This exploratory analysis of a randomized clinical trial investigates the effect of KIT and PDGFRA mutations on recurrence-free survival in a population of patients with gastrointestinal stromal tumors treated with surgery and adjuvant imatinib.
Key Points
Question
Does the duration of adjuvant imatinib treatment (1 year or 3 years) influence the prognostic significance of KIT proto-oncogene receptor tyrosine kinase (KIT) and platelet-derived growth factor receptor α genotypes in the treatment of patients with gastrointestinal stromal tumors?
Findings
In this exploratory analysis of a randomized clinical trial of 400 patients, 149 with a deletion in KIT exon 11 benefitted from the 3-year duration, whereas patients with other genotypes had little or no benefit. KIT exon 11 deletions were prognostic in the 1-year group but not in the 3-year group.
Meaning
The duration of adjuvant imatinib treatment influences the risk of gastrointestinal stromal tumor recurrence associated with KIT deletion mutations, and mutation analysis aids in patient selection for adjuvant imatinib.
Abstract
Importance
Little is known about whether the duration of adjuvant imatinib influences the prognostic significance of KIT proto-oncogene receptor tyrosine kinase (KIT) and platelet-derived growth factor receptor α (PDGFRA) mutations.
Objective
To investigate the effect of KIT and PDGFRA mutations on recurrence-free survival (RFS) in patients with gastrointestinal stromal tumors (GISTs) treated with surgery and adjuvant imatinib.
Design, Setting, and Participants
This exploratory study is based on the Scandinavian Sarcoma Group VIII/Arbeitsgemeinschaft Internistische Onkologie (SSGXVIII/AIO) multicenter clinical trial. Between February 4, 2004, and September 29, 2008, 400 patients who had undergone surgery for GISTs with a high risk of recurrence were randomized to receive adjuvant imatinib for 1 or 3 years. Of the 397 patients who provided consent, 341 (85.9%) had centrally confirmed, localized GISTs with mutation analysis for KIT and PDGFRA performed centrally using conventional sequencing. During a median follow-up of 88 months (completed December 31, 2013), 142 patients had GIST recurrence. Data of the evaluable population were analyzed February 4, 2004, through December 31, 2013.
Main Outcomes and Measures
The main outcome was RFS. Mutations were grouped by the gene and exon. KIT exon 11 mutations were further grouped as deletion or insertion-deletion mutations, substitution mutations, insertion or duplication mutations, and mutations that involved codons 557 and/or 558.
Results
Of the 341 patients (175 men and 166 women; median age at study entry, 62 years) in the 1-year group and 60 years in the 3-year group), 274 (80.4%) had GISTs with a KIT mutation, 43 (12.6%) had GISTs that harbored a PDGFRA mutation, and 24 (7.0%) had GISTs that were wild type for these genes. PDGFRA mutations and KIT exon 11 insertion or duplication mutations were associated with favorable RFS, whereas KIT exon 9 mutations were associated with unfavorable outcome. Patients with KIT exon 11 deletion or insertion-deletion mutation had better RFS when allocated to the 3-year group compared with the 1-year group (5-year RFS, 71.0% vs 41.3%; P < .001), whereas no significant benefit from the 3-year treatment was found in the other mutational subgroups examined. KIT exon 11 deletion mutations, deletions that involved codons 557 and/or 558, and deletions that led to pTrp557_Lys558del were associated with poor RFS in the 1-year group but not in the 3-year group. Similarly, in the subset with KIT exon 11 deletion mutations, higher-than-the-median mitotic counts were associated with unfavorable RFS in the 1-year group but not in the 3-year group.
Conclusions and Relevance
Patients with KIT exon 11 deletion mutations benefit most from the longer duration of adjuvant imatinib. The duration of adjuvant imatinib modifies the risk of GIST recurrence associated with some KIT mutations, including deletions that affect exon 11 codons 557 and/or 558.
Trial Registration
clinicaltrials.gov Identifier: NCT00116935
Introduction
Gastrointestinal stromal tumor (GIST) is a common type of sarcoma. Most GISTs are local when detected and are treated with surgery. Their malignancy potential varies, with the most important factors associated with prognosis after surgery being tumor mitotic rate and size, site in the gastrointestinal tract, and presence of tumor rupture.
