Key Points
Question
Does fenofibrate reduce cardiovascular disease risk in statin-treated patients with type 2 diabetes?
Findings
In this posttrial follow-up of the Action to Control Cardiovascular Risk in Diabetes Lipid Study, fenofibrate therapy was associated with reduced cardiovascular disease in study participants with dyslipidemia, defined as triglyceride levels greater than 204 mg/dL and high-density lipoprotein cholesterol levels less than 34 mg/dL.
Meaning
Extended follow-up of ACCORD-lipid trial participants confirms the original neutral effect of fenofibrate in the overall study cohort; the continued observation of heterogeneity of treatment response by baseline lipids suggests that fenofibrate therapy may reduce CVD in patients with diabetes with hypertriglyceridemia and low high-density lipoprotein cholesterol.
Abstract
Importance
Patients with type 2 diabetes are at high risk of cardiovascular disease (CVD) in part owing to hypertriglyceridemia and low high-density lipoprotein cholesterol. It is unknown whether adding triglyceride-lowering treatment to statin reduces this risk.
Objective
To determine whether fenofibrate reduces CVD risk in statin-treated patients with type 2 diabetes.
Design, Setting, and Participants
Posttrial follow-up of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid Study between July 2009 and October 2014; 5 years of follow-up were completed for a total of 9.7 years at general community and academic outpatient research clinics in the United States and Canada. Of the original 5518 ACCORD Lipid Trial participants, 4644 surviving participants were selected based on the presence of type 2 diabetes and either prevalent CVD or CVD risk factors and high-density lipoprotein levels less than 50 mg/dL (<55 mg/dL for women and African American individuals).
Interventions
Passive follow-up of study participants previously treated with fenofibrate or masked placebo.
Main Outcomes and Measures
Occurrence of cardiovascular outcomes including primary composite outcome of fatal and nonfatal myocardial infarction and stroke in all participants and in prespecified subgroups.
Results
The 4644 follow-on study participants were broadly representative of the original ACCORD study population and included significant numbers of women (n = 1445; 31%), nonwhite individuals (n = 1094; 21%), and those with preexisting cardiovascular events (n = 1620; 35%). Only 4.3% of study participants continued treatment with fenofibrate following completion of ACCORD. High-density lipoprotein and triglyceride values rapidly equalized among participants originally randomized to fenofibrate or placebo. Over a median total postrandomization follow-up of 9.7 years, the hazard ratio (HR) for the primary study outcome among participants originally randomized to fenofibrate vs placebo (HR, 0.93; 95% CI, 0.83-1.05; P = .25) was comparable with that originally observed in ACCORD (HR, 0.92; 95% CI, 0.79-1,08; P = .32). Despite these overall neutral results, we continued to find evidence that fenofibrate therapy effectively reduced CVD in study participants with dyslipidemia, defined as triglyceride levels greater than 204 mg/dL and high-density lipoprotein cholesterol levels less than 34 mg/dL (HR, 0.73; 95% CI, 0.56-0.95).
Conclusions and Relevance
Extended follow-up of ACCORD-lipid trial participants confirms the original neutral effect of fenofibrate in the overall study cohort. The continued observation of heterogeneity of treatment response by baseline lipids suggests that fenofibrate therapy may reduce CVD in patients with diabetes with hypertriglyceridemia and low high-density lipoprotein cholesterol. A definitive trial of fibrate therapy in this patient population is needed to confirm these findings.
Trial Registration
clinicaltrials.gov Identifier: NCT00000620.
This follow-up study of the ACCORD lipid trial examines whether fenofibrate is associated with reduced cardiovascular risk in statin-treated patients with type 2 diabetes.
Introduction
Cardiovascular disease (CVD) risk is increased in patients with type 2 diabetes, particularly in older patients and those with other risk factors for CVD. Compared with their counterparts without diabetes, the relative risk of fatal and nonfatal CVD events can be 2- to 3-fold and 3- to 4-fold higher, respectively, in men and women with diabetes.1,2,3,4 Increased risk of CVD in type 2 diabetes is attributable in part to the high prevalence of associated risk factors including hypertension and diabetic dyslipidemia, the latter characterized by elevated plasma triglyceride levels and low plasma levels of high-density lipoprotein cholesterol (HDL-C).5,6 The Action to Control Cardiovascular Risk in Diabetes (ACCORD) was a randomized, multicenter, partial double 2 × 2 factorial trial that enrolled 10 251 individuals with type 2 diabetes mellitus who were at high risk of CVD events. The ACCORD study tested the effects of intensive control of blood glucose, blood pressure, and plasma lipids on CVD risk in patients with type 2 diabetes.7
The ACCORD-Lipid was conducted in a subset of 5518 ACCORD participants and tested the hypothesis that combination statin-fibrate therapy would more effectively reduce CVD risk compared with statin alone in patients with type 2 diabetes. Although triglycerides and HDL-C are widely recognized as biomarkers of CVD risk,8 it is uncertain whether pharmacologic therapy directed toward lowering triglyceride levels and raising HDL-C effectively reduces that risk, particularly when added to statin therapy. In ACCORD-Lipid, following a mean 4.7 years of treatment, the rate of occurrence of the composite primary outcome measure of myocardial infarction, stroke, and fatal CVD was not significantly lower in participants randomized to fenofibrate therapy compared with those randomized to placebo.9 There were also no significant differences between the 2 study groups for any of the prespecified secondary outcomes, including fatal cardiovascular events, nonfatal MI, or nonfatal stroke.9 In contrast to these findings, prespecified subgroup analyses in ACCORD-Lipid detected significant heterogeneity in treatment effect by baseline lipids suggesting benefit for those with dyslipidemia, predefined as having both high triglycerides and low HDL-C levels at baseline. Heterogeneity in fenofibrate response was also noted by sex, with evidence of benefit for men vs possible harm in women.
