Abstract
Aneurinibacillus aneurinilyticus strain CKMV1 was isolated from rhizosphere of Valeriana jatamansi and possessed multiple plant growth promoting traits like production of phosphate solubilization (260 mg/L), nitrogen fixation (202.91 nmol ethylene mL−1 h−1), indole-3-acetic acid (IAA) (8.1 μg/mL), siderophores (61.60%), HCN (hydrogen cyanide) production and antifungal activity. We investigated the ability of isolate CKMV1 to solubilize insoluble P via mechanism of organic acid production. High-performance liquid chromatography (HPLC) study showed that isolate CKMV1 produced mainly gluconic (1.34%) and oxalic acids. However, genetic evidences for nitrogen fixation and phosphate solubilization by organic acid production have been reported first time for A. aneurinilyticus strain CKMV1. A unique combination of glucose dehydrogenase (gdh) gene and pyrroloquinoline quinone synthase (pqq) gene, a cofactor of gdh involved in phosphate solubilization has been elucidated. Nitrogenase (nif H) gene for nitrogen fixation was reported from A. aneurinilyticus. It was notable that isolate CKMV1 exhibited highest antifungal against Sclerotium rolfsii (93.58%) followed by Fusarium oxysporum (64.3%), Dematophora necatrix (52.71%), Rhizoctonia solani (91.58%), Alternaria sp. (71.08%) and Phytophthora sp. (71.37%). Remarkable increase was observed in seed germination (27.07%), shoot length (42.33%), root length (52.6%), shoot dry weight (62.01%) and root dry weight (45.7%) along with NPK (0.74, 0.36, 1.82%) content of tomato under net house condition. Isolate CKMV1 possessed traits related to plant growth promotion, therefore, could be a potential candidate for the development of biofertiliser or biocontrol agent and this is the first study to include the Aneurinibacillus as PGPR.
Keywords: Valeriana jatamansi, Phosphate solubilization, Nitrogen fixation, Glucose dehydrogenase, Aneurinibacillus aneurinilyticus, PGPR
Introduction
Soil is the reservoir for a variety of living organisms comprising microorganisms, plants, and animals, which do not live in isolation, but form a complex interactive network. A very special ecological niche, the rhizosphere is present around the roots of the plants that support a group of metabolically versatile microorganisms.1 Some of these rhizospheric bacteria that are beneficial to plants are often referred as plant growth promoting rhizobacteria (PGPR). Microbial inoculants or biofertilizers are used to hasten biological activity to improve availability of plant nutrients by fixing atmospheric nitrogen, making insoluble phosphate soluble and decomposing farm wastes, which result in the release of nutrients and antagonize various pathogenic fungi by producing siderophore, HCN (hydrogen cyanide), Chitinase, β-1,3-glucanase and a variety of different antibiotics.2
Phosphorus (P) is one of the major essential macronutrients for biological growth and development but it is commonly deficient in most natural soils since it is fixed as insoluble iron and aluminium phosphates in acidic soils or calcium phosphates in alkaline soils. It is generally accepted that the major mechanism of mineral phosphate solubilization is by the action of organic acids synthesized by soil microorganisms yet the exact biochemical basis of these transformations are not completely understood but can be explored. The concentration of soluble P in soil is usually very low, normally at levels of 1 ppm or less (10 M H2PO4−). The cell might take up several P forms but the greatest part is absorbed in the forms of HPO42− H2PO4−.3, 4
The genetic basis of mineral phosphate solubilization phenotype of bacteria is not well understood. It is believed that organic acids produced by the action of glucose dehydrogenase, which require pyrroloquinoline quinone (PQQ) as cofactor and it is a primary mechanism behind phosphate solubilization; this assumption has been confirmed by the cloning of gene involved in gluconic acid production viz. gdh and PQQ. PQQ-dependent glucose dehydrogenase (GDH) is capable of oxidizing glucose to gluconate. Gluconate is further oxidized to 2-ketogluconic acid by gluconate dehydrogenase (GADH). Glucose dehydrogenase (GDH) is a member of quinoproteins, catalysing the oxidation of glucose to gluconic acid, requiring PQQ and also metal ions such as Ca2+ (or Mg2+ in vitro) for its activity. Glucose, gluconate, manitol, and glycerol are among the possible inducers of the halo enzyme activity, but the information regarding the synthesis of this holoenzyme (GDH-PQQ) is not available.5
Microbial communities are a main component of ecosystems that play critical roles in the biochemical transformations of elements including nitrogen fixation.6 Therefore, nitrogen that is available to plants grown for many years without N fertilizers is considered to be due to biological fixation.7 This process catalysed by nitrogenase enzymes is essential for maintaining fertility in many ecosystems.8 The ability to fix nitrogen is widely distributed among diverse groups of Bacteria and Archae in different ecosystems. A substantial molecular diversity of N2 fixing bacteria has been detected in field grown rice and maize based on retrieval of nif H or nif D gene fragments from root DNA.9, 10
Therefore, the present study aims to understand the possible mechanism and to characterize the genes involved in the phosphate solubilization and nitrogen fixation by Aneurinibacillus aneurinilyticus isolated from the rhizosphere of medicinal plant Valeriana jatamansi. Information about genus A. aneurinilyticus with plant growth promoting attributes from any medicinal plants is limited. Hence, the results could be useful for better understanding of mechanism of plant growth promoting traits, which can help us to engineer this PGPR for agronomic interest.
Material and methods
Isolation and characterization of PGPR from Valeriana jatamansi
Sixty rhizobacteria with multiple PGP traits were obtained by using serial dilution technique from the rhizospheric soils of V. jatamansi, located in Chamba, Himachal Pradesh, India. A potential isolates were screened and selected on the basis of halo zone produced in Pikovskaya's (PVK) agar for phosphate-solubilization and chrome azurol S medium for siderophore production and growth on nitrogen free medium (NFb) for N2-fixing ability. Among sixty isolated strains, CKMV1 showed maximum effectiveness of multifarious plant growth-promoting attributes, i.e. phosphate-solubilization, IAA production, nitrogen fixing ability, HCN production, siderophore production and broad spectrum of antagonistic activity against common phytopathogenic fungi, i.e. Sclerotium rolfsii, Rhizoctonia solani, Phytopthora sp., Alternaria sp., Fusarium oxysporum and Dematophora necatrix.
Phenotypic characterization of the bacterial isolate was done based on their colony morphology, microscopic observations, and biochemical tests.11 The isolate was tested for the utilization of different carbon sources using KB009 Hi carbohydrate™ kit (Himedia, Mumbai). The isolate was identified based on whole cell fatty acids derivatized to methyl esters and analysed by gas chromatography at IMTECH, Chandigarh and by 16S rDNA sequence analysis.