Treatment with at least 3 years of imatinib is frequently recommended for high-risk patients with GISTs. This recommendation is based on the randomized Scandinavian Sarcoma Group (SSG) XVIII/Arbeitsgemeinschaft Internistische Onkologie (AIO) trial that compared 3 years to 1 year of adjuvant imatinib. The 3-year treatment improved recurrence-free survival (RFS) and overall survival (OS). The American College of Surgeons Oncology Group Z9001 trial that compared 1 year of adjuvant imatinib with placebo and the European Organisation for Research and Treatment of Cancer trial that compared 2 years of adjuvant imatinib to observation (each trial performed in part in a lower-risk patient population) found adjuvant imatinib to improve RFS but not OS. The reasons why the 3-year adjuvant imatinib treatment resulted in an OS benefit whereas the shorter treatments did not have remained speculative.
Activating mutations in KIT proto-oncogene receptor tyrosine kinase (KIT) (encodes KIT protein) (OMIM 164920) and less frequently in platelet-derived growth factor receptor α (PDGFRA) (encodes platelet-derived growth factor receptor α) (OMIM 173490) are considered the major molecular drivers of most GISTs. The resulting aberrant receptor tyrosine kinases are excellent targets for therapy with tyrosine kinase inhibitors. Most KIT mutations are deletion mutations in exon 11, and these deletions frequently span the critical codons 557 and/or 558. KIT exon 11 mutations that involve codons 557 and/or 558 are associated with poor prognosis in patient populations treated with surgery. Some other KIT mutations, such as exon 11 insertion and duplication mutations and PDGFRA mutations, are generally associated with favorable survival. However, the prognosis associated with single KIT or PDGFRA mutations may vary greatly, depending on the tumor mitotic rate, suggesting that further genetic aberrations besides KIT and PDGFRA mutations contribute to the clinical behavior of GIST.
Mutation analysis of KIT and PDGFRA and possibly also of other genes, such as B-Raf proto-oncogene, serine/threonine kinase (BRAF) (OMIM 164757) and succinate dehydrogenase (SDH) A (OMIM 600857), B (OMIM 185470), C (OMIM 602413), and D (OMIM 602690), is recommended also for estimation of GIST drug responsiveness. In particular, PDGFRA exon 18 mutation D842V confers imatinib resistance, whereas most KIT exon 11 deletion mutations are highly sensitive. Patients with advanced GIST with KIT exon 9 mutation likely benefit from a dose of imatinib that is higher than the standard 400 mg.
Little is known about whether the duration of adjuvant imatinib influences the prognostic significance of KIT and PDGFRA mutations. We studied this in the patient population of the SSGXVIII/AIO trial that compared 2 durations of adjuvant imatinib.
Methods
Participants
The SSGXVIII/AIO is an open-label, randomized, multicenter, phase 3 trial in which the participants were randomized for intention to treat in a 1:1 ratio to receive 400 mg/d of adjuvant imatinib orally for 1 or 3 years. The study, a protocol-based prespecified analysis, was approved by the Operative Ethics Committee of the Helsinki University Central Hospital and conducted according to the Good Clinical Practice guidelines. The participants provided written informed consent before study entry. All data were deidentified.
Patients 18 years or older with Eastern Cooperative Oncology Group performance status of 2 or less and GISTs completely excised macroscopically at open surgery were eligible. Patients with metastatic, inoperable, or recurrent GISTs and those with prior neoadjuvant imatinib therapy were excluded. Patients who were rendered free from all detectable GIST metastases with surgery were allowed to participate until October 2006, when the study protocol was amended and such patients were excluded.
The diagnosis of GISTs was made based on histologic assessment and the expression of KIT on immunohistochemical analysis. The estimated risk of recurrence was required to be high according to the modified National Institutes of Health consensus criteria. High risk was defined as tumor diameter greater than 10.0 cm, more than 10 mitoses per 50 high-power fields (HPFs), diameter greater than 5.0 cm and mitotic count higher than 5, or presence of tumor rupture.