The neutral overall CVD outcomes of the glycemia, blood pressure, and lipid treatment arms of ACCORD, along with the findings of heterogeneity of the effect of fenofibrate on cardiovascular outcomes in ACCORD-Lipid,9,10,11,12 supported the need for additional follow-up of participants to detect emergence of long-term “legacy” effects of the interventions and to explore the findings of heterogeneity by baseline dyslipidemia and sex. The ACCORD Follow-On Study (ACCORDION) was designed and conducted for this purpose. In this study, we describe the outcome of extended observational follow-up of ACCORD-Lipid participants in ACCORDION. The extended follow-up findings of the ACCORD blood pressure and glycemia intervention groups have been or will be reported separately.13
Methods
ACCORD and ACCORDION Study Design, Eligibility, and Conduct
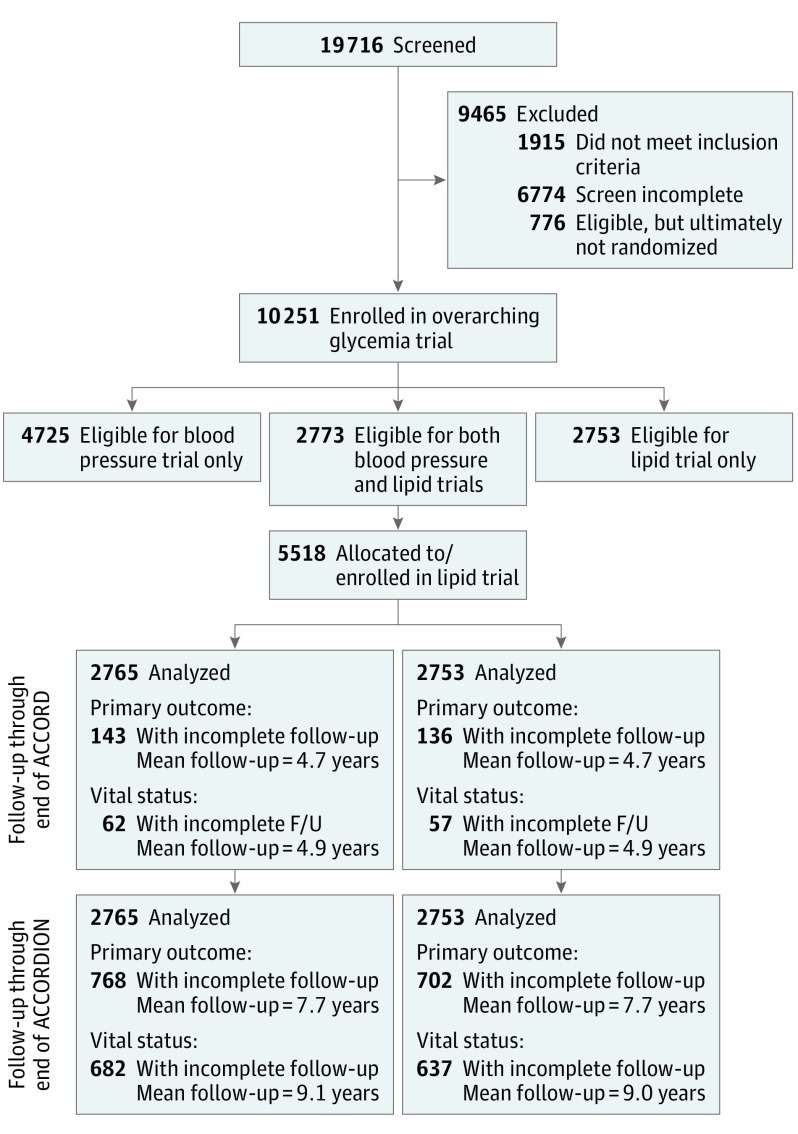
The rationale, design, and primary results of ACCORD were previously reported.9,10,11,12,14 Briefly, all participants underwent 2 sequential randomizations, the first to intensive vs standard glucose-lowering therapy in the overarching glycemia trial and the second to either intensive vs standard blood pressure or lipid therapy in the ACCORD-Blood Pressure or ACCORD-Lipid trials, respectively, in a partial double 2 × 2 design (Figure 1). The primary outcome for all 3 trials was the first occurrence of a nonfatal myocardial infarction (MI), nonfatal stroke, or death from a cardiovascular cause. Participants were recruited from 77 clinical sites across the United States and Canada between January 2001 and October 2005. Follow-up of ACCORD ended in June of 2009.
Figure 1. Action to Control Cardiovascular Risk in Diabetes (ACCORD)/ACCORD Follow-On Study (ACCORDION) CONSORT Diagram.
In the ACCORD study, eligible participants underwent 2 sequential randomizations, the first to intensive vs standard glycemia therapy followed by a second randomization to intensive vs standard blood pressure or lipid treatment in a double 2 × 2 factorial design. In the ACCORD lipid trial, participants were randomized to either fenofibrate or placebo on a background of statin therapy. Following completion of ACCORD, a total of 4644 surviving Lipid trial participants agreed to extended passive (nontreatment) follow-up in ACCORDION. Rates of occurrence of cardiovascular end points during the original study and during extended follow-up were assessed in all study participants with censoring for the last date of follow-up.
The ACCORD-Lipid trial, conducted in 5518 participants, was a randomized, placebo-controlled, double-blind treatment arm of ACCORD in which all participants received simvastatin to attain contemporary guideline-based low-density lipoprotein cholesterol (LDL-C) treatment goals7,15 and were randomly assigned to receive either fenofibrate or matched placebo. In addition to fulfilling the overall ACCORD eligibility criteria, participants were specifically eligible for ACCORD-Lipid if they also met the following: (1) LDL-C levels between 60 mg/dL and 180 mg/dL (to convert to millimoles per liter, multiply by 0.0259), inclusive; (2) HDL-C levels less than 55 mg/dL for women and African American individuals less than 50 mg/dL for all other groups (to convert to millimoles per liter, multiply by 0.0259); and (3) triglyceride levels less than 750 mg/dL if not receiving a lipid medication or less than 400 mg/dL if receiving a lipid medication (to convert to millimoles per liter, multiply by 0.0113). All participants provided written informed consent. Open-labeled simvastatin therapy began at the randomization visit, and the dose was modified over time in response to changing guidelines.15 The masked fenofibrate/placebo medication was fenofibrate was 160 mg/d in participants with normal renal function and 48 mg/d for those with an estimated glomerular filtration rate less than 50 mL/min/1.73 M.215
The ACCORD closeout visits were completed by June 2009. Following approval by the coordinating center (Wake Forest University) and participating clinical site institutional review board approvals, consenting participants were invited at these final trial visits to participate in the posttrial, nontreatment, observation-only ACCORDION study. Participant contacts were scheduled approximately every 6 months. These consisted of 2 in-clinic with 4 additional telephone visits annually. Information was collected regarding CVD events, hospitalizations, and medication usage. In-clinic visits also included a physical examination and, at the first and last visits, collection of urine and blood samples for analysis, a standardized electrocardiogram recording, and health-related quality of life data. Follow-up ended on October 31, 2014, or 60 months post-ACCORD, for a total of 5 years of posttrial observation. More detailed information can be found in the ACCORDION trial protocol (Supplement 2). A complete listing of the ACCORD/ACCORDION study group is provided in Supplement 1.
