This community-based cohort study assesses which blood pressure components best determine incident cardiovascular disease events in young adults and determines whether these associations vary by race and age at blood pressure measurement.
Key Points
Question
Which blood pressure components best determine incident premature cardiovascular events in young adults and does the association vary by race and age at blood pressure measurement?
Findings
This community-based cohort of 4880 young adults at baseline followed up for up to 28 years observed that in young adults (mean age, 25 years) systolic blood pressure is the most robust indicator of premature cardiovascular disease risk in black individuals, whereas diastolic blood pressure performed better than systolic blood pressure in determining premature cardiovascular disease in white individuals. In middle-aged adults (mean age, 40 years), systolic blood pressure instead of diastolic blood pressure better determined incident cardiovascular disease in both races.
Meaning
Our data question the classic view that diastolic blood pressure more often identifies cardiovascular disease events than systolic blood pressure in all individuals younger than 50 years.
Abstract
Importance
Data are sparse regarding which blood pressure (BP) components in young adulthood optimally determine cardiovascular disease (CVD) by middle age.
Objectives
To assess which BP components best determine incident CVD events in young adults and determine whether these associations vary by race and age at BP measurement.
Design, Setting, and Participants
Using data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, this study assessed the longitudinal race-stratified associations between BP and cardiovascular outcomes. CARDIA is a community-based cohort that recruited black and white individuals (age range, 18-30 years) from March 26, 1985, through June 7, 1986. CARDIA followed up participants for up to 28 years, and 94% of the surviving cohort completed at least 1 telephone interview or examination from August 2009 through August 2014.
Exposures
Blood pressures measubred at baseline (Y0) and 15 years later (Y15).
Main Outcomes and Measures
Composite CVD events, including coronary heart disease, stroke, heart failure, and other vascular diseases.
Results
A total of 4880 participants participated in the study (mean [SD] age, 24.9 [3.6] years at Y0 and 25.0 [3.6] years at Y15; 2223 male [45.6%] at Y0 and 1800 [44.2%] at Y15; 2657 female [54.4%] at Y0 and 2277 [55.8%] at Y0; 2473 black individuals [50.7%] at Y0 and 1994 [48.9%] at Y15; and 2407 white individuals [49.3%] at Y0 and 2083 [51.1%] at Y15). The mean SBP/DBP was 112/69 mm Hg in blacks and 109/68 mm Hg in whites at Y0 and 117/77 mm Hg in blacks and 110/72 mm Hg in whites at Y15. During a 25-year follow-up from Y0, 210 CVD events occurred (twice as many events in blacks [n = 140] compared with whites), of which 131 (87 in blacks) occurred after Y15. With adjustments for covariates, results from Cox proportional hazards models, including SBP and DBP, jointly suggested that, at Y0, SBP (hazard ratio [HR] per 1-SD increase, 1.32; 95% CI, 1.09-1.61) but not DBP (HR, 1.05; 95% CI, 0.88-1.26) was associated with CVD risk in blacks, whereas DBP (HR, 1.74; 95% CI, 1.21-2.50) but not SBP (HR, 0.82; 95% CI, 0.57-1.18) was associated with CVD risk in whites. At Y15, SBP was the strongest indicator of CVD in blacks (HR, 1.64; 95% CI, 1.25-2.16) and whites (HR, 1.67; 95% CI, 1.02-2.69).
Conclusions and Relevance
This study questions the classic view that DBP is more able to identify future CVD events than SBP in all individuals younger than 50 years. In young adulthood, SBP in black individuals and DBP in white individuals were the most robust indicators of future CVD. In middle-age, SBP in both races identified risk of incident CVD.
Introduction
Younger adults (≤50 years of age) are increasingly prone to stroke, chronic kidney disease, and worsening cardiovascular disease (CVD) mortality. An alarming increase in the prevalence of high blood pressure (BP) in young adulthood may underlie these adverse trends. However, which specific BP components in young adulthood may optimally identify cardiovascular outcomes by middle age and whether these associations vary by race and age at BP measurement are poorly understood.
Existing studies have found that the BP components that best identify CVD risk vary by age at BP measurement. In the Framingham Heart Study (FHS) and other studies with largely white study populations, among those younger than 50 years at BP measurement, diastolic BP (DBP) was a stronger indicator of incident CVD, whereas systolic BP (SBP) dominated at older ages. As a result, national guidelines encourage DBP as a treatment target in young adults. However, observations in those aged 18 to 30 years are sparse, and whether these results are consistent across race groups is unknown. Given the earlier development of hypertension and arterial stiffness in black compared with white adults, we hypothesized that SBP instead of DBP would be more strongly associated with CVD risk in black individuals, even at a young age. Using data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, which enrolled black and white adults in young adulthood and followed them up for 28 years, we assessed which BP components best identified incident CVD events and whether these associations varied by race and age at BP measurement.