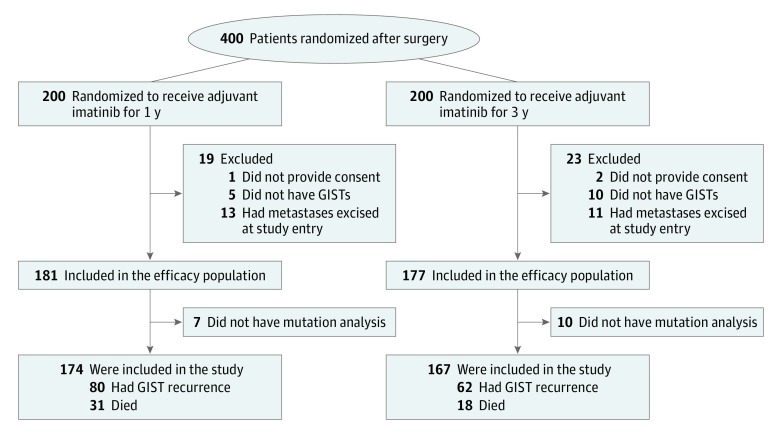
Between February 4, 2004, and September 29, 2008, a total of 400 patients were accrued from 24 study sites. Three patients who had been randomized without signing informed consent forms were excluded from the analyses, and we also excluded 15 patients who did not have GISTs at central pathology review of the tumor tissue specimens, 24 patients with GIST metastases resected at surgery, and 17 patients who did not have mutation analysis of KIT and PDGFRA performed centrally (Figure 1). The median follow-up time after the date of randomization of the 341 patients in the final study cohort was 88 months (range, 0.1-114 months). All patients were available for follow-up.
Figure 1. Profile of the Study.
GISTs indicate gastrointestinal stromal tumors.
Procedures
Randomization was performed centrally. Staging was performed before starting adjuvant imatinib with contrast-enhanced computed tomography or magnetic resonance imaging of the abdomen and the pelvis and with computed tomography or radiography of the chest. Computed tomography or magnetic resonance imaging of the abdomen was performed at 6-month intervals until month 84 of the study and then annually. Physical examination, blood cell counts, and blood biochemical analyses were assessed periodically.
Central Histopathologic Review
Local pathologists made the histologic diagnosis of GISTs, and GIST risk stratification was based on this assessment. Tumor histologic findings were reviewed centrally during the study by 1 of 2 pathologists with special interest in sarcoma pathology. At central pathology review, 15 patients were found not to have GISTs. The central pathologists also counted tumor mitoses from 50 HPFs (the total HPF areas were 11.24 and 12.50 mm2).
Mutation Analysis
Mutation analysis of KIT and PDGFRA was not required for study entry. KIT exons 9, 11, 13, and 17 and PDGFRA exons 12 and 18 were sequenced centrally during the study using conventional sequencing.
We focused on KIT exon 11 deletion mutations because these are the most common KIT aberrations in GISTs. We classified these mutations in 4 different ways: (1) presence of any deletion or insertion-deletion (indel) mutation in KIT exon 11, (2) presence of a mutation that involves exon 11 codon 557 and/or codon 558 (a deletion or indel mutation or a substitution mutation), (3) presence of a deletion or indel mutation that involves codon 557 and/or codon 558, and (4) a deletion of codons 557 and/or 558 only (leading to p.Trp557_Lys558del).
Statistical Analysis
We selected RFS as the survival end point for this exploratory study because RFS was also the primary objective in the SSGXVIII/AIO trial and more RFS events occurred compared with OS events, thus providing more statistical power (Figure 1). We defined RFS as the interval between the date of randomization and the date of first documentation of GIST recurrence or death, whichever occurred first, censoring patients who were alive without recurrence on the date of the last follow-up visit. Overall survival was calculated from the date of randomization to the date of death.
Survival between groups was compared using the Kaplan-Meier life-table method and an unstratified log-rank test. Frequency tables were analyzed using the χ2 test. Continuous distributions between groups were compared with the Mann-Whitney test. The P values are 2-sided and not adjusted for multiple testing. P < .05 indicates a significant finding. The data from the evaluable population were analyzed from February 4, 2004, through December 31, 2013, using an SPSS statistical software, version 22.0 (SPSS Inc).
Results
KIT and PDGFRA Mutations
Of the 341 patients (median age at study entry, 62 years [interquartile range (IQR), 52-68 years]) in the 1-year group and 60 years [IQR, 50-68 years] in the 3-year group; 92 males [52.6%] in the 1-year group and 83 [47.4%] in the 3-year group), 274 (80.4%) had GISTs with a KIT mutation, 43 (12.6%) had GISTs that harbored a PDGFRA mutation, and 24 (7.0%) had GISTs that were wild type for these genes (Table). A total of 149 (54.4%) of the KIT mutations were exon 11 deletion or indel mutations. A total of 111 (74.5%) involved codon 557, 558, or both, and 30 (20.1%) were deletions of 557 and 558, leading to p.Trp557_Lys558del. Of all 274 KIT mutations of any kind, 121 (44.2%) involved codons 557 and/or 558, 22 (8.0%) were exon 11 duplication or insertion mutations, 68 (24.8%) were exon 11 substitution mutations, and 26 (9.5%) were exon 9 duplication mutations (all leading to p.Ala502_Tyr503dup). The highest median mitotic counts were present in GISTs that had deletion of codons 557 and 558, leading to p.Trp557_Lys558del (17 mitoses per 50 HPFs), and the lowest counts were in tumors with PDGFRA mutation (1 mitosis per 50 HPFs). GISTs with a KIT exon 11 deletion or indel mutation harbored a median of 8 mitoses per 50 HPFs (eTable 1 in the Supplement).