P-solubilization and organic acid production
For qualitative estimation of P solubilization, isolates were spot inoculated on PVK agar plates for 72 h. The P-solubilization was exhibited with a clear zone formed around the colony. Further quantitative estimation of phosphorus was done in PVK broth amended with 5.0 g/L tricalcium phosphate (TCP) by the vanadomolybdate method.12
For the analysis of organic acids, bacterial culture was filtrated through 0.2 μm filter (Millipore, GTBP) and 20 μl of filtrates were injected to HPLC (Waters 996 HPLC) equipped with photodiode array detector. The organic acid separation was carried out on RP-18 column (Merck, Germany) with 0.1% orthophosphoric acid (Merck, Germany) as mobile phase. Retention time of each signal was recorded at a wavelength of 210 nm and compared with the three standard organic acids [gluconic acid (Sigma–Aldrich, USA), 2-keto gluconic acid (Sigma, USA) and formic acid (Supelco, USA)].
Nitrogenase activity
The diazotrophic character was first evaluated by the capability of strain to grow on nitrogen free medium and by determining the nitrogenase activity using acetylene reduction assay.13 Strain was cultivated in duplicate vials for 5 days at 28 °C. The vials contained 15 mL of gas phase each and were sealed with silicone rubber caps. This leakage presumably gave the proper tension required for acetylene-reducing activity.14 Duplicate cultures were exposed to atmospheres of either air or argon containing 10% acetylene. After 2 and 5 days of exposure to acetylene, 1 mL samples were withdrawn from the culture vials and analysed by gas chromatography with a hydrogen flame ionization detector. Reduction of acetylene to ethylene was determined by the peak height relative to a standard of 50 nmol of ethylene.
Production of indole acetic acid (IAA) and ACC deaminase
For the production of auxins, each isolate was grown in Luria–Bertani broth (amended with 5 mM l-tryptophan, 0.065% sodium dodecyl sulphate and 1% glycerol) for 72 h at 37 °C under shake conditions. Colorimetric estimation of IAA-like auxins was done using Salkowski reagent.15 The ability of the strain to show ACC deaminase activity was carried by growing the strains in a rich medium (TS)13 and then transferring to DF (Dworkin and Foster)16 salt minimal medium with ACC as sole nitrogen source.15
Siderophore, hydrocyanic acid and antifungal metabolites production
Siderophore activity was determined on Chrome-Azurol S (CAS) medium.17 CAS plates were spot-inoculated with bacterial strains and observed for development of orange halo against dark blue background around the colonies after 48 h of incubation at 28 °C. A change in colour from blue to orange (hydroxamate-type siderophore) or purple (catechol-type siderophore) was considered as a positive reaction. The production of siderophore in cell-free culture supernatant was determined using spectrophotometric method as described by Schwyn and Neilands.17 HCN production was inferred by the qualitative method of Baker and Schipper.18 The change in the colour of the filter paper previously dipped in 2% sodium carbonate prepared in 0.05% picric acid from yellow to dark brown was rated visually depending on the intensity of the colour change. The rhizobacterial isolate was tested for their ability to inhibit the growth of diverse soil-borne fungal pathogens using the in vitro dual-culture analysis. In vitro screening of biocontrol PGPR was done by determining the inhibitory effect of isolates against fungus using agar diffusion technique, i.e. dual plate method. Percent growth inhibition was calculated using the formula given by Vincent.19
where I is the percentage of growth inhibition; C, the growth of fungus in control; T, the growth of fungus in treatment.
The antifungal activity of cell-free supernatants of the selected isolate was evaluated against the target fungi F. oxysporum, Phytophthora sp., D. necatrix, S. rolfsii and Phythium aphanidermatum.
Cloning of gdh, pqq genes responsible for phosphate solubilization and nif H gene for nitrogen fixation from genomic DNA of Aneurinibacillus aneurinilyticus CKMV1
Primers for amplifying the partial sequences of protein genes were designed according to the conserved protein sequences (GHGTHVAGT and TATNTISGTSMA) of gdh, pqq and nif H already published. A pair of universal degenerate primers20 Zehr-nif Hf (5′-TGYGAYCCNAARGCNGA-3′) and Zehr-nif Hr (5′-ANDGCCATCATYTCNCC-3′) were used to amplify Partial nif H gene from genomic DNA. The pair of complete and degenerate designed primers as listed in Table 1 for pqq and gdh genes was used for amplification.
Table 1.
PCR primers for detection of genes, i.e. gdh, pqq, and nif H in Aneurinibacillus aneurinilyticus CKMV1.
| Genes for PGP traits | Forward primer sequence | Tm (°C) | Reverse primer sequence | Tm (°C) | Size (bp) | Reference |
|---|---|---|---|---|---|---|
| Genes for phosphate solubilization | ||||||
| gdh | 5′-ATGTATCCGGATTTAAAAGG-3′ | 54 | 5′-TTAACCGCGGCCTGCCTGG-3′ | 64 | 800 | Self designed |
| pqq | 5′-GGCTGCTGGCCGAACTGACTT-3′ | 64 | 5′GGCCGCAAGAAGCATTATTAG-3′ | 60 | 1000 | Self designed |
| Genes for nitrogen fixation | ||||||
| nif H | 5′-TGYGAYCCNAARGCNGA-3′ | 52 | 5′-ANDGCCATCATYTCNCC-3′ | 54 | 350 | Zehr and McReynolds20 |
Briefly, the genomic DNA was isolated by using conventional method.21 The genomic DNA were amplified in a PCR mixture containing 0.2 mM of each deoxynucleoside triphosphate, 20 pmol of each primer, 2.5 U of Taq DNA polymerase with 1× buffer in a 50 μl reaction volumes with initial denaturation at 94 °C for 2 min, 40 cycles of denaturation at 94 °C for 1 min, primer annealing of 54 °C for 45 s (for gdh), 58 °C for 45 s (for pqq synthase) and 50 °C for 2 min (for nif H) and extension of 72 °C for 2 min on PCR-System Thermal Cycler (LabNet). The PCR products were subjected to agarose (2.0%, w/v) gel electrophoresis in TBE buffer, followed by excising from the gel and purifying by using gel extraction kit (Real genomics, RBC). The purified fragments were ligated into pGEM-T cloning vector before transformation into chemically competent cells of Escherichia coli strain DH5α. Transformants were grown at 37 °C on Luria Broth (LB) agar containing ampicillin (100 μg/mL), IPTG (50 mM) and X-gal (80 μg/mL) for blue/white screening of recombinant colonies. Transformed E. coli strains were confirmed for the presence of insert of gdh, pqq and nif H using respective designed primers followed by sequencing (Xceleris labs, Ahmedabad). The obtained sequences were then subjected to BLAST and ORF Finder programme at NCBI to assemble the whole sequence of the targeted gene. The sequences thus obtained were also submitted to EMBL database. Sequences of amplified genes were aligned and bootstrapped with the help of PHYLIP and phylogenetic tree was constructed using Neighbour-Joining (J) method of mathematical averages and viewed in TREEVIEW software.