Methods
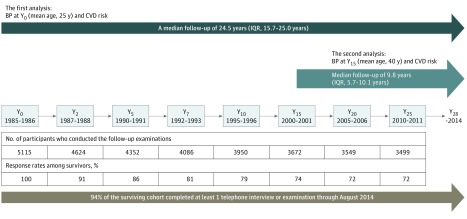
The CARDIA study recruited patients from January 1, 1985, through December 31, 1986, enrolling 5115 black and white adults 18 to 30 years old (eMethods in the Supplement). Serial follow-up examinations were conducted at years 2 (Y2), 5 (Y5), 7 (Y7), 10 (Y10), 15 (Y15), 20 (Y20), and 25 (Y25) after baseline (Y0) (Figure 1). A total of 94% of the surviving cohort completed at least 1 telephone interview or examination from August 2009 through August 2014. All participants provided written informed consent, and institutional review boards at each field center (University of Alabama at Birmingham; University of Minnesota, Minneapolis; Northwestern University, Chicago, Illinois; and Kaiser Permanente, Oakland, California) and the CARDIA Coordinating Center approved the study annually.
Figure 1. Timeline for the Coronary Artery Risk Development in Young Adults (CARDIA) Study and Analytic Plan in the Current Study.
Visits and numbers of participants are tabulated. In CARDIA, serial follow-up examinations were conducted at years 2 (Y2), 5 (Y5), 7 (Y7), 10 (Y10), 15 (Y15), 20 (Y20), and 25 (Y25). In the primary analyses, the association between blood pressure (BP) at Y0 and cardiovascular disease (CVD) risk during 25-year follow-up from Y0 was assessed. In the secondary analyses, the association between BP at Y15 and CVD risk during 10-year follow-up from Y15 was assessed. IQR indicates interquartile range.
BP and Other Measurements
From the Y0 to Y15 examinations, trained research staff measured brachial artery BP 3 times at 1-minute intervals after the participant had been sitting in a quiet room for 5 minutes, using a random-zero sphygmomanometer (Hawksley). The details are described in the eMethods in the Supplement. The mean of the second and third measurements was used for the analysis.
The BP components evaluated included SBP, DBP, mean arterial pressure (MAP), mid-BP, and pulse pressure (PP). The MAP was defined as (SBP + 2 × DBP)/3. The PP was defined as SBP − DBP. Mid-BP, calculated as (SBP + DBP)/2, was highly correlated with MAP (r = 0.99; P < .001); thus, we only report MAP. Hypertension was defined as an SBP of 140 mm Hg or higher, a DBP of 90 mm Hg or higher, or use of antihypertensive medication. Data on educational level, smoking, medication use, A Priori Diet Quality Score, clinical history of CVD, and fasting laboratory values were collected using standardized protocols and quality control procedures across study centers (eMethods in the Supplement).
Cardiovascular Outcomes
We recorded incident CVD events through September 31, 2013, by scheduled study examinations and yearly telephone interviews (eMethods in the Supplement). Composite CVD included coronary heart disease, hospitalization for heart failure, stroke, transient ischemic attack, intervention for peripheral artery disease, or death from cardiovascular causes. Participants who did not have events and who did not drop out of the study were censored at 28 years after the Y0 examination.
Statistical Analysis
Our analyses were performed with 2 approaches (Figure 1). In the first analysis, we examined the associations between BP components at Y0 and incident CVD events experienced throughout the entire follow-up period. The CVD events were ascertained annually during a 28-year follow-up period. Using Cox proportional hazards models, we first estimated race-specific multivariable-adjusted hazard ratios (HRs) and 95% CIs of incident CVD associated with each BP component. The proportionality assumption for the Cox analyses was confirmed graphically and with the inclusion of a time by BP component interaction. All BP components were analyzed per 1 SD to allow for direct comparison among components. Covariates included the sociodemographic characteristics of age, sex, educational level, and study site and the clinical characteristics at Y0 of smoking, body mass index (BMI), fasting glucose level, total cholesterol and high-density lipoprotein cholesterol levels, and antihypertensive medication use. These covariates were selected a priori because they have known correlations with SBP and DBP and are risk factors for incident CVD. Next, in race-combined analyses, analyses of the interaction between each BP component and race in association with incident CVD were performed with inclusion of multiplicative interaction terms. The likelihood ratio χ2, Akaike information criterion (AIC), and Bayes information criterion (BIC) estimates were used to assess model fit. Comparison of the discriminative ability of each model was conducted by Harrell C statistics (with 95% CIs, calculated by bootstrapping) as described by Newson. In sensitivity analyses, we examined the following: (1) the interaction between sex or baseline antihypertensive medication use and each BP component in association with CVD risk; (2) the Y0 BP–CVD risk association by excluding participants taking antihypertensive medications at Y0; (3) whether the Y0 BP–CVD risk association is modified by an adjustment for the A Priori Diet Quality Score; and (4) quartile analyses of BP.
In the second analysis, we examined the associations between BP components at Y15 and incident CVD events experienced after the Y15 examination. The rationale in selecting Y15 BP to represent middle age includes the following: (1) age distribution at Y15 (ie, 33 to 45 years of age) is separate from that at Y0 (18 to 30 years of age); (2) because CARDIA follow-up extends for 30 years at this point, use of follow-up after Y15 bisects the follow-up interval; and (3) the number of clinical events experienced after the Y20 examination was half as many as after the Y15 examination. We excluded those who had a CVD event or were censored before the Y15 examination. We used clinical characteristics at Y15 as covariates, in which we imputed missing data using multiple imputation chained equations with 20 iterations as described by Raghunathan et al (eFigure 1 in the Supplement). We then followed them up until they developed CVD or were censored. Time-to-event methods were used based on the Cox proportional hazards models with the time defined as the number of days from the Y15 examination to the first diagnosis of CVD events. All statistical analyses were performed with STATA software, version 12.1 (StataCorp). Statistical significance was defined as P < .05 using 2-sided tests.
