Abstract
Introduction
Tuberculosis, particularly multi-drug-resistant tuberculosis, is a major cause of morbidity and mortality worldwide. To the best of our knowledge, however, no study to date has assessed the combined use of the four available drugs for tuberculosis treatment, which is an issue of great clinical relevance.
Objective
To determine whether the four-drug fixed-dose combination is safer or more effective than separate drugs for treatment of pulmonary tuberculosis.
Methods
A systematic review of the literature was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results
In pooled results from five randomized controlled trials with 3502 patients across Africa, Asia, and Latin America, four-drug fixed-dose combination therapy was no better than separate drugs therapy in terms of culture conversion after 2 and 6 months of treatment. There were no significant differences between the groups in overall incidence of adverse effects. However, the meta-analytic measure (log odds ratio) revealed that separate drugs treatment had a 1.65 [exp (0.5) = 1.65] increased chance of gastrointestinal adverse effects compared to four-drug fixed-dose combination treatment.
Conclusions
The reviewed studies showed that four-drug fixed-dose combination therapy provides greater patient comfort by reducing the number of pills and the incidence of gastrointestinal adverse effects, as well as simplifying pharmaceutical management at all levels.
Introduction
Tuberculosis (TB) continues to be a major cause of morbidity and mortality worldwide, with 9 million new cases of TB diagnosed and 1.5 million TB-related deaths recorded globally in 2013. Approximately 95% of the estimated numbers of TB cases occur in low-income countries, with 82% of these cases being concentrated in 22 countries, among which Brazil ranks 17th.1 This TB burden is increased by human immunodeficiency virus (HIV) infection, which impairs the immune system and allows progression to active TB disease in large numbers of people.2
Furthermore, the global burden of drug-resistant TB is growing. In 2010, an estimated 650,000 cases of drug-resistant TB were reported worldwide.3 Incidence of drug-resistant TB has been on the rise in Brazil, according to data obtained in the Second Brazilian National Survey on Anti-TB Drug Resistance 2007–2008.4 In 2014, the Brazilian Ministry of Health delivered 148 GeneXpert instrument systems to all 92 municipalities that comprise the Rapid TB-Test Network, which covers all Brazilian states. These instrument systems are capable of diagnosing TB in 2 h, while simultaneously identifying the sensitivity profile to rifampicin, one of the main drugs for TB treatment.5 Alongside the rising prevalence of drug-resistant TB, there has been an increase in the spread of cases due to direct contact with drug-resistant TB patients. Consequently, drug-resistant TB has become an epidemic itself, especially in high-burden settings.6, 7 Multidrug resistance is a further threat to TB control. Development of drug- or multi-drug-resistant (MDR) TB is caused by inadequacies in treatment, such as in the number of drugs in the regimen to which the bacilli are susceptible, the dose or dosing frequency, the drug quality, or the treatment adherence.3, 8, 9
Fixed-dose combinations (FDCs) of drugs for TB treatment have been advocated internationally to prevent the emergence of drug resistance attributable to inappropriate drug intake.10, 11 Use of FDCs can reduce the risk of an incorrect dosage, simplify drug procurement, and aid in ensuring adherence without changing the drug dosage. In 2010, Brazil's National TB Program altered their traditional anti-TB treatment (2RHZ/RH regimen), which comprised rifampicin (R), isoniazid (H), and pyrazinamide (Z) for 2 months followed by R and H for 4 months. The change followed a report by the Second Brazilian National Survey on Anti-TB Drug Resistance (2007–2008), which showed that primary resistance to H or H + R had increased from 4.4% to 6.0% and from 1.1% to 1.4%, respectively, compared to data from the First Brazilian Survey (1995–1997). In the new 2RHZE/4RH regimen, a fourth drug, ethambutol (E), was added to the intensive phase (first 2 months) of TB treatment. Capsules containing R and H, administered with Z tablets, were replaced by FDC tablets containing R, H, Z, and E. In the new formulation, H and Z were administered at lower doses compared to the traditional 2RHZ/RH regimen. Pharmacological presentation of this scheme is a tablet containing a FDC of four drugs: 150 mg of R, 75 mg of H, 400 mg of Z, and 275 mg of E. The 2RHZE/RH scheme is still recommended for children under 10 years of age.4
The basic treatment of TB with four drugs is used worldwide, showing excellent effectiveness, particularly among patients with good treatment adherence. With the addition of a fourth drug, it is expected that treatment success will improve, preventing any further increase in resistance to H with or without R. FDC regimens have advantages such as improved patient comfort and treatment adherence (by reducing the number of pills) and simplified pharmaceutical management at all levels.4 The aims of this new approach were to increase treatment adherence and prevent drug resistance.12
Over the years, problems have been found with the quality of the 2RHZE/4RH regimen, such as a reduced bioavailability of R, instability of the formulation, toxic/allergic AEs, and development of resistance. Several studies have been conducted to assess the bioavailability, acceptability, and microbiological efficacy of R and H, with or without Z, administered as a FDC for daily or intermittent use.13, 14, 15, 16, 17 However, in patients with newly diagnosed TB, the use of four drugs in a fixed-dose combination (4-FDC) in the first 2 months of treatment has not been assessed for safety and efficiency relative to the administration of separate drugs (SDs).
To frame recent studies within the broader evidence base, we systematically reviewed randomized clinical trials (RCTs) that provided clinical data regarding the efficacy and safety of 4-FDC drugs in the treatment of pulmonary TB. This study aimed to determine whether the administration of 4-FDC is safer or more effective than SD regimens for the treatment of pulmonary TB.
Methods
A systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18 The protocol for this review was recorded on 23 May 2013 in the International Register of Prospective Systematic Reviews (PROSPERO) under registration no. CRD42013003217.