Efficacy of Aneurinibacillus aneurinilyticus CKMV1 on plant growth promotion
Tomato plant (Solanum lycopersicum variety Him sona) was used as model system to monitor the plant growth promotion and nutrient uptake by strain CKMV1 in net house. The sand, soil and farm yard manure (FYM) was mixed in a ratio of 1:1:1 in order to make the potting mixture. The mixture was then filled in the pots and moistened to one third saturation capacity. Twenty-five seeds untreated or treated with cell suspension of 1.5 O.D were sown at equidistance and covered with moss grass till emergence of plumule. After 3–4 days of seedlings emergence thinning was done and 3 plants per pot were maintained. Six replicate pots per treatment, with 3 plants in each pot were placed in a randomized block design in net house. The controls were designed in the same way but in this case, the tomato seeds were mock-inoculated with sterilized water. Tomato seedlings obtained from net house were analysed for shoot and root characteristics, root–shoot ratio, and nitrogen, phosphorus and potassium (NPK) analysis of plant biomass. The vigour index was calculated using the following formula as described by Babi and Anderson.22
Germination energy index (GEI) was computed using the formula:
where A1, A2, A3 is number of seeds newly germinated up to nth day respectively, N is total number of seeds used for treatment, n is number of days of observations.
Statistical analysis
Data were statistically analysed by analysis of variance using the general linear model developed by the SAS Institute (version 9.1; Cary, NC), and means were compared using the least significant difference (LSD) method; P ≤ 0.05 was considered significant.
Results
Isolation and characterization of the rhizobacterial isolate
Sixty bacterial isolates in total; thirty-eight from rhizosphere; and twenty two from endo rhizosphere of V. jatamansi were screened for multiple plant growth promoting (PGP) traits. Amongst them isolate CKMV1 exhibited multifarious plant growth promoting traits, viz., HCN production; P-solubilization (260 mg/L); IAA production (8.1 μg/mL); siderophore production (61.6%) and broad antifungal activity, i.e. F. oxysporum (64.3%), R. solani (91.6%), S. rolfsii (93.6%), Phytophthora spp. (71.4%) and Alternaria spp. (71.1%) (Table 2).
Table 2.
Screening of selected P-solubilizing bacterial isolate Aneurinibacillus aneurinilyticus CKMV1 for multifarious plant growth promoting traits.
| PGP traits | Activity |
|---|---|
| P-solubilization index | 3.1 |
| P-solubilization (mg/L) | 260 |
| Siderophore unit (%) | 61.6 |
| IAA (μg/mL) | 8.1 |
| ACC deaminase activities (n mg−1 h−1) | + |
| HCN production | + |
| Nitrogenase activity (nmol ethylene mL−1 h−1) | 202.91 |
| Antifungal activity (%) against | |
| F. oxysporum | 64.3 |
| R. solani | 91.6 |
| S. rolfsii | 93.6 |
| Alternaria sp. | 71.1 |
| Phytophthora sp. | 71.4 |
Isolate CKMV1 was Gram's positive, motile rods, Cream, irregular, raised elevation and erose margin. The isolate was positive for catalase, nitrate reduction, arginine dehydrolase, esculin hydrolysis, tyrosine utilization and acid from fructose, sucrose, inositol, l-malate, l-serine, glutarate, sorbitol and negative for citrate utilization, Voges-Proskauer's, indole production, starch hydrolysis and acid production from lactose, xylose, dextrose galactose, raffinose, trehalose, melibose sucrose, sodium glucanate, dulcitol, arabitol, mannitol, erythritol, α-methyl-d-glucoside, rhamnose, cellobiose, melezilose-d-mannoside, xylitole, d-arabinose, utilization, ONPG, nitrate reduction, arginine dehydrolase, starch hydrolysis, esculin hydrolysis.
The fatty acids Iso-C14:0, C14:0, Iso-C15:1F, Iso-C15:0, Anteiso-C15:0, C16:1 w7c alcohol, Iso-C16:0, Iso-C17:0, Sum in Feature 3, C16:0, Iso-C 17:1 w10c, Sum in Feature 4 and C16:1 w11c were present in the isolate (Table 3). The analysis showed the highest similarity of the isolate with A. aneurinilyticus as per MIDI system (Microbial Identification System, Inc.).
Table 3.
Cellular fatty acid composition of A. aneurinilyticus strain CKMV1 by FAME analysis.
| Fatty acids | CKMV1 (%) |
|---|---|
| Iso-C14:0 | 2.2 |
| C14:0 | 1.4 |
| Iso-C15:1F | 0.8 |
| Iso-C15:0 | 55.6 |
| Anteiso-C15:0 | 4.2 |
| C16:1 w7c alcohol | 0.9 |
| Iso-C16:0 | 2.9 |
| C16:1 w11c | 11.2 |
| Sum in Feature 3a | 5.2 |
| C16:0 | 5.2 |
| Iso-C 17:1 w10c | 5.1 |
| Sum in Feature 4b | 2.1 |
| Iso-C17:0 | 3.3 |
Strain CKMV1 is showing similarity with Aneurinibacillus sp. as per MIDI.
Summed Feature 3 comprised 16:1 w7c/15 iso 2OH.
Summed Feature 4 comprised 17:1 ISO I/ANTEI B which could not be resolved by MIDI.
The identification of isolate was further confirmed by comparison of 16S rDNA sequences in the NCBI database using a BLASTn analysis. An amplicon of size 1241 bp representing 16S rDNA sequence of strain CKMV1 was obtained. 16S rDNA sequence of isolate CKMV1 showed maximum sequence similarity with Aneurinibacillus (AB271755). Thus, by sequence comparison with available data in the Gen Bank using Blast confirmed the strain as A. aneurinilyticus. The sequence were deposited to EMBL data base and assigned accession number, i.e. AB271755 for 16S rRNA sequence for the isolate CKMV1.
