Abstract
Importance
Quantitative measurements based on optical coherence tomographic angiography (OCTA) may have value in managing diabetic retinopathy (DR), but there is limited information on the ability of OCTA to distinguish eyes with DR.
Objective
To evaluate the ability of measurements of retinal microvasculature using OCTA to distinguish healthy eyes from eyes with DR.
Design, Setting, and Participants
In this prospective cross-sectional study, OCTA was used to examine the eyes of participants with type 2 diabetes with or without DR and the eyes of participants without diabetes from September 17, 2015, to April 6, 2016. Density maps based on superficial retinal layer (SRL) and deeper retinal layer (DRL) images were generated after a method to remove decorrelation tails was applied to the DRL images.
Exposures
Both eyes of each participant were examined by means of a 3-mm OCTA scan and 7-field fundus photography using the Diabetic Retinopathy Severity Scale.
Main Outcomes and Measures
Two measures were examined: perfusion density, based on the area of vessels, and vessel density, based on a map with vessels of 1-pixel width. The size of the foveal avascular zone was also calculated automatically, and so was the area under the receiver operating characteristic curve.
Results
A total of 50 eyes from 26 participants with diabetes (10 women and 16 men; mean [SD] age, 64.9 [7.5] years) and 50 healthy eyes from 25 participants without diabetes (14 women and 11 men; mean [SD] age, 64.0 [7.1] years) were imaged. All participants were white. Vessel density measured in the SRL had the highest area under the receiver operating characteristic curve (0.893 [95% CI, 0.827-0.959]), compared with perfusion density in the SRL (0.794 [95% CI, 0.707-0.881]), foveal avascular zone area (0.472 [95% CI, 0.356-0.588]), and vessel density in the DRL (0.703 [95% CI, 0.601-0.805]). Vessel density in the SRL negatively correlated with best-corrected visual acuity (r = –0.28; P = .05) and severity of DR (r = –0.46; P = .001). Density metrics correlated with age. No correlation was detected between vascular density or foveal avascular zone metrics and hemoglobin A1C or duration of diabetes.
Conclusions and Relevance
Vessel density measured by OCTA provides a quantitative metric of capillary closure that correlates with severity of DR and may allow staging, diagnosis, and monitoring that do not require subjective evaluation of fundus images.
This cross-sectional study evaluates the ability of measurements of retinal microvasculature using optical coherence tomographic angiography to distinguish healthy eyes from eyes with diabetic retinopathy.
Key Points
Question
Can quantitative metrics based on optical coherence tomographic angiography be used to distinguish healthy eyes from diabetic eyes?
Findings
In this cross-sectional study, vessel density measured in the superficial retina distinguished healthy eyes from diabetic eyes and correlated with clinically relevant measures such as stage of disease, visual acuity, and central retinal thickness.
Meaning
These data suggest that quantification of the microvasculature in the retina correlates with existing clinical observations and may be an improvement over the current standard because it is objective and continuous rather than subjective and categorical.
Introduction
Optical coherence tomography (OCT) is a noninvasive imaging modality that allows detailed structural visualization of the retina. Optical coherence tomographic angiography (OCTA) is a functional extension of OCT that detects motion or blood flow contrast. Optical coherence tomographic angiography imaging has emerged as a noninvasive strategy to visualize the retinal and choroidal microvasculature without the use of an exogenous intravenous dye injection. The technique is based on the principle of identifying the temporal evolution of the OCT signal caused by the motion of scattering particles such as erythrocytes within the vessels. This technique can be used to generate information on 3-dimensional blood flow for visualization of the retinal and choroidal vasculature. Fluorescein angiography and indocyanine green angiography are currently the criterion standards for visualization of vasculature. Fluorescein angiography provides functional information regarding leakage of dye to provide information on the permeability of vessels, as well as the ability to stain structures such as fibrous tissue or subretinal fluid, which is not available with OCTA. However, fluorescein angiography and indocyanine green angiography are invasive, have occasional adverse effects, and have limited ability to distinguish signals from different axial depths within the retina.
