Abstract
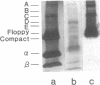
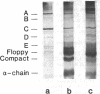
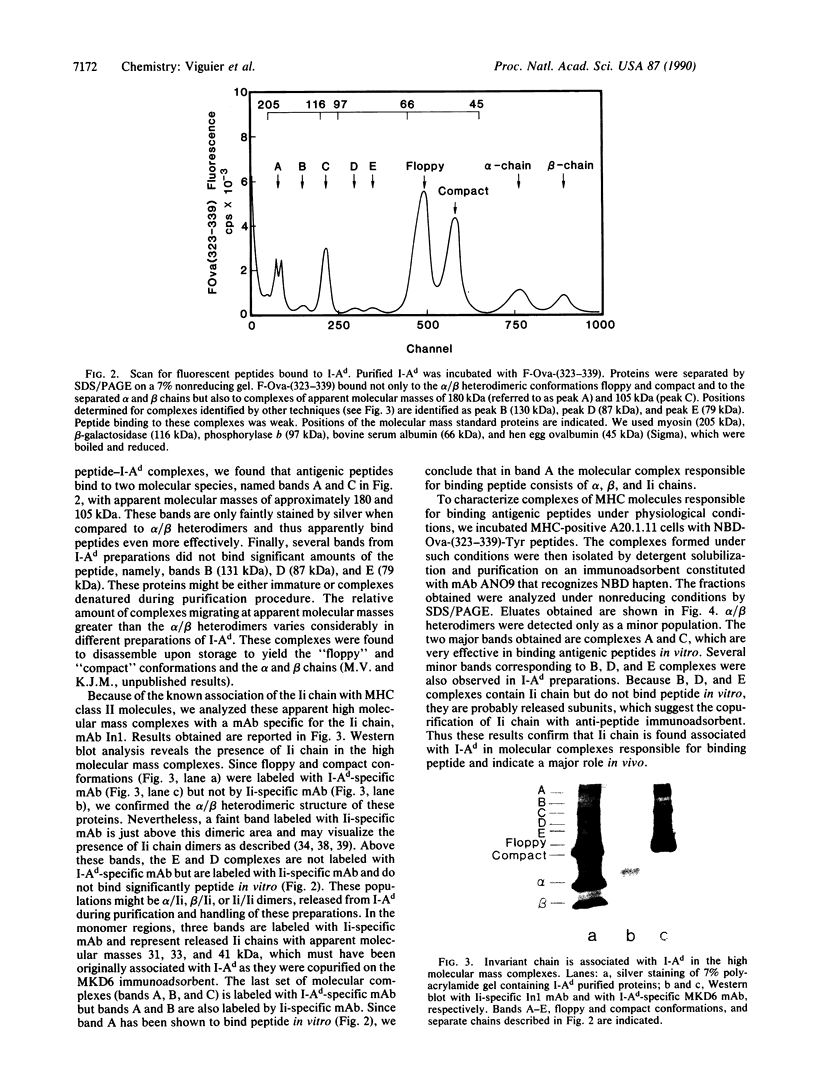
The binding of a chicken ovalbumin peptide (residues 323-339), Ova-(323-339), to I-Ad molecules was investigated in vitro and in vivo. By using antigenic peptides labeled either with a hapten or with fluorescein, complexes formed in vitro between I-Ad and antigenic peptides were detected by Western blot analysis with an antibody recognizing the hapten 7-nitrobenzo-2-oxa-1,3-diazole and by scanning gels for fluorescence emitted by fluoresceinated peptide. Both techniques reveal that Ova-(323-339) binds not only to I-Ad alpha/beta heterodimers and separated alpha and beta chains but also to complexes of higher molecular mass. Additional analysis shows that one of these additional complexes contains I-Ad heterodimers, antigenic peptides, and also invariant chain. To explore the physiological role of these complexes, cells were incubated with haptenated peptide and the I-Ad-peptide complexes formed in vivo were purified by affinity chromatography using hapten-specific antibody. The complexes formed migrate with a significantly higher apparent molecular mass than the alpha/beta heterodimers. A band at 180 kDa contained the alpha/beta heterodimer, the antigenic peptide, and the invariant chain. These results show that in vivo high molecular mass complexes formed by the I-Ad heterodimer and the invariant chain bind antigenic peptides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson S. Z., Lipscomb M. F., Tucker T. F., Uhr J. W. Specific binding of T lymphocytes to macrophages. II. Role of macrophage-associated antigen. J Immunol. 1977 Oct;119(4):1493–1500. [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Buus S., Colon S., Smith C., Freed J. H., Miles C., Grey H. M. Interaction between a "processed" ovalbumin peptide and Ia molecules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3968–3971. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Grey H. M. Autologous peptides constitutively occupy the antigen binding site on Ia. Science. 1988 Nov 18;242(4881):1045–1047. doi: 10.1126/science.3194755. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. P., Parham P. Direct binding of influenza peptides to class I HLA molecules. Nature. 1989 Feb 23;337(6209):743–745. doi: 10.1038/337743a0. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Requirements for the processing of antigens by antigen-presenting B cells. I. Functional comparison of B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2382–2388. [PubMed] [Google Scholar]
- Clayberger C., Dekruyff R. H., Aisenberg J., Cantor H. Hapten-reactive inducer T cells. I. Definition of two classes of hapten-specific inducer cells. J Exp Med. 1983 Jun 1;157(6):1906–1919. doi: 10.1084/jem.157.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. G., Uhr J. W., Capra J. D., Vitetta E. S. Structural studies on the murine IA alloantigens. II. Molecular weight characterization of the products of the I-A and I-E/C subregions. J Immunol. 1978 Dec;121(6):2205–2212. [PubMed] [Google Scholar]
- Cresswell P. Intracellular class II HLA antigens are accessible to transferrin-neuraminidase conjugates internalized by receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8188–8192. doi: 10.1073/pnas.82.23.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornmair K., Rothenhäusler B., McConnell H. M. Structural intermediates in the reactions of antigenic peptides with MHC molecules. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):409–416. doi: 10.1101/sqb.1989.054.01.050. [DOI] [PubMed] [Google Scholar]