Prespecified Outcomes, Subgroups, Event Ascertainment
The prespecified primary outcome for ACCORDION-Lipid was the same as for ACCORD: the first postrandomization occurrence of a major cardiovascular event, specifically nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes.7 Secondary outcomes included an expanded composite macrovascular outcome, a major coronary heart disease event, nonfatal myocardial infarction, nonfatal stroke, total fatal/nonfatal stroke, all-cause mortality, cardiovascular mortality, total fatal/nonfatal congestive heart failure, and CVD-free survival. The consistency of intervention effects was also examined across the same prespecified baseline subgroups examined in ACCORD including sex, age, race/ethnicity, baseline CVD history, hemoglobin A1c, glycemia treatment assignment, tertiles of LDL-C, HDL-C ,and triglyceride, as well as in those with and without dyslipidemia at baseline (defined as the combination of the highest tertile of triglyceride and lowest tertile of HDL-C).
Based on findings of concordance between outcomes reported by investigators and adjudication of outcomes by a centralized endpoint committee in ACCORD (eTable 1 in Supplement 3) The ACCORDION analyses were conducted using outcomes reported by site investigators during both the active (trial) and passive follow-up period, regardless of the original adjudication classification.
Statistical Analysis
Statistical analyses were done by the ACCORDION Coordinating Center using SAS, version 9.4 (SAS Institute), according to a prespecified plan that was finalized before any analyses began. A nominal 2-tailed level of significance of P < .05 was used for all analyses without adjustment for multiple testing.
Baseline characteristics of participants enrolled in ACCORD-Lipid and a comparison of those who consented to the posttrial passive follow-up were summarized using means, standard deviations, and percentages. Effects of the original interventions on lipid levels during the active treatment phase and subsequent follow-up were estimated by calculating the mean lipid levels and 95% confidence intervals at follow-up visits by treatment group from the date of randomization through the end of the trial and then beyond through the end of ACCORDION. Follow-up for each participant was defined as the time from randomization until the last date for which the participant’s health status was available (Figure 1). All comparisons of intervention groups were performed according to the intention-to-treat principle.16
Analyses were conducted using both the original mean 4.7 years of treatment (ACCORD) and with addition of the 5 years of passive posttrial follow-up in ACCORDION, for a mean of 9.7 years of follow-up. The number and annual percentage of participants who had a postrandomization event was determined using Kaplan-Meier estimates. Cox proportional hazards regression analyses were used to estimate the long-term effect of allocation to either fenofibrate or placebo on the primary and secondary outcomes, using a χ2 statistic from a likelihood ratio test obtained from proportional hazards models with and without the term for intervention arm. Hazard ratios and 95% confidence intervals were calculated after accounting for variables that were prespecified in the prior ACCORDION analyses. Analyses were conducted on all 5518 ACCORD-Lipid trial participants with censoring of outcomes on the date of the last available information. The consistency of the effect of the study group assignment of the primary outcome and mortality within prespecified subgroups was assessed with the use of statistical tests of interactions between the treatment effect and the subgroup within the Cox models.
Results
Of the original 5518 men and women enrolled in the ACCORD-Lipid Trial,9 4644 provided written consent to be followed up during the posttrial period, representing 90% of surviving participants. The baseline characteristics at the time of entry into ACCORD among those consenting to participate in ACCORDION were similar to those of the entire original ACCORD-Lipid cohort including plasma lipoproteins and prevalence of preventive therapies such as statins (Table 1). In contrast, the 874 ACCORD participants who did not consent to post-ACCORD follow-up, including those who died during ACCORD, were older, more likely to be nonwhite, and more likely to have had a prior cardiovascular event, a lower prevalence of statin therapy, and a higher LDL-C on entry into ACCORD (Table 1).
Table 1. Comparison of ACCORD Baseline Characteristics Between Those Who Consented for Post-ACCORD Follow-up and Those Who Did Not Consent.