Search strategy and selection criteria
Articles were searched in the following databases: Cumulative Index to Nursing and Allied Health Literature (CINAHL – http://www.cinahl.com), Cochrane Library (http://www.update-software.com/cochrane), Latin American and Caribbean Literature in Health Sciences (LILACS; http://lilacs.bvsalud.org/), MEDLINE (http://www.nlm.nih.gov), Scientific Electronic Library Online (SciELO; http://www.scielo.br), Scopus (http://www.scopus.com), Web of Science (http://www.webofknowledge.com), Science Direct (http://www.sciencedirect.com), ExcerptaMedica Database (EMBASE; http://www.embase.com), CAPES Theses Database, and public domain internet databases (http://www.periodicos.capes.gov.br). All databases were searched from inception through 10 September 2013 for articles in English, French, and Spanish. We sought to compare results from RCTs involving patients with newly diagnosed TB who were administered a 4-FDC or SD regimen in the first 2 months of treatment for pulmonary TB. Therefore, a search strategy was developed by combining the following search terms: tuberculosis; treatment; and rifampicin; and isoniazid; and ethambutol; and pirazinamide; and not HIV, as exploded Medical Subject Headings (MeSH) and free-text terms. Additionally, abstracts from the following conferences until September 2013 were searched: Union World Conference on Lung Health, Interscience Conference on Antimicrobial Agents and Chemotherapy, Society of General Microbiology, British Thoracic Society, European Respiratory Society, and the American Thoracic Society. Bibliographies of all relevant articles were also reviewed.
Data extraction and management
Two independent reviewers (GCL and EVS) and a third reviewer (JSN) resolved any disagreements on selected studies. Efficacy and safety of the treatment were the primary and secondary outcomes, respectively. Treatment outcomes were recorded according to definitions adapted from those given in WHO guidelines.19 Briefly, treatment success was defined as the number of patients who were cured or who completed combined treatment. Default was defined as failure of the patient to attend the healthcare service for over 30 consecutive days after the scheduled return date. Safety was defined as the number of AEs.
The quality of studies was assessed with the Jadad scale.20 A predefined data extraction form (in EXCEL) was used to extract data from each study selected for review. The following information was recorded: study characteristics, including authors, setting, study design, and hospitalization; patient characteristics, including age, population, availability of drug susceptibility testing, sputum conversion and default rates at the beginning and end of treatment, observed AEs, previous TB regimens, HIV status, and other comorbidities; and treatment characteristics, including the number of patients receiving 4-FDC and SD regimens, and treatment outcomes.
Data analysis
When possible, statistical calculations were performed with the R software package, version 3.1.1.21 Because all evaluated outcome measures were dichotomous, odds ratios (ORs) were calculated, with the uncertainty of the result expressed by the estimate of the 95% confidence interval (CI) around this measure. Individual studies were grouped by either the fixed- or random-effects method, depending on the results of the test for homogeneity. Homogeneity among studies was evaluated by the Cochran Q test, with p > 0.05 indicating statistically significant data homogeneity.
Results
Study selection results
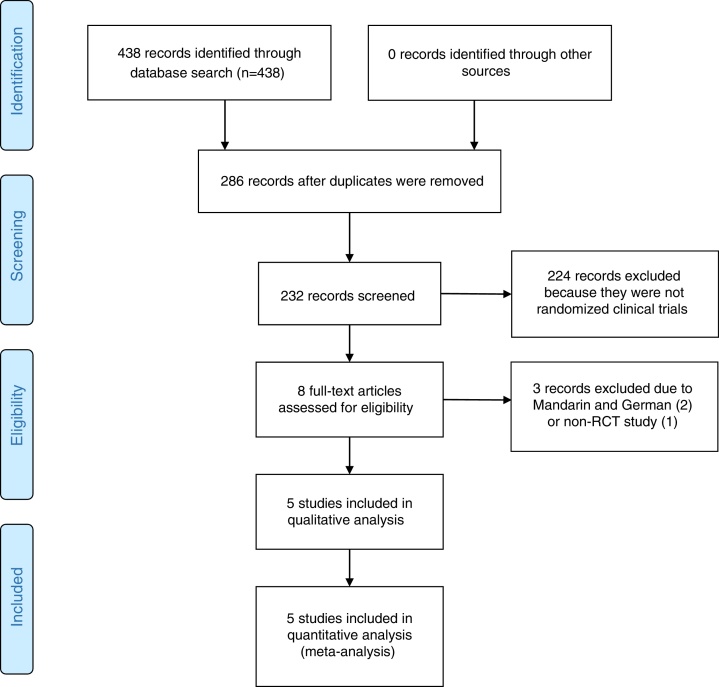
The search strategy retrieved 438 potential articles (Fig. 1). Two articles, one published in Mandarin and one in German, were excluded due to language. One article was excluded for failure to meet the criteria of an RCT. Eight articles were screened as full-text articles, and five articles were subsequently analyzed.14, 22, 23, 24, 25 All studies were conducted and published in countries with a high incidence of TB. The five studies that were considered for analysis included 3502 patients across Africa, Asia and Latin America.
Fig. 1.
Flow diagram.
Study and patient characteristics
Study and treatment characteristics are summarized in Table 1. All studies were RCTs. Of the 3502 included patients, 2072 were men (59.2%). Only three studies detailed the mean patient age, with the result of 35 ± 15.3 years. In one study, patients were randomly assigned to three groups (A, B, C): patients in groups A and B were given 4-FDC, and patients in group C were given SD formulations.25 Patients in the other studies were randomized into two groups (4-FDC and SD). All patients in the studies received the 4-FDC during the intensive phase of treatment.
Table 1.