Optical coherence tomographic angiography offers the possibility of quantification of features of interest. In particular, the density of vessels in the macula and the size of the foveal avascular zone (FAZ) are known to be affected by the presence of diabetic retinopathy (DR). The fovea and the FAZ have been well studied using OCT, adaptive optics, and fluorescein angiography, and quantification of the density of vessels in the macula has been reported in recent studies using OCTA. Results of these studies demonstrate a difference between the observations in healthy eyes and those in diabetic eyes; however, several issues remain to be addressed before such metrics can be used in a meaningful way. Since numerous metrics can be conceived and tested, it is helpful to be able to compare among these, using a standard related to current clinical practice. One way to compare different metrics is to use the area under the receiver operating characteristic (ROC) curve. If a common population and a common criterion standard are used, it offers a quantitative comparison among methods. It is also helpful to study methods that are available commercially; for that reason, in this article, we focus on quantifying the density of vasculature within the layers available in a commercial instrument.
The purpose of this study was to evaluate multiple quantitative measures of the microvasculature in eyes of patients with type 2 diabetes, as well as healthy eyes imaged with OCTA, and to determine the ability to use characteristics of the microvasculature to distinguish healthy eyes from the eyes of patients with DR.
Methods
In this prospective cross-sectional study (clinicaltrials.gov identifier NCT02391558), participants with diabetes with or without DR and individuals without diabetes recruited from Associação para Investigação Biomédica em Luz e Imagen staff volunteers and from companions who accompanied the patient during their imaging visit underwent OCTA imaging and a full ophthalmologic examination from September 17, 2015, to April 6, 2016. The tenets of the Declaration of Helsinki were followed, approval was obtained from the National Ethics Committee for Clinical Research, and written informed consent to participate in the study was obtained from all individuals after all procedures were explained.
Exclusion criteria for the participants without diabetes included any history of ocular injury; ocular surgery; ocular disease such as age-related macular degeneration, glaucoma, or vitreomacular disease; or systemic disease such as diabetes that could affect the eye. The information about previous medical conditions and ocular treatments was obtained by interviewing the participants. Only individuals with a spherical error between –6 and +2 diopters were included. Images were evaluated at the time of acquisition for quality, including having a signal strength greater than 6, minimal motion artifacts, and minimal evidence of defocus or blur. No eyes had to be excluded because acceptable images could not be obtained.
Optical coherence tomographic angiography imaging was performed with a Cirrus high-definition–OCT prototype AngioPlex instrument using the Optical Micro Angiography algorithm (Carl Zeiss Meditec, Inc). Both eyes of each participant were imaged with a scan comprising 245 clusters of B-scans repeated 4 times, where each B-scan consisted of 245 A-scans. The resulting OCT volume scan had dimensions of 3 × 3 × 2 mm. The effect of eye motion–related artifacts was minimized by the use of tracking. The Optical Micro Angiography algorithm was applied to the volumetric data sets, and the images were extracted as described previously.
The averaged OCT B-scans were also examined in the usual manner of OCT data to show the retinal tissue. This volume of data was segmented using the CIRRUS inner-limiting membrane and retinal pigment epithelium segmentation algorithms. From these layers, estimates were derived to subdivide the inner retina into 2 distinct physiologic layers: a superficial retinal layer (SRL) and a deeper retinal layer (DRL). The inner retina was estimated as being the tissue between the inner-limiting membrane and an offset from the retinal pigment epithelium of 110 µm. The SRL was defined as the inner 70% of the inner retina, and the DRL was the remaining 30% of the inner retina. The layer estimates were applied to the 3-dimensional Optical Micro Angiography algorithm motion contrast data set. A maximum projection method within the layer of interest was used to generate the en face images. A model-based method was used to remove the decorrelation tails from the DRL en face image. All these steps are performed within the software commercially available on the Cirrus high-definition–OCT with AngioPlex (Carl Zeiss Meditec, Inc).
To calculate the perfusion density and the vessel density, a thresholding algorithm was applied to the SRL or DRL en face images to create a binary slab that assigns to each pixel a 1 (perfused) or 0 (background). From this slab, a skeletonized slab was created, representing vessels with a trace of 1 pixel in width. We define the perfusion density as the total area of perfused vasculature per unit area in a region of measurement, calculated by taking the mean of the binary slab within a desired region of interest. We define the vessel density as the total length of perfused vasculature per unit area in a region of measurement. A similar length-based metric has been used as a measurement of road density. We calculate the vessel density by taking the mean of the skeletonized slab within a desired region of interest and scaling the result by the distance between pixels (in this case, 512 pixels per 3 mm). The mean of the skeletonized slab is only a first-order estimate of the length of perfused vasculature. A more accurate calculation would require considering the relationship between neighboring pixels with a value of 1 in the skeletonized slab.