| Baseline Characteristic | All Lipid Trial Participants (n = 5518) | Consented for Post-ACCORD Follow-up (n = 4644) | Did Not Consent for Post-ACCORD Follow-up (n = 874) | Difference (n = 5518) | P Value for Difference |
|---|---|---|---|---|---|
| Age, mean (SD), y | 62.3 (6.8) | 62.0 (6.6) | 63.9 (7.5) | −1.9 | <.001 |
| Female sex, No./total No. (%) | 1694/5518 (30.7) | 1445/4644 (31.1) | 249/874 (28.5) | 2.6 | .12 |
| Race/ethnicity,a No./total No. (%) | |||||
| White | 3612/5518 (65.5) | 3067/4644 (66.0) | 545/874 (62.4) | 3.6 | <.001 |
| Black | 826/5518 (15.0) | 655/4644 (14.1) | 171/874 (19.6) | −5.5 | |
| Hispanic | 407/5518 (7.4) | 339/4644 (7.3) | 68/874 (7.8) | −0.5 | |
| Education, No./total No. (%) | |||||
| Less than high school | 750/5515 (13.6) | 588/4641 (12.7) | 162/874 (18.5) | −5.8 | <.001 |
| High school graduate or GED | 1433/5515 (26.0) | 1184/4641 (25.5) | 249/874 (28.5) | −3.0 | |
| Some college | 1827/5515 (33.1) | 1537/4641 (33.1) | 290/874 (33.2) | −0.1 | |
| College degree or higher | 1505/5515 (27.3) | 1332/4641 (28.7) | 173/874 (19.8) | 8.9 | |
| Previous cardiovascular event, No./total No. (%) | 2016/5518 (36.5) | 1620/4644 (34.9) | 396/874 (45.3) | −10.4 | <.001 |
| Previous congestive heart failure, No./total No. (%) | 291/5508 (5.3) | 198/4644 (4.3) | 93/864 (10.8) | −6.5 | <.001 |
| Cigarette-smoking status, No./total No. (%) | |||||
| Current | 793/5510 (14.4) | 640/4638 (13.8) | 153/872 (17.5) | −3.7 | <.001 |
| Former | 2546/5510 (46.2) | 2121/4638 (45.7) | 425/872 (48.7) | −3.0 | |
| Never | 2161/5510 (39.2) | 1867/4638 (40.3) | 294/872 (33.7) | 6.6 | |
| Weight, mean (SD), kg | 94.8 (18.7) | 94.9 (18.7) | 94.7 (18.4) | 0.2 | .84 |
| Body mass index, mean (SD)b | 32.3 (5.4) | 32.3 (5.4) | 32.4 (5.4) | −0.1 | .72 |
| Blood pressure, mean (SD), mm Hg | |||||
| Systolic | 133.9 (17.8) | 133.4 (17.5) | 136.6 (6.6) | −3.2 | <.001 |
| Diastolic | 74.0 (10.8) | 73.9 (10.7) | 74.2 (11.3) | −0.3 | .45 |
| Medications, No./total No. (%) | |||||
| Insulin | 1836/5518 (33.3) | 1511/4644 (32.5) | 325/874 (37.2) | −4.7 | .01 |
| Metformin | 3420/5518 (62.0) | 2943/4644 (63.4) | 477/874 (54.6) | 8.8 | <.001 |
| Any sulfonylurea | 2892/5518 (52.4) | 2448/4644 (52.7) | 444/874 (50.8) | 1.9 | .30 |
| Any thiazolidinedione | 973/5518 (17.6) | 840/4644 (18.1) | 133/874 (15.2) | 2.9 | .04 |
| Angiotensin-converting-enzyme inhibitor | 2967/5518 (53.8) | 2499/4644 (53.8) | 468/874 (53.5) | 0.3 | .89 |
| Angiotensin-receptor-blocker | 838/5518 (15.2) | 709/4644 (15.3) | 129/874 (14.8) | 0.5 | .70 |
| Aspirin | 3106/5518 (56.3) | 2626/4644 (56.5) | 480/874 (54.9) | 1.6 | .37 |
| β-Blocker | 1798/5518 (32.6) | 1488/4644 (32.0) | 310/874 (35.5) | −3.5 | .05 |
| Any thiazide diuretic | 1473/5518 (26.7) | 1251/4644 (26.9) | 222/874 (25.4) | 1.5 | .35 |
| Statin | 3299/5518 (59.8) | 2819/4644 (60.7) | 480/874 (54.9) | 5.8 | .001 |
| Any lipid-lowering agent | 3558/5518 (64.5) | 3036/4644 (65.4) | 522/874 (59.7) | 5.7 | .001 |
| Duration of diabetes, mean (SD), y | 10.6 (7.5) | 10.6 (7.5) | 10.9 (7.7) | −0.3 | .26 |
| Glycated hemoglobin, % | |||||
| Mean (SD) | 8.3 (1.0) | 8.25 (1.01) | 8.43 (1.15) | −0.18 | <.001 |
| Median (IQR) | 8.1 (7.6 to 8.8) | 8.1 (7.5 to 8.8) | 8.2 (7.7 to 9.0) | −0.1 | .001 |
| Fasting plasma glucose, mean (SD), mg/dL | 175.8 (54.9) | 176.1 (54.5) | 174.2 (57.1) | 1.9 | .34 |
| Amputation owing to diabetes, No./total No. (%) | 110/5518 (2.0) | 85/4644 (1.8) | 25/874 (2.9) | −1.1 | .05 |
| Potassium, mean (SD), mg/dL | 4.5 (0.4) | 4.47 (0.42) | 4.49 (0.46) | −0.02 | .41 |
| Serum creatinine, mean (SD), mg/dL | 0.9 (0.2) | 0.92 (0.22) | 0.97 (0.26) | −0.05 | <.001 |
| eGFR (mL/min/1.73m2) | |||||
| 30-49 mL/min/1.73m2 | 141/5488 (2.6) | 102/4621 (2.2) | 39/867 (4.5) | −2.3 | <.001 |
| >50 mL/min/1.73m2 | 5347/5488 (97.4) | 4519/4621 (97.8) | 828/867 (95.5) | 2.3 | |
| Total plasma cholesterol, mean (SD), mg/dL | 175.2 (37.3) | 174.7 (36.9) | 177.6 (39.4) | −2.9 | .04 |
| Plasma LDL-C, mean (SD), mg/dL | 100.6 (30.7) | 99.9 (30.3) | 103.9 (32.5) | −4.0 | .001 |
| Plasma HDL-C, mean (SD), mg/dL | |||||
| Women | 41.4 (7.7) | 41.6 (7.8) | 40.6 (7.6) | 1.0 | .07 |
| Men | 36.6 (7.3) | 36.7 (7.2) | 36.3 (7.8) | 0.4 | .21 |
| Plasma triglycerides, mg/dL | |||||
| Mean (SD) | 187.6 (112.6) | 188.0 (113.4) | 185.3 (108.3) | 2.7 | .53 |
| Median (IQR) | 162 (113 to 229) | 162 (113 to 230) | 162 (112 to 228) | 0.0 | .69 |
Abbreviations: ACCORD, Action to Control Cardiovascular Risk in Diabetes; ACCORDION, ACCORD Follow-On Study; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 76.25; to convert glucose to millimoles per liter, multiply by 0.0555; to convert glycated hemoglobin to proportion of total hemoglobin, multiply by 0.01; to convert HDL-C to millimoles per liter, multiply by 0.0259; to convert LDL-C to millimoles per liter, multiply by 0.0259; to convert potassium to millimoles per liter, multiply by 1; to convert triglycerides to millimoles per liter, multiply by 0.0113.
Participants could have selected more than 1 racial/ethnic group.
Calculated as weight in kilograms divided by height in meters squared.