Characteristics of randomized clinical trials, comparing FDC with SD regimens for treatment of TB, that were included in the systematic review.
| Study | No. of patients | Setting | Follow-up (mo) | Eligibility criteria | Study treatments, initial phase (2 mo)a | Jadad scale |
|---|---|---|---|---|---|---|
| Su, 200214 | 105 | Taiwan | 6 | 18+ yr with active pulmonary TB confirmed by smear or culture and no history of previous treatment for TB | FDC = INH 50 mg + RIF 120 mg + PZA 250 mg + EMB 1200 mg; SD = INH 300 mg + RIF 450 mg + PZA 150 mg + EMB 120 mg | 2 |
| Gravendeel et al., 200323 | 360 | Indonesia | 36 | Sputum positive, weight of 33–50 kg, and provided written consent for participation | FDC = INH 75 mg + RIF 150 mg + PZA 400 mg + EMB 275 mg; SD = INH 300 mg + RIF 450 mg + PZA 500 mg + EMB 250 mg | 2 |
| Zaka et al., 200825 | 293 | Pakistan | 6 | 15–55 yr, sputum positive, no kidney, liver or heart disease, and not pregnant | Group A (FDC) = INH 75 mg + RIF 120 mg + PZA 350 mg + EMB 250 mg; Group B (FDC) = INH 60 mg + RIF 120 mg + PZA 300 mg + EMB 225 mg; Group C (SD) = INH 100 mg + RMP 450 mg and 150 mg + PZA 500 mg | 3 |
| Bartacek et al., 200922 | 1159 | Egypt, India, Pakistan, Philippines, and Thailand | 21 | 15+ yr, ≥2 positive sputum samples or 1 positive sputum sample and a chest X-ray, and received treatment for TB for ≤1 mo | FDC = INH 75 mg + RIF 150 mg + PZA 400 mg + EMB 275 mg; SD = INH 75 mg + RIF 150 mg + PZA 400 mg + EMB 275 mg | 3 |
| Lienhardt et al., 201124 | 1585 | Africa, Asia, and Latin America | 30 | New diagnosis of TB confirmed by sputum smear-positive, received treatment for TB for ≤4 wk, fixed address, agreed to receive visitors, and provided written informed consent | FDC = INH 75 mg + RIF 150 mg + PZA 400 mg + EMB 275 mg; SD = INH 100 mg + RIF 150 mg + PZA 400 mg + EMB 400 mg | 3 |
EMB, ethambutol; FDC, fixed-dose combination; INH, isoniazid; PZA, pirazinomide; RIF, rifampin; SD, single dose; TB, tuberculosis.
Doses were administered on the basis of body weight according to WHO and The European Union recommendations.
Two studies provided information regarding HIV status.22, 24 In one study,24 77 of the 1168 patients (6.6%) were HIV positive. In another included study,22 only six out of 1159 patients (0.5%) were HIV positive (one in the FDC group, five in the SD group). In four studies, clinical efficacy was monitored by regular chest X-rays.14, 22, 24, 25
Two studies provided data from drug sensitivity tests.14, 24 In one study,14 four patients in the FDC group and six patients in the SD group had PZA-resistant bacilli. Two patients in the SD group had EMB-resistant bacilli. In another study,24 1132 patients (573 FDC, 559 SD) were tested for drug sensitivity. Fully sensitive organisms were identified in 508 FDC patients (88.2%) and 497 SD patients (88.9%). Non-MDR, H-resistant isolates were observed in 65 FDC patients (11.3%) and 62 SD patients (11.1%).
Only one study evaluated or reported weight gain, a decrease in the erythrocyte sedimentation rate, and an increase in hemoglobin in the initial and continuation phases of therapy.25 In another study, one patient in the FDC group (1/51, 2%) experienced bacterial relapse after 5 months of successful completion of the initial course of treatment.14
Adverse events
Table 2 summarizes the AEs reported by the included studies. The most common AEs were gastrointestinal disorders (nausea, vomiting), which were reported by all studies. However, dermatological, rheumatologic, and hepatic problems, and even death, were cited. All studies found that the difference between the 4-FDC and SD groups in the overall number of drug-related AEs was no longer significant after general disorders were excluded. Three studies reported patient death. One study,24 reported eight deaths, which were most likely (2/591 in FDC and 2/579 in SD group) or possibly due to TB (2/591 in FDC and 2/579 in SD group). In another study, only one death was reported, which was in the SD group.25 Bartacek et al. reported 15 deaths (11 in FDC and 4 in SD group), but only two of them (two hepatitis cases, both in FDC group) were considered to be drug-related.22
Table 2.
Adverse events (AEs) reported in the included studies.
