Abstract
We report the safety and immunogenicity of a double lysine and pantothenate auxotroph of Mycobacterium tuberculosis in mice. The ΔlysA ΔpanCD mutant is completely attenuated in immunocompromised SCID and gamma interferon knockout mice yet induces short-term and long-term protection in immunocompetent and CD4-deficient mice following single-dose subcutaneous vaccination.
Tuberculosis (TB) remains a major global health problem, and the increased incidence of TB cases in human immunodeficiency virus (HIV)-infected and AIDS patient populations underlines the need for an improved and safe vaccine (12). The currently used Mycobacterium bovis BCG vaccine has several limitations, including variable protective efficacy in adults (1a, 16), waning of protective immunity over time (4, 15), and safety concerns in immunocompromised individuals (1, 6, 25, 38). In addition, comparative genomic analyses have identified more than 100 coding sequences that are missing from BCG but present in M. tuberculosis (5, 8, 26), including the secreted protein ESAT-6, which has been shown to be a protective antigen in several animal studies (7, 31). These missing regions may encode potential antigenic determinants that could increase the immunogenicity of a vaccine if it were derived from an attenuated strain of M. tuberculosis.
Live, attenuated vaccines offer a powerful means to prevent several important human diseases; a number of these live preparations are in routine use, including attenuated vaccines for polio, measles, mumps, and rubella. The advantages of live vaccines include the ability to replicate within host tissues, thereby facilitating the generation of prolonged memory T-cell responses. Several attenuated M. tuberculosis vaccine candidates that were constructed by deleting genes required for growth in mice (20, 21, 35) have been shown to confer protection against infection with virulent M. tuberculosis. We have previously reported the safety and immunogenicity of the single M. tuberculosis ΔlysA (30) and ΔpanCD (32) auxotrophs in mice. In an attempt to further enhance the safety of a live, attenuated M. tuberculosis vaccine strain, we introduced the panCD deletion into an unmarked ΔlysA mutant of M. tuberculosis by specialized transduction (2). The deletions of the lysA and panCD genes were confirmed by Southern blotting (Fig. 1A). Strain mc26020 (ΔlysA ΔpanCD mutant) is strictly auxotrophic for lysine and pantothenate, and no growth of the mutant was observed in the absence of lysine and pantothenate supplementation. No revertants were recovered when 1011 CFU of strain mc26020 were plated on minimal medium or on medium containing either pantothenate or lysine, demonstrating the mutations to be highly stable and nonrevertible. Similarly, no revertants were observed following serial passage of the mutant or when it was cultured from infected mice.
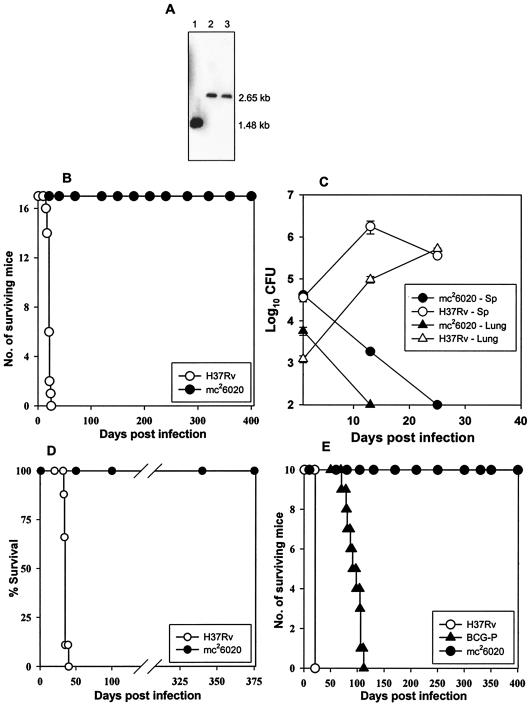
FIG. 1.
ΔlysA ΔpanCD mutant of M. tuberculosis (mc26020) is severely attenuated in immunocompromised and immunocompetent mice. (A) Southern blot demonstrating the loss of the panCD loci from an unmarked ΔlysA mutant of M. tuberculosis. Lane 1 represents the genomic DNA from an unmarked ΔlysA auxotroph of M. tuberculosis, and lanes 2 and 3 represent the genomic DNAs from two independent lysine-pantothenate double auxotrophs of M. tuberculosis. The sizes of the expected hybridization products are shown on the right of the blot. (B) Survival of immunocompetent BALB/c mice (n = 17) infected intravenously with H37Rv (○) or mc26020 (•). (C) Growth of mc26020 in the spleens (Sp) and lungs of immunocompetent C57BL/6 mice during the early phase of infection. (D) Survival of SCID mice (n = 10) infected intravenously with H37Rv (○) or mc26020 (•). (E) Survival of GKO C57BL/6 mice (n = 10) infected intravenously with H37Rv (○), the mc26020 mutant (•), or M. bovis BCG-P (▴).
