Key Points
Question
In patients with cirrhosis at high risk for hepatocellular carcinoma (HCC), is surveillance for HCC with magnetic resonance imaging (MRI) superior to surveillance by ultrasonography (US)?
Findings
In this prospective surveillance study of 407 patients with cirrhosis and a risk for developing HCC greater than 5% per year, screening MRI with liver-specific contrast resulted in a significantly higher HCC detection rate and lower a false-positive rate compared with US. Using MRI screening, most HCC cases detected were at a very early stage, which was associated with a high chance of curative treatments.
Meaning
In high-risk patients with cirrhosis, surveillance by MRI with liver-specific contrast may allow earlier detection of HCC than US, improving clinical outcomes.
This clinical trial examines the hepatocellular carcinoma detection rate using ultrasonography compared with magnetic resonance imaging in patients with cirrhosis who are at high risk for hepatocellular carcinoma.
Abstract
Importance
Current recommendations for patients with cirrhosis are to undergo surveillance for hepatocellular carcinoma (HCC) with ultrasonography (US) every 6 months. However, the sensitivity of US screening to detect early-stage HCC is suboptimal. Magnetic resonance imaging (MRI) with liver-specific contrast may detect additional HCCs missed by US in high-risk patients with cirrhosis.
Objective
To compare the HCC detection rate of US and MRI in patients with cirrhosis who are at high risk for HCC.
Design, Setting, and Participants
A prospective surveillance study of 407 patients with cirrhosis and an estimated annual risk of HCC greater than 5% who underwent 1 to 3 biannual screening examinations with paired US and liver-specific contrast-enhanced MRI at a tertiary care hospital between November 2011 and August 2014. All patients were followed-up with dynamic computed tomography (CT) at 6 months after the study. The confirmation of HCC was based on the results of histologic examination and/or typical CT images of HCC.
Main Outcomes and Measures
HCC detection rates and false-positive findings of US vs MRI.
Results
A total of 407 eligible patients received 1100 screenings with paired US and MRI. Hepatocellular carcinomas were diagnosed in 43 patients: 1 detected by US only, 26 by MRI only, 11 by both, and 5 were missed by both. The HCC detection rate of MRI was 86.0% (37/43), significantly higher than the 27.9% (12/43) of US (P < .001). Magnetic resonance imaging showed a significantly lower rate of false-positive findings than US (3.0% vs 5.6%; P = .004). Of the 43 patients with HCC, 32 (74.4%) had very early-stage HCC (a single nodule <2 cm), and 29 (67.4%) received curative treatments. The 3-year survival rate of the patients with HCC (86.0%) was not inferior to those without HCC (94.2%; hazard ratio, 2.26; 95% CI, 0.92-5.56; P = .08).
Conclusions and Relevance
In patients with cirrhosis at high-risk of HCC, screening that used MRI with liver-specific contrast resulted in a higher HCC detection rate and lower false-positive findings compared with US. With MRI screening, most of the cancers detected were at very early stage, which was associated with a high chance of curative treatments and favorable survival of patients. Whether surveillance with MRI would reduce mortality from HCC in high-risk patients requires further investigation.
Trial Registration
clinicaltrials.gov Identifier: NCT01446666
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second largest cause of cancer mortality in the world. Hepatocellular carcinoma has been the fastest-rising cause of cancer-related deaths in Western countries during the past 2 decades and is expected to increase further in the next decade. Hepatocellular carcinoma usually develops in patients with cirrhosis. With the decrease in mortality by complications of cirrhosis, HCC is becoming the leading cause of death among patients with cirrhosis.
The prognosis of patients with HCC is extremely poor with the 5-year survival rate below 20%. The prognosis largely depends on tumor stage, and curative treatments are available only for patients diagnosed when the cancer is at an early stage. Even for patients with early stage HCC, the chance of liver transplantation is often limited owing to donor shortage, and surgical resection is seldom possible because of considerable portal hypertension. Thus, the only curative treatment option is local ablation in many cases, which highlights the importance of surveillance to detect HCCs at a very early stage (a single lesion <2 cm).
Currently, ultrasonography (US) at 6-month intervals is recommended for the surveillance of patients at risk to detect HCC at an early stage. However, the sensitivity of US is suboptimal. Moreover, the sensitivity of US may be particularly impaired in those at highest risk of developing HCC because of the very nodular liver.
Given the limited efficacy of US, other imaging techniques, such as computed tomography (CT) or magnetic resonance imaging (MRI) have been suggested for the surveillance of HCC in patients at high risk. In diagnostic setting, MRI using a liver-specific contrast agent, gadoxetic acid, has been shown to be superior to dynamic CT or MRI enhanced by other types of contrast agents for detecting and characterizing liver lesions in the intrahepatic staging workup of HCC. However, to our knowledge, there is no data on the use of MRI in the screening or surveillance setting.