| Study | No. of patients in FDC/SD group | AEs (no. of patients) in FDC group | AEs (no. of patients) in SD group | Default | Relapse | TB drug-related death |
|---|---|---|---|---|---|---|
| Su, 200214 | 26/25 | Hyperuricemia (8), skin itching (4), skin rash (2), drug fever (1), abnormal liver function (3) | Hyperuricemia (7), skin itching (7), skin rash (2), abnormal liver function (5), gastrointestinal disorders (5), blurred vision (2), sensation of numbness (1) | Not evaluated | 1/57FDC | Not evaluated |
| Gravendeel et al., 200323 | 198/162 | Gastrointestinal disorders (81), skin reaction (83), muscle-joints (64) | Gastrointestinal disorders (89), skin reaction (67), muscle-joints (73) | 1/198 FDC, 2/162 SD | Not evaluated | Not evaluated |
| Zaka et al., 200825 | 194/99 | Gastrointestinal disorders (28), skin reaction (8), muscle-joints (3) | Gastrointestinal disorders (23), skin reaction (3), muscle-joints (0), death (1) | 53/197 FDC, 30/99 SD | 0/293 FDC and SD | 1 case in group C with suspected TB meningitis |
| Bartacek et al., 200922 | 558/564 | Skin disorders (40); asthenia, headache, fever (29); musculo-skeletal disorders (20); hepatic and biliary disorders (14); others (19) | Skin disorders (30); asthenia, headache, fever (5); musculo-skeletal disorders (22); liver and biliary disorders (21); others (8) | 25/558 FDC, 15/564 SD | 6/344 FDC, 3/360 SD | 2/558 FDC cases of hepatitis |
| Lienhardt et al., 201124 | 798/787 | Rheumatological (7); dermatological (16); hepatic (5); and gastrointestinal (6) disorders; others (3) | Rheumatological (11); dermatological (15); hepatic (1); and gastrointestinal (11) disorders; others (4). | 40/798 FDC, 39/787 SD | 23/591 FDC, 19/579 SD | 4/591 FDC, 4/579 SD |
One study reported less frequent muscle-joint effects during the intensive phase of treatment in a patient receiving the FDC regimen.23 Only one study evaluated patient acceptability of medication, in terms of taste, number of tablets, and problems with swallowing. The paper reported significantly improved patient acceptability of the regimen in the 4-FDC group.22
Study quality
To evaluate the methodological quality of included trials, a Jadad scale based on three questions was used. Scores greater than 3 are indicative of high-quality studies, and scores of 2 or less are indicative of low-quality studies.20 Three studies had a high quality score, and two studies were considered low quality.
Treatment outcomes
A meta-analysis was developed for each variable in the study. Tested variables included the following: rates of sputum conversion in the initial phase of treatment (assessed within 2 months of treatment initiation), sputum conversion in the final phase of treatment, default, number of patients with AEs, and number of patients with gastrointestinal AEs. For each variable, the following null hypothesis was tested:
H0
The event of interest (variable) has the same chance of occurring in both treatment groups (4-FDC and SD).
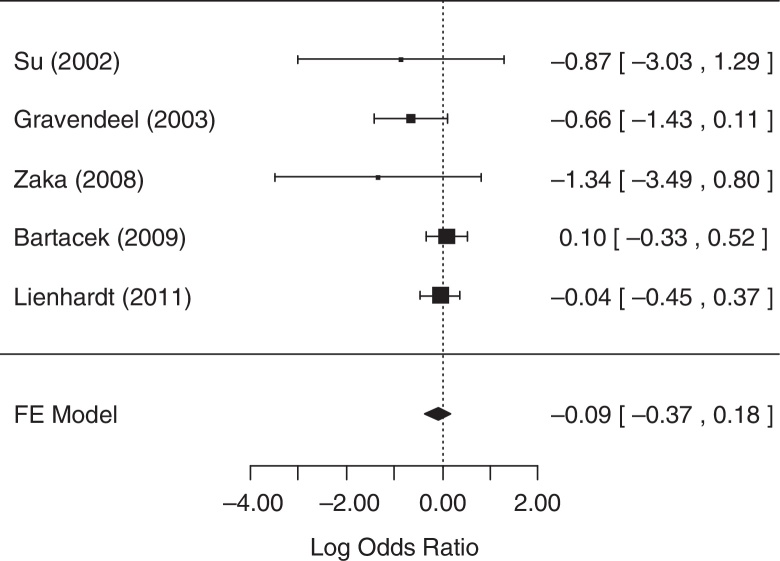
For the analysis of sputum conversion in the initial phase of treatment (≤2 months), all five studies collected related data and were considered in the analysis. The fixed-effects model was chosen because heterogeneity was not identified (p = 0.3169). The null hypothesis was not rejected (p = 0.4922), suggesting that there was no statistical evidence that the rate of sputum conversion in the initial phase of treatment differed between treatment groups. A forest plot (Fig. 2) showed that the 95% CI range for the log OR contained zero (log OR: −0.09, 95% CI: −0.37 to 0.18), indicating that the OR between treatments was statistically equal to one. Therefore, meta-analysis results did not reveal a statistically significant difference between 4-FDC and SD treatments in terms of the rate of sputum conversion in the initial phase of treatment.
Fig. 2.
Forest plot for rate of sputum conversion in the initial phase of treatment (≤2 months after start of treatment).
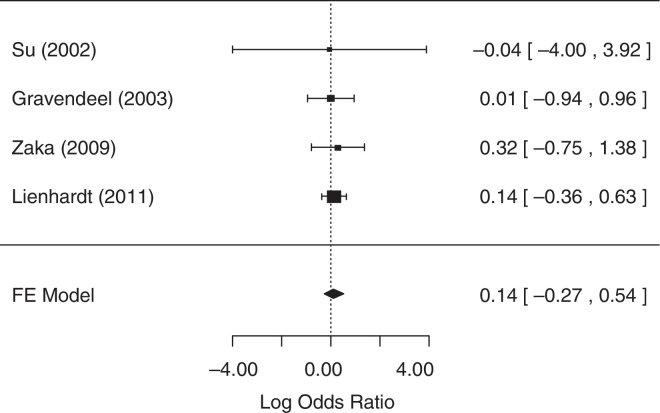
For the analysis of sputum conversion in the final phase of treatment, one study,25 did not collect related data and was excluded from the analysis. The fixed-effects model was chosen because heterogeneity was not identified (p = 0.98). The null hypothesis was not rejected (p = 0.5037), suggesting that there was no statistical evidence that sputum conversion in the final phase of treatment differed between treatment groups. A forest plot (Fig. 3) showed that the 95% CI range for the log OR contained zero (log OR: 0.14, 95% CI: −0.27 to 0.54), indicating that the OR between treatments was statistically equal to one. Therefore, meta-analysis results did not reveal a statistically significant difference between 4-FDC and SD treatments in terms of sputum conversion in the final phase of treatment.