Nitrogen fixation and ACC deaminase determination
The diazotrophic character was first evaluated by the capability of the isolate to grow in the nitrogen free selective medium. The strain CKMV1 was tested for its capacity to fix nitrogen by assaying its nitrogenase activity. A. aneurinilyticus CKMV1 was confirmed as authentic nitrogen fixing bacteria by the effectively reduction of acetylene approx to 202.91 nmol ethylene mL−1 h−1. A. aneurinilyticus CKMV1 was also found to be positive for the ACC deaminase activity by its ability to grow on DF salt medium (Table 2).
In vitro determination of P-solubilizing activity of A. aneurinilyticus CKMV1 and detection of organic acids by gas chromatography
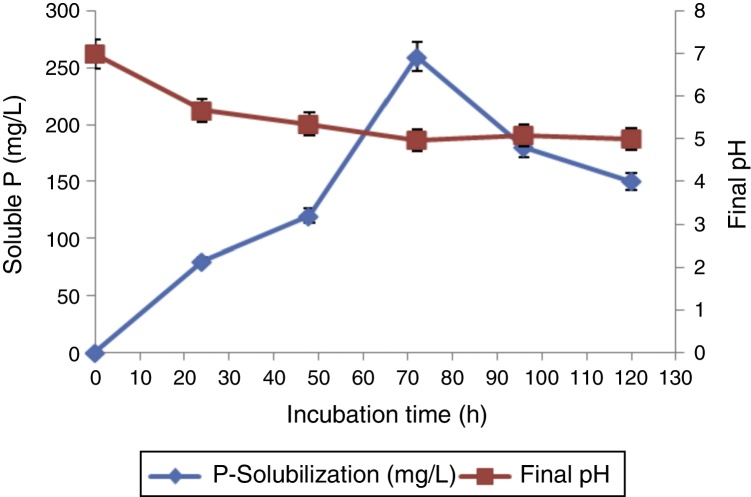
In order to study the relationships between pH, organic acid production and insoluble P-solubilization, we grew A. aneurinilyticus CKMV1 in the PVK medium upto 120 h. The effect of time course on final pH and soluble P in the PVK broth are shown in Fig. 1. P-solubilization was increased with increase of culture time, reaching a maximum (260 mg/L) at 72 h. On the other hand, the culture pH decreased from 7 to 4.97 during the period of the linear increase of P-solubilization (Fig. 1).
Fig. 1.
Changes in P-solubilization and pH of medium with time by A. aneurinilyticus (CKMV1).
The organic acids were identified by HPLC in the cultural filtrates after 72 h of incubation. HPLC analysis of the culture filtrate revealed three major peaks and rest of the peaks might represent the unknown organic acids for which standards were not used (data not shown). The three major peaks were identified as gluconic acid, formic acid and 2-ketogluconic acid by comparing their retention times with the authentic standards. As shown in Table 4, gluconic acid (1.34%) was detected as the major organic acids with small percentages of 2-ketogluconic acid (0.24%) and formic acid (0.10%) along with unknown organic acids in minor concentrations during TCP solubilization by the test isolate. The production of gluconic acid and subsequent decrease in pH of the medium by A. aneurinilyticus CKMV1 strain states that possible mechanism of phosphate solubilization by A. aneurinilyticus CKMV1 could be by the production of gluconic acid.
Table 4.
Organic acid production by Aneurinibacillus aneurinilyticus CKMV1 in Pikovskaya's broth after 72 h of incubation.
| Organic acid | Retention time (min) | Percentagea |
|---|---|---|
| Gluconic acid | 7.48 | 1.34 |
| 2-Ketogluconic acid | 14.76 | 0.24 |
| Formic acid | 9.81 | 0.10 |
.
Relationship of TCP solubilization (Y) with final pH, IAA (X), % siderophore unit (X), % growth inhibition against F. oxysporum, Phytophthora sp., D. necatrix, S. rolfsii, P. aphanidermatum (X) in PVK medium
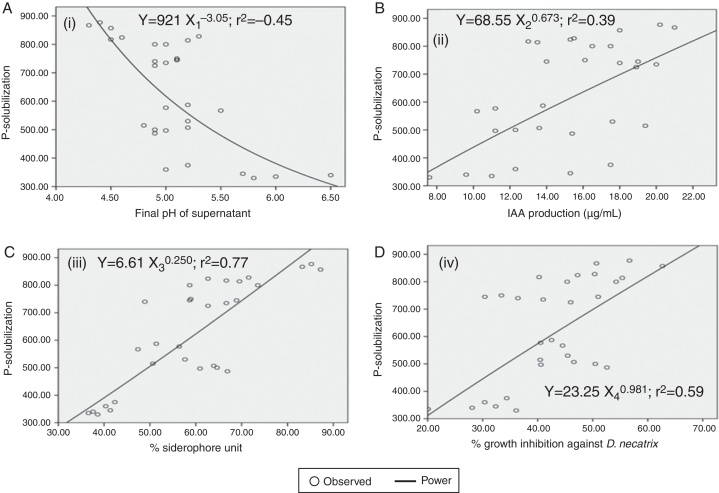
To know the overall impact of P-solubilization (Y) on final pH (X1), IAA (X2), percent siderophore unit (X3), percent growth inhibition against D. necatrix (X4), a non-linear regression analysis were carried out. It was found that rYX1, rYX2, rYX3 and rYX4 were significant at 5 percent level of significance. To find out the probable value of P-solubilization (Y) for given value of final pH (X1), IAA (X2), percent siderophore unit (X3), percent growth inhibition against D. necatrix (X4); the data obtained on the variable were subjected to the fitting of various mathematical functions, viz., linear, quadratic, cubic, power and inverse functions. On the basis of observed and expected frequencies and finally on the basis of r2 values, it was observed that power function in all the cases gave the best fitted equation significant at 5% level of significance. To find out the probable value of P-solubilization (Y) for given fitted regression equations along with standard error (S.E.) of regression coefficients and r2 values have been presented below. Thus, present results revealed strong negative relation between P-solubilization and final pH of PVK medium. On the other hand, positive correlation of P-solubilization with IAA, siderophore production and percent growth inhibition against D. necatrix was observed. Their graphical representations have also been shown in Fig. 2A–D.
Fig. 2.
Relationship between phosphate solubilization and (A) final pH, (i) (S.E. of the regression coefficient = 0.267); (B) IAA production, (ii) (S.E. of the regression coefficient = 0.245); (C) percent % siderophore unit, (iii) (S.E. of the regression coefficient = 0.156); (D) percent (%) growth inhibition of Dematophora necatrix, (iv) (S.E. of the regression coefficient = 0.278).