The mean duration of follow-up during ACCORD-Lipid was 4.7 years for the primary outcome and 5.0 years for all-cause mortality. With the addition of posttrial follow-up, the overall mean duration of follow-up was 7.7 years for the primary outcome and 9.1 years for all-cause mortality. The maximum length of follow-up for individual participants was more than 12 years. At the final ACCORD study visit, 4754 of the participants (86.0%) remained on a statin and 2137 participants (77.3%) originally assigned to fenofibrate remained on fenofibrate.9 Following completion of ACCORD, further lipid treatment was guided by primary care clinicians who continued to prescribe statin therapy in 2476 ACCORDION participants (74.1%). In contrast, only 144 ACCORDION participants (4.3%) were continued or started on fibrate therapy following completion of ACCORD.
Plasma Lipids
At the time of entry into ACCORD, fasting plasma lipids at baseline were similar between the participants assigned to fenofibrate and placebo9 (eFigure 1 in Supplement 3). At entry into ACCORD, 3299 ACCORD-Lipid trial participants (59.8%) were already on statin therapy, and mean LDL was approximately 100 mg/dL (Table 1). During ACCORD, all participants were treated with simvastatin at a dose of 20 mg/d to 40 mg/d.9 Low-density lipoprotein cholesterol levels progressively decreased to a mean of 80 mg/dL in the placebo group and 81.1 mg/dL in the fenofibrate group over the course of the trial because statin therapy was initiated in all participants and was intensified in response to accrual of safety information and in response to evolving guidelines9,15,17 (eFigure 1 in Supplement 3). During the posttrial follow-up period, LDL-C levels declined slightly from a mean of 80.2 mg/dL at the first follow-up visit to an average of 77 mg/dL in both groups. During ACCORD, triglyceride levels were reduced by 22%, from a mean of 187 mg/dL to 145 mg/dL in participants randomized to fenofibrate and declined 8.7%, from a mean of 186.2 mg/dL to 170 mg/dL in those randomized to placebo9 (eFigure 1 in Supplement 3). During the posttrial period, triglyceride levels continued to decline in the placebo group and increased in the fenofibrate group to a mean of 160.8 mg/dL in both groups, reflecting high rates of discontinuation of fibrate therapy following completion of ACCORD (eFigure 1 in Supplement 3). During ACCORD, HDL-C increased 8.4% in the fenofibrate group (from 38.0 mg/dL to 41.2 mg/dL) and 6.0% in the placebo group (from 38.2 mg/dL to 40.5 mg/dL).9 During the posttrial period, HDL-C levels declined to a mean level of 40.5 mg/dL in participants originally randomized to fenofibrate to levels comparable with those in participants originally randomized to placebo (eFigure 1 in Supplement 3).
Clinical Outcomes
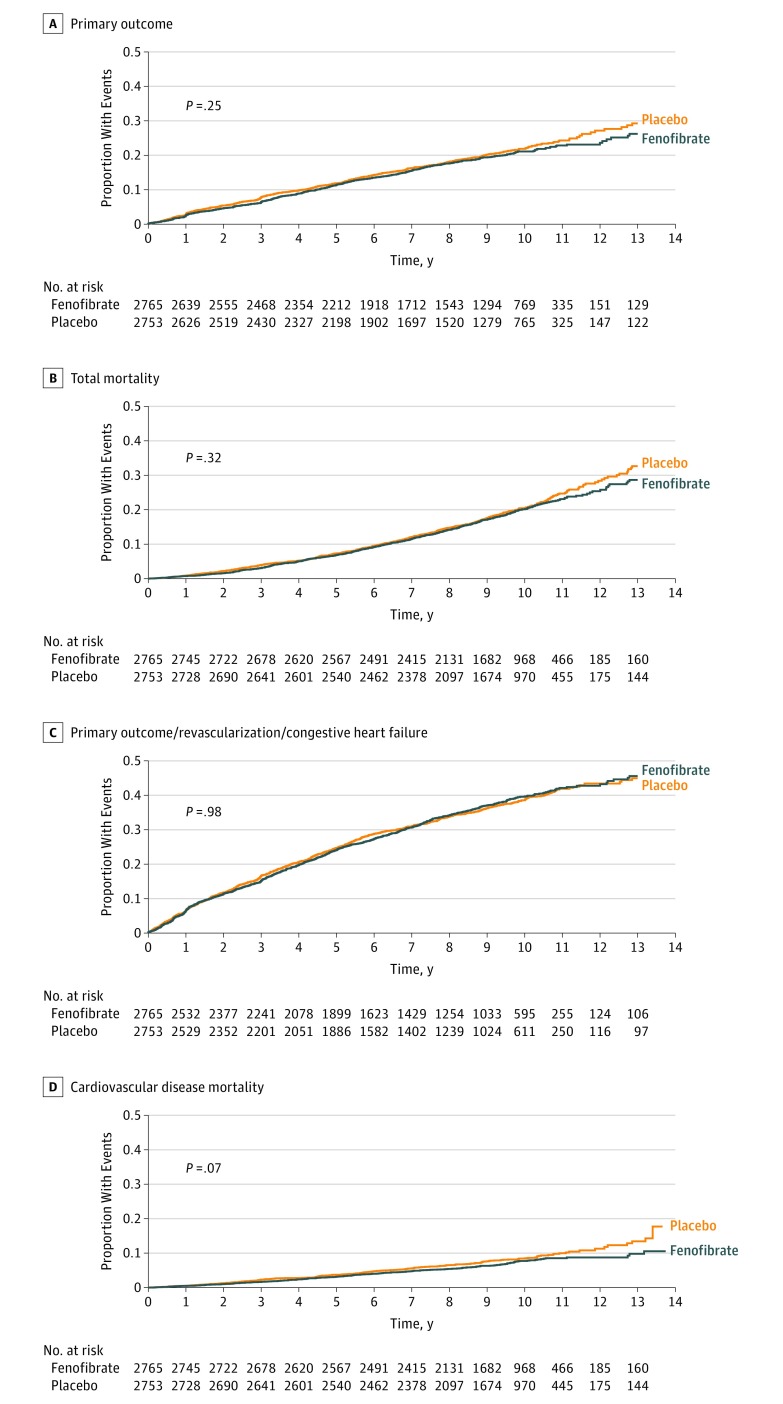
Rates of occurrence by treatment assignment and hazard ratios for investigator-reported primary and secondary cardiovascular outcome measures during the entire study period, including extended follow-up, are presented in Table 2. Following a mean of 9.0 total years of follow-up, 508 primary end point events occurred in the fenofibrate group vs 539 in the placebo group (hazard ratio [HR], 0.93; 95% CI, 0.83-1.05; P = .25) (Table 2). The annual primary outcome rate was 2.38% among participants randomized to fenofibrate vs 2.55% among those randomized to placebo. The HR for the primary end point during extended follow-up in ACCORDION was essentially identical to that observed during the 4.7-year active treatment phase of ACCORD (HR, 0.92; 95% CI, 0.79-1.08; P = .32) (Table 2). Thus, the additional 5 years of follow-up did not change the original neutral findings of the ACCORD study.9 Similarly, the hazard ratios for the secondary outcomes, including the individual components of the primary outcome, were not statistically different between treatment groups and were comparable with those observed during ACCORD (Table 2). Kaplan-Meier curves describing the almost 10-year accumulation of major cardiovascular events in the 2 groups visually confirm the comparable rates of accrual of outcomes in the 2 treatment groups (Figure 2).
