Abstract
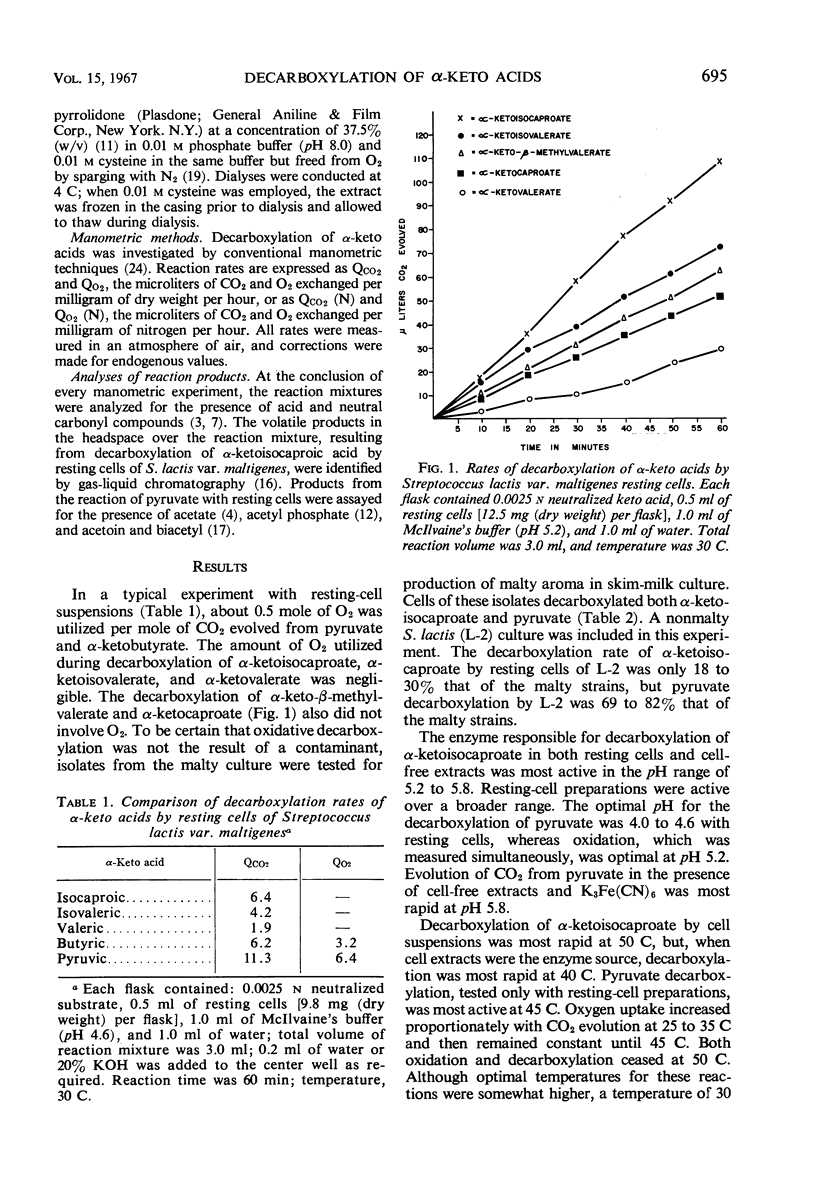
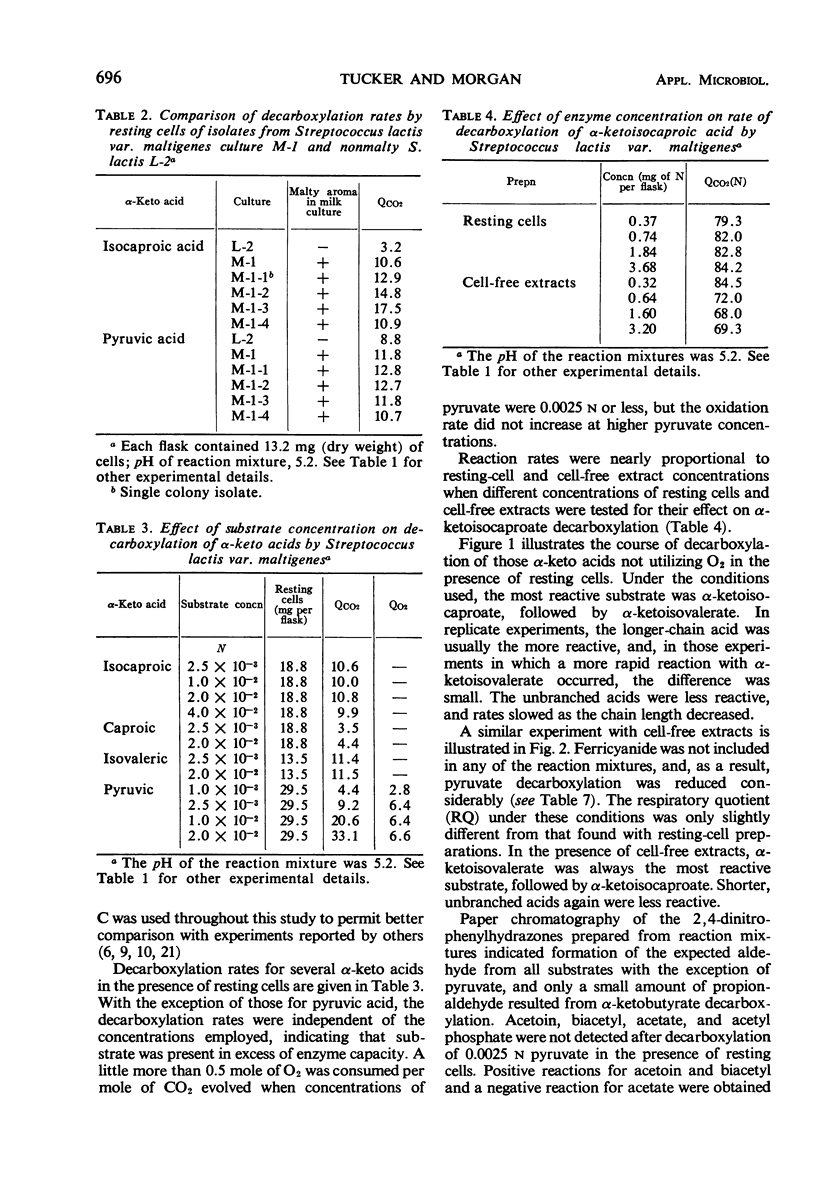
Decarboxylation rates for a series of C-3 to C-6 α-keto acids were determined in the presence of resting cells and cell-free extracts of Streptococcus lactis var. maltigenes. The C-5 and C-6 acids branched at the penultimate carbon atom were converted most rapidly to the respective aldehydes in the manner described for α-carboxylases. Pyruvate and α-ketobutyrate did not behave as α-carboxylase substrates, in that O2 was absorbed when they were reacted with resting cells. The same effect with pyruvate was noted in a nonmalty S. lactis, accounting for CO2 produced by some “homofermentative” streptococci. Mixed substrate reactions indicated that the same enzyme was responsible for decarboxylation of α-ketoisocaproate and α-ketoisovalerate, but it appeared unlikely that this enzyme was responsible for the decarboxylation of pyruvate. Ultrasonic disruption of cells of the malty culture resulted in an extract inactive for decarboxylation of pyruvate in the absence of ferricyanide. Dialyzed cell-free extracts were inactive against all keto acids and could not be reactivated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN W. C., SANDINE W. E., ELLIKER P. R. Lysis of lactic acid bacteria by lysozyme and ethylenediaminetetraacetic acid. J Bacteriol. 1962 Mar;83:697–698. doi: 10.1128/jb.83.3.697-698.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVALLINI D., FRONTALI N. Quantitative determination of keto-acids by paper partition chromatography. Biochim Biophys Acta. 1954 Mar;13(3):439–445. doi: 10.1016/0006-3002(54)90351-0. [DOI] [PubMed] [Google Scholar]