To determine the extent to which MRI with liver-specific contrast detects additional HCCs missed by US screening, we screened 407 high-risk patients with paired US and MRI at 6-month intervals for 3 rounds.
Methods
Study Population
This prospective study was conducted at Asan Medical Center, an academic tertiary care center in Korea (The PRIUS study, ClinicalTrials.gov ID NCT01446666). Study participants were recruited between November 2011 and August 2012. The inclusion criteria for participation were an age of 20 years or older and the presence of cirrhosis with an estimated annual HCC risk of more than 5%. Cirrhosis was diagnosed histologically and/or radiologically. The risk of HCC was estimated by using a model with some modifications as follows: risk index = 1.41 (if the age is ≥50 years) + 1.65 (if the prothrombin activity is ≤75%) + 0.92 (if the platelet count is <100 × 103/mm3) + 0.74 (if anti-hepatitis C virus antibody or hepatitis B virus surface antigen test is positive). The risk index greater than 2.33 was estimated to correspond to an annual risk of developing HCC of more than 5%.
Other eligibility criteria included Eastern Cooperative Oncology Group performance status of 0 or 1, and absence of previous history or current suspicion of HCC. The absence of HCC had been evaluated by US, dynamic CT scan, or MRI within 6 months before enrollment. Patients with Child-Pugh class C liver function or an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 were excluded.
The study was approved by the institutional review board of Asan Medical Center. All participants gave written informed consent and they were not compensated for participating. The trial protocol is provided in Supplement 1.
Study Protocol
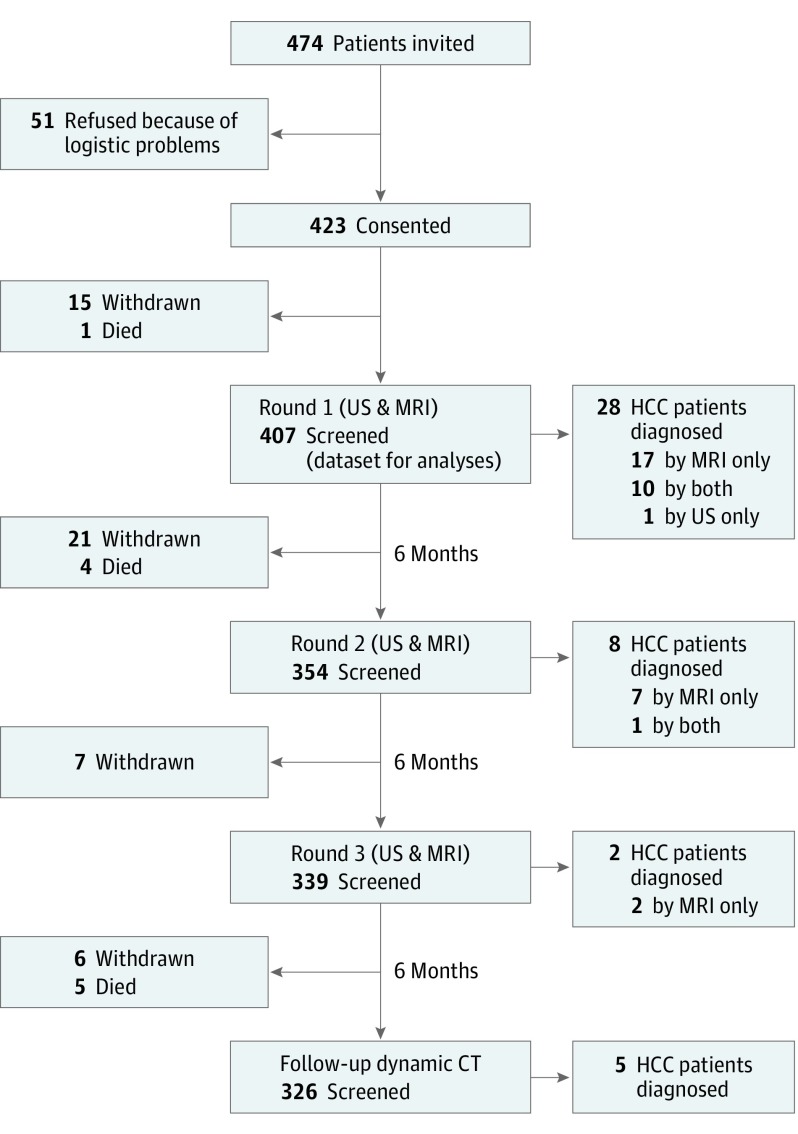
The patients were evaluated by 3 rounds of screening tests with paired US and gadoxetic acid-enhanced MRI at 6-month intervals (Figure 1). The first screening round was performed at 6 months after their most recent imaging session before enrollment.