Fig. 3.
Forest plot for sputum conversion in the final phase of therapy.
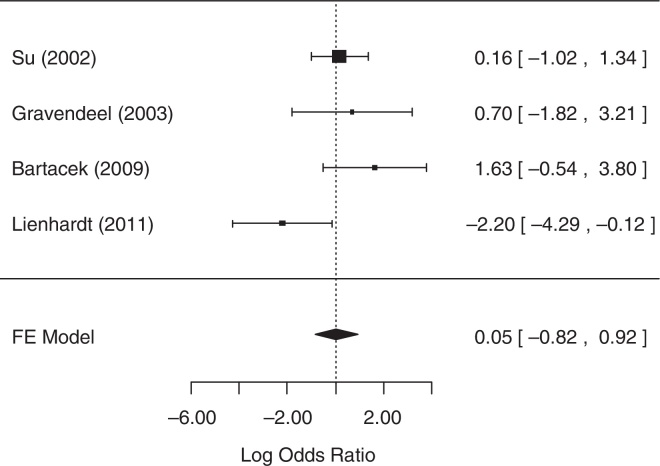
For the analysis of default, one study,25 did not collect related data. As it was not possible to contact the authors, this study was excluded from the analysis. The fixed-effects model was chosen because heterogeneity was not identified (p = 0.0775). The null hypothesis was not rejected (p = 0.9092), suggesting that there was no statistical evidence that the default differed between treatment groups. A forest plot (Fig. 4) showed that the 95% CI range for the log OR contained zero (log OR: 0.05, 95% CI: −0.82 to 0.92), indicating that the OR between treatments was statistically equal to one. Therefore, meta-analysis results did not reveal a statistically significant difference between 4-FDC and SD treatments in terms of default.
Fig. 4.
Forest plot for default.
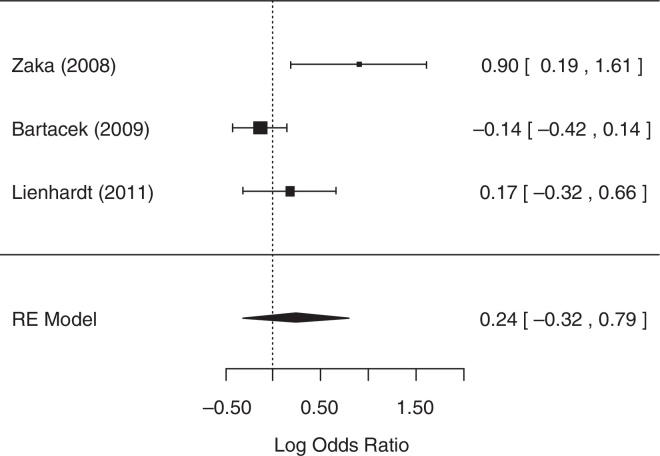
For the analysis of the number of patients with AEs, two studies,14, 23 did not collect related data and were excluded from the analysis, despite reasonable attempts to contact the authors of these studies. The random-effects model was chosen because heterogeneity was identified (p = 0.0246 and I2 = 75.85%). The null hypothesis was not rejected (p = 0.4091), suggesting that there was no statistical evidence that the number of patients with AEs differed between treatment groups. A forest plot (Fig. 5) showed that the 95% CI range for the log OR contained zero (log OR: 0.24, 95% CI: −0.32 to 0.79), indicating that the OR between treatments was statistically equal to one. Therefore, meta-analysis results did not reveal a statistically significant difference between 4-FDC and SD treatments in terms of the number of patients with AEs.
Fig. 5.
Forest plot for number of patients with adverse effects.
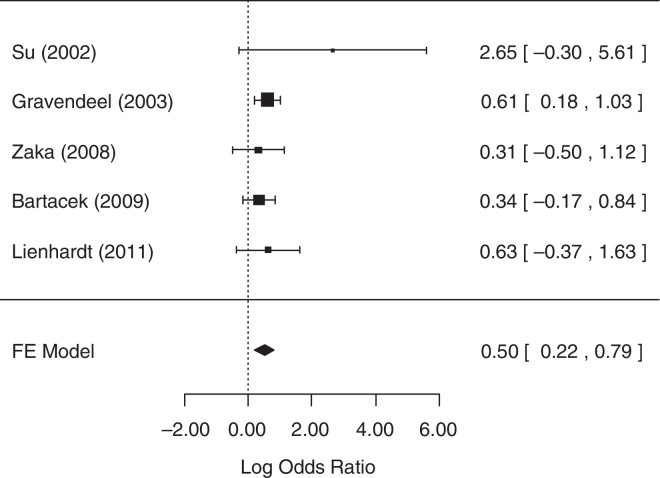
For the analysis of the number of patients with gastrointestinal AEs, all five studies collected related data and were included in the analysis. The fixed-effects model was chosen because heterogeneity was not identified (p = 0.5656). The null hypothesis was rejected (p = 0.0006), suggesting that there was statistical evidence that the chance of occurrence of gastrointestinal AEs differed between treatment groups. A forest plot (Fig. 6) showed that the 95% CI range for the log OR did not contain zero (log OR: 0.50, 95% CI: 0.22–0.79), indicating that the OR between treatments was statistically different from one. The meta-analytic measure (log OR) revealed that the SD treatment was associated with a 1.65-fold [i.e., exp (0.5) = 1.65] greater likelihood of gastrointestinal AEs than the 4-FDC treatment.
Fig. 6.
Forest plot for number of patients with gastrointestinal adverse effects.