Table. Characteristics of the 341 Patients in the Scandinavian Sarcoma Group VIII/Arbeitsgemeinschaft Internistische Onkologie Trial With Mutation Analysis for KIT and PDGFRA Availablea.
| Characteristic | 1 y of Adjuvant Imatinib Treatment (n = 174) |
3 y of Adjuvant Imatinib Treatment (n = 167) |
|---|---|---|
| Age at study entry, median (IQR), y | 62 (52-68) | 60 (50-68) |
| Sex | ||
| Male | 92 (52.6) | 83 (47.4) |
| Female | 82 (49.4) | 84 (50.6) |
| Tumor diameter, median (IQR), cm | 9.5 (6-13) | 10 (7-13) |
| Tumor location | ||
| Gastric | 87 (47.5) | 96 (52.5) |
| Nongastric | 86 (55.1) | 70 (44.9) |
| Not available | 1 | 1 |
| Tumor mitotic countb | ||
| <6 | 79 (50.0) | 79 (50.0) |
| 6-10 | 26 (50.0) | 26 (50.0) |
| >10 | 66 (54.1) | 56 (45.9) |
| Not available | 3 | 6 |
| Tumor rupture before or at surgery | ||
| No | 144 (53.3) | 126 (46.7) |
| Yes | 30 (42.3) | 41 (57.7) |
| KIT | ||
| Exon 9 | 12 (46.2) | 14 (53.8) |
| Exon 11 | ||
| Deletion | 45 (44.1) | 57 (55.9) |
| Insertion-deletion | 26 (55.3) | 21 (44.7) |
| Duplication or insertion | 13 (59.1) | 9 (40.9) |
| Substitution | 38 (55.9) | 30 (44.1) |
| PDGFRA | ||
| Exon 12 | 3 (60.0) | 2 (40.0) |
| Exon 18 | 20 (52.6) | 18 (47.4) |
| Other mutationc | 3 (33.3) | 6 (66.7) |
| Wild type for KIT and PDGFRA | 14 (58.3) | 10 (41.7) |
Abbreviations: IQR, interquartile range; KIT, KIT proto-oncogene receptor tyrosine kinase; PDGFRA, platelet-derived growth factor receptor α.
Data are presented as number (percentage) of patients unless otherwise indicated.
Counts per 50 high-power fields of the microscope at central review of the tumor specimens.
Includes 4 tumors with a KIT exon 13 mutation, 2 tumors with a KIT exon 11 duplication or insertion mutation plus a substitution mutation, 1 with a KIT exon 11 insertion mutation plus an insertion-deletion mutation, 1 with a KIT exon 11 deletion mutation plus a KIT exon 11 insertion mutation, and 1 with an unspecified KIT mutation.
Mutation Type and RFS
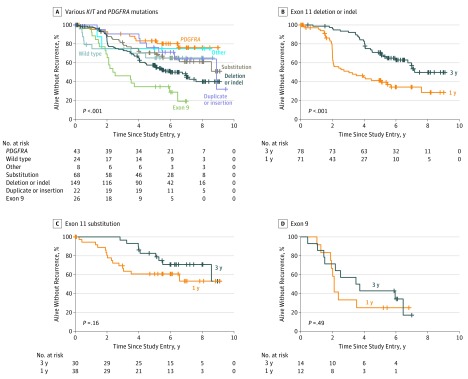
During the follow-up, 80 RFS events occurred in the 1-year group and 62 in the 3-year group. Patients whose GISTs harbored a PDGFRA mutation, a KIT exon 11 duplication or insertion mutation, or a KIT exon 11 substitution mutation had more favorable RFS than patients with a KIT exon 11 deletion or indel mutation, and patients with a KIT exon 9 mutation had the worst outcome (Figure 2A). Patients with a KIT exon 11 deletion or indel mutation treated with 3 years of adjuvant imatinib treatment had substantially better RFS compared with patients treated with 1 year of imatinib treatment (5-year RFS, 71.0% vs 41.3%; P < .001), whereas no significant difference in RFS was observed in the other subgroups studied (5-year RFS, 82.6% at 3 years and 60.8% at 1 year in patients with KIT exon 11 substitution mutation and 42.9% at 3 years and 25.0% at 1 year in patients with KIT exon 9 mutation) (Figure 2B-D and eFigure 1 in the Supplement).
