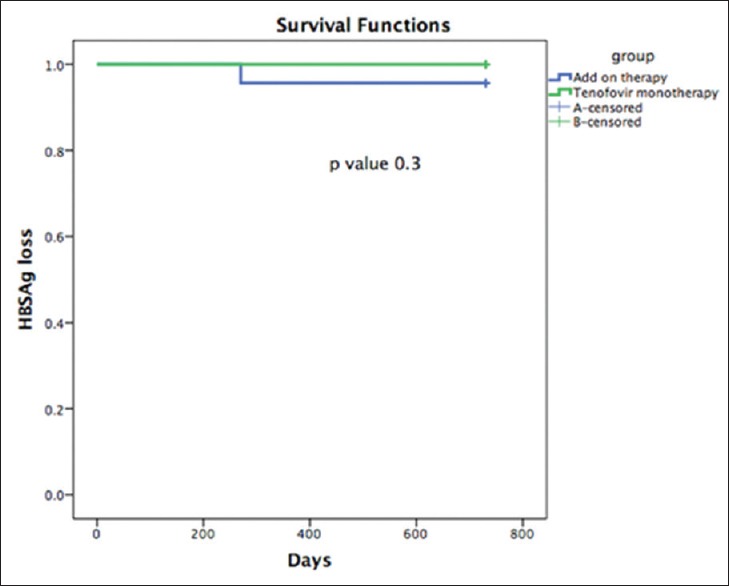
During the study period, HBsAg loss and seroconversion was observed in one patient out of 23 in the add-on therapy as compared to zero patients out of 25 in the tenofovir monotherapy group
By the end of two years, the mean HBsAg decreased significantly within the add-on therapy group (from 4753 IU/ml to 2402; P = 0.03). At the end of two years, the mean HBsAg decreased from 5957IU/ml to 4198 (P = 0.09) in the tenofovir monotherapy. A paired t-test was used to calculate the difference in HBsAg in the group from the base line to end of two years (Data given in Table 2). Median and interquartile values of HBsAg in IU/ml, before the randomization and at each 6 months are given in Table 3.
Table 2.
Baseline and on-treatment HBsAg levels at different time-points in the two patient groups
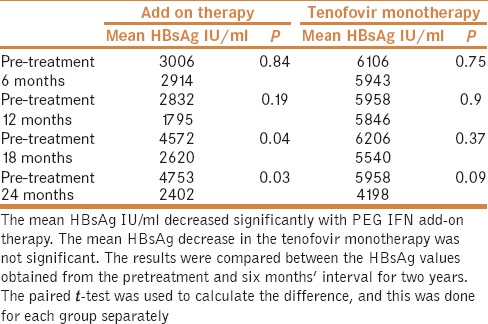
Table 3.
The median and interquartile values of HBsAg values in IU/ml before randomization and during the treatment

Serological and virological response during the treatment period is shown in Table 4. It shows the number of patients with HBsAg and HBeAg before randomization and changes occurred during the two years of study period. Two patients in the tenofovir group had HBeAg loss, and one patient had seroconversion. In the add-on group, one patient who was HBeAg non-reactive at the time of randomization converted to HBeAg reactive status.
Table 4.
Describes the number of patients who have lost HBsAg, HBeAg and or seroconverted with respective antibody formation during the study period. Data are given as number of patients

At the end of two years, 35% of the patients in the add-on treatment group had <500 IU/ml of HBsAg compared to 20% of the patients in the tenofovir monotherapy. Two patients in the add-on therapy and one patient in tenofovir group had >1 log reduction of HBsAg at the end of two years.
After the recruitment, in the add-on group, three patients' HBV viral load increased to >2000 IU/ml and reverted to <15 by the end of the study. From the tenofovir group, three patients' HBV viral load increased to >2000 IU/ml during the treatment and reverted to <15 IU/ml within months. The increase in the HBV viral load for patients was attributed to non-compliance to the treatment.