Discussion
On the basis of the pooled results of the RCTs, 4-FDC therapy failed to show benefits over the SD regimen in culture conversion after 2 or 6 months of treatment. However, the results did not demonstrate complete inferiority of FDC compared to SD regimens when using the strict definition applied in this review. Except for one study that identified better treatment satisfaction,22 none of the included studies identified improved patient adherence among TB patients treated with 4-FDC compared to those treated with SD formulations.
Most of the side effects that were reported by the studies in this review were not considered serious and could be managed through symptomatic palliation in both groups of patients (4-FDC and SD). Even in a study that reported 176 patients (86%) with at least one AE associated with treatment, only two patients abandoned the study because of AEs.26 Gastrointestinal side effects, such as diarrhea and malabsorption, can hinder achievement of optimal blood concentrations of anti-TB drugs in patients co-infected with HIV.27 This observation suggests that 4-FDC therapy, by causing fewer gastrointestinal side effects, would benefit co-infected patients. Some patients reported stopping medication because of AEs,28 whereas others indicated that they were not informed about side effects or what to do to counter them.29, 30, 31 No ophthalmic AEs (ocular toxic effects) were reported that could be associated with the new drug (EMB). Retrobulbar optic neuritis, the main AE to EMB, is rare in the doses and exposure times commonly used for TB treatment.32
Despite the potential for providing the highest level of evidence in therapeutic intervention research, RCTs have been criticized because of their limited generalizability. RCTs are often conducted under optimal medical care and may underestimate the potential benefit of using 4-FDC formulations to enhance adherence in settings where malpractice or unmonitored therapies are common. Important differences in adherence have been found in many RCTs.33 Therefore, pragmatic clinical trials, which are conducted in a way that more closely resembles typical clinical practice, may be more appropriate to obtain a better estimate of treatment effectiveness.34, 35
At the beginning of 2013, a systematic review was published in Canada to evaluate the risk of treatment failure or disease relapse, acquired drug resistance, bacterial conversion after 2 months of treatment, AEs, adherence, and treatment satisfaction associated with treatment of active TB using FDC or SD formulations.36 This study concluded that, although FDC formulations simplify TB therapy, the current evidence did not indicate that these formulations improve treatment outcomes among patients with active TB. However, that systematic review included studies of both four-drug and two-drug combinations and, therefore, differs from the present one in the number of retrieved articles. These differences justify the need for a revision to compare precisely the effect of 4-FDC versus SD formulations.
The World Health Organization has recommended 4-FDC treatments since 1999. Combined treatments prevent drug selection by the patient (monotherapy) by providing all of the drugs in the same tablet.12, 34, 35, 37 Due to their simplified and standardized nature, 4-FDC regimens facilitate dosage calculation and prevent prescription errors. However, one of the most relevant features of 4-FDC formulations, the prevention of drug resistance, was not addressed in those studies. Nevertheless, based on their similar efficacies, user-friendliness, lower costs, and operational and logistical advantages, generalized use of 4-FDC formulations should continue to be recommended.
One limitation of this meta-analysis is that the included studies did not investigate adherence to the prescribed treatment. Moreover, the impact of the Directly Observed Treatment Short-Course (DOTS) strategy on the outcomes of TB treatment was not assessed, which resulted in less precise estimates. Another limitation is the inconsistency in ascertainment of the time of relapse in the different studies; because of the heterogeneous methods, we did not pool the study results for this variable. We could not assess mortality as an outcome because this term was defined differently in the studies (all-cause vs. TB-specific mortality), measured over different follow-up periods and, in some studies, was not reported or not attributed to the treatment group. Finally, small differences in drug concentrations existed between studies. Regardless of these limitations, this systematic review has several strengths. Lack of significant heterogeneity of the estimates of sputum conversion in the initial and final phases of therapy and of default in the different trials permitted pooling and increased the precision of our results regarding treatment efficacy.
By the end of 2009, Brazil was the only country with a high burden of TB to use a three-drug treatment regimen. Despite a free-of-charge treatment, the mean default rate was approximately 9.3% and reached 14% in some states.38 In a Brazilian descriptive study based on prospective data obtained from the medical records of adult TB patients treated with 4-FDC tablets, the obtained cure rates were similar to those obtained with SD treatments. However, the rate of treatment abandonment was much higher (17.5%) than that considered appropriate (≤5%). These data strongly suggest that the use of FDC tablets does not have a significant impact on adherence to treatment. Therefore, measures to improve adherence, such as supervised treatment, should not be neglected.11 In addition, studies conducted in Brazil have demonstrated the association between lower rates of treatment abandonment and supervised treatment.39, 40, 41
The new 4-FDC regimen was expected to result in lower default rates and higher effectiveness of treatment by preventing drug selection and the further appearance of resistant pathogens. To ensure success of the new treatment, better care and attention to patients, including expansion of DOTS strategy in Brazil, are needed. In the analysis of the Brazilian case, Zuim et al. said that the success of TB control, as with other health problems, goes beyond the availability of diagnostic tests and drugs, requiring measures related to the establishment of links between health professionals and health system users.42 Corroborating that idea, in Taiwan, a prospective RCT was conducted using the DOTS strategy to compare the safety and efficacy of two types of anti-TB regimens (FDC versus SD) for pulmonary TB treatment. No significant difference in safety or efficacy was found between the groups when the DOTS strategy was used.43
Of the 22 high TB-burden countries, Brazil is the last to adopt the 4-FDC regimen.38 Gemal et al. stated that the maintenance of low resistance rates in Brazil compared to other countries might be because medicines are distributed exclusively by public health services, in accordance with the logistics system of the Ministry of Health.44
Conclusion
Among the five variables, only gastrointestinal AEs differed significantly between treatments (SD and 4-FDC), with a meta-analytic measurement equal to 0.50 and a p-value of less than 0.001. All of the studies showed that 4-FDC therapy provides greater patient comfort by reducing the number of pills and the incidence of gastrointestinal AEs, which are the most-reported side effects, in addition to simplifying pharmaceutical management at all levels. Therefore, 4-FDC therapy is an important evolution in TB treatment. These therapies should be implemented with simultaneous pharmacovigilance studies and pragmatic trial designs to simulate real-world clinical practice, associated with new technologies and measures to establish links between health professionals and health system users.