Figure 2. Associations of KIT Proto-oncogene Receptor Tyrosine Kinase (KIT) and Platelet-Derived Growth Factor Receptor α (PDGFRA) Mutation Types With Recurrence-Free Survival.
A, Different types of KIT and PDGFRA mutations. B, KIT exon 11 deletion or insertion-deletion (indel) mutations. C, KIT exon 11 substitution mutations. D, KIT exon 9 mutations.
KIT Exon 11 Deletions That Involve Codon 557 and/or 558
We next investigated in more detail the largest subgroup of mutations: KIT exon 11 deletions. When the patients who had any KIT exon 11 deletion or indel mutation (n = 149) were compared with the rest of the patients, patients with a KIT exon 11 deletion or indel mutation had unfavorable RFS (5-year RFS, 57.5% vs 68.4%; P = .03) (eFigure 2 in the Supplement). Similarly, 30 patients who had deletion of the KIT exon 11 codons 557 and 558 had less favorable RFS than the rest of the patients (5-year RFS, 48.3% vs 65.2%; P = .001), whereas no significant difference in RFS was found between patients who had any type of KIT exon 11 mutation involving codon 557 or 558 (5-year RFS, 60.6% vs 65.3%; P = .32) or between patients who had a KIT exon 11 deletion or indel mutation that involved 557 and/or 558 and the rest of the patients (5-year RFS, 57.7% vs 66.4%; P = .07).
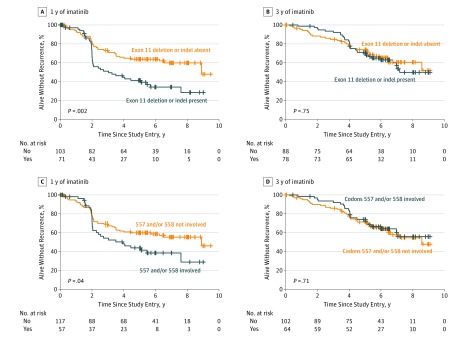
The prognostic significance of the different types of KIT exon 11 mutations for RFS depended on the duration of adjuvant imatinib administered. Presence of a KIT exon 11 deletion or indel mutation, a KIT exon 11 mutation that involved codons 557 and/or 558, an exon 11 deletion or indel mutation that involved codons 557 and/or 558, and a deletion mutation of codons 557 and 558 were each significantly associated with unfavorable RFS compared with the rest of the patients in the subgroup of patients assigned to 1 year of imatinib treatment, but no such associations were present among patients who were assigned to 3 years of imatinib treatment (Figure 3 and eFigure 3 in the Supplement). Because the assumption for proportionality was not valid, the interaction between the duration of adjuvant imatinib and the presence of KIT deletion mutation was not tested in a linear multivariable model. The main prognostic factors were generally similarly distributed between the 1-year and 3-year arms in the subgroups of patients with different types of KIT exon 11 deletion mutations (eTables 2, 3, and 4 in the Supplement).
Figure 3. Associations of KIT Proto-oncogene Receptor Tyrosine Kinase (KIT) Exon 11 Deletion Mutations With Recurrence-Free Survival.
A and B, Deletion and insertion-deletion (indel) mutations. C and D, KIT exon 11 mutations that do or do not involve codons 557 and/or 558.
Patients with KIT exon 11 deletion or indel mutation had better OS than the rest of the patients in the entire cohort (5-year OS, 93.5% vs 87.3%; P = .04), but no significant difference was found in the 1-year (5-year OS, 88.9% vs 85.7%; P = .27) and 3-year (5-year OS, 97.3% vs 89.1%; P = .06) subsets.
