Abstract
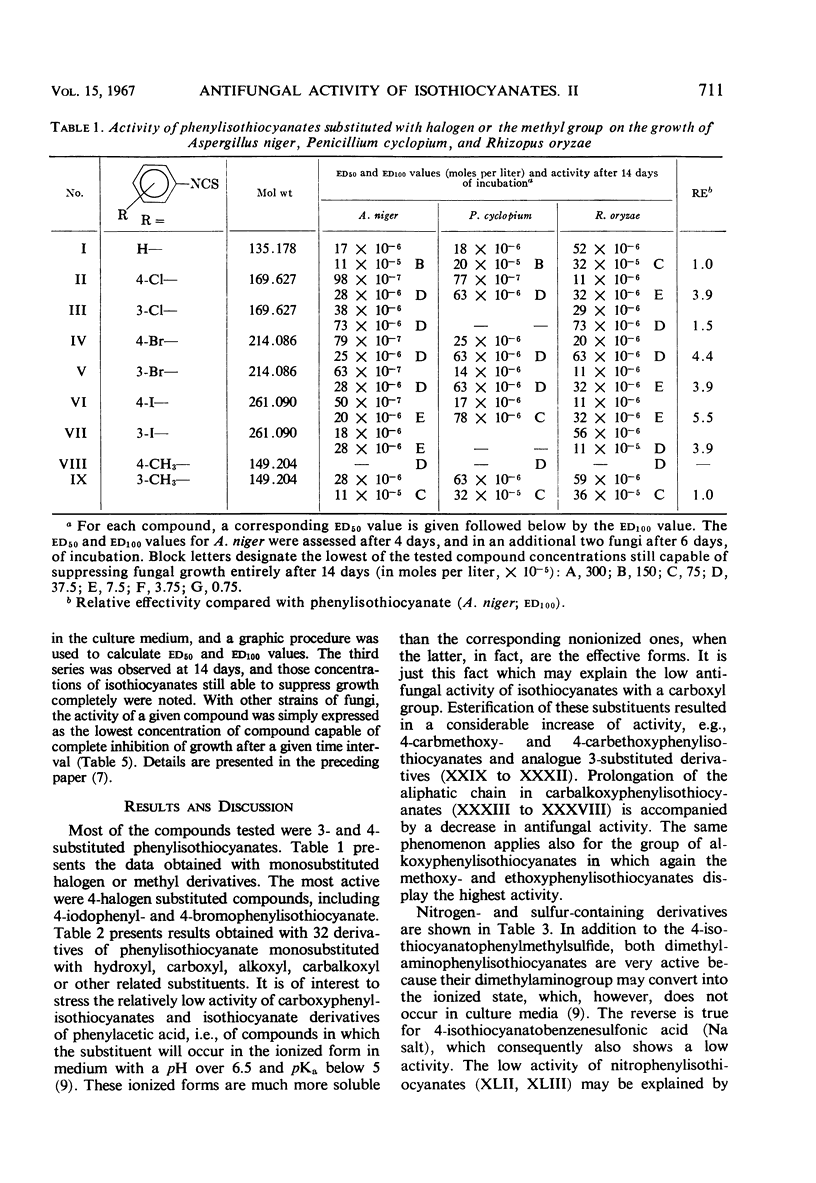
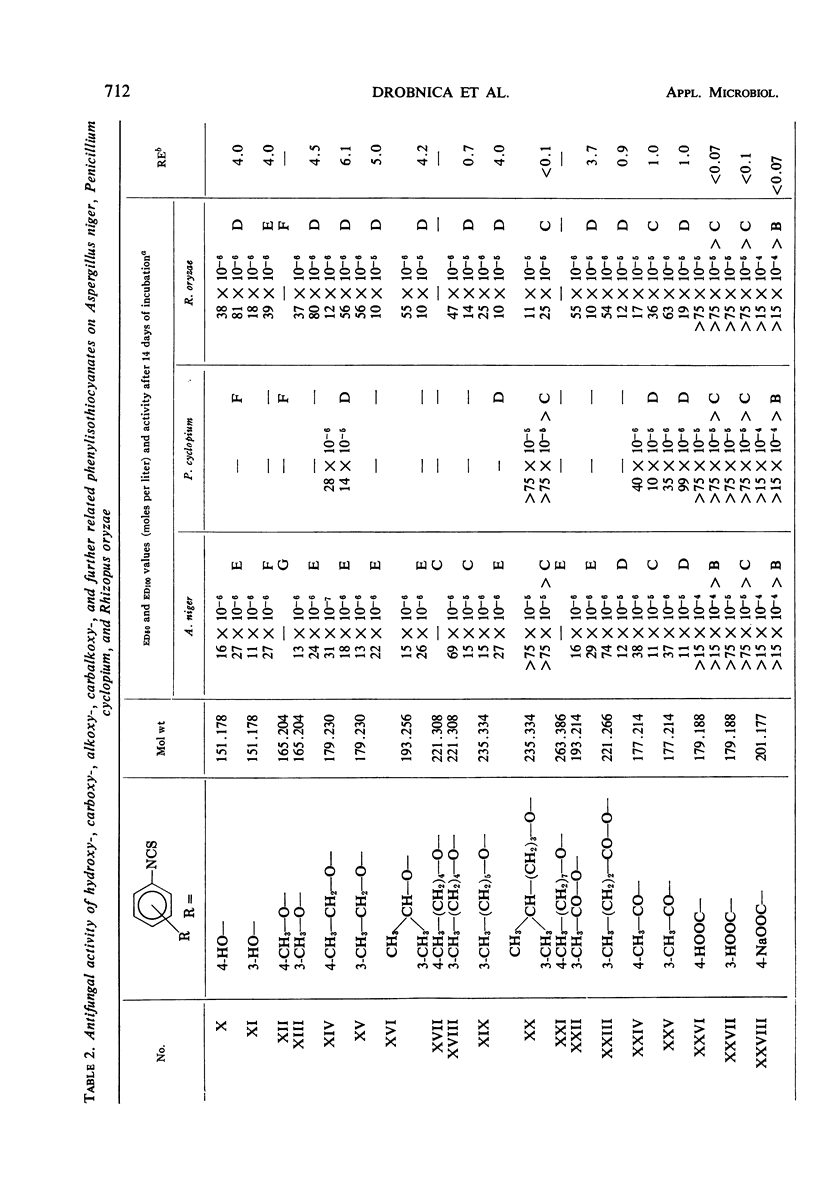
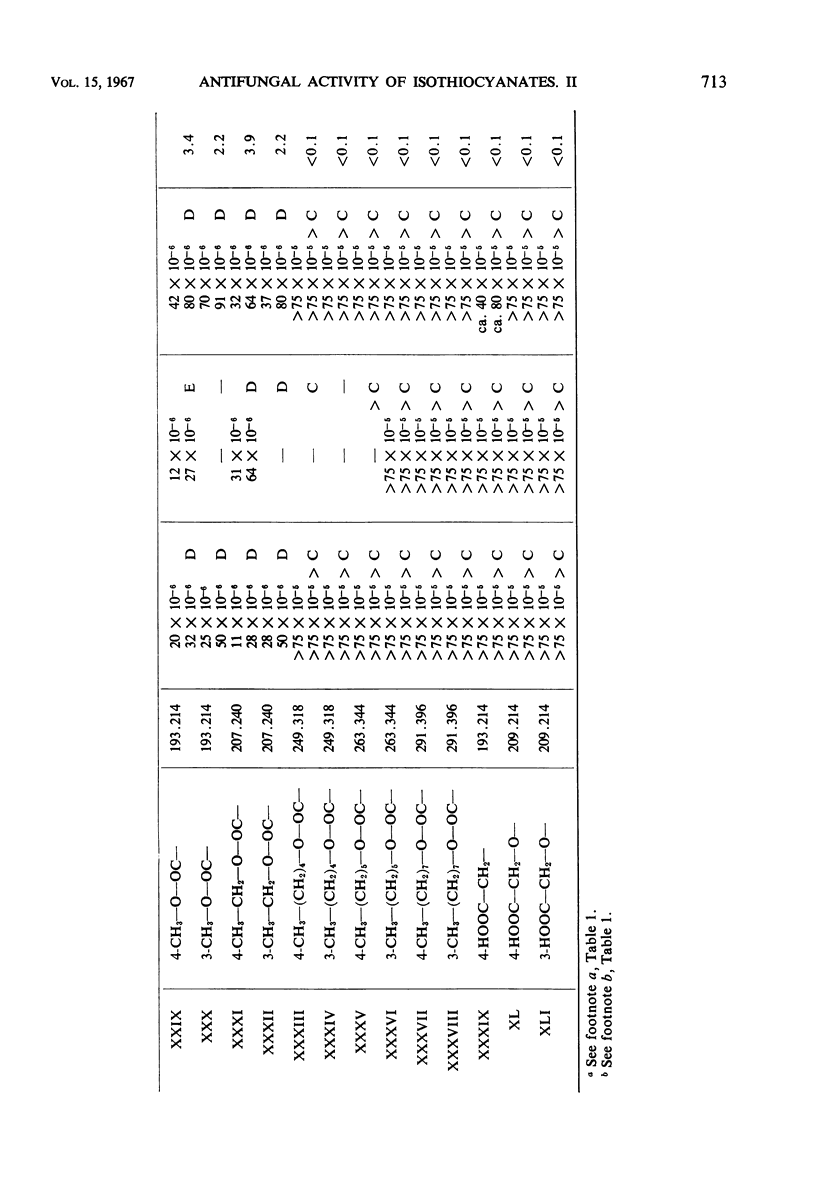
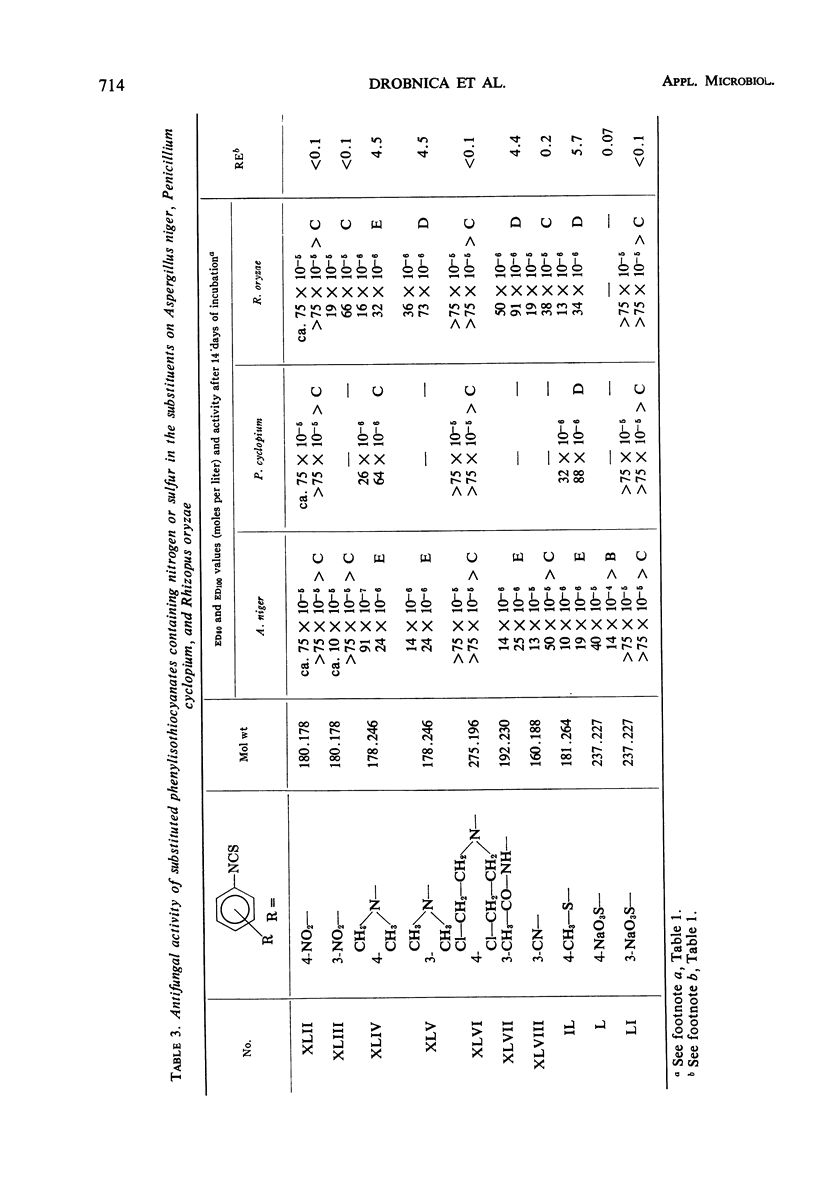
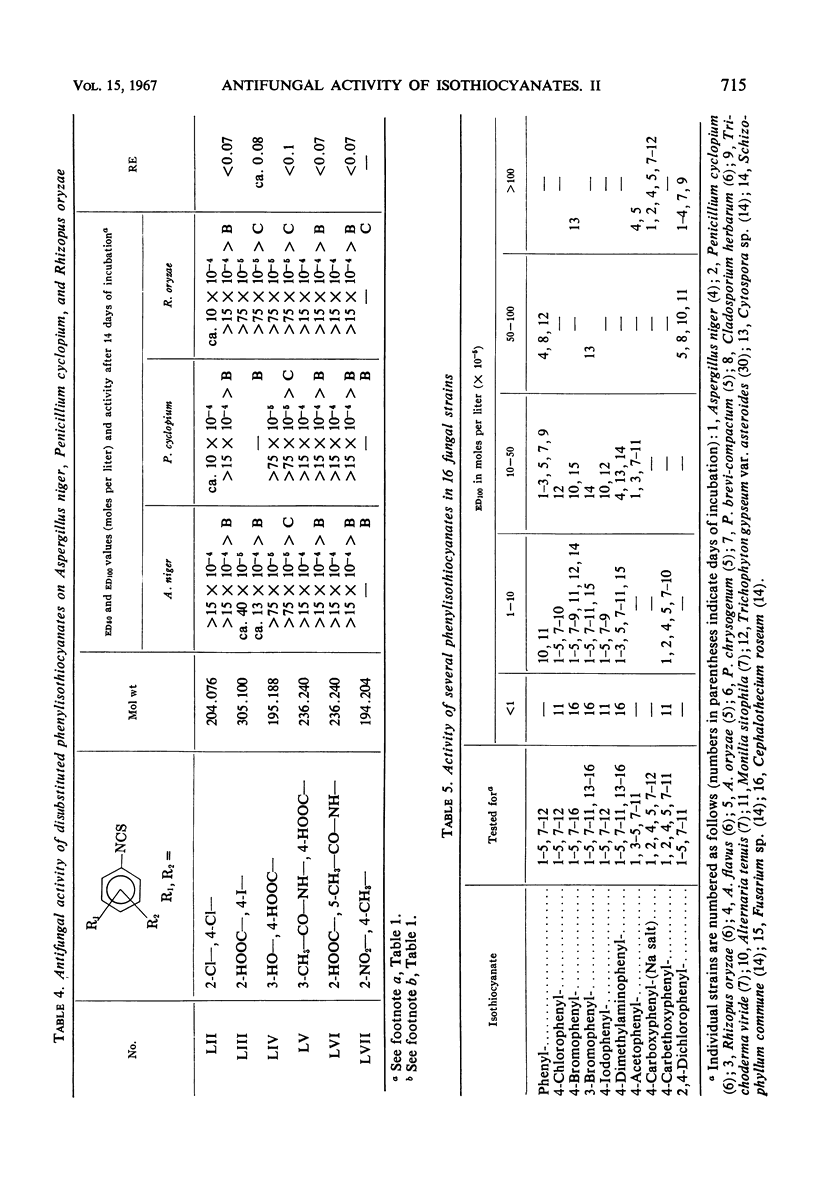
Antifungal activity on Aspergillus niger, Penicillium cyclopium, and Rhizopus oryzae, as well as on additional saprophytic and parasitic fungi, was determined in 57 substituted derivatives of phenylisothiocyanate. Most of the investigated compounds displayed rather equal activity