Figure 1. Study Profile.
After enrollment, a total of 49 patients withdrew from further participation owing to logistic problems (n = 38), refusal of MRI (n = 7), breathing difficulty (n = 2) and claustrophobia (n = 1) during MRI scanning, and injection site complications (n = 1).
CT indicates computed tomography; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; US, ultrasonography.
Both US and MRI were performed on the same day whenever possible, or within 7 days of one another. Liver MRI was performed with a 1.5-T scanner (Magnetom Avanto; Siemens). Gadoxetic acid (Primovist; Bayer) was administered at a dose of 0.025 mmol/kg. Axial T1-weighted images of the arterial, portal, delayed, and hepatobiliary phases were obtained at 4-mm slice thickness.
Ultrasonographic examinations and MRI interpretations were allocated by an independent research coordinator (D.K.K.) to different radiologists (S.Y.K., S.J.L., H.J.W., or J.H.B.) who specialized in liver imaging with substantial expertise. Radiologists were blinded to the findings of the other imaging modality of the same and previous screening rounds. The findings of MRI and US were recorded in a predefined standardized way on a 5-point scale for MRI or 4-point scale for US, indicating the likelihood of HCC: highly suggestive, suspicious, equivocal, probably benign, or definitely benign/negative, corresponding to categories 5, 4, 3, 2, and 1, respectively (eTable 1 and 2 in Supplement 2).
The positive screening criterion was a category 5 or 4 on MRI or US. When an MRI or US examination detected a nodule scored as category 5 or 4, a recall process with dynamic 4-phase CT scan was performed within 3 months. Cases that were suspicious for HCC on CT imaging underwent biopsy under US guidance whenever possible. If a lesion was detected only by MRI, a targeted second-look ultrasound was performed under the real-time US-CT fusion imaging guidance to help guide the biopsy. The confirmation of HCC was based on the results of histologic examination and/or typical CT images (nodule >1 cm with arterial hypervascularity and portal/delayed-phase washout) as recommended by practice guidelines.
Hepatocellular carcinoma stages were defined by the Barcelona Clinic Liver Cancer (BCLC) staging system: very early stage as single nodule smaller than 2 cm; early stage HCC as a single 2- to 5-cm lesion or 2 to 3 lesions each smaller than 3 cm.
Follow-up
At 6-months after the last screening round, all study patients were followed up with dynamic CT scans to exclude false-negative findings of the last screening examinations. After then, study participants were followed up for at least 2 years for vital status. Study participants who completed at least 1 paired screening examination but who left the study for any reason before completing 3 screening rounds were also followed up.
Statistical Analysis
The primary end point was detection rates of US and MRI examinations for patients with HCC. A sample size of 380 (including 19 predicted HCC incidences) was calculated to achieve 81% power for the difference in HCC detection rate of 70% for US and 92% for MRI. Assuming a maximum drop-out rate of 10%, the required sample size was 423.
The results have been calculated on the basis of data on patients with HCC that were detected during the 3 rounds of screening tests and by follow-up dynamic CT scan 6 months after the last screening round. The HCC detection rate was defined as the number of patients with HCC detected by a given modality divided by the total number of patients with HCC detected by all modalities and by follow-up dynamic CT scan. The false-positive rate was defined as the number of tests with positive findings by a specific imaging modality in patients without an HCC; and the positive predictive value was the number of true-positive test results in patients with the positive tests in a specific modality.
Differences in the relative HCC detection rate and false-positive rate of each modality were compared using the McNemar test. Survival of the patients was calculated from the date of first screening examination to the date of death or of last follow-up (June 30, 2015).
All reported P values are 2-sided and are not adjusted for multiple testing. All statistical analyses were performed using R software (v3.1.1, R project).
Results
Study Population
Among 423 patients with cirrhosis who consented to the study, 407 patients who received at least 1 pair of screening tests with US and MRI constituted the study cohort (Figure 1). The median (interquartile range [IQR]) age at entry was 56 (52-62) years, and the proportion of patients with Child-Pugh class A was 78.6% (Table 1). After enrollment, 49 patients withdrew from further participation mostly owing to logistic problems (n = 38), and 10 died of liver failure without HCC.