Using an existing algorithm available on the Cirrus device, we used the fovea position as the starting point for an iterative region-growing algorithm that identified the FAZ. The area and perimeter of this zone were calculated, and the circularity index was calculated as 4πA/P, where A is the area and P is the perimeter. The FAZ measures were based only on the SRL because there is only expected to be a single capillary plexus at the border of the FAZ.
Continuous variables were summarized for both the group without diabetes and the group with DR using the following statistics: the mean (SD), minimum, maximum, median, first quartile, and third quartile values. The frequency and percentages are reported for the categorical measures. A 2-sided t test was performed to compare the measurements of healthy eyes with the measurements of the eyes of patients with DR, assuming heteroscedastic conditions. The t test was also performed to compare density measurements made on the DRL with decorrelation tail correction with the measurements without such correction, and to compare the distribution among women with the distribution among men in both populations.
Age, duration of diabetes, and hemoglobin A1c (HbA1c) level were noted for each participant from the patient record. The DR severity level was determined by a single grader (C.N.) within the context of an experienced reading center and was based on the 7-field protocol using the Diabetic Retinopathy Severity Scale. Best-corrected visual acuity (BCVA) was measured for each eye using the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol and precision vision charts at 4 m. A Cirrus OCT scan of the macula consisting of 128 B-scans with 512 A-scans each (Macular Cube 512 × 218 scan) was taken of each eye, and the central retinal thickness (CRT) was measured using the standard macular thickness analysis.
The nonparametric Spearman correlation coefficient, based on the ranked values for each variable rather than the raw data, was determined to evaluate the monotonic association between each measurement and age, duration of diabetes, HbA1c level, CRT, and BCVA.
Receiver operating characteristic analysis was used to determine the diagnostic efficacy of the measurements. The ROC curve was plotted by computing the sensitivity and specificity using each symmetric value of the rating variable as a possible cut point. A point was plotted on the graph for each of the cut points; these plotted points were joined by straight lines to form the ROC curve, and the area under the curve (AUC) was computed using the trapezoidal rule. All statistical analyses were performed with Stata version 12.1 (StataCorp LP). P < .05 was considered significant.
Results
A total of 50 eyes from 26 patients with diabetes and 50 healthy eyes from 25 participants without diabetes were imaged (eFigure in the Supplement). A summary of the characteristics of the participants and their eyes is shown in Table 1. The populations did not differ significantly in mean (SD) age (with diabetes, 64.9 [7.5] years; without diabetes, 64.0 [7.1] years), but they did differ in distribution of sex (with diabetes, 10 women and 16 men; without diabetes, 14 women and 11 men). All participants were white. The group with diabetes included individuals with no DR and individuals with mild to severe nonproliferative DR. The following distribution was considered based on DR ETDRS level: (1) early stage, ETDRS levels 10 to 20 (12 eyes [24%]); (2) mild nonproliferative DR, ETDRS level 35 (27 eyes [54%]); and (3) moderate to severe nonproliferative DR, ETDRS levels 43 to 53 (11 eyes [22%]).
Table 1. Distribution of Characteristics of Eyes and Participants.
| Characteristic | Healthy Group (50 Eyes) |
Diabetic Group (50 Eyes) |
|---|---|---|
| Age, y | ||
| Mean (SD) [range] | 64.0 (7.1) [50-75] | 64.9 (7.5) [52-76] |
| Median (IQR) | 64 (60-70) | 66 (59-71) |
| Sex, No./total No. (%) of participants | ||
| Female | 14/25 (56) | 10/26 (38) |
| Male | 11/25 (44) | 16/26 (62) |
| BCVA, No. (%) | ||
| 20/16 | NA | 9 (18) |
| 20/20 | NA | 13 (26) |
| 20/25 | NA | 26 (52) |
| 20/32 | NA | 2 (4) |
| Central retinal thickness, µm | ||
| Mean (SD) [range] | NA | 283 (36) [239-423] |
| Median (IQR) | NA | 278 (263-290) |
| Diabetes duration, y | ||
| Mean (SD) [range] | NA | 18.7 (6.5) [7-33] |
| Median (IQR) | NA | 17 (15-24) |
| Hemoglobin A1c, % | ||
| Mean (SD) [range] | NA | 7.62 (1.76) [6.10-14.10] |
| Median (IQR) | NA | 7.05 (6.60-8.00) |
Abbreviations: BCVA, best-corrected visual acuity; IQR, interquartile range; NA, not applicable.