Results
BP Measured at Y0 and CVD Risk
A total of 4880 participants participated in the study (mean [SD] age, 24.9 [3.6] years at Y0 and 25.0 [3.6] years at Y15; 2223 male [45.6%] at Y0 and 1800 [44.2%] at Y15; 2657 female [54.4%] at Y0 and 2277 [55.8%] at Y0; 2473 black individuals [50.7%] at Y0 and 1994 [48.9%] at Y15; and 2407 white individuals [49.3%] at Y0 and 2083 [51.1%] at Y15). Of the initial 5115 participants, we excluded 145 participants lost to follow-up (2.8%), 2 participants who underwent gender reassignment, and 1 participant who withdrew study consent. Of the remaining 4967 participants, 152 participants had missing covariates at baseline; Y2 examination data for 65 of these participants were substituted for baseline values (1 BP observation, 33 smoking observations, 11 BMI observations, 18 alcohol consumption observations, and 2 antihypertensive medication use observations) because there were no CVD events from the Y0 to Y2 examinations. The remaining participants were excluded, leaving a sample of 4880 participants for analysis (eFigure 1 in the Supplement).
Race-specific clinical characteristics of the included participants are given in Table 1. The SBP, MAP, and PP were higher in black individuals than white individuals at Y0, but the DBP was similar between races. The distribution of SBP and DBP at Y0 according to race is shown in eFigure 2 in the Supplement. In both races, male sex and higher BMI were associated with higher SBP, DBP, and MAP (eTable 1 and eTable 2 in the Supplement). The correlation between SBP and DBP is similar overall between black and white individuals (eTable 1 in the Supplement).
Table 1. Clinical Characteristics of Study Cohort by Race at Baselinea.
| Characteristic | Black Individuals (n = 2473) |
White Individuals (n = 2407) |
P Valueb |
|---|---|---|---|
| Age, y | 24.3 (3.8) | 25.4 (3.4) | <.001 |
| Men, No. (%) | 1088 (44.0) | 1135 (47.2) | .03 |
| Educational level, y | 13.1 (1.8) | 14.6 (2.4) | <.001 |
| Body mass indexc | 25.3 (5.7) | 23.6 (4.1) | <.001 |
| Smoking status, No. (%) | |||
| Current smokers | 835 (33.8) | 639 (26.5) | <.001 |
| Former smokers | 220 (8.9) | 433 (18.0) | |
| Never smokers | 1418 (57.3) | 1335 (55.5) | |
| A Priori Diet Quality Scored | 56.8 (10.4) | 68.8 (12.5) | <.001 |
| Fasting glucose, mg/dL | 82 (20) | 83 (13) | .12 |
| Total cholesterol, mg/dL | 177 (35) | 176 (32) | .41 |
| High-density lipoprotein cholesterol, mg/dL | 54 (13) | 52 (13) | <.001 |
| Hypertension, % | 5.4 | 2.8 | <.001 |
| Antihypertensive medication, % | 3.2 | 1.4 | <.001 |
| Pressures, mean (SD), mm Hg | |||
| SBP | 111.5 (11.0) | 109.3 (10.8) | <.001 |
| DBP | 68.9 (9.9) | 68.4 (9.2) | .07 |
| MAP | 83.1 (9.2) | 82.0 (8.8) | <.001 |
| Mid-BP | 90.2 (9.2) | 88.8 (9.0) | <.001 |
| PP | 42.7 (10.0) | 40.9 (8.9) | <.001 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555; total cholesterol and high-density lipoprotein cholesterol to millimoles per liter, multiply by 0.0259.
Data are presented as mean (SD) unless otherwise indicated.
P values were calculated by unpaired t test or χ2 test.
Calculated as weight in kilograms divided by height in meters squared.
A higher A Priori Diet Quality Score indicates better diet quality.
eFigure 3 in the Supplement shows the cause-specific incidence rate of cardiovascular outcomes, and the race-specific Kaplan-Meier cumulative incidence of CVD is shown in eFigure 4 in the Supplement. In black individuals, during a median follow-up of 24.5 years (49 201 person-years) (interquartile range [IQR], 15.7-25.0 years), 140 CVD events occurred (2.85 per 1000 person-years; 95% CI, 2.41-3.36). In white individuals, 70 CVD events occurred (1.37 per 1000 person-years; 95% CI, 1.09-1.73) during a median follow-up of 24.7 years (51 039 person-years) (IQR, 20.1-25.0). All CVD events occurred before 58 years of age.