Table 1. Patient Characteristics at Study Entry.
| Characteristic | Value |
|---|---|
| No. of patients | 407 |
| Age, median (IQR), y | 56 (52-62) |
| Male | 230 (56.5) |
| Female | 177 (43.5) |
| BMI | 24.3 (22.4-26.8) |
| Cause of cirrhosis, No. (%) | |
| Hepatitis B virus | 288 (70.8) |
| Hepatitis C virus | 37 (9.1) |
| Alcohol | 52 (12.8) |
| Others | 30 (7.4) |
| Diagnosis of cirrhosis, No. (%) | |
| Histological | 15 (3.7) |
| Radiological | 392 (96.3) |
| Liver stiffness, median (IQR), kPaa | 14.5 (10.5-18.7) |
| Alanine aminotransferase, median (IQR), IU/mL | 24 (18-32) |
| Albumin, median (IQR), g/dL | 3.8 (3.5-4.2) |
| INR, median (IQR) | 1.2 (1.1-1.3) |
| Total bilirubin, median (IQR), mg/dL | 1.4 (1.1-4.0) |
| Creatinine, median (IQR), mg/dL | 0.8 (0.7-0.9) |
| Alpha-fetoprotein, median (IQR), ng/mL | 3 (2-5) |
| Platelet, median (IQR), ×1000/mm3 | 73 (52-89) |
| Model for end-stage liver disease, median (IQR) | 9.8 (8.3-12.0) |
| Child-Pugh, median (IQR) | |
| Score | 5 (5-6) |
| Class | |
| A, No. (%) | 320 (78.6) |
| B, No. (%) | 87 (21.4) |
| Preceding test before the study, No. (%) | |
| Ultrasonography | 267 (65.6) |
| Dynamic computed tomography | 137 (33.7) |
| Dynamic magnetic resonance imaging | 3 (0.7) |
| Performance status, No. (%)b | |
| 0 | 382 (93.9) |
| 1 | 25 (6.1) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range.
Liver stiffness was measured by shear-stress ultrasound elastography. The test results were not obtainable in 17 (4.2%) patients. F4 fibrosis (>9.5 kPa) was observed in 333 (81.0%) of patients.
Performance status was estimated using the Eastern Cooperative Oncology Group scale.
Of 1100 screening rounds, 762 (69.3%) of the US and MRI examinations were done on the same day, and only 10 (0.9%) were more than a week apart (maximum 13 days).
HCC Detection
In 1100 screening rounds of paired US and MRI, HCC was diagnosed in 38 patients. In addition, 5 patients were diagnosed with HCC at follow-up CT scan 6 months after negative findings on the last screening round with US and MRI (Figure 1). There were no interval cancers that were detected by clinical symptom or by unscheduled examinations between 2 rounds of screening after negative findings on screening. Thus, the total number of patients with HCC was 43 during median follow-up of 1.5 years, and the overall incidence rate of HCC was 8.5 per 100 patient-years.
Of the 43 patients with HCC, 39 showed typical features of HCC on dynamic CT images, and others were diagnosed pathologically. Pathological diagnosis of HCC was possible in 20 patients. Biopsy was unobtainable in the others because the nodules were not visualized by US or the location was deemed risky to target.
Of the 43 patients, 32 (74.4%) had very early-stage (single nodule <2 cm) and 10 (23.3%) had early-stage HCC (single 2-5 cm or 2-3 lesions each <3 cm). Only 1 patient had advanced stage HCC of 3.5 cm size with portal vein invasion that was detected by both of US and MRI at the first screening round (Table 2).
Table 2. Characteristics of Patients With Hepatocellular Carcinoma at Diagnosis.
| Characteristics | Patients With HCC (n = 43) |
|---|---|
| Age at diagnosis, median (range), y | 59 (55-65) |
| Male, No. (%) | 29 (67.4) |
| Female | 14 (32.6) |
| Cause of cirrhosis, No. (%) | |
| Hepatitis B virus | 32 (74.4) |
| Hepatitis C virus | 5 (11.6) |
| Alcohol | 1 (2.3) |
| Others | 5 (11.6) |
| Liver stiffness, median (IQR), kPaa | 17.0 (12.1-19.4) |
| Albumin, median (IQR), g/dL | 3.6 (3.3-3.8) |
| INR, median (IQR) | 1.23 (1.15-1.32) |
| Total bilirubin, median (IQR), mg/dL | 1.5 (1.1-2.6) |
| Ascites, No. (%) | 6 (14.0) |
| Child-Pugh score, median (IQR) | 6 (5-7) |
| Preceding test before the study, No. (%) | |
| Ultrasonography | 32/267 (74.4) |
| Dynamic computed tomography | 11/137 (25.6) |
| Dynamic magnetic resonance imaging | 0/3 (0) |
| Pathologic diagnosis | 20 (46.5) |
| Alpha-fetoprotein, median (IQR), ng/mL | 5 (3-10) |
| Tumor size, median (IQR), cmb | 1.5 (1.1-1.8) |
| No. of tumors, No. (%) | |
| 1 | 39 (90.7) |
| 2 | 3 (7.0) |
| 3 | 1 (2.3) |
| Vascular invasion, No. (%) | 1 (2.3) |
| HCC stage, No. (%)c | |
| Very early | 32 (74.4) |
| Early | 10 (23.3) |
| Advanced | 1 (2.3) |
| Initial treatment, No. (%) | |
| Transplantation | 4 (9.3) |
| Surgical resection | 4 (9.3) |
| Local ablation | 21 (48.8) |
| TACE | 12 (27.9) |
| Systemic chemotherapy | 0 |
| No treatment | 2 (4.7) |
Abbreviations: HCC, hepatocellular carcinoma: INR, international normalized ratio; IQR, interquartile range; TACE, transarterial chemoembolization.