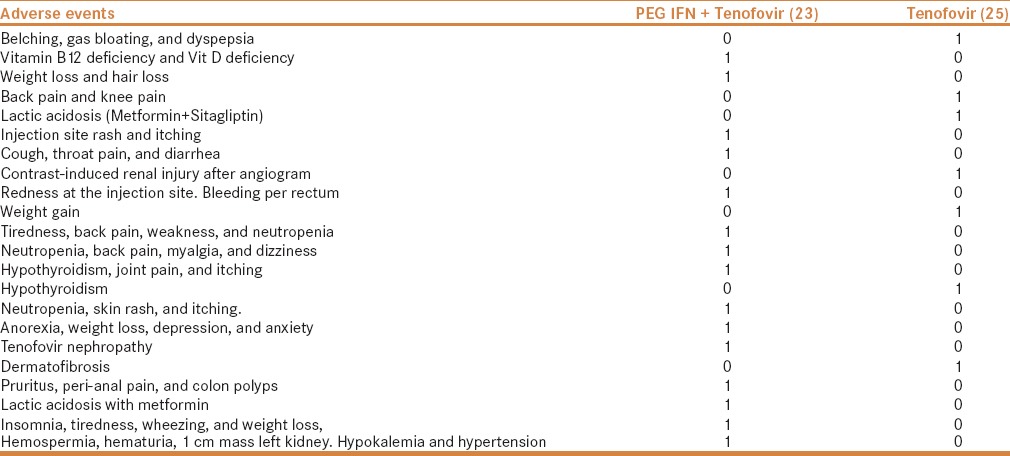
More number of patients had treatment discontinuation or interruption during the study period in the add-on treatment group [Table 5]. Adverse events that occurred during the study trial period are given in Table 6.
Table 5.
Treatment discontinuation or interruption was observed more in patients treated with PEG IFN and tenofovir compared to tenofovir monotherapy
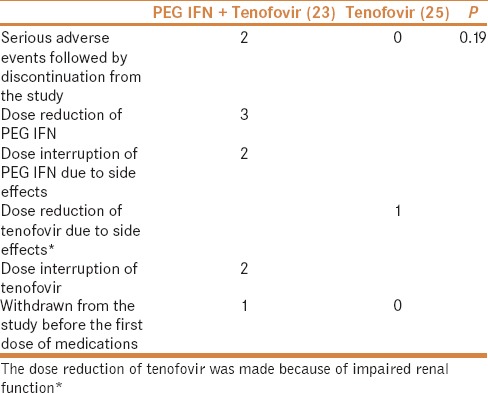
Table 6.
Adverse events were more often noted in the PEG IFN and tenofovir group
DISCUSSION
In this randomized controlled trial, we found that in chronic hepatitis B patients with a combination of PEG IFN to tenofovir as an add-on therapy, HBsAg clearance and seroconversion was 4.3% in comparison to tenofovir monotherapy. The HBsAg had decreased significantly in patients treated with PEG IFN and tenofovir. The adverse events and discontinuation of treatment were observed more in the add-on treatment group.
This study specifically tried to look at the benefit of the combination of PEG IFN and tenofovir to clear the HBsAg. We randomized patients only when they had a virological response to tenofovir. Hence, we did not aim for the virological response, though sustained viral suppression was the treatment objective. It is well known that most CHB patients on NAs require a long-term treatment course and have adherence problems and viral resistance.
A theoretical advantage of adding PEG IFN and NAs has been explained earlier: it might restore both arms of the immune system, NK cells as well as CD8+ T cells that are typically dysfunctional during chronic HBV infection and only partially restored by either therapy. This phenomenon, in turn, may help in eliminating infected cells; induce hepatocyte proliferation; cccDNA loss and HBsAg loss.[17,18] Because of this theoretical background, we randomized patients who have been treated with tenofovir for more than six months. Only 12.5% of the randomized patients had HBV DNA 15 to 2000 IU/ml. We had expected more number of patients to achieve HBsAg clearance with combination therapy because of the immunomodulatory actions of PEG IFN and potent anti-viral actions of tenofovir.