These fitted regression lines can be successfully employed to work out the value of P-solubilization for a given value of final pH, IAA, % siderophore unit and % growth inhibition against inhibition against tested fungal pathogens, and hence, these regression lines can be regarded as an estimation equation.
Cloning of phosphate solubilizing and nitrogen fixing gene of A. aneurinilyticus CKMV1
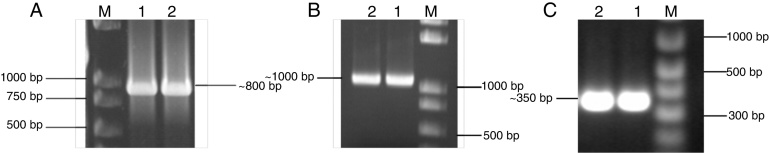
PCR amplification produced a reliable fragment of about ∼800 bp for complete gdh (Fig. 3A), ∼1000 bp for partial pqq (Fig. 3B) and ∼350 bp for nif H (Fig. 3C). Sequencing of cloned complete gdh and partial nif H concluded that the complete gdh gene from A. aneurinilyticus CKMV1 was 786 bp long encoding 261 amino acid proteins and partially amplified nif H is 324 bp long encoding a 108 amino acids corresponding to nif H gene.
Fig. 3.
Agarose gel electrophoresis showing the amplification of complete glucose dehydrogenase (gdh), partial pqq synthase and partial nif H gene from A. aneurinilyticus (CKMV1). Lane M: 100 bp Marker. (A) Lanes 1 and 2: showing ∼800 bp amplification corresponding to complete glucose dehydrogenase (gdh) gene from A. aneurinilyticus (CKMV1). (B) Lanes 1 and 2: showing ∼1000 bp amplification corresponding to PQQ synthase gene from A. aneurinilyticus (CKMV1). (C) Lanes 1 and 2: showing ∼350 bp amplification corresponding to nif H gene from A. aneurinilyticus (CKMV1).
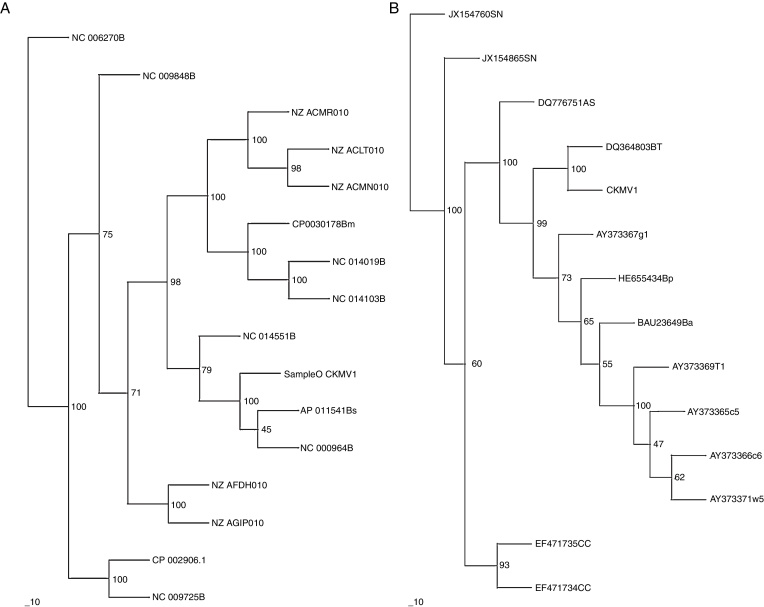
Sequence of complete gdh gene of A. aneurinilyticus CKMV1 was analysed with corresponding sequences of 15 different (gdh) gene of Bacillus spp. reported from different parts of the world. Sequence analysis revealed that gdh gene of A. aneurinilyticus CKMV1 showed maximum homology (99%) with gdh gene of Bacillus subtilis (Acc. No. AP011541 and Acc. No. NC000964) (Fig. 4).
Fig. 4.
Neighbour-joining tree based on complete glucose dehydrogenase gene sequences (A) and partial nif H gene sequences (B) showing the phylogenetic relationship of strain CKMV1. The numbers at the nodes indicate the levels of bootstrap support based on data for 1000 replicates; values inferred greater than 50% are only presented. The scale bar indicates 100 substitutions per nucleotide position.
Sequence of partial nif H gene of A. aneurinilyticus CKMV1 was also analysed with corresponding sequences of 13 different (nif H) gene of Bacillus spp. reported from different parts of the world. Sequence analysis revealed that nif H gene of A. aneurinilyticus CKMV1 showed maximum homology (97.5%) with nif H gene of Bacillus sp. strain BT97 (Acc. No. DQ36803) reported from China and least homology (68.8%) with nif H gene of Bacillus azotofixans (Acc No. BAU23649) reported from USA. It was also confirmed by phylogenetic analysis (Fig. 4) as nif H gene of A. aneurinilyticus CKMV1 clustered closely with nif H gene of other reported isolates of Bacillus sp.
The sequences were deposited to EMBL database and assigned with accession number for complete gdh (HG515387) and partial nif H (HG515388).
Growth response of seed treatment with liquid formulation of bacterial isolate CKMV1 on tomato seedlings in net house
The plant growth promotion potential of A. aneurinilyticus strain CKMV1 was determined in tomato seeds. A significant influence on growth was resulted with treatment of A. aneurinilyticus strain CKMV1 in tomato seedlings grown in pots under net house conditions (Table 5). Higher percent germination (88.7%), and vigour index (2768.57) were recorded for seeds treated with isolate CKMV1 which was significantly higher than that of untreated seeds. It was observed that the bacterized seedlings recorded 22.6% and 13.8% higher root and shoot lengths, respectively, compared to uninoculated control. Seed bacterization resulted in greater enhancement of the root growth, as compared to the shoot growth. Increase in shoot dry weight (26.8%) and root dry weight (12.9%) was also observed.
Table 5.
Effect of inoculation of plant growth promoting rhizobacteria Aneurinibacillus aneurinilyticus CKMV1 on the plant growth promoting parameters and nutrient uptake of tomato seedlings.
| Control | CKMV1 | T(cal) | |
|---|---|---|---|
| Growth parameters | |||
| Shoot length (cm) | 18.82 | 21.82 | 53.03 |
| Shoot dry weight (g) | 2.89 | 3.95 | 48.49 |
| Length of roots (cm) | 7.04 | 9.10 | 46.89 |
| Root dry weight (g) | 0.62 | 0.70 | 5.11 |
| Nutrient uptake | |||
| NPK content of soil (kg/ha) | N = 206.98 | N = 382.59 | 67.87 |
| P = 123.20 | P = 123.63 | 27.50 | |
| K = 215.00 | K = 262.36 | 52.89 | |
| NPK content of shoot biomass (%) | N = 0.64 | N = 0.74 | 6.39 |
| P = 0.33 | P = 0.36 | 2.91 | |
| K = 1.53 | K = 1.82 | 18.54 | |
T(tab) = 2.353.