With adjustments for covariates, results from Cox proportional hazards models suggested that both SBP and DBP, when considered alone, were positively associated with CVD risk in black individuals, whereas only DBP was associated in white individuals (Table 2). When SBP and DBP were considered jointly, SBP but not DBP retained statistical significance in black individuals, whereas DBP but not SBP was associated with CVD risk in white individuals. When MAP and PP were analyzed jointly, MAP was consistently associated with CVD in both races. Results were similar when A Priori Diet Quality Score was included as an adjustment factor (eTable 3 in the Supplement). In race-combined analyses, significant interactions were found between race and SBP (P = .02) and PP (P = .02) but not DBP (P = .052).
Table 2. Association Between Each BP Component at Y0 and CVD Risk: Race-Specific HRs and Changes in Model Fit and Discrimination for Incident CVD Eventsa.
| Component | Black Individuals (n = 2473) |
White Individuals (n = 2407) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) Per 1 SD of BP Component | Likelihood Ratio χ2b | AIC | BIC | C Statistics (95% CI)b | HR (95% CI) Per 1 SD of BP Component | Likelihood Ratio χ2a | AIC | BIC | C Statistics (95% CI)b | |
| Base model | NA | 104.62 | 2006.55 | 2076.31 | 0.694 (0.636-0.752) | NA | 93.71 | 987.22 | 1056.66 | 0.735 (0.650-0.820) |
| Base model plus single BP measurement (Y0) | ||||||||||
| SBP | 1.36 (1.14-1.61)c | 115.51d | 1997.66 | 2073.23 | 0.707 (0.642-0.772) | 1.16 (0.88-1.53) | 94.77 | 988.16 | 1063.38 | 0.733 (0.649-0.818) |
| DBP | 1.19 (0.99-1.41) | 108.48e | 2004.69 | 2080.26 | 0.699 (0.637-0.760) | 1.54 (1.17-2.03)c | 103.08c | 979.85 | 1055.07 | 0.746 (0.664-0.828) |
| MAP | 1.28 (1.08-1.51)e | 112.29 | 2000.87 | 2076.45 | 0.702 (0.638-0.766) | 1.42 (1.09-1.85)c | 104.46c | 982.47 | 1057.69 | 0.742 (0.659-0.824) |
| PP | 1.10 (0.95-1.28) | 106.21c | 2006.96 | 2082.53 | 0.697 (0.639-0.755) | 0.73 (0.54-0.99)e | 97.97e | 984.96 | 1060.18 | 0.742 (0.659-0.824) |
| Base model plus dual BP measurement (Y0) | ||||||||||
| Model 1 | ||||||||||
| SBP | 1.32 (1.09-1.61)b | 115.79c | 1999.38 | 2080.76 | 0.707 (0.642-0.772) | 0.82 (0.57-1.18) | 104.19c | 980.74 | 1061.75 | 0.748 (0.667-0.829) |
| DBP | 1.05 (0.88-1.26) | NA | NA | NA | NA | 1.74 (1.21-2.50)c | NA | NA | NA | NA |
| Model 2 | ||||||||||
| MAP | 1.31 (1.11-1.55)c | 115.79d | 1999.38 | 2080.77 | 0.707 (0.642-0.772) | 1.40 (1.08-1.83)e | 104.19c | 980.73 | 1061.74 | 0.748 (0.667-0.829) |
| PP | 1.17 (0.99-1.36) | NA | NA | NA | NA | 0.74 (0.54-1.01) | NA | NA | NA | NA |
Abbreviations: AIC, Akaike information criterion; BIC, Bayes information criteria; BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure; HR, hazard ratio; MAP, mean arterial pressure; NA, not applicable; PP, pulse pressure; SBP, systolic blood pressure; Y0, year 0.
Race-specific adjusted HRs (95% CIs) for risk of incident CVD with a 1-SD increment in SBP, DBP, MAP, and PP are shown. A 1-SD increment of each BP parameter at Y0 is as follows: SBP, 11 mm Hg; DBP, 10 mm Hg; MAP, 9 mm Hg; and PP, 10 mm Hg. Base model includes the following sociodemographic characteristics: age, sex, educational level, and study site (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). The following clinical characteristics were included at Y0: smoking, body mass index, fasting glucose level, total cholesterol and high-density lipoprotein cholesterol levels, and antihypertensive medication use. Statistical significance was defined as P < .05.
The differences from base model were statistically tested. The Harrell C was used to calculate C statistics.
P < .01.
P < .001.
P < .05.
The Y0 BP component demonstrating the best model fit (ie, likelihood ratio χ2, AIC, and BIC) and discriminative ability (ie, C statistic) to identify incident CVD was SBP in black individuals and DBP in white individuals (Table 2). These indexes were not improved when combining SBP with DBP in either race.
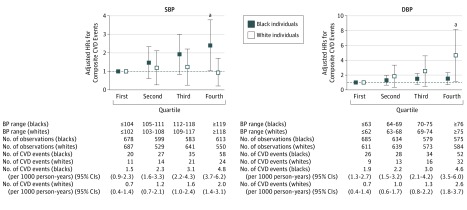
In both races, there was no evidence of interaction between each BP component and sex or baseline antihypertensive medication use in association with CVD risk. We repeated Cox proportional hazards analyses in those without receiving hypertensive medication at Y0, and results were similar (eTable 4 in the Supplement). Quartile analyses observed that, using a reference of the first quartile group of SBP (<105 mm Hg), CVD risk in the highest quartile group (SBP ≥119 mm Hg) was significant in black individuals but not in white individuals. The CVD risk in the highest quartile group of DBP (≥75 mm Hg), compared with the reference group (DBP <63 mm Hg), was significant in white individuals but not in black individuals (Figure 2).