SI conversion factor: To convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
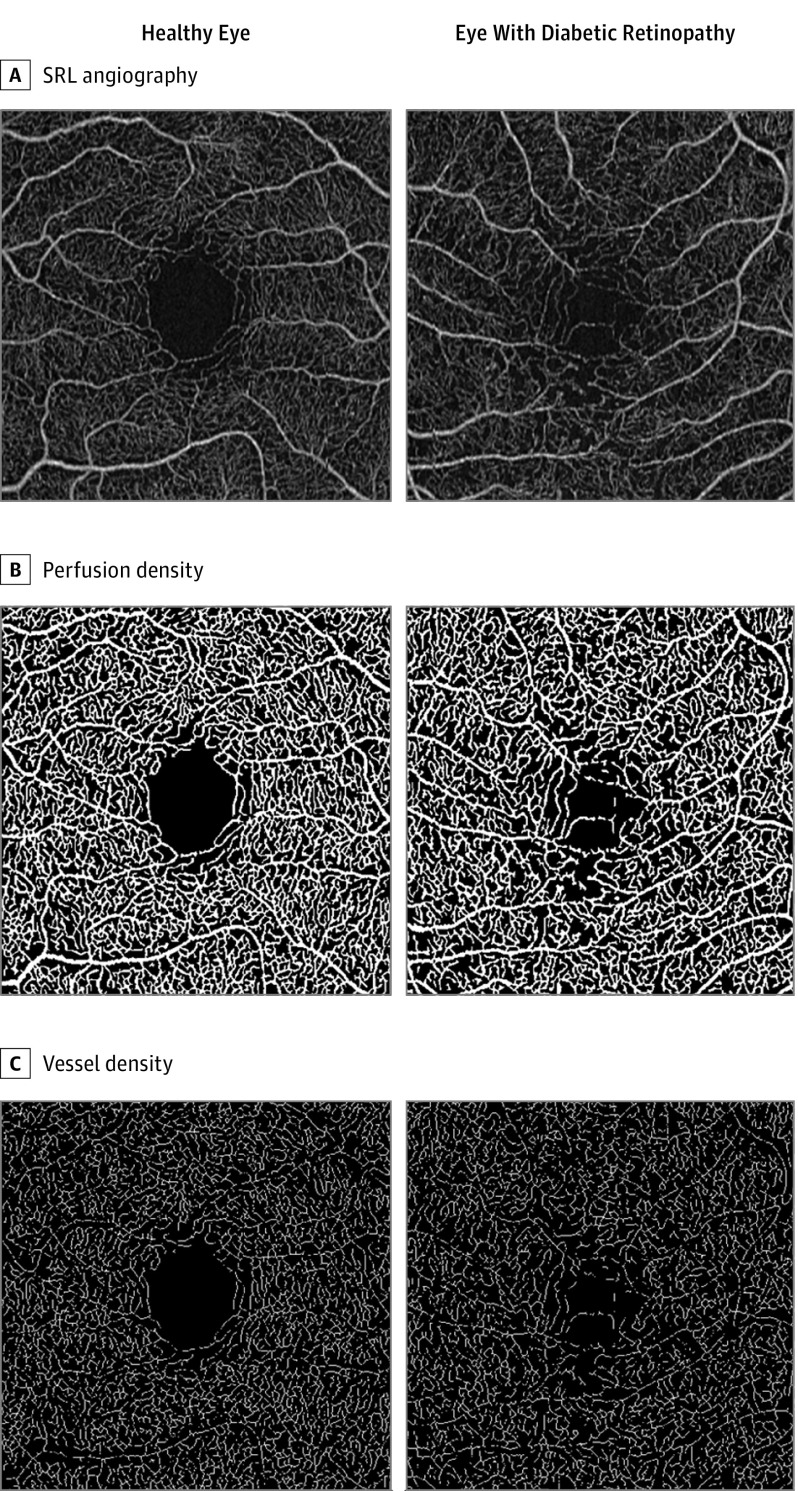
The steps in the quantification process leading to the different measurements for 1 eye with DR and 1 healthy eye are shown in Figure 1. A difference in the overall appearance of the vasculature can be appreciated, with identifiable microvascular abnormalities, including microaneurysms and capillary closure in the eye with DR.