Impact of seed treatment on nitrogen, phosphorus and potassium content of soil in net house
Effect of seed treatment with bacterial strains in net house on nitrogen (N), phosphorus (P) and potassium content (K) of soil was determined from two months old tomato seedlings. NPK content of the soil before sowing of tomato seed was 194.4 kg/ha, 108.0 kg/ha and 201.9 kg/ha, respectively. The results for NPK content of soil before and after experiment are given in Table 5. In net house, higher nitrogen (382.59 kg/ha), phosphorus (123.63 kg/ha) and potassium content (262.36 kg/ha) was observed in case of CKMV1 treated soil than the untreated soil which was kept as a control (206.98 kg/ha; 123.20 kg/ha; 215.0 kg/ha), respectively.
Impact of seed treatment on plant nutrient uptake in net house
It was observed that NPK content of whole shoot system of treated seedlings with A. aneurinilyticus strain CKMV1 was significantly higher than the untreated seedlings (Table 5). Percent nitrogen (0.74), percent phosphorus (0.36) and percent potassium content (1.82) was higher in case of CKMV1 seedling as compared to untreated control.
Discussion
Many soil bacteria and especially rhizosphere bacteria can stimulate plant growth through a number of direct and indirect pathways. The present investigation was carried out on a strain of A. aneurinilyticus (CKMV1) isolated from rhizosphere of medicinal plant V. jatamansi revealed its multiple plant growth promoting properties. This comprised its ability for solubilization of complex insoluble phosphate, production of IAA, and siderophore production, nitrogen fixation and antifungal activity. Significant phosphate solubilization (260.0 mg/L) and decrease in pH from 7.0 to 4.96 was obtained for isolate CKMV1, after 72 h of incubation. Different bacterial isolates have different capabilities for phosphate solubilization, Pseudomonas fluorescens and Bacillus megatarium was reported to solubilize 400 mg/L of phosphate, with reduction in pH from 7 to 4.23 698 mg/L, 360 mg/L, 230 mg/L of soluble phosphate was also reported to be produced by Pantoea agglomerans24; Penicillium radicum and Rahnella aquatilis,25 respectively. Significance of IAA production by PGPR isolates has been realized and worked out by several researchers in recent years.26, 27, 28 Strain CKMV1 maximum produced IAA (8.1 μg/mL) after 72 h of incubation. IAA production by microbes promotes the root growth by directly stimulating plant cell elongation or cell division (Table 2).15
Biocontrol potential of a PGPR was associated with production of number of secondary metabolites and antibiotics, siderophore production and HCN production were some of them.29, 30 Isolate CKMV1 showed siderophore production (61.60%) along with HCN production. Along with this it also showed antifungal activity against different fungal pathogens of tomato, viz., F. oxysporum (64.3%), R. solani (91.6%), S. rolfsii (93.6%), Phytophthora spp. (71.4%), Alternaria spp. (71.1%) and D. necatrix (52.7%) (Table 2). This might be due to the production of siderophore by PGPR as it makes iron available to the plant an unavailable to pathogens thus effective in controlling plant pathogens.31, 32
Biochemical characterization, FAME analysis (Table 3), 16S rDNA amplification and sequencing was successfully used to identify the CKMV1 up to species level, and sequencing data validated the strain CKMV1 as A. aneurinilyticus, first time isolated from medicinal plant rhizosphere with multiple PGP traits. Earlier there was a report of isolation of A. aneurinilyticus from sludge of pulp and paper mill.33
Nitrogen fixation was also recorded for the first time in PGPR belonging to genus Aneurinibacillus by performing acetylene reduction assay for estimation of nitrogenase activity. PCR amplification of nif H along with estimation of nitrogenase activity of Rhizobia and Bacillus has been extensively used to confirm the nitrogen fixing ability of diazotrophs and Bacillus sp.34, 35 In nitrogen-fixing bacteria, nitrogenase is encoded by a set of operons which includes regulatory genes (such as nif LA), structural genes (such as nif HDK) and other supplementary genes. Nif DK encodes for dinitrogenase reductase and nif H for Fe protein subunit.36 Nif genes are found in many bacteria besides rhizobia and are a part of genome that are considered as part of the normal bacterial genome.37
Phosphate solubilization was significantly correlated with final pH of supernatant, percent siderophore unit, IAA and percent growth inhibition against D. necatrix. Several authors attributes P solubilization of inorganic phosphate to the production and release of organic acids.4 However, others suggested some additional mechanisms as is confirmed by weak or even lacking linear correlation between pH and the amount of P-solubilized. Strong negative correlation between pH and P solubilized (r = −0.45) in the present study (Fig. 2) is similar to negative correlation between pH and P-solubilized as reported by Alam et al.38 The inverse correlation between the pH value of the culture and released of P suggested that the solubility of phosphate is directly correlated with acid production but are not the only possible mechanisms for P-solubilization.
Organic acid profiling of A. aneurinilyticus CKMV1 revealed the production of four organic acids with gluconic acid on top with 1.34% (Table 4).39 The presence of organic acid in culture filterate provided first clue that the possible mechanism of phosphate solubilization by A. aneurinilyticus CKMV1 is via production of organic acids in the medium. Production of multiple organic acids has also been demonstrated for Bacillus species.40, 41 However, there was no previous report in literature on gluconic acid production by the bacteria belonging to genus Aneurinibacillus to which CKMV1 belongs. Since the production of organic acids is considered to be the principal mechanism for mineral phosphate solubilization, it could be assumed that any gene involved in organic acid synthesis might have an effect on this character.42 Gluconic acid production is catalyzed by periplasmic oxidation of glucose by membrane bound glucose dehydrogenase (GDH).42 In Gram-negative bacteria, the synthesis of gluconic acid has been shown to be dependent on pyrroloquinoline quinine (PQQ) as an enzymatic cofactor of the gdh.43, 44 However, studies concerning with detection of GDH encoding gene (gdh) involved in P-solubilization has been conducted in Bacillus sp. isolated from rhizosphere apple but not in Aneurinibacillus. Genetics of phosphate solubilization and mineralization could be studied once the biochemical mechanism of solubilization is known. A. aneurinilyticus CKMV1 accumulate 260 mg/L of phosphorus from TCP (Table 2). The results also showed a negative relation between pH of supernatant and P-solubilization from tri-calcium phosphate which further confirms that organic acids has been produced in liquid media that lowers the pH of media. Therefore, (gdh) gene encoding the enzyme glucose dehydrogenase responsible for gluconic acid production and (pqq) gene encoding the enzyme PQQ synthase were characterized from A. aneurinilyticus CKMV1 by using designed primers. The complete gdh gene from CKMV1 was also cloned and sequenced and sequence analysis revealed that the complete gdh gene was 786 bp in length encoding 261 amino acids with 99% homology to gdh gene sequence from Bacillus subtilis (NC00964 and AP011541) (Fig. 3, Fig. 4). The conservation of the gdh gene across the Bacillus spp. indicated the significance of enzyme in sugar metabolism. But in order to confirm the role of glucose dehydrogenase in acid production, it was important to confirm quinoprotein nature of gdh from A. aneurinilyticus which was confirmed by the amplification of pqq synthase gene of ∼1000 bp from strain CKMV1 by using designed primers. The presence of pqq synthase along with glucose dehydrogenase (gdh) in A. aneurinilyticus strain CKMV1 confirmed the quinoprotein nature of gdh and also confirmed its role in acid production followed by phosphate solubilization which was also confirmed through HPLC data.