Figure 2. Adjusted Hazard Ratios (HRs) for Risk of Composite Cardiovascular Disease (CVD) Events by Systolic Blood Pressure (SBP) or Diastolic Blood Pressure (DBP) Quartiles.
Race-specific adjusted HRs of composite CVD risk by quartiles of SBP or DBP. The first quartile group was defined as reference. As adjustment factors, sociodemographic characteristics (age, sex, educational level, and study site), clinical characteristics at baseline (Y0) (smoking, body mass index, fasting glucose level, total cholesterol and high-density lipoprotein cholesterol levels, and antihypertensive medication use), and DBP at Y0 (left figure) or SBP at Y0 (right figure) were used. Error bars indicate 95% CIs.
aP < .05.
BP Measured at Y15 and CVD Risk
Of the 4967 participants included in the analysis on baseline BP (Y0) values, we excluded 87 participants who had a CVD event and 803 who were censored before the Y15 examination. Among the 4077 remaining participants, 3547 attended the Y15 examination, of whom 3534 had BP measurements. We imputed BP measurements for the 13 participants who attended the Y15 examination and who had no BP measurement and for the 530 participants who did not attend the Y15 examination but had at least 1 follow-up contact after the Y15 examination, leaving a sample of 4077 participants for analysis. Their mean (SD) age was 40.2 (3.6) years, 56% were women, and 49% were black (eTable 5 in the Supplement). Approximately 8% of the entire cohort and 46% of patients with hypertension reported using antihypertensive medications. Mean (SD) BP using imputation was similar to that without imputation (eTable 6 in the Supplement). In black individuals, during a median follow-up of 9.7 years (16 241 person-years) (IQR, 5.6-10.0 years), 87 CVD events occurred (5.36 per 1000 person-years; 95% CI, 4.34-6.61). In white individuals, 44 CVD events occurred (2.49 per 1000 person-years; 95% CI, 1.86-3.35) during a median follow-up of 9.8 years (17 639 person-years) (IQR, 5.9-10.1 years). Results from Cox proportional hazards models suggested that SBP was consistently associated with CVD risk in both races when SBP and DBP were considered singly and jointly (Table 3), whereas the association of DBP with CVD incidence was attenuated with adjustment for SBP. The PP was positively associated with CVD risk in black individuals only. Results were similar when we excluded those receiving hypertensive medication at Y15 (eTable 7 in the Supplement). Analyses without imputing missing BP at Y15 (n = 3534) had similar results (eTable 8 in the Supplement).
Table 3. Association Between Each BP Component at Y15 and CVD Risk: Race-Specific HRs for Incident CVD Eventsa.
| Component | HRs (95% CI) per 1 SD of BP Component | |
|---|---|---|
| Black Individuals (n = 1994) |
White Individuals (n = 2083) |
|
| Single BP measurement (Y15) | ||
| SBP | 1.61 (1.35-1.93)b | 1.85 (1.30-2.61)c |
| DBP | 1.47 (1.18-1.82)c | 1.63 (1.16-2.29) |
| MAP | 1.56 (1.29-1.88)b | 1.78 (1.28-2.49)c |
| PP | 1.40 (1.17-1.68)b | 1.26 (0.91-1.74) |
| Dual BP measurements (Y15) | ||
| Model 1 | ||
| SBP | 1.64 (1.25-2.16)b | 1.67 (1.02-2.69)d |
| DBP | 0.97 (0.71-1.33) | 1.16 (0.73-1.84) |
| Model 2 | ||
| MAP | 1.45 (1.18-1.78)b | 1.75 (1.25-2.44)c |
| PP | 1.26 (1.03-1.53)d | 1.20 (0.88-1.65) |
Abbreviations: BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure; HR, hazard ratio; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; Y15, year 15.
Race-specific adjusted HRs (95% CIs) for risk of incident CVD with a 1-SD increment in SBP, DBP, MAP, and PP are shown. A 1-SD increment of each BP parameter at Y15 is as follows: SBP, 15 mm Hg; DBP, 12 mm Hg; MAP, 12 mm Hg; and PP, 10 mm Hg. In single BP measurement, each BP component was analyzed separately. In dual BP measurements, SBP and DBP or MAP and PP were analyzed jointly. As adjustment factors, all models include the following sociodemographic characteristics: age, sex, educational level, and study site (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). The following clinical characteristics were included at Y15: fasting glucose level, total cholesterol and high-density lipoprotein cholesterol levels, and antihypertensive medication use. Statistical significance was defined as P < .05.
P < .001.
P < .01.
P < .05.
Discussion
In this community-based cohort of young adults at baseline followed up for up to 28 years, BP components that best indicated CVD risk varied by race and age. Twice as many premature (before 58 years of age) CVD events occurred in black individuals compared with white individuals. In young adults with a mean age of 25 years (at Y0), SBP was the most robust indicator of premature CVD risk in blacks, whereas DBP performed better than SBP in identifying premature CVD in white individuals. Young black adults with prehypertensive SBP levels are at increased risk for CVD events, independent of their DBP levels. Conversely, white individuals with prehypertensive DBP levels are at increased risk for CVD events, independent of their SBP levels. In middle-aged adults with a mean age of 40 years (at Y15), SBP instead of DBP better identified incident CVD in both races. The PP at Y15 was positively associated with CVD risk in black individuals but not white individuals.