Figure 1. Image Processing Steps in Microvascular Density Quantification.
Each row shows an example of one aspect of the quantitative measurements for a healthy eye (55-year-old woman) and an eye with diabetic retinopathy (54-year-old man; Early Treatment Diabetic Retinopathy Study grade 35C; type 2 diabetes for 12 years; hemoglobin A1c, 7.2%). A, AngioPlex superficial retinal layer (SRL) angiography en face for a 3 × 3-mm scan. B, Binarized slab of the SRL image, used for perfusion density; mean perfusion density is 0.422 for the healthy eye and 0.411 for the eye with diabetic retinopathy. C, Skeletonized image used for vessel density; mean vessel density of 22.9 mm–1 for the healthy eye and 21.8 mm–1 for the eye with diabetic retinopathy.
A summary of the descriptive statistics for each variable measured from the SRL, DRL, and FAZ is shown in Table 2. There was substantial overlap between healthy eyes and eyes with DR for all variables, but the eyes with DR had a lower mean (SD) vascular density compared with healthy eyes (21.1 [1.0] vs 22.5 [0.7] mm–1). The mean (SD) FAZ area (0.26 [0.10] vs 0.25 [0.10] mm2) and perimeter (2.32 [0.59] vs 2.05 [0.53] mm) were larger for diabetic eyes than for healthy eyes, whereas the mean (SD) FAZ circularity index was lower (0.78 [0.09] vs 0.82 [0.06]).
Table 2. Descriptive Statistics for Density Metrics and Foveal Avascular Zone (FAZ) Area in the Superficial Retinal Layer (SRL) and the Deeper Retinal Layer (DRL) for Both Groups.
| Statistic | Perfusion Densitya | Vessel Density, mm-1 | FAZ Area, mm2 | FAZ Perimeter, mm | FAZ Circularity Index | ||
|---|---|---|---|---|---|---|---|
| SRL | DRLb | SRL | DRLb | ||||
| Healthy eye group | |||||||
| Mean (SD) [range] | 0.419 (0.007) [0.401-0.436] |
0.337 (0.034) [0.234-0.380] |
22.5 (0.7) [21.3-23.9] |
17.7 (1.9) [11.8-20.5] |
0.25 (0.10) [0.04-0.49] |
2.05 (0.53) [0.15-2.89] |
0.82 (0.06) [0.67-0.94] |
| Median (IQR) | 0.420 (0.413-0.425) |
0.346 (0.323-0.363) |
22.5 (22.2-22.9) |
18.1 (16.7-18.9) |
0.24 (0.16-0.33) |
2.10 (1.72-2.48) |
0.83 (0.79-0.86) |
| Diabetic group | |||||||
| Mean (SD) [range] | 0.409 (0.009) [0.384-0.425] |
0.320 (0.033) [0.224-0.376] |
21.2 (1.0) [19.3-23.4] |
16.4 (1.9) [11.3-19.6] |
0.26 (0.10) [0.06-0.63] |
2.32 (0.59) [1.13-4.05] |
0.78 (0.09) [0.55-1.08] |
| Median (IQR) | 0.410 (0.405-0.415) |
0.325 (0.293-0.347) |
21.2 (20.5-21.9) |
16.2 (15.0-17.9) |
0.26 (0.19-0.31) |
2.27 (2.04-2.58) |
0.78 (0.72-0.84) |
| P valuec | <.001 | .009 | <.001 | <.001 | .28 | .01 | .12 |
A unitless measure.
After removal of decorrelation tail artifact.
For 2-tailed, 2-population heteroscedastic comparison between groups.
The eTable in the Supplement shows descriptive statistics for density measurements of the DRL in both groups, before and after decorrelation tail correction, and compares the measurements of the eyes with DR with those of healthy eyes, as well as the measurements without correction for decorrelation tails. The mean (SD) measurement of density in the DRL is different between healthy eyes and the eyes from participants with DR for both perfusion density (healthy eyes, 0.337 [0.034]; DR, 0.320 [0.033]) and vessel density (healthy eyes, 17.7 [1.9]; DR, 16.4 [1.9]), and the utility of measures of density in the DRL may be improved by the use of a correction for decorrelation tail artifacts.