Funding
This work has not received any funding.
Conflicts of interest
The authors declare no conflicts of interest.
Associate Editor: Agnes Marie Sá Figueiredo
References
- 1.The World Health Organization . WHO Press; Geneva, Switzerland: 2014. Treatment Outcomes. The Global Tuberculosis Report. [Google Scholar]
- 2.Skrahin A., Ahmed R., Ferrara K. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: an open-label phase 1 safety trial. Lancet Respir Med. 2014;2(2):108–122. doi: 10.1016/S2213-2600(13)70234-0. [DOI] [PubMed] [Google Scholar]
- 3.The World Health Organization . WHO Press; Geneva, Switzerland: 2012. The Burden of Disease Caused by TB. The Global Tuberculosis Report; pp. 8–28. [Google Scholar]
- 4.BRAZIL, Ministry of Health . 2009. Secretary of Health Surveillance Department of Epidemiological Surveillance. National Tuberculosis Control Programme. Technical Note on the Changes in the Treatment of Tuberculosis in Brazil for Adults and Teenagers – Version 2. Available from: http://portal.saude.gov.br/portal/arquivos/pdf/nota_tecnica_versao_28_de_agosto_v_5.pdf [cited 10.11.14] [Google Scholar]
- 5.BRAZIL, Ministry of Health . 2013. Department of Management and Incorporation of Technologies in Health Secretary of Science. Technology and Supplies Strategic – DGITS/SCTIE. National Technology Incorporation Commission on SUS (CONITEC). Report No. 49. Available from: http://www.fundacaoataulphodepaiva.com.br/wp-content/uploads/2013/03/Relatorio-XpertMTBRIF-CP5.pdf [acessed 22.10.15] [Google Scholar]
- 6.Keshavjee S., Farmer P.E. Picking up the pace-scale-up of MDR tuberculosis treatment programs. N Engl J Med. 2010;363:1781–1784. doi: 10.1056/NEJMp1010023. [DOI] [PubMed] [Google Scholar]
- 7.The World Health Organization . WHO Press; Geneva, Switzerland: 2015. Global Tuberculosis Report. [Google Scholar]
- 8.Wright A., Zignol M., Van Deun A. Global Project on Anti-Tuberculosis Drug Resistance Surveillance Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373:1861–1873. doi: 10.1016/S0140-6736(09)60331-7. [DOI] [PubMed] [Google Scholar]
- 9.Malangu N., Mngomezulu M. Evaluation of tuberculosis infection control measures implemented at primary health care facilities in Kwazulu-Natal province of South Africa. BMC Infect Dis. 2015;15:117. doi: 10.1186/s12879-015-0773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Union Anti-tuberculosis Regimens of Chemotherapy Recommendations from the Committee on Treatment of the International Union against Tuberculosis and Lung Disease. Bull Int Union Tuberc Lung Dis. 1988;63(2):60–64. [PubMed] [Google Scholar]
- 11.Ferreira A.C., Silva Junior J.L., Conde M.B., Rabahi M.F. Clinical treatment outcomes of tuberculosis treated with the basic regimen recommended by the Brazilian National Ministry of Health using fixed-dose combination tablets in the greater metropolitan area of Goiania, Brazil. J Bras Pneumol. 2013;39(1):76–83. doi: 10.1590/S1806-37132013000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blomberg B., Fourie B. Fixed-dose combination drugs for tuberculosis: application in standardised treatment regimens. Drugs. 2003;63:535–553. doi: 10.2165/00003495-200363060-00002. [DOI] [PubMed] [Google Scholar]
- 13.Acocella G., Nonis A., Gialdroni-Grassi G., Grassi C. Comparative bioavailability of isoniazid, rifampin, and pyrazinamide administered in free combination and in a fixed triple formulation designed for daily use in antituberculosis chemotherapy. I. Single-dose study. Am Rev Respir Dis. 1988;138:882–885. doi: 10.1164/ajrccm/138.4.882. [DOI] [PubMed] [Google Scholar]
- 14.Su W.J., Perng R.P. Fixed-dose combination chemotherapy (Rifater/Rifinah) for active pulmonary tuberculosis in Taiwan: a two-year follow-up. Int J Tuberc Lung Dis. 2002;6(11):1029–1032. [PubMed] [Google Scholar]
- 15.Gohel M.C., Sarvaiya K.G. A novel solid dosage form of rifampicin and isoniazid with improved functionality. AAPS PharmSciTech. 2007;8:E68. doi: 10.1208/pt0803060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhutani H., Mariappan T.T., Singh S. The physical and chemical stability of anti-tuberculosis fixed-dose combination products under accelerated climatic conditions. Int J Tuberc Lung Dis. 2004;8:1073–1080. [PubMed] [Google Scholar]
- 17.Hiremath P.S., Saha R.N. Oral matrix tablet formulations for concomitant controlled release of anti-tubercular drugs: design and in vitro evaluations. Int J Pharm. 2008;362:118–125. doi: 10.1016/j.ijpharm.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.The World Health Organization . 4th ed. WHO Press; Geneva, Switzerland: 2010. Treatment of Tuberculosis: Guidelines for National Programmes. [Google Scholar]
- 20.Jadad A.R. BMJ Books; 1998. Randomized Controlled Trials. Available from: http://www.bmjpg.com/rct/maex.htlm [accessed 03.10.13] [Google Scholar]
- 21.Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. Available from: http://www.r-project.org/ [Google Scholar]