In present study, we investigated the effectiveness of PGPR isolate CKMV1 on increase in seed germination rate as well as growth of seedlings. The evaluation of results of the plant growth promotion of tomato seedling under net house indicated that CKMV1 as PGPR strain capable of promoting growth of tomato. CKMV1 significantly increased plant height, root length and production of dry matter from shoot and root of tomato seedlings (Table 5). Germination percentage and vigour index in CKMV1 treated seeds was significantly higher than that of control. Plant inoculation with phosphate solubilizing microorganisms such as Pseudomonas was beneficial to produce the lateral radicles.45 PGPRs enhanced the growth of seedlings root by inducing the production of phytohormones such as auxins (IAA). There were many reports regarding the enhancement of root and plant growth by applying bacteria.6, 28
Conclusion
In conclusion, the present study is the first report on A. aneurinilyticus strain CKMV1 isolated from the rhizosphere of V. jatamansi, showing P-solubilization, N-fixation, IAA, siderophore, HCN production, ACC deaminase activity and biocontrol traits against phytopathogenic fungi, i.e. F. oxysporum, Phytophthora sp., D. necatrix, S. rolfsii. This report also proved that decrease in final pH was occurred during solubilization of phosphorus in the medium which may be due to production of different organic acid which were simultaneously detected by HPLC during P-solubilization and their production was further confirmed by amplification and sequencing of genes such as gdh and pqq responsible for organic acid production. Identification of genes for PGP traits can open new avenues for further manipulation of this strain. Further the inoculation of bacterial strain not only improves growth of seedlings but also increases the uptake of shoot NPK contents which were significantly more in case of inoculated seedlings than the untreated control and the increase may be caused by nitrogen fixation and phosphorous solubilization ability of PGPR. Hence, it is concluded that the A. aneurinilyticus strain CKMV1, isolated from rhizosphere of V. jatamansi, can be used as potential biofertilizer for tomato and various vegetable crops not being crop specific.
Conflicts of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We thank the All India Network Project on Soil Biodiversity and Biofertilizer (ICAR) for providing necessary funds to carry out this research work.
Associate Editor: Welington Luiz de Araújo
References
- 1.Raaijmakers J.M., Vlami M., De Souza J.T. Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek. 2002;81:537. doi: 10.1023/a:1020501420831. [DOI] [PubMed] [Google Scholar]
- 2.Mehta P., Walia A., Kakkar N., Shirkot C.K. Tricalcium phosphate solubilisation by new endophyte Bacillus methylotrophicus CKAM isolated from apple root endosphere and its plant growth-promoting activities. Acta Physiol Plantarum. 2014;36:2033–2045. [Google Scholar]
- 3.Ehrlich H.L. Mikrobiologische and biochemische Verfahrenstechnik. In: Einsele A., Finn R.K., Samhaber W., editors. Geomicrobiology. Weinheim; VCH Verlagsgesellschaft: 1990. [Google Scholar]
- 4.Perez E., Sulbaran M., Ball M.M., Yarza B.L.A. Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the South eastern Venezuelan region. Soil Biol Biochem. 2007;39:2905–2914. [Google Scholar]
- 5.Rodriguez H., Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17(4–5):319–339. doi: 10.1016/s0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A., Guleria S., Mehta P., Walia A., Chauhan A., Shirkot C.K. Plant growth promoting traits of phosphate solubilizing rhizobacteria isolated from seabuckthorn growing in cold desert region of trans-Himalayas and evaluating their potential on growth of tomato seedlings. Acta Physiol Plantarum. 2015;37(3):1–12. [Google Scholar]
- 7.James E.K. Nitrogen fixation in endophytic and associative symbiosis. Field Crops Res. 2000;65:197–209. [Google Scholar]
- 8.Vitousek P.M., Howarth R.W. Nitrogen limitation on land and on the sea. Biogeochemistry. 1991;3:87–115. [Google Scholar]
- 9.Ueda T., Suga Y., Yahiro N., Matsuguchi T. Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nif H gene sequences. J Bacteriol. 1995;177:1414–1417. doi: 10.1128/jb.177.5.1414-1417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding Y., Wang J., Liu Y., Chen S. Isolation and identification of nitrogen-fixing bacilli from plant rhizospheres in Beijing region. J Appl Microbiol. 2005;99:1271–1281. doi: 10.1111/j.1365-2672.2005.02738.x. [DOI] [PubMed] [Google Scholar]
- 11.Holt J.G., Krieg N.R., Sneathm P.H.A., Staley J.T., Williams S.T. Williams and Williams; Baltimore, MD: 1994. Bergey's Manual of Determinative Bacteriology. [Google Scholar]
- 12.Sundara Rao W.V.B., Sinha M.K. Phosphate dissolving micro-organisms in the soil and rhizosphere. Indian J Agric Sci. 1963;33:272–278. [Google Scholar]
- 13.Donate-Correa J., León-Barrios M., Perez-Galdona R. Screening for plant growth-promoting rhizobacteria in Chemaecytisus proliferus (tagasaste), a forage tree-shrub legume endemic to the canary islands. Plant Soil. 2004;266:261–272. [Google Scholar]
- 14.Hardy R.W.F., Holsten R.D., Jackson E.K., Burns R.C. The acetylene-ethylene assay for Nz fixation: laboratory and field evaluation. Plant Physiol. 1968;43:1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glick B.R. The enhancement of plant growth by free living bacteria. Can J Microbiol. 1995;41:109–117. [Google Scholar]