KIT Exon 11 Deletion Mutations and Mitotic Counts
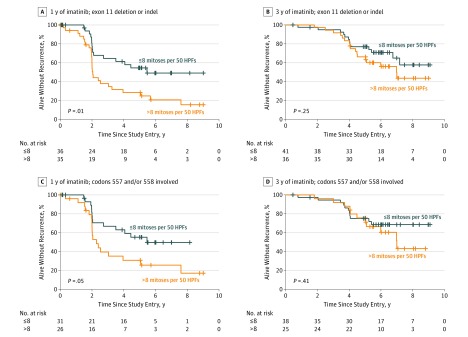
To further examine the associations between KIT deletion mutations and the duration of adjuvant imatinib treatment, we next investigated how the duration of adjuvant imatinib influences the prognostic significance of the tumor mitotic count in subsets of patients with different types of KIT deletion mutations. In these analyses, we used the median mitotic count of GISTs with KIT deletion mutation in the central histopathologic review as the cutoff value (8 mitoses per 50 HPFs). The tumor mitotic count was significantly associated with RFS in the subsets of patients who had KIT deletion or indel mutation (5-year RFS, 54.5% vs 28.4%; P = .01) or who had any KIT mutation that involved codons 557 and/or 558 (5-year RFS, 55.2% vs 30.8%; P = .05) when the patients had been assigned to the 1-year group of the trial, but mitotic count was not significantly associated with RFS in these subgroups when the patients had been assigned to the 3-year arm (Figure 4 and eFigure 4 in the Supplement).
Figure 4. Associations of Gastrointestinal Stromal Tumor Mitotic Count With Recurrence-Free Survival in Patients With KIT Proto-oncogene Receptor Tyrosine Kinase (KIT) Exon 11 Deletion Mutation.
A and B, KIT exon 11 deletion and insertion-deletion (indel) mutations. C and D, KIT exon 11 mutations that involve codons 557 and/or 558. HPFs indicates high-power fields.
Discussion
Few data are available on how the duration of adjuvant imatinib treatment influences the risk of recurrence related to different GIST genotypes. We found that patients whose GIST harbors KIT exon 11 deletion mutation benefit most from the 3-year adjuvant treatment, whereas patients with other mutations had less or no benefit. KIT exon 11 deletion mutations, KIT exon 11 mutations that involved the codons 557 and/or 558, and deletions that led to p.Trp557_Lys558del were each significantly associated with unfavorable RFS in the subset of patients randomized to 1 year of imatinib treatment but not among those randomized to 3 years. When we examined the prognostic significance of tumor mitotic count in the subgroup of patients with KIT exon 11 deletion mutations, mitotic count was significantly associated with unfavorable RFS among only patients assigned to the 1-year group but not among those assigned to the 3-year group.
To our knowledge, the observation that the duration of adjuvant imatinib treatment influences the clinical significance of important prognostic factors, such as tumor mitotic count and tumor genotype, is novel. In the adjuvant setting, the duration of imatinib administration may thus influence profoundly the molecular biology of some GISTs. This might not be as evident in advanced GISTs, in which the rates of GIST progression may not differ markedly after imatinib therapy discontinuation after variable durations of imatinib treatment. In the preplanned subgroup analyses of the SSGXVIII/AIO trial, patients who had GISTs with a high mitotic count (>10 mitoses per 50 HPFs) and those with KIT exon 11 deletion mutation benefited most from the 3-year adjuvant treatment, whereas patients whose GISTs had a lower count (<10 mitoses) did not benefit. These findings suggest that longer adjuvant imatinib treatment eradicates more efficiently GIST cells that harbor imatinib-sensitive KIT mutations and have a high cell proliferation rate than the shorter treatment.
The finding that patients with KIT exon 11 deletion mutation benefit most from the longer adjuvant treatment is in accordance with prior observations. Patients with advanced GISTs with KIT exon 11 mutation respond frequently to imatinib and have longer progression-free survival compared with patients with exon 9 KIT mutation or those whose GISTs lack KIT and PDGFRA mutations. In one study, patients with KIT exon 11 mutation that involved codons 557 and/or 558 responded to imatinib more frequently than patients with a KIT exon 11 mutation that did not involve these codons. In the American College of Surgeons Oncology Group Z9001 trial that compared 1 year of adjuvant imatinib to 1 year of placebo, patients with a KIT exon 11 deletion mutation had higher RFS when randomized to imatinib, whereas no significant RFS benefit was found among patients with KIT exon 11 insertion or substitution mutation, KIT exon 9 mutation, PDGFRA mutation, or GISTs that were wild type for KIT and PDGFRA.