Previously, different combinations of PEGINF and NAs have been tried. The trials have tested the efficacy of adding both PEGINF and NAs simultaneously or sequentially. In sequential therapy, either PEG IFN is started first and later NAs is added or initially NAs is started and then later PEG IFN is added. Once the second medication is added, we have the option of continuing both PEG IFN and NAs or discontinuing one of them.[6,11]
Most simultaneous or sequential combination trials of PEG IFN and NAs have been able to produce HBsAg loss in the range of 2 to 9 percent.[9,10,12,13,19,20] We also got a result consistent with others; the HBsAg loss and seroconversion was 4.3% in our study.
All the recent guidelines agree that from NAs, tenofovir and entecavir are preferred because of their potency and minimal risk of resistance.[1,2,3] The combination trials experimented NAs and PEG IFN. Entecavir was tried in ARES trial and OSS trial.[11,21] Tenofovir was used by Marcellin et al. in their multinational study.[9] Therefore, we selected patients who have been treated with the most potent NU tenofovir. Before these patients were given tenofovir, 73% of them had received one or more other anti-viral treatments.
In our study, HBsAg levels decreased during the study period but this decline, by paired t-test results, was statistically more with add-on therapy. Will HBsAg level decrease further with time and will more patients treated with PEG IFN and tenofovir attain HBsAg clearance? Only a long-term follow-up can give us the answer.
In HBeAg negative genotype D patients, the calculated expected time for HBsAg clearance was 17 years to 63 years; and shortest was 17 years with tenofovir.[22]
Quantification of HBsAg in patients being treated with PEG IFN has been found useful in predicting treatment response.[23] Failure of decline in HBsAg at six months can be used for stopping PEG IFN. Baseline HBsAg levels are significantly lower in patients who achieve an SVR than in non-responders. This has been found true in both HBeAg-positive and HBeAg-negative patients.[24] Week 24 HBsAg titers <1500 IU/ml are associated with higher rates of treatment response after PEGIFN and HBsAg loss after 6 months' follow-up than HBsAg levels >1500 IU/ml, in both HBeAg-positive and negative patients.[25] From a retrospective study of 55 HBeAg-positive patients, 7 (63.6%) out of 11 patients with HBsAg level <1500 IU/ml achieved a virological response at week 48.[26]
We observed, at the end of two years, 35% of the patients in add-on treatment have <500 IU/ml of HBsAg compared to 20% in the tenofovir monotherapy. Two patients in add-on therapy and one patient in tenofovir group had >1 log reduction of HBsAg at the end of two years. Earlier, low levels of HBsAg and >1log10 reduction of HBsAg has been observed with increased HBsAg clearance.[27,28]
End of the treatment HBsAg level is a favorable factor for HBsAg clearance. In one study, of the 198 patients, 16 (8%) had cleared HBsAg by the three-years post treatment analysis. Of the 23 patients with an HBsAg level ≤ 10 IU/mL, 12 (52%) had cleared HBsAg three years after treatment compared with only four (2.3%) of the 171 patients with an HBsAg level ≥10 IU/mL at the end of treatment (P < 0.0001).[27]
Another factor that favors HBsAg loss is a decrease in HBsAg level by the end of treatment. In a French study, 97 HBeAg-positive patients were retrospectively evaluated. During a median follow-up of 14 years (range, 5–20 years), 28 patients (29%) developed HBsAg seroconversion. Most patients who lost HBsAg during post-treatment follow-up had a ≥1 log10 IU/ml decrease in HBsAg levels at the end of 48 weeks.[28]
Another study showed that longer duration of combination might be a favorable factor for HBsAg clearance. In that trial, 47 patients with HBeAg-positive CHB received either PEG IFNalpha-2a (135 mug once weekly) plus lamivudine (100 mg daily) or adefovir (10 mg daily). All patients completed 96 weeks of therapy and were followed up for a further 24 weeks. Researchers found that HBsAg seroconversion rate, which was 6.4% at 48 weeks, increased to 21.3% at 96 weeks, and 27.7% at 120 weeks.[29]
As expected, more patients in the add-on treatment arm faced discontinuation of treatment, dose adjustments, and adverse events. Notably, two patients developed lactic acidosis with tenofovir, and both patients were already on metformin. In both patients, lactic acid levels reversed into normal with discontinuation of metformin.