Table 3 shows Spearman correlations for age, BCVA, CRT, HbA1c level, duration of diabetes, and DR severity for participants with diabetes. The Spearman coefficient is most appropriate for ranked outcomes such as BCVA and DR severity, but we applied it for age and CRT for comparison with the other variables. There was a weak correlation between BCVA and vessel density measured in the SRL (Spearman coefficient = –0.28; P = .05). For CRT, there are correlations with both perfusion density and vessel density in the SRL (Spearman coefficient = 0.45, P = .001 for perfusion density, and Spearman coefficient = 0.36, P = .01 for vessel density). For DR severity, there were correlations with both perfusion density and vessel density in the SRL (Spearman coefficient = –0.36, P = .01 for perfusion density, and Spearman coefficient = –0.46, P = .001 for vessel density). Table 3 shows correlation results for DRL, but these had very small Spearman coefficients and large P values, indicating poor correlation for all variables except age. None of the microvascular measures correlated with HbA1c level or duration of diabetes. A t test comparing the results for men with the results for women found no significant differences in either the healthy or diabetic groups.
Table 3. Spearman Coefficients for Association of Variables With Density and Foveal Avascular Zone (FAZ) Measurements in Eyes With Diabetic Retinopathy (DR).
| Variable | FAZ Area | FAZ Perimeter | FAZ Circularity Index | Perfusion Density in SRL | Vessel Density in SRL | No Decorrelation Tail Removal | Decorrelation Tail Removal | ||
|---|---|---|---|---|---|---|---|---|---|
| Perfusion Density in DRL | Vessel Density in DRL | Perfusion Density in DRL | Vessel Density in DRL | ||||||
| Age | 0.04 | 0.17 | −0.25 | −0.30 | −0.10 | −0.44 | −0.38 | −0.49 | −0.43 |
| P value | .80 | .25 | .08 | .04 | .49 | .001 | .01 | <.001 | .002 |
| BCVA | 0.140 | 0.08 | −0.06 | −0.21 | −0.28 | 0.01 | −0.04 | −0.05 | −0.10 |
| P value | .34 | .57 | .68 | .14 | .05 | .97 | .80 | .75 | .50 |
| CRT | −0.24 | −0.17 | −0.11 | 0.45 | 0.36 | 0.24 | 0.27 | 0.24 | 0.25 |
| P value | .10 | .23 | .43 | .001 | .01 | .09 | .06 | .10 | .08 |
| Duration of diabetes | −0.02 | 0.01 | −0.01 | −0.04 | −0.13 | −0.06 | −0.04 | −0.05 | −0.05 |
| P value | .91 | .96 | .94 | .77 | .38 | .66 | .78 | .75 | .73 |
| Hemoglobin A1c | 0.05 | 0.03 | −0.05 | −0.05 | −0.16 | 0.06 | 0.03 | 0.05 | 0.01 |
| P value | .76 | .82 | .71 | .74 | .27 | .69 | .83 | .74 | .93 |
| DR severity | 0.190 | 0.26 | −0.16 | −0.36 | −0.46 | −0.17 | −0.21 | −0.20 | −0.28 |
| P value | .19 | .07 | .28 | .01 | .001 | .24 | .13 | .17 | .05 |
Abbreviations: BCVA, best-corrected visual acuity; CRT, central retinal thickness; DRL, deeper retinal layer; SRL, superficial retinal layer.
We found that age had a small effect on all measures of density but not on FAZ size, which suggests that there are other contributing sources of variability in vessel density in the presence of DR. For perfusion density in the SRL, as well as both perfusion density and vessel density measured in the DRL, age-related changes were observed in both healthy eyes and eyes with DR.