These current findings have several consequences. They may help to explain why an OS benefit emerged in the SSGXVIII/AIO trial in favor of the 3-year group, whereas no OS benefit was observed in the 2 randomized trials that evaluated shorter durations of adjuvant imatinib and that also accrued lower-risk patients. The current findings suggest that longer adjuvant treatments tend to result in less steep slopes of the RFS curves when adjuvant imatinib is discontinued, which may be the case (eFigure 5 in the Supplement). The present findings further suggest that patients with GISTs treated with long durations of adjuvant imatinib may need to be followed up longer for tumor recurrence because some of the recurrent tumors may surface late despite the presence of high-risk features at the time of the diagnosis. More important, studies that evaluate the prognostic factors of localized GISTs now not only need to consider whether adjuvant imatinib was administered but also need to take into account the duration of adjuvant imatinib.
Limitations
A limitation of this explorative study is that the mutational subgroups were not predefined in the trial protocol. To reduce the risk for a type I error, we focused on commonly used mutational subgroups. Variable definitions for the aberrations that affect KIT exon 11 codons 557 and/or 558 have been used, and, therefore, we classified these mutations using 3 categories. Because the size of some mutational subgroups was small, the statistical power to detect a treatment effect may have remained insufficient. Mutation analysis was not available for 17 (4.7%) of the 358 patients in the SSGXVIII/AIO trial efficacy population, but no bias in the frequency of the most important prognostic factors was found between patients with and without mutation analysis available. Patients with KIT exon 9 mutation might have benefited from an imatinib dose higher than 400 mg/d, but this was unknown at the time the trial was designed.
Conclusions
The duration of adjuvant imatinib influences the risk of GIST recurrence associated with KIT deletion mutations. The adverse prognostic influence of some frequent mutations, such as deletion mutations that involve KIT exon 11 codons 557 and/or 558, was no longer detectable in patients treated with adjuvant imatinib for 3 years. Tumor mutation analysis aids in the selection of patients for adjuvant imatinib treatment because patients with KIT deletion mutations benefit more from 3 years of adjuvant imatinib than patients with other mutations, and some GISTs, notably those with PDGFRA D842V mutation, are considered imatinib resistant. Two ongoing randomized trials are currently comparing 5 vs 3 years and 6 vs 3 years of adjuvant imatinib treatment to evaluate whether still longer adjuvant treatments will provide further benefits.
eTable 1. KIT and PDGFRA Mutation Type and Tumor Mitotic Count
eTable 2. Characteristics of the 149 Patients Who Had GIST With KIT Exon 11 Deletion or Deletion-Insertion (Indel) Mutation
eTable 3. Characteristics of the 121 Patients Who Had GIST With KIT Exon 11 Mutation Involving Codons 557 and/or 558
eTable 4. Characteristics of the 111 Patients Who Had GIST With KIT Exon 11 Deletion or Deletion-Insertion (Indel) Mutation Involving Codons 557 and/or 558
eFigure 1. Association of KIT Exon 11 Insertion/Duplication Mutations, Absence of KIT and PDGFRA Mutations, PDGFRA Mutations, and PDGFRA Exon 18 Substitution Mutation D842V on Recurrence-Free Survival
eFigure 2. Associations of KIT Exon 11 Deletion Mutations With Recurrence-Free Survival in the Study Cohort
eFigure 3. Associations of KIT Exon 11 Deletion Mutations With Recurrence-Free Survival in the Random Allocation Groups
eFigure 4. Associations of GIST Mitotic Count With Recurrence-Free Survival in Patients With KIT Exon 11 Deletion Mutation That Involve Codons 557/558
eFigure 5. Recurrence-Free Survival (RFS) of Patients With Localized GIST in Three Randomized Trials
References
- 1.Ducimetière F, Lurkin A, Ranchère-Vince D, et al. . Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6(8):e20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382(9896):973-983. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: soft tissue sarcoma. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Published 2011. Accessed April 1, 2016.