The advantage of the study was that it was a randomized controlled trial. We addressed the specific question of HBsAg clearance as our primary objective. We used the most potent anti-viral nucleotide analog tenofovir to induce viral suppression for an adequate period and randomized patients with viral response to tenofovir. The trials results were analyzed based on intention to treat analysis, and all randomized patients were assessed.
The sample size was one of the limitations of the trial. Though we had expected to enroll more patients, within the limited recruitment time, we were able to randomize only 48 patients. Also, the trial was a single-center study.
CONCLUSION
Contrary to our expectations, only 4.3% of patients in the add-on treatment had HBsAg clearance. HBsAg level decreased significantly more in add-on treatment patients. The patients receiving PEG IFN had adverse events, and they required more monitoring. With no significant HBsAg clearance, the utility of this combination regimen is questionable.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Seto WK, Hui AJ, Wong VW, Wong GL, Liu KS, Lia CL, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: A multicentre prospective study. Gut. 2015;64:667–72. doi: 10.1136/gutjnl-2014-307237. [DOI] [PubMed] [Google Scholar]
- 5.Chi H, Hansen BE, Yim C, Arends P, Abu-Amara M, van der Eijk AA, et al. Reduced risk of relapse after long-term nucleos(t)ide analogue consolidation therapy for chronic hepatitis B. Aliment Pharmacol Ther. 2015;41:867–76. doi: 10.1111/apt.13150. [DOI] [PubMed] [Google Scholar]
- 6.Wong GL, Wong VW, Chan HL. Combination therapy of interferon and nucleotide/nucleoside analogues for chronic hepatitis B. J Viral Hepat. 2014;21:825–34. doi: 10.1111/jvh.12341. [DOI] [PubMed] [Google Scholar]
- 7.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annual Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 8.Boni C, Penna A, Ogg GS, Bertoletti A, Pilli M, Cavallo C, et al. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: New perspectives for immune therapy. Hepatology. 2001;33:963–71. doi: 10.1053/jhep.2001.23045. [DOI] [PubMed] [Google Scholar]
- 9.Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, et al. Combination of Tenofovir Disoproxil Fumarate and Peginterferon alfa-2a Increases Loss of Hepatitis B Surface Antigen in Patients with Chronic Hepatitis B. Gastroenterology. 2016;150:134–44. doi: 10.1053/j.gastro.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Marcellin P, Lau GK, Bonino F, Farci P, Jin R, Piratvisuth T, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–17. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer WP, Xie Q, Sonneveld MJ, Zhang N, Zhang Q, Tabak F, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study) Hepatology. 2015;61:1512–22. doi: 10.1002/hep.27586. [DOI] [PubMed] [Google Scholar]
- 12.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–95. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 13.Chan HL, Leung NW, Hui AY, Wong VW, Liew CT, Chim AM, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: Comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann Intern Med. 2005;142:240–50. doi: 10.7326/0003-4819-142-4-200502150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lampertico P. The royal wedding in chronic hepatitis B: The haves and the have-nots for the combination of pegylated interferon and nucleos(t)ide therapy. Hepatology. 2015;61:1459–61. doi: 10.1002/hep.27731. [DOI] [PubMed] [Google Scholar]