Table 2. Prespecified Primary and Secondary Outcomes by Original Treatment Assignment During ACCORDION Extended Follow-up vs During ACCORD Double-Blind Treatment Phase.
| Outcome | Treatment Effect During ACCORDa (Fenofibrate/Placebo) | Hazard Ratios During ACCORDION (Fenofibrate/Placebo) | Treatment Effect During Extended Follow-up ACCORD + ACCORDION (Fenofibrate/Placebo)b | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Primary outcomec | 0.92 (0.79-1.08) | .32 | 0.93 (0.76-1.34) | .47 | 0.93 (0.83-1.05) | .25 |
| Secondary outcomes | ||||||
| Primary outcome plus revascularization or hospitalization for congestive heart failure | 0.94 (0.85-1.05) | .30 | 1.12 (0.95-1.32) | .20 | 1.00 (0.92-1.10) | .98 |
| Major coronary disease event d | 0.92 (0.79-1.07) | .26 | 0.95 (0.77-1.17) | .61 | 0.91 (0.81-1.03) | .13 |
| Nonfatal myocardial infarction | 0.91 (0.74-1.12) | .39 | 1.05 (0.76-1.44) | .78 | 0.93 (0.78-1.10) | .37 |
| Nonfatal Stroke | 1.17 (0.76-1.78) | .48 | 0.93 (0.65-1.32) | .67 | 1.12 (0.87-1.43) | .38 |
| Fatal or nonfatal stroke | 1.05 (0.71-1.56) | .80 | 1.01 (0.71-1.42) | .97 | 1.12 (0.89-1.42) | .33 |
| All-cause mortality | 0.91 (0.75-1.10) | .34 | 0.96 (0.83-1.11) | .57 | 0.94 (0.84-1.06) | .32 |
| Cardiovascular mortality | 0.86 (0.66-1.12) | .26 | 0.82 (0.63-1.07) | .14 | 0.84 (0.69-1.01) | .07 |
| Fatal or nonfatal congestive heart failure | 0.82 (0.65-1.05) | .10 | 0.85 (0.67-1.06) | .15 | 0.86 (0.71-1.05) | .14 |
| Nonfatal myocardial Infarction, nonfatal stroke or all cause mortalitye | NRa | NRa | 0.98 (0.84-1.13) | .74 | 0.97 (0.88-1.07) | .58 |
Abbreviations: ACCORD, Action to Control Cardiovascular Risk in Diabetes; ACCORDION, ACCORD Follow-On Study; NR, not reported by Ginsberg et al9 because it was not an ACCORD Protocol Outcome.
Hazard ratio of events occurring during ACCORD, ACCORDION alone, and combined. Number and event rate during combined follow-up (ACCORD and ACCORDION) in study participants originally randomized to fenofibrate vs placebo.18
Rates per 100 person-years during ACCORD (active treatment phase) and ACCORDION (extended poststudy passive follow-up period).
Primary outcome for original ACCORD study, combined occurrence of nonfatal myocardial infarction, nonfatal stroke, and fatal cardiovascular event.
A major coronary disease event was defined as a fatal coronary event, nonfatal myocardial infarction, or unstable angina.
New outcome measure; all other outcomes are an original ACCORD protocol outcome.
Figure 2. Kaplan-Meier Analyses of the Primary Outcome, Expanded Macrovascular Outcome, and Death.
The cumulative incidence of the primary outcome (nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes) (A), the expanded macrovascular outcome (a combination of the primary outcome plus revascularization or hospitalization for congestive heart failure) (B), and death from any cause (C) or from cardiovascular causes (D) during follow-up.
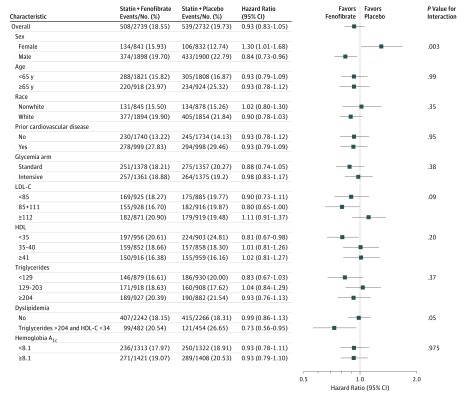
In contrast to the overall neutral effect of fenofibrate therapy in the entire ACCORD cohort, heterogeneity in the effect of fenofibrate on the primary cardiovascular outcome continued to be observed during extended follow-up in those with dyslipidemia at study entry. During the combined trial plus posttrial period, the primary outcome in study participants with dyslipidemia who were randomized to fenofibrate was 27% lower than among those with dyslipidemia randomized to placebo but only 1% lower in nondyslipidemic study participants (HR, 0.73; 95% CI, 0.56-0.95 vs HR, 0.99; 95% CI, 0.86-1.13; P = .05 for dyslipidemic vs non-dyslipidemic, respectively) (Figure 3). Persistent heterogeneity in fenofibrate response was also observed in men vs women, and the primary outcome in the fenofibrate treatment group was 16% lower for men but 30% higher for women (HR, 0.84; 95% CI, 0.73-0.96 vs HR, 1.30; 95% CI, 1.10-1.68; P = .003 for men vs women, respectively) (Figure 3). These HRs are nearly identical to those observed in the original ACCORD trial (eTable 2 in Supplement 3).9
Figure 3. Hazard Ratios for the Primary Outcome in Prespecified Subgroups.