- 22.Bartacek A., Schutt D., Panosch B., Borek M., Rimstar, FDC Study Group Comparison of a four-drug fixed-dose combination regimen with a single tablet regimen in smear-positive pulmonary tuberculosis. Int J Tuberc Lung Dis. 2009;13(6):760–766. [PubMed] [Google Scholar]
- 23.Gravendeel J.M., Asapa A.S., Becx-Bleumink M., Vrakking H.A. Preliminary results of an operational field study to compare side-effects, complaints and treatment results of a single-drug short-course regimen with a four-drug fixed-dose combination (4FDC) regimen in South Sulawesi, Republic of Indonesia. Tuberculosis (Edinb) 2003;83:183–186. doi: 10.1016/s1472-9792(02)00053-7. [DOI] [PubMed] [Google Scholar]
- 24.Lienhardt C., Cook S.V., Burgos M. Efficacy and safety of a 4-drug fixed-dose combination regimen compared with separate drugs for treatment of pulmonary tuberculosis: the Study C randomized controlled trial. JAMA. 2011;305:1415–1423. doi: 10.1001/jama.2011.436. [DOI] [PubMed] [Google Scholar]
- 25.Zaka-Ur-Rehman Z., Jamshaid M., Chaudhry A. Clinical evaluation and monitoring of adverse effects for fixed multidose combination against single drug therapy in pulmonary tuberculosis patients. Pak J Pharm Sci. 2008;21:185–194. [PubMed] [Google Scholar]
- 26.Leimane V., Riekstina V., Holtz T.H. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–326. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 27.Zumla A., Chakaya J., Centis R. Tuberculosis treatment and management—an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med. 2015;3:220–234. doi: 10.1016/S2213-2600(15)00063-6. [DOI] [PubMed] [Google Scholar]
- 28.Munro S.A., Lewin S.A., Smith H.J., Engel M.E., Fretheim A., Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4:e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaiswal A., Singh V., Ogden J.A. Adherence to tuberculosis treatment: lessons from the urban setting of Delhi, India. Trop Med Int Health. 2003;8(July (7)):625–633. doi: 10.1046/j.1365-3156.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- 30.Edginton M.E., Sekatane C.S., Goldstein S.J. Patients’ beliefs: do they affect tuberculosis control? A study in a rural district of South Africa. Int J Tuberc Lung Dis. 2002;6(December (12)):1075–1082. [PubMed] [Google Scholar]
- 31.Wares D.F., Singh S., Acharya A.K., Dangi R. Non-adherence to tuberculosis treatment in the eastern Tarai of Nepal. Int J Tuberc Lung Dis. 2003;7:327–335. [PubMed] [Google Scholar]
- 32.American Thoracic Society, CDC, and Infectious Diseases Society of America Treatment of Tuberculosis. MMWR. 2003;52(RR-11):1–82. Erratum: MMWR. 2005;53(51–52): 1195–222. [PubMed] [Google Scholar]
- 33.Haynes R.B., Ackloo E., Sahota N., McDonald H.P., Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;2:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Chalkidou K., Tunis S., Whicher D., Fowler R., Zwarenstein M. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials. 2012;9:436–446. doi: 10.1177/1740774512450097. [DOI] [PubMed] [Google Scholar]
- 35.Tunis S.R., Stryer D.B., Clancy C.M. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 36.Albanna A.S., Smith B.M., Cowan D., Menzies D. Fixed-dose combination antituberculosis therapy: a systematic review and meta-analysis. Eur Respir J. 2013;42:721–732. doi: 10.1183/09031936.00180612. [DOI] [PubMed] [Google Scholar]
- 37.Blomberg B., Spinaci S., Fourie B., Laing R. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis. Bull World Health Organ. 2001;79:61–68. [PMC free article] [PubMed] [Google Scholar]
- 38.Dalcolmo M.P. Tratamento da TB sensível e resistente. Pulmão RJ. 2012;21(1):55–59. [Google Scholar]
- 39.Ferreira S.M., Silva A.M., Botelho C. Noncompliance with treatment for pulmonary tuberculosis in Cuiaba, in the State of Mato Grosso – Brazil. J Bras Pneumol. 2005;31(5):427–435. [Google Scholar]
- 40.Souza M.S., Pereira S.M., Marinho J.M., Barreto M.L. Characteristics of healthcare services associated with adherence to tuberculosis treatment. Rev Saude Publica. 2009;43(6):997–1005. doi: 10.1590/s0034-89102009005000085. [DOI] [PubMed] [Google Scholar]
- 41.Vieira A.A., Ribeiro S.A. Compliance with tuberculosis treatment after the implementation of the directly observed treatment, short-course strategy in the city of Carapicuíba, Brazil. J Bras Pneumol. 2011;37(2):223–231. doi: 10.1590/s1806-37132011000200013. [DOI] [PubMed] [Google Scholar]
- 42.Zuim R., Menezes A., Trajman A. The Brazilian experience of implementing RHZE fixed-dose combination for tuberculosis treatment. Epidemiol Serv Saude. 2014;23:537–540. [Google Scholar]
- 43.Zhang H.Q., Xi X.E., Wang Y.L., Han W., Zhang C.X., Jiao J.H. Side effects of tuberculosis treatment with fixed-dose combinations. J Biol Regul Homeost Agents. 2015;29(2):379–388. [PubMed] [Google Scholar]
- 44.Gemal A., Keravec J., Menezes A., Trajman A. Can Brazil play a more important role in global tuberculosis drug production? An assessment of current capacity and challenges. BMC Public Health. 2013;13:279. doi: 10.1186/1471-2458-13-279. [DOI] [PMC free article] [PubMed] [Google Scholar]