Liver stiffness was measured by shear-stress ultrasound elastography. The test results were not obtainable in 4 (9.3%) patients.
Maximum tumor diameter.
HCC stages were defined by the BCLC staging system: very early stage as single nodule smaller than 2 cm; early stage HCC as single 2- to 5-cm lesion or 2 to 3 lesions each smaller than 3 cm, with no vascular invasion or distant metastases. One patient had advanced stage HCC of 3.5 cm size with portal vein invasion that was detected by both US and MRI at first screening round.
Performance of the Screening Methods
The positive screening criterion was category 4 or 5 on US or MRI. Of the 43 patients with HCCs: 1 was detected by US only, 26 by MRI only, 11 by both, and 5 were missed by both and were detected by follow-up CT scan (Table 3). The overall HCC detection rates of US and MRI were 27.9% and 86.0%, respectively (P < .001). For very early stage cancers only, the respective HCC detection rates were 27.3% and 84.8% (P < .001). The false-positive rates were 5.6% (59/1057) for US, which was significantly higher than the 3.0% (32/1057) for MRI (P = .004). Among 71 positive findings by US, 12 patients were confirmed to have HCC, giving a positive predictive value of 16.9%. Among 69 positive findings by MRI, 37 cases were confirmed as having HCC, giving a positive predictive value of 53.6%.
Table 3. Hepatocellular Carcinoma Detection Rate, False-Positive Rate, and Positive Predictive Value of the 2 Surveillance Methodsa.
| Surveillance Method and Category | Tests, No. | Patients With HCC, No. | Cumulative Total of Tests, No. | Cumulative True-Positive Results, No. | Detection Rate for Any HCC (Sensitivity), % | Detection Rate for Very Early and Early Stage HCC (Sensitivity), % | Detection Rate Very Early Stage HCC (Sensitivity), % | Specificity, % | False-Negative Rate, % | False-Positive Rate, % | PPV, % | Biopsy Procedures Performed, No. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US | ||||||||||||
| 4 (Suspicious) | 71 | 12 | 71 | 12 | 27.9 | 26.2 | 27.3 | 94.4 | 72.1 | 5.6 | 16.9 | 4 |
| 3 (Equivocal) | 5 | 0 | 76 | 12 | 27.9 | 26.2 | 27.3 | 93.9 | 72.1 | 6.1 | 15.8 | 0 |
| 2 (Probably benign) | 32 | 2 | 108 | 14 | 32.6 | 31.0 | 33.3 | 91.1 | 67.4 | 8.9 | 13.0 | 2 |
| 1 (Definitely benign/ negative) | 992 | 29 | 1100 | 43 | 100 | 100 | 100 | 0.0 | 0.0 | 100 | 3.9 | 14 |
| MRI | ||||||||||||
| 5 (Highly suggestive) | 33 | 26 | 33 | 26 | 60.5 | 59.5 | 54.5 | 99.3 | 39.5 | 0.7 | 78.8 | 12 |
| 4 (Suspicious) | 36 | 11 | 69 | 37 | 86.0 | 85.7 | 84.8 | 97.0 | 14.0 | 3.0 | 53.6 | 6 |
| 3 (Equivocal) | 15 | 1 | 84 | 38 | 88.4 | 88.1 | 84.8 | 95.6 | 11.6 | 4.4 | 45.2 | 1 |
| 2 (Probably benign) | 92 | 0 | 176 | 38 | 88.4 | 88.1 | 84.8 | 86.9 | 11.6 | 13.1 | 21.6 | 0 |
| 1 (Definitely benign/ negative) | 924 | 5 | 1100 | 43 | 100 | 100 | 100 | 0.0 | 0.0 | 100 | 3.9 | 1 |
Abbreviations: HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; PPV, positive predictive value; US, ultrasonography.