The horizontal bars represent 95% confidence intervals, and the vertical dashed line indicates the overall hazard ratio. P values are for tests for interaction. HDL-C indicates high-density lipoprotein and LDL-C indicates low-density lipoprotein cholesterol.
To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.0112.
Discussion
Up to 35% of patients with type 2 diabetes are at increased risk of atherosclerotic CVD events related to the presence of diabetic dyslipidemia, defined as hypertriglyceridemia and the associated accumulation of remnant particles, low HDL-C, and small dense LDL.8,19 However, it is unclear whether pharmacologic therapy directed toward reversing these abnormalities will result in reduced risk of CVD. Although the cardiovascular efficacy of niacin and fibrate (gemfibrozil) monotherapy was clearly established by prestatin-era clinical trials,20,21,22 subsequent trials failed to demonstrate efficacy of newer fibrates, specifically fenofibrate and bezafibrate either alone23,24 or in combination with statin.9 Furthermore, between 2007 and 2016, a number of trials testing various triglyceride-lowering and HDL-C–raising medical therapies have failed to demonstrate benefit of add-on therapy in statin-treated patients.25,26,27,28 These clinical trial outcomes are reflected in treatment guidelines promulgated by the American Heart Association and the American College of Cardiology that focus on recommendations for statin therapy but do not clearly advocate the use of triglyceride-lowering therapy for CVD prevention.29
In the case of fenofibrate, 2 cardiovascular end point trials conducted within the last decade failed to show benefit with administration of fenofibrate in patients with type 2 diabetes either alone in the FIELD study24 or as add-on therapy to statin in the ACCORD study.9 Among the possible reasons for the neutral outcome of FIELD was a disproportionately higher drop in statin therapy in the fenofibrate group.24 In ACCORD, one possible reason for failure to demonstrate benefit of add-on fenofibrate therapy was that the treatment duration of 4.7 years was not sufficient to detect a treatment effect. The goal of ACCORDION was to extend the study with an additional 5.0 years of passive follow-up to detect emergence of a “legacy” effect of fenofibrate treatment, similar to that observed in the niacin arm of the Coronary Drug Project30 and with glucose lowering in both the United Kingdom Prospective Diabetes Study and the 2008 Veterans Affairs Diabetes Trial.31,32 A legacy effect did not emerge during extended follow-up in ACCORDION, thus confirming the original overall ACCORD observations. On the other hand, the lower cardiovascular event rates observed among the subgroup of participants with hypertriglyceridemia and low HDL-C who were randomized to fenofibrate therapy during the active treatment phase of ACCORD continued to be observed during the extended follow-up period. These findings support the hypothesis that individuals with diabetic dyslipidemia may benefit from add-on fenofibrate therapy. This hypothesis is supported by the comparable findings of similar subgroup analyses of several major fibrate trials including the FIELD study,33 the Helsinki Heart Study (HHS),34 Bezafibrate Infarction Prevention Trial (BIP),23 and VA-HDL Intervention Trial (VA-HIT).35 Insofar as the triglyceride-lowering effect of fibrates is greatest among patients with hypertriglyceridemia,34 it is not totally unexpected that individuals with hypertriglyceridemia would be most likely to benefit from fibrate therapy. This was clearly evident in the lipid response to fenofibrate in the hypertriglyceridemia/low–HDL-C subset of ACCORD-Lipid participants (eFigure 2 and eFigure 3 in Supplement 3). However, in ACCORD-Lipid, this subset comprised only 17% of all participants (n = 941).9
The sex differences in fenofibrate response observed in ACCORD9 were also observed during extended follow-up in ACCORDION. The observation of sex differences in cardiovascular outcomes with fenofibrate treatment, with men appearing to benefit vs evidence of possible harm in women in ACCORD9 and now with extended follow-up in ACCORDION, is both unexpected and unexplained, particularly because similar heterogeneity in fenofibrate treatment effect by sex was not observed in the FIELD trial.24 These differences may be attributed to lower numbers of women participants in ACCORD vs FIELD as well as unexpectedly low event rates among placebo-treated women in ACCORD.36 Therefore, the sex difference may be a chance finding.
It is important to note in the context of our findings that the safety profile of combination therapy with fenofibrate and statin appears to be acceptable. Specifically, in ACCORD, fenofibrate was used in combination with simvastatin in more than 2500 patients for a mean of 4.7 years without increased incidence of muscle or liver toxicity.9 This is in distinct contrast to the increased risk of myopathy that occurs with coadministration of the fibrate gemfibrozil and statin owing to a known pharmacokinetic interaction.37,38 It is also important to note that in ACCORD, fenofibrate treatment slowed progression of diabetic microvascular disease including retinopathy and nephropathy.9,18 On the other hand, reversible increases in creatinine and paradoxical lowering of HDL-C were also observed with increased frequency in those randomized to fenofibrate in ACCORD-Lipid.39,40
Limitations
It is also important to note that these prespecified subgroup analyses can only be considered hypothesis-generating and in some cases are based on a relatively small number of events. Although analyses beyond the original predefined primary outcome measure cannot be considered definitive and therefore not suitable for guideline formulation or product labeling, they inform refinement of our original hypothesis for further testing and provide useful information to clinical practitioners regarding possible treatment for diabetic dyslipidemia.
Conclusions
In conclusion, an additional 5 years of follow-up of surviving ACCORD-Lipid study cohort members extends the original overall neutral outcome of the ACCORD study and provides additional support for possible benefit of fenofibrate therapy in patients with type 2 diabetes in whom triglycerides remain elevated and HDL-C levels remain low despite statin therapy. Our findings support the hypothesis that patients with diabetic dyslipidemia may derive some benefit from add-on triglyceride-lowering therapy. Randomized trials testing the cardiovascular efficacy of fibrate as well as other triglyceride-lowering treatments in this specific patient population are needed.
List of ACCORD/ACCORDION Study Group Members.