- 16.Dworkin M., Foster J.W. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 1958;75(5):592–603. doi: 10.1128/jb.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwyn B., Nellands J.B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 18.Baker A.W., Schippers B. Microbial cyanide production in the rhizosphere in relation to potato yield production and Pseudomonas spp. mediated plant growth stimulation. Soil Biol Biochem. 1987;19:451–457. [Google Scholar]
- 19.Vincent J.M. Distortion of fungal hyphae in the presence of certain inhibitors. Nature. 1947;150:850. doi: 10.1038/159850b0. [DOI] [PubMed] [Google Scholar]
- 20.Zehr J.P., McReynolds L.A. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1989;55:2522–2526. doi: 10.1128/aem.55.10.2522-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory; New York: 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 22.Babi A.A., Anderson J.D. Vigour determination in soybean seed by multiple criteria. Crop Sci. 1973;13:630–633. [Google Scholar]
- 23.Jeon J.S., Lee S.S., Kim H.Y., Ahn T.S., Song H.G. Plant growth promotion in oil by some inoculated microorganism. J Microbiol. 2003;41(4):271–276. [Google Scholar]
- 24.Tate R.L. John Wiley & Sons, Inc.; New York: 2000. Soil Microbiology; pp. 149–152. [Google Scholar]
- 25.Kim K.Y., Jordan D., Krishnan H.B. Rahnella aquatilis, a bacterium isolated from soybean rhizosphere, can solubilize hydroxyapatite. FEMS Microbiol Lett. 1997;153:273–277. [Google Scholar]
- 26.Boiero L., Perrig D., Masciarelli O., Penna C., Cassan F., Luna V. Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl Microbiol Biotechnol. 2007;74:874–880. doi: 10.1007/s00253-006-0731-9. [DOI] [PubMed] [Google Scholar]
- 27.Swain M.R., Naskar S.K., Ray R.C. Indole-3-acetic acid production and effect on sprouting of yam (Dioscorea rotundata L.) minisetts by Bacillus subtilis isolated from culturable cowdung microflora. Pol J Microbiol. 2007;56:103–110. [PubMed] [Google Scholar]
- 28.Sharma R., Sharma P., Chauhan A., Walia A., Shirkot C.K. Plant growth promoting activities of rhizobacteria isolated from Podophyllum hexandrum growing in North-West region of Himalayas. Proc Natl Acad Sci, India Sec B: Biol Sci. 2016 [Google Scholar]
- 29.Ahemed M., Khan M.S. Functional aspects of plant growth promoting rhizobacteria: recent advancements. Insight Microbiol. 2011;1(3):39–54. [Google Scholar]
- 30.Mehta P., Walia A., Chauhan A., Kulshrestha S., Shirkot C.K. Phosphate solubilization and plant growth promoting potential by stress tolerant Bacillus sp. isolated from rhizosphere of apple orchards in trans Himalayan region of Himachal Pradesh. Ann Appl Biol. 2013;163:430–443. [Google Scholar]
- 31.Koo S.Y., Hong S.H., Ryu H.W., Cho K.S. Plant growth promoting trait of rhizobacteria isolated from soil contaminated with petroleum and heavy metals. J Microbiol Biotechnol. 2010;20(3):587–593. [PubMed] [Google Scholar]
- 32.Ramos-Solano B., García J.A.L., Garcia-Villaraco A., Algar E., Gracia-Cristobal J., Manero F.J.G. Siderophore and chitinase producing isolates from the rhizosphere of Nicotiana glauca Graham enhance growth and induce systemic resistance in Solanum lycopersicum L. Plant Soil. 2010;334:189–197. [Google Scholar]
- 33.Raj A., Chandra R., Reddy M.M.K., Purohit H.J., Kapley A. Biodegradation of kraft lignin by a newly isolated bacterial strain Aneurinibacillus aneurinilyticus from the sludge of a pulp paper mill. World J Microbiol Biotechnol. 2007;23:793–799. [Google Scholar]
- 34.Pandey A., Sharma E., Palni L.M.S. Influence of bacterial inoculation on maize in upland farming systems of the Sikkim Himalaya. Soil Biol Biochem. 1998;30:379–384. [Google Scholar]
- 35.Chowdhury S.P., Schmid M., Hartmann A., Tripathi A.K. Diversity of 16S rRNA and nif H genes derived from rhizosphere soil and roots of an endemic drought tolerant grass, Lasiurus sindicus. Eur J Soil Biol. 2009;45:114–122. [Google Scholar]
- 36.Dean D.R., Jacobson M.R. Biochemical genetics of nitrogenase. In: Stacey G., Burris R.H., Evans H.J., editors. Biological Nitrogen Fixation. New York; Chapman and Hall: 1992. pp. 763–834. [Google Scholar]
- 37.Young P.W., Haukka K. Diversity and phylogeny of rhizobia. New Phytol. 1996;133:87–94. [Google Scholar]
- 38.Alam S., Khalil S., Ayub N., Rashid M. In vitro solubilization of inorganic phosphate by phosphate solubilizing micro-organisms (PSM) from maize rhizosphere. Int J Agric Biol. 2002;4(4):454–458. [Google Scholar]
- 39.Mohammadi K. Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. Resour Environ. 2012;2(1):80–85. [Google Scholar]
- 40.Chen Y.P., Rekha P.D., Arun A.B., Shen F.T., Lai W.A., Young C.C. Phosphate solubilizing bacteria from subtropical soil and their tri-calcium phosphate solubilizing abilities. Appl Soil Ecol. 2006;34:33–41. [Google Scholar]
- 41.Mehta P., Walia A., Shirkot C.K. Functional diversity of phosphate solubilizing plant growth promoting rhizobacteria isolated from apple trees in the trans Himalayan region of Himachal Pradesh, India. Biol Agric Hortic. 2015;31(4):265–288. [Google Scholar]
- 42.Ahmed N., Shahab S. Phosphate solubilization: their mechanism genetics and application. Internet J Microbiol. 2009;9(1):1–19. [Google Scholar]
- 43.Choi O., Kim J., Kim J.G. Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol. 2008;146:657–668. doi: 10.1104/pp.107.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sashidhar B., Podile A.R. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol. 2010;109:1–12. doi: 10.1111/j.1365-2672.2009.04654.x. [DOI] [PubMed] [Google Scholar]
- 45.Antoun H., Kloepper J. Plant growth promoting rhizobacteria. In: Brenner S., Miller J., editors. Encyclopedia of Genetics. Academic Press; 2001. pp. 1477–1480. [Google Scholar]