The results have been calculated on the basis of data on patients with HCC that were detected during the 3 rounds of screening tests and by follow-up dynamic CT scan 6 months after the last screening round. No interval cancer was detected between the screening rounds and before the follow-up CT scan. The positive screening criterion was a category 5 or 4 on US or MRI. The cumulative number of true positive results is the number of patients with HCC found in a specific imaging category or higher; the HCC detection rate is the percentage of patients with HCC with a positive test result in a specific category or higher (the cumulative number of true positive results divided by the total number of patients with HCC); the false positive rate is the percentage of positive test results in patients without a cancer; and the PPV is the percentage of true positive test results in patients with the positive tests in a specific imaging category or higher (the cumulative number of true positive test results divided by the cumulative number of tests).
The area under the receiver operating characteristic curve (AUROC) for MRI was 0.93 (95% CI, 0.87-0.98), which was significantly higher than that for US (0.62; 95% CI, 0.55-0.69; P < .001) (eFigure 1 in Supplement 2).
Among the 59 false-positive findings on US, 27 lesions were confirmed as pseudolesions that did not have any matched lesions on follow-up CT and MRI, 20 were cirrhosis-related nodules, 4 were exophytic hepatic parenchyma, 3 were complicated cysts, and 1 was abnormal vasculature. Out of the 32 false-positive findings on MRI, 12 were abnormal vasculature, 8 were cirrhosis-related nodules, 7 were pseudolesions that did not have any matched lesions on follow-up CT and MRI, 3 were considered as inflammatory lesions, and 2 were hemangiomas.
Among several clinical factors and imaging characteristics, subcapsular location of the HCC was the only significant factor for the false-negative findings by US (P = .02) (eTable 3 in Supplement 2). Among 20 HCC nodules located at subcapsule, only 2 were detected by US. A representative case is shown in eFigure 2 in Supplement 2.
Six patients with HCC had false-negative findings by MRI, and 1 case was detected by US. The lesion was located close to the inferior vena cava and right hepatic vein, and could be identified on a retrospective review of the MRI images, suggesting misinterpretation by a human error (eFigure 3 in Supplement 2). The other 5 lesions were missed by MRI and US, and were identified later on follow-up CT scans obtained 6 months after the study. On a retrospective review of MRI images, 4 of the corresponding lesions were detectable, but none of them showed typical vascular patterns of HCC on the last round of MRI (eFigures 4 and 5 in Supplement 2).
Treatment and Survival of Patients With HCC
Of the 43 patients with HCC, 29 (67.4%) received potentially curative treatments, such as liver transplantation, surgical resection, and local ablation (Table 2).
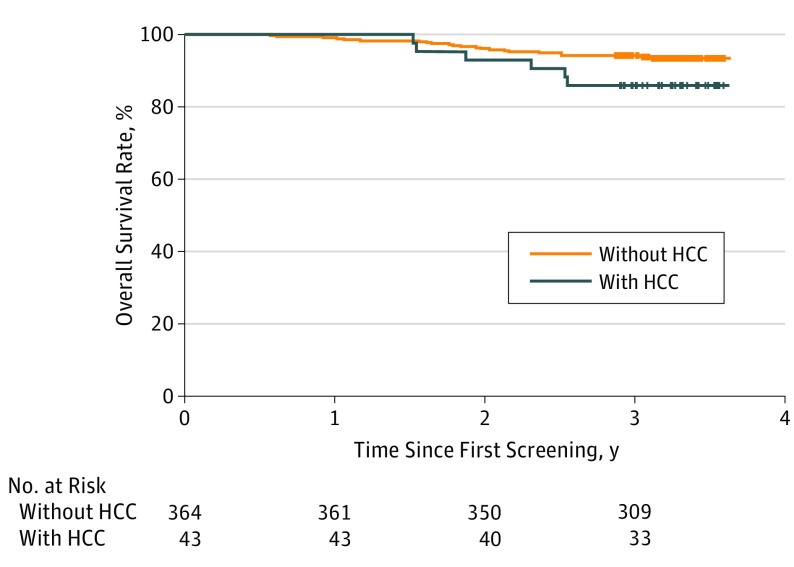
Patients were followed up for a mean of 3.3 years from the date of first screening examination to monitor survival outcome. Among 43 patients with HCC, 6 died: 1 by the progression of HCC that was detected at advanced stage and 5 by liver failure. The estimated 3-year overall survival rate of the 43 patients with HCC was 86.0%, which was not significantly lower than 94.2% of the 364 patients without HCC (hazard ratio [HR], 2.26; 95% CI, 0.92-5.56; P = .08) (Figure 2).
Figure 2. Survival Curves for Study Patients With and Without HCC.