against the three mentioned fungi, in contradistinction to the analogues of natural benzyl- and β-phenylethylisothiocyanate with their characteristic low activity against R. oryzae. Differences occurred in the type of activity of compounds in which the —NCS group is directly bound on the aromatic moiety, as compared with those compounds in which this group is bound to the aliphatic radical or to the aromatic moiety indirectly by means of the methyl group or by a longer aliphatic chain. The results obtained confirm the negative influence of ionized substituents on the aromatic moiety, i.e., of —COOH, —CH2— COOH, and —SO3H groups, as well as of substituents which cause an extreme increase in reactivity of the —NCS group resulting in a high instability of the entire isothiocyanate molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacíková D., Nemec P., Drobnica L., Antos K., Kristián P., Hulka A. Antiworm activity of some natural and synthetic compounds. I. Effect of aliphatic and mononuclear aromatic isothiocyanates on Turbatrix aceti. J Antibiot (Tokyo) 1965 Jul;18(4):162–170. [PubMed] [Google Scholar]
- Drobnica L., Zemanová M., Nemec P., Antos K., Kristián P., Stullerová A., Knoppová V., Nemec P., Jr Antifungal activity of isothiocyanates and related compounds. I. Naturally occurring isothiocyanates and their analogues. Appl Microbiol. 1967 Jul;15(4):701–709. doi: 10.1128/am.15.4.701-709.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