Trial Protocol.
eTable 1. Comparison of Investigator Reported Outcomes in ACCORD Versus Adjudicated Outcomes Reported by the Endpoints Committee.
eTable 2. Comparison of CVD Outcomes in Pre-specified Subgroups Sex and Dyslipidemia in ACCORD and With Addition of Extended Follow-up in ACCORDION.
eFigure 1. Lipid Levels By ACCORD Treatment Arm from Randomization through the End of ACCORDION.
eFigure 2. Lipid Response to Fenofibrate in Hypertriglyceridemic/Low HDL-C Subgroup vs all others in ACCORD: HDL-C and Triglyceride.
eFigure 3. Lipid Response to Fenofibrate in Hypertriglyceridemic/Low HDL-C Subgroup vs all others in ACCORD: LDL-C and Non-HDLC.
References
- 1.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164(13):1422-1426. [DOI] [PubMed] [Google Scholar]
- 2.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434-444. [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229-234. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen H, Lehto S, Salomaa V, et al. ; The FINMONICA Myocardial Infarction Register Study Group . Impact of diabetes on mortality after the first myocardial infarction. Diabetes Care. 1998;21(1):69-75. [DOI] [PubMed] [Google Scholar]
- 5.Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35(3):491-510, vii-viii. [DOI] [PubMed] [Google Scholar]
- 6.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998;316(7134):823-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buse JB, Bigger JT, Byington RP, et al. ; ACCORD Study Group . Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12A):21i-33i. [DOI] [PubMed] [Google Scholar]
- 8.Miller M, Stone NJ, Ballantyne C, et al. ; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease . Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg HN, Elam MB, Lovato LC, et al. ; ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerstein HC, Miller ME, Byington RP, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cushman WC, Evans GW, Byington RP, et al. ; ACCORD Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstein HC, Miller ME, Genuth S, et al. ; ACCORD Study Group . Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ACCORD Study Group Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39(5):701-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goff DC Jr, Gerstein HC, Ginsberg HN, et al. ; ACCORD Study Group . Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99(12A):4i-20i. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg HN, Bonds DE, Lovato LC, et al. ; ACCORD Study Group . Evolution of the lipid trial protocol of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99(12A):56i-67i. [DOI] [PubMed] [Google Scholar]
- 16.Harmonised Tripartite Guideline ICH. ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Stat Med. 1999;18(15):1905-1942. [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Merz CN, et al. ; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association . Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239. [DOI] [PubMed] [Google Scholar]
- 18.Chew EY, Ambrosius WT, Davis MD, et al. ; ACCORD Study Group; ACCORD Eye Study Group . Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29(3):531-537. [DOI] [PubMed] [Google Scholar]
- 20.Clofibrate and niacin in coronary heart disease. JAMA. 1975;231(4):360-381. [PubMed] [Google Scholar]
- 21.Rubins HB, Robins SJ, Collins D, et al. ; Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group . Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418. [DOI] [PubMed] [Google Scholar]
- 22.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237-1245. [DOI] [PubMed] [Google Scholar]
- 23.Bezafibrate Infarction Prevention (BIP) study Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102(1):21-27. [DOI] [PubMed] [Google Scholar]
- 24.Keech A, Simes RJ, Barter P, et al. ; FIELD study investigators . Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GG, Olsson AG, Abt M, et al. ; dal-OUTCOMES Investigators . Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089-2099. [DOI] [PubMed] [Google Scholar]
- 26.Boden WE, Probstfield JL, Anderson T, et al. ; AIM-HIGH Investigators . Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255-2267. [DOI] [PubMed] [Google Scholar]
- 27.Landray MJ, Haynes R, Hopewell JC, et al. ; HPS2-THRIVE Collaborative Group . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203-212. [DOI] [PubMed] [Google Scholar]
- 28.Barter PJ, Caulfield M, Eriksson M, et al. ; ILLUMINATE Investigators . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109-2122. [DOI] [PubMed] [Google Scholar]
- 29.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. [DOI] [PubMed] [Google Scholar]
- 30.Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8(6):1245-1255. [DOI] [PubMed] [Google Scholar]
- 31.Hayward RA, Reaven PD, Wiitala WL, et al. ; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206. [DOI] [PubMed] [Google Scholar]
- 32.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589. [DOI] [PubMed] [Google Scholar]
- 33.Scott R, O’Brien R, Fulcher G, et al. ; Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study Investigators . Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32(3):493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manninen V, Elo MO, Frick MH, et al. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. JAMA. 1988;260(5):641-651. [PubMed] [Google Scholar]
- 35.Robins SJ, Collins D, Wittes JT, et al. ; VA-HIT Study Group. Veterans Affairs High-Density Lipoprotein Intervention Trial . Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285(12):1585-1591. [DOI] [PubMed] [Google Scholar]
- 36.d’Emden MC, Jenkins AJ, Li L, et al. ; FIELD Study Investigators . Favourable effects of fenofibrate on lipids and cardiovascular disease in women with type 2 diabetes: results from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2014;57(11):2296-2303. [DOI] [PubMed] [Google Scholar]
- 37.Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95(1):120-122. [DOI] [PubMed] [Google Scholar]
- 38.Prueksaritanont T, Tang C, Qiu Y, Mu L, Subramanian R, Lin JH. Effects of fibrates on metabolism of statins in human hepatocytes. Drug Metab Dispos. 2002;30(11):1280-1287. [DOI] [PubMed] [Google Scholar]
- 39.Mychaleckyj JC, Craven T, Nayak U, et al. Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care. 2012;35(5):1008-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linz PE, Lovato LC, Byington RP, et al. Paradoxical reduction in HDL-C with fenofibrate and thiazolidinedione therapy in type 2 diabetes: the ACCORD Lipid Trial. Diabetes Care. 2014;37(3):686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerstein HC, Miller ME, Byington RP, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of ACCORD/ACCORDION Study Group Members.
Trial Protocol.
eTable 1. Comparison of Investigator Reported Outcomes in ACCORD Versus Adjudicated Outcomes Reported by the Endpoints Committee.
eTable 2. Comparison of CVD Outcomes in Pre-specified Subgroups Sex and Dyslipidemia in ACCORD and With Addition of Extended Follow-up in ACCORDION.
eFigure 1. Lipid Levels By ACCORD Treatment Arm from Randomization through the End of ACCORDION.
eFigure 2. Lipid Response to Fenofibrate in Hypertriglyceridemic/Low HDL-C Subgroup vs all others in ACCORD: HDL-C and Triglyceride.
eFigure 3. Lipid Response to Fenofibrate in Hypertriglyceridemic/Low HDL-C Subgroup vs all others in ACCORD: LDL-C and Non-HDLC.