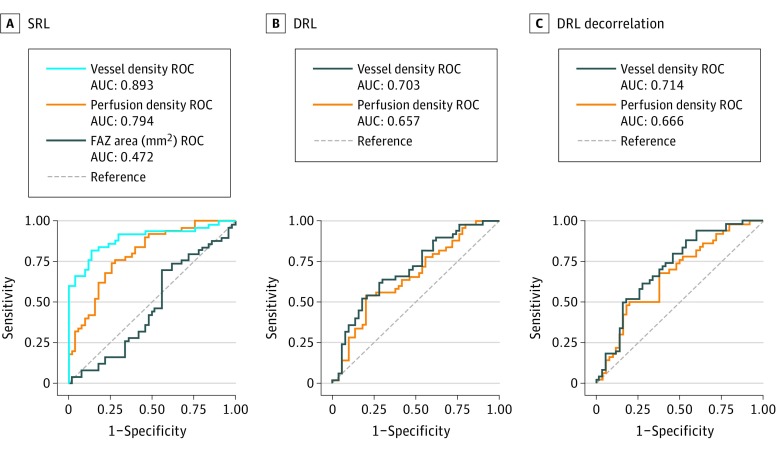
Figure 2 shows the ROC curve for the FAZ, and for the perfusion and vessel density as averaged over the cube. The FAZ area shows no diagnostic efficacy, with an AUC of 0.472 (95% CI, 0.356-0.588), but both of the density metrics show the ability to distinguish healthy eyes from eyes with DR in this population, with AUCs of 0.893 (95% CI, 0.827-0.959) for vessel density and 0.794 (95% CI, 0.707-0.881) for perfusion density in the SRL. Sensitivity at 95% specificity was 66% for vessel density, 32% for perfusion density, and 4% for FAZ for the SRL. Regarding density measures in the DRL, with or without decorrelation tail removal, the diagnostic efficacy is poorer than density measurements of the SRL but better than the FAZ.
Figure 2. Receiver Operating Characteristic (ROC) Curves.
A, Foveal avascular zone (FAZ) area, vessel density, and perfusion density in the superficial retinal layer (SRL). B, Vessel density and perfusion density in the deeper retinal layer (DRL). C, Vessel density and perfusion density in the DRL with decorrelation tail removal. AUC indicates area under the curve.
Discussion
Several recent studies have evaluated the use of OCTA for DR, analyzing different variables with special emphasis on capillary perfusion and FAZ metrics. In this study, we evaluated the clinical utility of quantitative measures of microvasculature in OCTA. Although several studies have demonstrated the potential value of measures of microvasculature in the management of DR, our study uses the ROC curve to compare the overall value of different approaches. In this age-matched population with a range of disease, the mean vessel density measured in the SRL had the highest AUC, indicating that it is best among the methods tested at differentiating healthy eyes from eyes with DR. There were not enough participants to break down the comparison by stage of disease, but the prevalence of initial stages of disease in this population (30 of 50 eyes [78%] were either in the mild stage or had no DR) is consistent with a goal of being sensitive to early signs of disease. As expected, the FAZ showed a wide range in the healthy eyes, and the effectiveness of its use was no better than chance at distinguishing eyes with DR from the healthy population.
Measurements in the DRL are affected by decorrelation tails, and the results of our study show that removing decorrelation tail artifacts improves the utility of density measurements made in this layer. However, with current technology, measurements of density in the DRL do not appear to have the same diagnostic efficacy as measurements in the SRL. Although we found that there is lower density in the eyes with DR than in healthy eyes in the DRL, which is consistent with other studies, a comparison using the AUC shows the SRL to be more practically efficacious, at least as a diagnostic tool. Future improvements to layer definition, sampling density, and decorrelation tail removal may increase the value of measurements in this layer.
There was a range of density observed within each ETDRS grade level, and the best variable with respect to AUC (vessel density) was also correlated with BCVA and severity of DR. These facts suggest that capillary closure may provide relevant information regarding progression of retinopathy in individual patients with diabetes. This study did not detect a correlation between density or FAZ and HbA1c level or duration of diabetes, which suggests that there is no strong correlation between microvascular measurements in the retina and systemic disease, at least in this small population. The correlation of vessel density in the SRL with visual acuity suggests that it may be a potential indicator for vision loss.
Microvascular perfusion of the macula is particularly relevant regarding DR progression and vision loss. The 2 vision-threatening complications of DR, proliferative retinopathy and center-involving macular edema, have been shown to be directly associated with poor microvascular perfusion and increased leakage, respectively. Alterations of microvascular perfusion have been associated with retinal microthrombosis, vascular remodeling, and increased microaneurysm turnover. Such alterations may occur predominantly in a specific phenotype of DR associated with more rapid progression to sight-threatening disease. Identification of eyes with DR that show lower vascular density in the macula may contribute to improved and individualized management of diabetic retinal disease, with closer follow-up periods and earlier and more timely treatment of the sight-threatening complications of diabetes.