- 4.ESMO/European Sarcoma Network Working Group Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii21-iii26. [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H, Eriksson M, Sundby Hall K, et al. . One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272. [DOI] [PubMed] [Google Scholar]
- 6.Dematteo RP, Ballman KV, Antonescu CR, et al. ; American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team . Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corless CL, Ballman KV, Antonescu CR, et al. . Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casali PG, Le Cesne A, Poveda Velasco A, et al. . Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group intergroup randomized trial in collaboration with the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol. 2015;33(36):4276-4283. [DOI] [PubMed] [Google Scholar]
- 9.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, von Mehren M, Blanke CD, et al. . Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472-480. [DOI] [PubMed] [Google Scholar]
- 11.Wardelmann E, Losen I, Hans V, et al. . Deletion of Trp-557 and Lys-558 in the juxtamembrane domain of the c-kit protooncogene is associated with metastatic behavior of gastrointestinal stromal tumors. Int J Cancer. 2003;106(6):887-895. [DOI] [PubMed] [Google Scholar]
- 12.Martín J, Poveda A, Llombart-Bosch A, et al. ; Spanish Group for Sarcoma Research . Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol. 2005;23(25):6190-6198. [DOI] [PubMed] [Google Scholar]
- 13.Dematteo RP, Gold JS, Saran L, et al. . Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008;112(3):608-615. [DOI] [PubMed] [Google Scholar]
- 14.Wozniak A, Rutkowski P, Schöffski P, et al. . Tumor genotype is an independent prognostic factor in primary gastrointestinal stromal tumors of gastric origin: a european multicenter analysis based on ConticaGIST. Clin Cancer Res. 2014;20(23):6105-6116. [DOI] [PubMed] [Google Scholar]
- 15.Joensuu H, Rutkowski P, Nishida T, et al. . KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich MC, Corless CL, Demetri GD, et al. . Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342-4349. [DOI] [PubMed] [Google Scholar]
- 17.Debiec-Rychter M, Sciot R, Le Cesne A, et al. ; EORTC Soft Tissue and Bone Sarcoma Group; Italian Sarcoma Group; Australasian GastroIntestinal Trials Group . KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42(8):1093-1103. [DOI] [PubMed] [Google Scholar]
- 18.Joensuu H, Eriksson M, Sundby Hall K, et al. . Adjuvant imatinib for high-risk GI stromal tumor: analysis of a randomized trial. J Clin Oncol. 2016;34(3):244-250. [DOI] [PubMed] [Google Scholar]
- 19.Wardelmann E, Merkelbach-Bruse S, Pauls K, et al. . Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res. 2006;12(6):1743-1749. [DOI] [PubMed] [Google Scholar]
- 20.Andersson J, Bümming P, Meis-Kindblom JM, et al. . Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology. 2006;130(6):1573-1581. [DOI] [PubMed] [Google Scholar]
- 21.Patrikidou A, Chabaud S, Ray-Coquard I, et al. ; French Sarcoma Group . Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol. 2013;24(4):1087-1093. [DOI] [PubMed] [Google Scholar]
- 22.Patrikidou A, Domont J, Chabaud S, et al. ; French Sarcoma Group . Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer. 2016;52:173-180. [DOI] [PubMed] [Google Scholar]
- 23.Cassier PA, Fumagalli E, Rutkowski P, et al. ; European Organisation for Research and Treatment of Cancer . Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res. 2012;18(16):4458-4464. [DOI] [PubMed] [Google Scholar]
- 24.clinicaltrials.gov Three Versus Five Years of Adjuvant Imatinib as Treatment of Patients With Operable GIST. NCT02413736. https://clinicaltrials.gov/ct2/show/NCT02413736. Accessed November 18, 2015.
- 25.clinicaltrials.gov Efficiency of Imatinib Treatment Maintenance or Interruption After 3 Years of Adjuvant Treatment in Patients With Gastrointestinal Stromal Tumours (GIST) (ImadGist). NCT02260505. https://clinicaltrials.gov/ct2/show/NCT02260505. Accessed November 18, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. KIT and PDGFRA Mutation Type and Tumor Mitotic Count
eTable 2. Characteristics of the 149 Patients Who Had GIST With KIT Exon 11 Deletion or Deletion-Insertion (Indel) Mutation
eTable 3. Characteristics of the 121 Patients Who Had GIST With KIT Exon 11 Mutation Involving Codons 557 and/or 558
eTable 4. Characteristics of the 111 Patients Who Had GIST With KIT Exon 11 Deletion or Deletion-Insertion (Indel) Mutation Involving Codons 557 and/or 558
eFigure 1. Association of KIT Exon 11 Insertion/Duplication Mutations, Absence of KIT and PDGFRA Mutations, PDGFRA Mutations, and PDGFRA Exon 18 Substitution Mutation D842V on Recurrence-Free Survival
eFigure 2. Associations of KIT Exon 11 Deletion Mutations With Recurrence-Free Survival in the Study Cohort
eFigure 3. Associations of KIT Exon 11 Deletion Mutations With Recurrence-Free Survival in the Random Allocation Groups
eFigure 4. Associations of GIST Mitotic Count With Recurrence-Free Survival in Patients With KIT Exon 11 Deletion Mutation That Involve Codons 557/558
eFigure 5. Recurrence-Free Survival (RFS) of Patients With Localized GIST in Three Randomized Trials