The 3-year survival rate of the patients with HCC (86.0%) detected by the surveillance tests was not inferior to those without HCC (94.2%; hazard ratio, 2.26; 95% CI, 0.92%-5.56%; P = .08). HCC indicates hepatocellular carcinoma.
Alpha-Feto Protein (AFP)
The median level of AFP at HCC detection was 5.3 ng/mL (IQR, 2.9-10.4 ng/mL). When the positive screening value of AFP was defined as >20 ng/mL, 5 (11.6%) out of 43 patients with HCC showed elevated levels of AFP. The false-positive rate of AFP was 1.9% (20/1057). Among 25 positive findings by AFP, 5 patients were confirmed to have HCC, giving a positive predictive value of 20.0%.
The AUROC for US in conjunction with AFP for the detection of HCC was significantly higher than that of US alone (0.65; 95% CI, 0.57-0.73 vs 0.62; 95% CI, 0.55-0.69; P = .049) (eFigure 6A in Supplement 2). In contrast, the AUROC of MRI in conjunction with AFP was not higher than that of MRI alone (0.93; 95% CI, 0.87-0.98 vs 0.93, 95% CI, 0.87-0.98; P = .99) (eFigure 6B in Supplement 2).
Discussion
The results of our prospective study support our hypothesis that MRI with liver-specific contrast is more sensitive than US to detect early stage HCC in high-risk patients with cirrhosis. For very early stage HCC (single lesion <2 cm), MRI screening yielded a detection rate of 84.8%, significantly higher than the 27.3% detected by US. Magnetic resonance imaging also showed a significantly lower false-positive results rate and a significantly higher positive predictive value than US.
The proportion of patients with very early stage and early stage HCC was strikingly high in our study population (97.7%). Consistent with that finding, the patients in our study group also had a high chance of receiving curative treatments (67.4%) and a favorable rate of survival. The 3-year survival rate of 86% in our study patients with HCC was obtained despite the severely limited access to liver transplantation.
Only 27.9% of the cancers were detected by US, which is far lower than the detection rates reported in previous meta-analyses (63%). A possible explanation would be that MRI screening was able to detect tumors far earlier in their development than US, thus reducing the apparent HCC detection rate of US compared with MRI, with which 74.4% of HCC patients were detected at very early stage (single nodule <2 cm). In fact, our results are similar to the previous prospective studies of patients with advanced cirrhosis, which reported that US detected only about 20% of cases of HCC at a very early stage. Another explanation for the poor sensitivity of US may be that patients at particularly high-risk of HCC usually have inherent distortions of the liver parenchyma by cirrhosis that may obscure or, conversely, falsely simulate HCC at US imaging. The fact that we recruited only patients with advanced cirrhosis, therefore, may be reflected in our results.
Another drawback of US screening was that it had a significantly higher false-positive rate than MRI, generating more findings that required unnecessary additional recall examinations with dynamic CT and/or biopsy, which caused additional cost and potential harm to patients. The addition of AFP marginally improved the discriminating capacity of US, but not that of MRI.
A major consideration in implementing an MRI surveillance program for HCC would be the cost-effectiveness of the intervention. In general, the cost-effectiveness of a cancer screening program relies on multiple factors including the characteristics of the population under surveillance, the test’s performance, the test’s cost, and the availability of treatments that substantially extend survival of the patients. With the limited availability of liver transplantation, surveillance for HCC would only be cost-effective in patients with Child-Pugh Class A. Considering these points, we excluded patients with Child-Pugh Class C.
Limitations
This study should be interpreted within its limitations. First, pathological confirmation of HCC was not possible in all patients, mostly because of the inability to obtain biopsy samples by US-guidance. Dynamic CT, which was used for the diagnosis of HCC, has lower sensitivity than MRI with liver-specific contrast. However, the CT diagnostic criteria we used (arterial hypervascularity and portal/delayed-phase washout) are highly specific for HCC in patients with cirrhosis, as recommended by practice guidelines. Therefore, there would be little possibility for false diagnosis or overdiagnosis of HCC in our study patients. Second, the high survival rate of our patients with HCC might be overestimated by lead-time bias. However, the possibility is low because high proportion of patients received curative treatment, and only 1 patient died from HCC progression during follow-up for more than 2 years. Third, our findings should also be viewed in the light of the different degrees of resources needed to offer MRI and US screening. MRI availability is limited and it requires more personnel, costly facilities, and more consumables than US. Despite the fact that MRI is in increasing use for HCC surveillance in clinical practice, our data do not support the use of MRI surveillance rather than US in broader populations of patients at intermediate or low risk for HCC. Lastly, our study may have limitations for generalization of the results. The predominant population (74.4%) included in this study had HBV-associated cirrhosis.