Limitations
The limitations of this study include the relatively small sample size, the large range of stages of DR, the use of estimates to define the positions of the SRL and DRL, and the cross-sectional design. The study was not large enough to account for confounding factors such as duration of diabetes, treatment, sex, or level of blood glucose control. Statistical significance was not adjusted to account for multiple testing or correlations between 2 eyes from the same individual because the comparisons in this study were exploratory.
Conclusions
Even with these limitations, our study demonstrates that vascular density measurements based on OCTA performed with Cirrus AngioPlex are associated with accepted clinical measures, and they provide metrics to monitor capillary closure in the parafoveal region that are likely to be clinically useful. Further study is warranted to determine the details of the distribution of these measurements in populations of interest, as well as the effect of time and progression of disease on these measurements in individual eyes. It may be that measurable microvascular changes occur before accepted clinical hallmarks are seen. Additional work might include establishing the characteristics of healthy eyes, including such effects as sex and age, so that small changes from a healthy state might be correlated in a longitudinal study to evaluate development of clinically observable disease.
eFigure. CONSORT Flow Diagram for Clinical Study
eTable. Descriptive Statistics for Density Measurements Based on the DRL With and Without Correcting for Decorrelation Tails, in Both Groups
References
- 1.Wang RK, An L, Francis P, Wilson DJ. Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography. Opt Lett. 2010;35(9):1467-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Opt Express. 2007;15(7):4083-4097. [DOI] [PubMed] [Google Scholar]
- 3.Mansour AM, Schachat A, Bodiford G, Haymond R. Foveal avascular zone in diabetes mellitus. Retina. 1993;13(2):125-128. [DOI] [PubMed] [Google Scholar]
- 4.Hwang TS, Gao SS, Liu L, et al. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016;134(4):367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015;35(11):2353-2363. [DOI] [PubMed] [Google Scholar]
- 6.Shahlaee A, Samara WA, Hsu J, et al. In vivo assessment of macular vascular density in healthy human eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016;165(5):39-46. [DOI] [PubMed] [Google Scholar]
- 7.Samara WA, Shahlaee A, Sridhar J, Khan MA, Ho AC, Hsu J. Quantitative optical coherence tomography angiography features and visual function in eyes with branch retinal vein occlusion. Am J Ophthalmol. 2016;166(6):76-83. [DOI] [PubMed] [Google Scholar]
- 8.Coscas F, Sellam A, Glacet-Bernard A, et al. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT211-OCT223. [DOI] [PubMed] [Google Scholar]
- 9.Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57(8):3907-3913. [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 11.Bagherinia H. A Novel Approach to Reducing Decorrelation Tail Artifacts in Optical Coherence Tomography Angiography. Seattle, WA: Association for Research in Vision and Ophthalmology; 2016. [Google Scholar]
- 12.Road density: USEPA Regional Vulnerability Assessment Program (ReVA). Environmental Protection Agency website. https://catalog.data.gov/harvest/object/b0ef510e-e6c7-4adf-8888-51577d7a7523/html/original. Accessed February 7, 2017.
- 13.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics: ETDRS report number 7. Ophthalmology. 1991;98(5)(suppl):741-756. [DOI] [PubMed] [Google Scholar]
- 14.Matsunaga DR, Yi JJ, De Koo LO, Ameri H, Puliafito CA, Kashani AH. Optical coherence tomography angiography of diabetic retinopathy in human subjects. Ophthalmic Surg Lasers Imaging Retina. 2015;46(8):796-805. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz DM, Fingler J, Kim DY, et al. Phase-variance optical coherence tomography: a technique for noninvasive angiography. Ophthalmology. 2014;121(1):180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Y, Bailey ST, Hwang TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A. 2015;112(18):E2395-E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160(1):35-44.e1. [DOI] [PubMed] [Google Scholar]
- 18.Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular complications of diabetes and therapeutic approaches. Biomed Res Int. 2016;2016:3801570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boeri D, Maiello M, Lorenzi M. Increased prevalence of microthromboses in retinal capillaries of diabetic individuals. Diabetes. 2001;50(6):1432-1439. [DOI] [PubMed] [Google Scholar]
- 20.Cunha-Vaz J, Ribeiro L, Lobo C. Phenotypes and biomarkers of diabetic retinopathy. Prog Retin Eye Res. 2014;41:90-111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. CONSORT Flow Diagram for Clinical Study
eTable. Descriptive Statistics for Density Measurements Based on the DRL With and Without Correcting for Decorrelation Tails, in Both Groups