Several potential mechanisms may underlie the observed racial differences. First, different BP components reflect distinctive hemodynamics and pathophysiologic mechanisms. Both MAP and DBP, at least up to 50 years of age, reflect a steady state load of BP and are representative of resistant vessel structure and function alterations. The PP is a measure of pulsatile pressure load and representative of large arterial (aortic) stiffness. The SBP is an integrated measure of steady and pulsatile pressure load. The association between SBP at Y0 and CVD risk in black individuals suggests that increased pulsatile pressure load on vascular structures is an important contributor to incident CVD in young black adults. By contrast, the association between DBP at Y0 and CVD risk in white individuals suggests that higher steady flow (attributable to higher vascular resistance) instead of pulsatile components of BP contributes to incident CVD in young whites. Second, SBP and PP, both reflecting aortic stiffness, were higher in blacks than whites at Y0. In CARDIA, SBP increased continuously from the age of 20 to 35 years in blacks, whereas in whites, SBP remained relatively constant until the age of 35 years. Even at a young age, blacks might have developed aortic stiffness to a greater extent than whites. Increased SBP in young blacks may be indicative of early vascular damage, reflecting not only measured (ie, BP) but also unmeasured risk factors (eg, environmental factors and sociopsychological stress) during a lifetime. Third, aortic SBP, reflecting the pressure experienced by perfused target organs (eg, the brain and heart), is reportedly lower up to 40 mm Hg than the corresponding value of brachial SBP in young adults. Brachial SBP may give an inaccurate estimation of the true aortic BP in young adults. This so-called pressure amplification from the ascending aorta to the brachial artery is smaller, partly because of earlier peripheral wave reflection, in young black than white adults. Indeed, higher aortic SBP is observed in young black compared with white men despite their brachial SBPs being similar. Brachial SBP might reflect aortic SBP more precisely in young black than white adults. Lastly, SBP was higher in blacks than whites, but DBP was similar between races at Y0. The informativeness of risk factors in models may be determined not only by their association with outcomes but also by their prevalence in the population.
Investigators from the FHS found that, among white individuals younger than 50 years, DBP performs better than SBP for identifying CVD risk, whereas SBP and PP instead of DBP better identify CVD in those 50 to 60 years. A shift from DBP to SBP as an indicator of adverse cardiovascular outcomes was observed at a younger age in whites of our study (ie, 40 years of age) than the FHS. Some mechanisms behind the inconsistency are suggested. First, BP in our study was assessed in 1985 and 2000, whereas the FHS took place in 1970, before the onset of the obesity epidemic in the United States. The results of the FHS may not account for the effect of that epidemic, including lifestyle and dietary habit changes. Second, the evaluation of BP was from only the baseline measurement in the FHS. In this study, we found a change with age from DBP to SBP as the primary indicator within the same people in whites. Third, the assessed outcomes were not consistent between the FHS and CARDIA. Nevertheless, caution is required in interpreting the Y15 BP–CVD risk association in whites. The BP component at Y15 that demonstrated the best model fit (ie, likelihood ratio χ2, AIC, and BIC) was SBP in whites. However, the C statistic change by adding SBP to the base model was modest with wide range in whites, which may be partly because of their low number of events. Whether SBP can best indicate incident CVD events in whites at approximately 40 years of age will need to be confirmed in larger studies or cohorts.
C statistic changes by adding SBP at Y0 to base models in black adults or by adding DBP in white adults were nonsignificant. However, these changes were statistically significant but modest in those who were not taking antihypertensive medication. This finding suggests that (1) the discriminative ability of BP might be affected by the presence of medication use, (2) large HRs (or relative risks) are required to achieve clinically meaningful C statistic change, and (3) there was a narrow distribution of BP at Y0 in this young healthy adult population. Discrimination overall is better in whites than blacks across all models. Traditional risk factors appear to be less predictive of premature CVD in blacks than whites. Even when the HRs are similar in blacks and whites, racial differences in the prevalence of risk factors can affect variables for model selection. This finding may contribute to differences in the overall performance of the risk model when it is applied to blacks vs whites.
Strengths and Limitations
Strengths of this study include the large, community-based biracial cohort; adjudication of suspected cardiovascular outcomes by a panel of physicians using detailed evaluation criteria; high retention; and the standardized data collection protocols and rigorous quality control in CARDIA. All events were premature CVD events, an important measure of a population's health but often understudied in older cohorts. However, there are limitations. First, possible residual confounding may be affecting the BP–CVD risk associations. Sodium intake may be one such example. Although a high A Priori Diet Quality Score tends to indicate lower sodium intake, the score itself cannot capture individual sodium intake well. Second, participants taking antihypertensive medication were included, which potentially leads to an underestimation of the true association between BP and CVD risk. However, results were similar when participants taking antihypertensive medications were excluded. Third, antihypertensive medication use by hypertensive CARDIA participants at Y15 might be higher than levels of use in the general population. Although the reason is unclear, the difference might result from research participation effects (ie, the Hawthorne effect), and participants in CARDIA might not be representative of the US general population. Fourth, our results may not be generalizable to other ethnic groups (eg, Asian and Hispanic).