Conclusions
This study shows that, in patients with cirrhosis at high risk of HCC, screening using MRI with liver-specific contrast resulted in a higher HCC detection rate and lower false-positive results compared with US. With MRI screening, most of the cancers detected were at very early stage, which was associated with a high chance of curative treatments and favorable survival of patients. Since the annual risk of developing HCC is not uniform across all patients with cirrhosis, the tailored surveillance strategy based on the individual HCC risks may enable delivery of precision medicine to patients and improve their clinical outcomes. Whether surveillance with liver-specific contrast-enhanced MRI would reduce mortality from HCC in high-risk patients requires further investigation.
Trial Protocol
eTable 1. Magnetic resonance imaging categories and scoring criteria for hepatocellular carcinoma.
eTable 2. Ultrasonography categories and scoring criteria for hepatocellular carcinoma.
eFigure 1. Receiver operating characteristic curves for US and MRI in the detection of overall and very early stage HCC.
eTable 3. Factors associated with the false-negative findings of US to detect HCC nodules.
eFigure 2. A representative patient with a HCC detected only by gadoxetic acidenhanced MRI.
eFigure 3. The patient with a HCC detected only by US.
eFigure 4. A representative patient with a HCC false negative both on US and MRI.
eFigure 5. A representative patient with a HCC false negative both on US and MRI.
eFigure 6. Receiver operating characteristic curves for US and MRI with or without AFP in the detection of HCC.
References
- 1.Fitzmaurice C, Dicker D, Pain A, et al. ; Global Burden of Disease Cancer Collaboration . The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245-1255. [DOI] [PubMed] [Google Scholar]
- 4.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5)(suppl 1):S35-S50. [DOI] [PubMed] [Google Scholar]
- 5.Allemani C, Weir HK, Carreira H, et al. ; CONCORD Working Group . Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman M. Hepatocellular carcinoma: screening and staging. Clin Liver Dis. 2011;15(2):323-334, vii-x. [DOI] [PubMed] [Google Scholar]
- 7.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47(1):82-89. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-943. [DOI] [PubMed] [Google Scholar]
- 11.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santi V, Trevisani F, Gramenzi A, et al. ; Italian Liver Cancer (ITA.LI.CA) Group . Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53(2):291-297. [DOI] [PubMed] [Google Scholar]
- 13.Singal AG, Nehra M, Adams-Huet B, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108(3):425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Poggio P, Olmos S, Ciccarese F, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(11):1927-1933 e2. [DOI] [PubMed] [Google Scholar]
- 15.Yu NC, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(2):161-167. [DOI] [PubMed] [Google Scholar]
- 16.Liu WC, Lim JH, Park CK, et al. Poor sensitivity of sonography in detection of hepatocellular carcinoma in advanced liver cirrhosis: accuracy of pretransplantation sonography in 118 patients. Eur Radiol. 2003;13(7):1693-1698. [DOI] [PubMed] [Google Scholar]
- 17.Kim HD, Lim YS, Han S, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology. 2015;148(7):1371-1382. [DOI] [PubMed] [Google Scholar]
- 18.Chou R, Cuevas C, Fu R, et al. Imaging Techniques for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;162(10):697-711. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275(1):97-109. [DOI] [PubMed] [Google Scholar]
- 20.Velázquez RF, Rodríguez M, Navascués CA, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37(3):520-527. [DOI] [PubMed] [Google Scholar]
- 21.Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014;33(4):227-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101(4):422-434. [DOI] [PubMed] [Google Scholar]
- 23.Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003;98(3):679-690. [DOI] [PubMed] [Google Scholar]
- 24.Hammerstingl R, Huppertz A, Breuer J, et al. ; European EOB-study group . Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18(3):457-467. [DOI] [PubMed] [Google Scholar]
- 25.Joshi K, Mendler M, Gish R, et al. Hepatocellular carcinoma surveillance: a national survey of current practices in the USA. Dig Dis Sci. 2014;59(12):3073-3077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Magnetic resonance imaging categories and scoring criteria for hepatocellular carcinoma.
eTable 2. Ultrasonography categories and scoring criteria for hepatocellular carcinoma.
eFigure 1. Receiver operating characteristic curves for US and MRI in the detection of overall and very early stage HCC.
eTable 3. Factors associated with the false-negative findings of US to detect HCC nodules.
eFigure 2. A representative patient with a HCC detected only by gadoxetic acidenhanced MRI.
eFigure 3. The patient with a HCC detected only by US.
eFigure 4. A representative patient with a HCC false negative both on US and MRI.
eFigure 5. A representative patient with a HCC false negative both on US and MRI.
eFigure 6. Receiver operating characteristic curves for US and MRI with or without AFP in the detection of HCC.