- Elliott W. L., Stille C. J., Thomas L. J., Humphreys R. E. An hypothesis on the binding of an amphipathic, alpha helical sequence in Ii to the desetope of class II antigens. J Immunol. 1987 May 1;138(9):2949–2952. [PubMed] [Google Scholar]
- Ellner J. J., Rosenthal A. S. Quantitative and immunologic aspects of the handling of 2,4 dinitrophenyl guinea pig albumin by macrophages. J Immunol. 1975 May;114(5):1563–1569. [PubMed] [Google Scholar]
- Giacoletto K. S., Sant A. J., Bono C., Gorka J., O'Sullivan D. M., Quaranta V., Schwartz B. D. The human invariant chain is the core protein of the human class II-associated proteoglycan. J Exp Med. 1986 Nov 1;164(5):1422–1439. doi: 10.1084/jem.164.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Peters J. H. Immunologic alterations in bovine serum albumin resulting from partial or complete reduction and alkylation. J Immunol. 1972 Mar;108(3):785–799. [PubMed] [Google Scholar]
- Guagliardi L. E., Koppelman B., Blum J. S., Marks M. S., Cresswell P., Brodsky F. M. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990 Jan 11;343(6254):133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Okudaira H., King T. P. Immunogenic properties of modified antigen E. II. Ability of urea-denatured antigen and alpha-polypeptide chain to prime T cells specific for antigen E. J Immunol. 1975 Jan;114(1 Pt 1):110–115. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Horowitz M., Dailey M. O., Hunter R. A., Wigzell H. Lipid-modified antigens. I. Specificity of guinea pig T lymphocyte responses and I-region restriction. J Immunol. 1979 Apr;122(4):1482–1488. [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. F., Auffray C., Korman A. J., Shackelford D. A., Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984 Jan;36(1):1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J. Structure of Ia antigens: identification of dimeric complexes formed by the invariant chain. J Immunol. 1982 Mar;128(3):1155–1158. [PubMed] [Google Scholar]
- Koch N., Koch S., Hämmerling G. J. Ia invariant chain detected on lymphocyte surfaces by monoclonal antibody. Nature. 1982 Oct 14;299(5884):644–645. doi: 10.1038/299644a0. [DOI] [PubMed] [Google Scholar]
- Koch N., Lauer W., Habicht J., Dobberstein B. Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii). An alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO J. 1987 Jun;6(6):1677–1683. doi: 10.1002/j.1460-2075.1987.tb02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leahy D. J., Rule G. S., Whittaker M. M., McConnell H. M. Sequences of 12 monoclonal anti-dinitrophenyl spin-label antibodies for NMR studies. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3661–3665. doi: 10.1073/pnas.85.11.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O. In search of a function for the invariant chain associated with Ia antigens. Surv Immunol Res. 1985;4(1):27–34. doi: 10.1007/BF02918583. [DOI] [PubMed] [Google Scholar]
- Long E. O. Intracellular traffic and antigen processing. Immunol Today. 1989 Jul;10(7):232–234. doi: 10.1016/0167-5699(89)90259-4. [DOI] [PubMed] [Google Scholar]