- 15.Ouzan D, Penaranda G, Joly H, Khiri H, Pironti A, Halfon P. Add-on peg-interferon leads to loss of HBsAg in patients with HBeAg-negative chronic hepatitis and HBV DNA fully suppressed by long-term nucleotide analogs. J Clin Virol. 2013;58:713–7. doi: 10.1016/j.jcv.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Kittner JM, Sprinzl MF, Grambihler A, Weinmann A, Schattenberg JM, Galle PR, et al. Adding pegylated interferon to a current nucleos(t)ide therapy leads to HBsAg seroconversion in a subgroup of patients with chronic hepatitis B. J Clin Virol. 2012;54:93–5. doi: 10.1016/j.jcv.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Thimme R, Dandri M. Dissecting the divergent effects of interferon-alpha on immune cells: Time to rethink combination therapy in chronic hepatitis B? J Hepatol. 2013;58:205–9. doi: 10.1016/j.jhep.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Petersen J, Dandri M. Optimal therapy for chronic hepatitis B: Hepatitis B virus combination therapy? Liver Int. 2015;35:114–20. doi: 10.1111/liv.12720. [DOI] [PubMed] [Google Scholar]
- 19.Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: A randomised trial. Lancet. 2005;365:123–9. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 20.Piccolo P, Lenci I, Demelia L, Bandiera F, Piras MR, Antonucci G, et al. A randomized controlled trial of pegylated interferon-alpha2a plus adefovir dipivoxil for hepatitis B e antigen-negative chronic hepatitis B. Antivir Ther. 2009;14:1165–74. doi: 10.3851/IMP1466. [DOI] [PubMed] [Google Scholar]
- 21.Ning Q, Han M, Sun Y, Jiang J, Tan D, Hou J, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: A randomised open-label trial (OSST trial) J Hepatol. 2014;61:777–84. doi: 10.1016/j.jhep.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 22.Boglione L, D'Avolio A, Cariti G, Gregori G, Burdino E, Baeitto L, et al. Kinetics and prediction of HBsAg loss during therapy with analogues in patients affected by chronic hepatitis B HBeAg negative and genotype D. Liver Int. 2013;33:580–5. doi: 10.1111/liv.12091. [DOI] [PubMed] [Google Scholar]
- 23.Martinot-Peignoux M, Asselah T, Marcellin P. HBsAg quantification to optimize treatment monitoring in chronic hepatitis B patients. Liver Int. 2015;35:82–90. doi: 10.1111/liv.12735. [DOI] [PubMed] [Google Scholar]
- 24.Chan HL, Wong VW, Chim AM, Chan HY, Wong GL, Sung JJ. Serum HBsAg quantification to predict response to peginterferon therapy of e antigen positive chronic hepatitis B. Aliment Pharmacol Ther. 2010;32:1323–31. doi: 10.1111/j.1365-2036.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- 25.Martinot-Peignoux M, Lapalus M, Asselah T, Marcellin P. HBsAg quantification: Useful for monitoring natural history and treatment outcome. Liver Int. 2014;34:97–107. doi: 10.1111/liv.12403. [DOI] [PubMed] [Google Scholar]
- 26.Chen GY, Zhu MF, Zheng DL, Bao YT, Wang J, Zhou X, et al. Baseline HBsAg predicts response to pegylated interferon-alpha2b in HBeAg-positive chronic hepatitis B patients. World J Gastroenterol. 2014;20:8195–200. doi: 10.3748/wjg.v20.i25.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, et al. Hepatitis B virus surface antigen levels: A guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141–50. doi: 10.1002/hep.22760. [DOI] [PubMed] [Google Scholar]
- 28.Moucari R, Korevaar A, Lada O, Martinot-Peignoux M, Boyer N, Mackiewicz V, et al. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: A long-term follow-up study. J Hepatol. 2009;50:1084–92. doi: 10.1016/j.jhep.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Cao ZH, Ma LN, Zhang HW, Liu YL, Chen XY. Extended treatment with peginterferon alpha-2a in combination with lamivudine or adefovir for 96 weeks yields high rates of HBeAg and HBsAg seroconversion. J Dig Dis. 2013;14:446–50. doi: 10.1111/1751-2980.12065. [DOI] [PubMed] [Google Scholar]