Conclusions
Our data question the classic view that DBP is more predictive of CVD events than SBP in all individuals younger than 50 years. Arterial aging is a complex and heterogeneous process beginning early in life; thus, one size may not fit all in the clinical management of BP for young adults. Different BP components reflect distinctive hemodynamics; therefore, refining BP components associated with CVD could lead to an optimization of diagnostic and therapeutic strategies. Specifically, for young black adults with high SBP, therapeutic strategies aimed at improving or slowing aortic stiffness, such as lifestyle changes, including dietary interventions (eg, reducing salt and weight loss) and using renin-angiotensin-aldosterone system inhibitors, may be beneficial in preventing premature CVD events. In contrast, for young white adults with high DBP, therapeutic strategies aimed at reducing peripheral vascular resistance (eg, exercise and using calcium channel blockers) may be efficacious. The hypothesis will need to be confirmed in interventional trials, with consideration of all the complex issues at play, including cost-effectiveness, utility (including potential adverse events), and patient perspectives.
eMethods. The Coronary Artery Risk Development in Young Adults (CARDIA) Study Methods
eFigure 1. Flowchart: Sample for the Analyses, CARDIA
eFigure 2. Histogram of BP at Y0 or Y15 in CARDIA
eFigure 3. Cause-Specific Incidence Rates of Cardiovascular Outcomes by Race
eFigure 4. Kaplan-Meier Curves of the Cumulative Incidence of CVD Event by Race
eTable 1. Race-Specific Correlations of BP Components With the Demographic Variables and Clinical Characteristics at Y0
eTable 2. Race-Specific Correlations of BP Components With the Demographic Variables and Clinical Characteristics at Y0
eTable 3. The Association Between Each BP Component at Y0 and CVD Risk: Race-Specific Hazard Ratios for Incident CVD Events
eTable 4. The Association Between Each BP Component at Y0 and CVD Risk in Participants Without Receiving Antihypertensive Medication at Y0: Race-Specific Hazard Ratios and Changes in Model Fit and Discrimination for Incident CVD Events
eTable 5. Clinical Characteristics of Study Cohort by Race at Y15
eTable 6. Mean (SD) of BP at Y15 With or Without Imputation
eTable 7. The Association Between Each BP Component at Y15 and CVD Risk in Participants Without Receiving Antihypertensive Medication at Y15: Race-Specific Hazard Ratios for Incident CVD Events
eTable 8. The Association Between Each BP Component at Y15 (Without Multiple Imputation) and CVD Risk: Race-Specific Hazard Ratios for Incident CVD Events (n = 3534)
References
- 1.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50(22):2128-2132. [DOI] [PubMed] [Google Scholar]
- 2.Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79(17):1781-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995-2008. Ann Neurol. 2011;70(5):713-721. [DOI] [PubMed] [Google Scholar]
- 4.US Renal Data System (USRDS) 2015 Annual Data Report: Volume 1: Chronic Kidney Disease in the United States and Volume 2: End-Stage Renal Disease in the United States. http://www.usrds.org/2015/view/Default.aspx. Accessed March 15, 2016.
- 5.Nguyen QC, Tabor JW, Entzel PP, et al. Discordance in national estimates of hypertension among young adults. Epidemiology. 2011;22(4):532-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suglia SF, Clark CJ, Gary-Webb TL. Adolescent obesity, change in weight status, and hypertension: racial/ethnic variations. Hypertension. 2013;61(2):290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannel WB, Wilson PWF. Cardiovascular risk factors and hypertension In: Izzo JL, Sica DA, Black HR, eds. Hypertension Primer: The Essentials of High Blood Pressure, Basic Science, Population Science and Clinical Management. 4th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008:244-248. [Google Scholar]
- 8.Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103(9):1245-1249. [DOI] [PubMed] [Google Scholar]
- 9.Khattar RS, Swales JD, Dore C, Senior R, Lahiri A. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation. 2001;104(7):783-789. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Wei FF, Thijs L, et al. ; International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators . Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation. 2014;130(6):466-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamler J, Neaton JD, Wentworth DN. Blood pressure (systolic and diastolic) and risk of fatal coronary heart disease. Hypertension. 1989;13(5)(suppl):I2-I12. [DOI] [PubMed] [Google Scholar]
- 12.Sundström J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ. 2011;342:d643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. [DOI] [PubMed] [Google Scholar]
- 14.Su S, Wang X, Kapuku GK, et al. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension. 2014;64(1):201-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris AA, Patel RS, Binongo JN, et al. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc. 2013;2(2):e002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffernan KS, Jae SY, Wilund KR, Woods JA, Fernhall B. Racial differences in central blood pressure and vascular function in young men. Am J Physiol Heart Circ Physiol. 2008;295(6):H2380-H2387. [DOI] [PubMed] [Google Scholar]
- 17.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105-1116. [DOI] [PubMed] [Google Scholar]
- 18.National Heart Lung and Blood Institute Coronary Artery Risk Development in Young Adults (CARDIA) Study Manuals of Operation. 1985. http://www.cardia.dopm.uab.edu/exam-materials2/manual-of-operations/year-0. Accessed July 21, 2016.