- McMillan M., Frelinger J. A., Jones P. P., Murphy D. B., McDevitt H. O., Hood L. Structure of murine Ia antigens. Two dimensional electrophoretic analyses and high pressure liquid chromatography tryptic peptide maps of products of the I-A and I-E subregions and of an associated invariant polypeptide. J Exp Med. 1981 Apr 1;153(4):936–950. doi: 10.1084/jem.153.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Germain R. N. Efficient cell surface expression of class II MHC molecules in the absence of associated invariant chain. J Exp Med. 1986 Nov 1;164(5):1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rothenhäusler B., Dornmair K., McConnell H. M. Specific binding of antigenic peptides to separate alpha and beta chains of class II molecules of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):352–354. doi: 10.1073/pnas.87.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., McConnell H. M. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature. 1989 Jan 19;337(6204):274–276. doi: 10.1038/337274a0. [DOI] [PubMed] [Google Scholar]
- Sant A. J., Cullen S. E., Giacoletto K. S., Schwartz B. D. Invariant chain is the core protein of the Ia-associated chondroitin sulfate proteoglycan. J Exp Med. 1985 Dec 1;162(6):1916–1934. doi: 10.1084/jem.162.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M., Chan C., Clement L. T. Nature of the antigenic complex recognized by T lymphocytes. VIII. Specific inhibition of the stimulatory capacity of antigen-pulsed hapten-modified peritoneal exudate cells by anti-hapten antibody. Eur J Immunol. 1982 Oct;12(10):819–824. doi: 10.1002/eji.1830121005. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Colon S., Kappler J. W., Marrack P., Grey H. M. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J Immunol. 1984 Oct;133(4):2067–2074. [PubMed] [Google Scholar]
- Simonis S., Cullen S. E. Fatty acylation of murine Ia alpha, beta, and invariant chains. J Immunol. 1986 Apr 15;136(8):2962–2967. [PubMed] [Google Scholar]
- Spiro R. C., Quaranta V. The invariant chain is a phosphorylated subunit of class II molecules. J Immunol. 1989 Oct 15;143(8):2589–2594. [PubMed] [Google Scholar]
- Stille C. J., Thomas L. J., Reyes V. E., Humphreys R. E. Hydrophobic strip-of-helix algorithm for selection of T cell-presented peptides. Mol Immunol. 1987 Oct;24(10):1021–1027. doi: 10.1016/0161-5890(87)90068-x. [DOI] [PubMed] [Google Scholar]
- Stockinger B., Pessara U., Lin R. H., Habicht J., Grez M., Koch N. A role of Ia-associated invariant chains in antigen processing and presentation. Cell. 1989 Feb 24;56(4):683–689. doi: 10.1016/0092-8674(89)90590-4. [DOI] [PubMed] [Google Scholar]
- Streicher H. Z., Berkower I. J., Busch M., Gurd F. R., Berzofsky J. A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubin M., Berte C., Mach B. Alternative splicing and alternative initiation of translation explain the four forms of the Ia antigen-associated invariant chain. EMBO J. 1986 Dec 20;5(13):3483–3488. doi: 10.1002/j.1460-2075.1986.tb04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung E., Jones P. P. The invariant chain of murine Ia antigens: its glycosylation, abundance and subcellular localization. Mol Immunol. 1981 Oct;18(10):899–913. doi: 10.1016/0161-5890(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Thomas D. W., Shevach E. M. Nature of the antigenic complex recognized by T lymphocytes. VI. The effect of anti-TNP antibody on T cell responses to TNP-conjugated macrophages. J Immunol. 1978 Sep;121(3):1145–1151. [PubMed] [Google Scholar]
- Watts T. H., McConnell H. M. High-affinity fluorescent peptide binding to I-Ad in lipid membranes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9660–9664. doi: 10.1073/pnas.83.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]