- GORDON D. F., Jr, MORGAN M. E., TUCKER J. S. Differentiation of Streptococcus lactis var. maltigenes from other lactic streptococci. Appl Microbiol. 1963 Mar;11:171–177. doi: 10.1128/am.11.2.171-177.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E. Evidence for a two-site mechanism for decarboxylation of alpha-keto acids by alpha-carboxylase. J Biol Chem. 1961 Aug;236:2302–2308. [PubMed] [Google Scholar]
- KING T. E., CHELDELIN V. H. Pyruvic carboxylase of Acetobacter suboxydase. J Biol Chem. 1954 Jun;208(2):821–831. [PubMed] [Google Scholar]
- LEMMEN L. J., TOURTELLOTTE W. W., GLIMN J. G., HIGGINS J. E., PARKER J. A. Study of cerebrospinal fluid proteins with paper electrophoresis. IV. Methods for concentrating dilute protein solutions. Med Bull (Ann Arbor) 1957 Apr;23(4):135–138. [PubMed] [Google Scholar]
- Morgan M. E., Lindsay R. C., Libbey L. M., Pereira R. L. Identity of additional aroma constituents in milk cultures of Streptococcus Lactis var. Maltigenes. J Dairy Sci. 1966 Jan;49(1):15–18. doi: 10.3168/jds.S0022-0302(66)87777-9. [DOI] [PubMed] [Google Scholar]
- OSTER M. O., WOOD N. P. FORMATE--PYRUVATE EXCHANGE REACTION IN STREPTOCOCCUS FAECALIS. II. REACTION CONDITIONS FOR CELL EXTRACTS. J Bacteriol. 1964 Jan;87:104–113. doi: 10.1128/jb.87.1.104-113.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENTHESHANUGANATHAN S. The mechanism of the formation of higher alcohols from amino acids by Saccharomyces cerevisiae. Biochem J. 1960 Mar;74:568–576. doi: 10.1042/bj0740568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUOMALAINEN H., OURA E. Cell permeability and decarboxylation of alpha-keto acids by intact yeast. Biochim Biophys Acta. 1958 Apr;28(1):120–127. doi: 10.1016/0006-3002(58)90437-2. [DOI] [PubMed] [Google Scholar]