- 19.Luepker RV, Apple FS, Christenson RH, et al. ; AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute . Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543-2549. [DOI] [PubMed] [Google Scholar]
- 20.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST (Trial of Org 10172 in Acute Stroke Treatment). Stroke. 1993;24(1):35-41. [DOI] [PubMed] [Google Scholar]
- 21.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2):152-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. [DOI] [PubMed] [Google Scholar]
- 23.Newson RB. Comparing the predictive powers of survival models using Harrell’s C or Somers’ D. Stata J. 2010;10(3):339-358. [Google Scholar]
- 24.Raghunathan TE, Lepkowski JM, van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27(1):85-95. [Google Scholar]
- 25.Vlachopoulos C, O’Rourke M. Diastolic pressure, systolic pressure, or pulse pressure? Curr Hypertens Rep. 2000;2(3):271-279. [DOI] [PubMed] [Google Scholar]
- 26.Avolio AP, Van Bortel LM, Boutouyrie P, et al. Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension. 2009;54(2):375-383. [DOI] [PubMed] [Google Scholar]
- 27.Safar ME. Arterial aging—hemodynamic changes and therapeutic options. Nat Rev Cardiol. 2010;7(8):442-449. [DOI] [PubMed] [Google Scholar]
- 28.Franklin SS, Gustin W IV, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308-315. [DOI] [PubMed] [Google Scholar]
- 29.Nichols WW, O’Rourke MF. McDonald’s Blood Flow in Arteries. Philadelphia, PA: Lea & Febiger; 1990. [Google Scholar]
- 30.Berne RM, Levy MN. Cardiovascular Physiology. 6th ed St Louis, MO: Mosby Year Book; 1992:135-151. [Google Scholar]
- 31.Kishi S, Teixido-Tura G, Ning H, et al. Cumulative Blood pressure in early adulthood and cardiac dysfunction in middle age: the CARDIA study. J Am Coll Cardiol. 2015;65(25):2679-2687. [DOI] [PubMed] [Google Scholar]
- 32.McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35(26):1719-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001;38(6):1461-1466. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121(15):1768-1777. [DOI] [PubMed] [Google Scholar]
- 35.Pencina MJ, D’Agostino RB Sr. Evaluating discrimination of risk prediction models: the C statistic. JAMA. 2015;314(10):1063-1064. [DOI] [PubMed] [Google Scholar]
- 36.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290(2):199-206. [DOI] [PubMed] [Google Scholar]
- 37.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46(3):454-462. [DOI] [PubMed] [Google Scholar]
- 39.Sacks FM, Svetkey LP, Vollmer WM, et al. ; DASH-Sodium Collaborative Research Group . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3-10. [DOI] [PubMed] [Google Scholar]
- 40.Shahin Y, Khan JA, Chetter I. Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: a meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis. 2012;221(1):18-33. [DOI] [PubMed] [Google Scholar]
- 41.Flack JM, Sica DA, Bakris G, et al. ; International Society on Hypertension in Blacks . Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56(5):780-800. [DOI] [PubMed] [Google Scholar]
- 42.Cléroux J, Kouamé N, Nadeau A, Coulombe D, Lacourcière Y. Aftereffects of exercise on regional and systemic hemodynamics in hypertension. Hypertension. 1992;19(2):183-191. [DOI] [PubMed] [Google Scholar]
- 43.Hansson L, Zanchetti A, Carruthers SG, et al. ; HOT Study Group . Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755-1762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. The Coronary Artery Risk Development in Young Adults (CARDIA) Study Methods
eFigure 1. Flowchart: Sample for the Analyses, CARDIA
eFigure 2. Histogram of BP at Y0 or Y15 in CARDIA
eFigure 3. Cause-Specific Incidence Rates of Cardiovascular Outcomes by Race
eFigure 4. Kaplan-Meier Curves of the Cumulative Incidence of CVD Event by Race
eTable 1. Race-Specific Correlations of BP Components With the Demographic Variables and Clinical Characteristics at Y0
eTable 2. Race-Specific Correlations of BP Components With the Demographic Variables and Clinical Characteristics at Y0
eTable 3. The Association Between Each BP Component at Y0 and CVD Risk: Race-Specific Hazard Ratios for Incident CVD Events
eTable 4. The Association Between Each BP Component at Y0 and CVD Risk in Participants Without Receiving Antihypertensive Medication at Y0: Race-Specific Hazard Ratios and Changes in Model Fit and Discrimination for Incident CVD Events
eTable 5. Clinical Characteristics of Study Cohort by Race at Y15
eTable 6. Mean (SD) of BP at Y15 With or Without Imputation
eTable 7. The Association Between Each BP Component at Y15 and CVD Risk in Participants Without Receiving Antihypertensive Medication at Y15: Race-Specific Hazard Ratios for Incident CVD Events
eTable 8. The Association Between Each BP Component at Y15 (Without Multiple Imputation) and CVD Risk: Race-Specific Hazard Ratios for Incident CVD Events (n = 3534)