To evaluate the synergistic effect of the ΔlysA and ΔpanCD mutations on bacterial virulence, immunocompetent BALB/c mice (6 to 8 weeks old, purchased from Jackson Laboratories, Bar Harbor, Maine) were infected intravenously with 5 × 106 CFU of mc26020 or wild-type M. tuberculosis H37Rv. All mc26020-infected mice survived for >400 days (Fig. 1B), compared to the rapid mortality of H37Rv-infected mice (average, 21 days). At 3 weeks postinfection, no mc26020 bacteria were recovered from the lungs, spleens, or livers of mice infected with the ΔlysA ΔpanCD mutant and only occasional, mild lung lesions composed of low numbers of macrophages and lymphocytic infiltrations in the interstitium were detected in these animals. By comparison, the mice infected with H37Rv exhibited severe fatal spreading pneumonia, markedly enlarged spleens with severe diffuse granulomatous splenitis, and hepatitis with large numbers of acid-fast bacilli. In mc26020-infected mice, the lung lesions were almost completely resolved at 8 weeks postinfection. To further evaluate the growth potential of this mutant during the early phase of infection, immunocompetent C57BL/6 mice were infected and the growth kinetics were followed for the first 4 weeks. The mc26020 bacterial numbers steadily declined in the lungs and spleen following intravenous infection with 105 CFU/ml, which were cleared from the lungs in 2 weeks but persisted in low numbers in the spleen until 4 weeks postinfection (Fig. 1C).
HIV-infected individuals have a 10% annual risk of developing TB; thus, a TB vaccine for this population would be extremely valuable in controlling the TB epidemic. However, given their immunocompromised state, any live, attenuated vaccine for use in the HIV-infected population would have to be much attenuated. In order to assess the degree of attenuation of this double-deletion mutant, severe combined immunodeficient (SCID) mice (6 to 8 weeks old, purchased from The Jackson Laboratory, Bar Harbor, Maine), a highly stringent model for safety, were infected intravenously with 105 CFU of mc26020 or 102 CFU of H37Rv. Mice infected with 102 CFU of H37Rv died within 5 weeks postinfection, whereas all mice infected with 105 CFU of mc26020 survived for 375 days, at which time the experiment was terminated (Fig. 1D). The mc26020 mutant was completely cleared from the lungs, spleens, and livers of infected SCID mice in 8 weeks (data not shown). To further evaluate the safety of this mutant, gamma interferon knockout (GKO) mice (6 to 8 weeks old, purchased from The Jackson Laboratory), which are extremely sensitive to tuberculous infection (11, 18), were infected intravenously with 105 CFU of M. tuberculosis H37Rv, BCG Pasteur (BCG-P), or strain mc26020. All of the GKO mice infected with H37Rv (mean survival time [MST], 21 days) or BCG-P (MST, 94.5 days) succumbed to the tuberculous infection, whereas all mice infected with mc26020 survived for 400 days (Fig. 1E). The extreme susceptibility of immunocompromised mice to M. tuberculosis and BCG infections is consistent with data from earlier studies with SCID (32, 40) and GKO (11) mice. Our data clearly demonstrate the enhanced safety of the mc26020 strain in the highly rigorous SCID and GKO mice in comparison with a BCG vaccine strain.
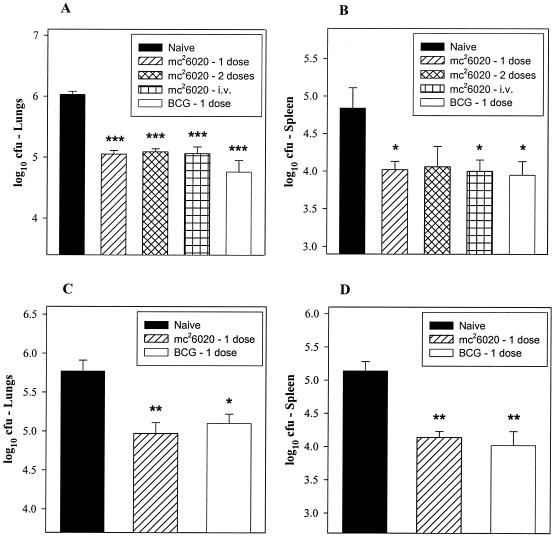
Having assessed the safety and growth kinetics of mc26020 in immunodeficient and immunocompetent mice, we further evaluated the ability of this highly attenuated strain to induce short-term and long-term protection in mice. C57BL/6 mice (6 to 8 weeks old, purchased from The Jackson Laboratory) were immunized subcutaneously or intravenously with 106 CFU of mc26020 and then challenged with a low dose of virulent M. tuberculosis Erdman (50 to 100 CFU) by the aerosol route. The aerosol challenge was performed at either 3 or 7 months postvaccination in order to assess the duration and persistence of the memory immune response. Following subcutaneous immunization, no mutant bacteria could be cultured from the spleens or lungs of mice at 8 and 12 weeks postvaccination. At 28 days post-aerosol challenge, mice immunized 3 months earlier with a single dose of mc26020 showed a significant reduction in bacterial CFU in the lungs (P < 0.001) and spleen (P < 0.05) relative to naive controls (Fig. 2A and B). Interestingly, in mice vaccinated 7 months prior to challenge, the bacterial numbers following aerosol challenge were also significantly decreased in the lungs (P < 0.01) and spleens (P < 0.01) of mice vaccinated with a single dose of mc26020 relative to those in the lungs and spleens of naive controls (Fig. 2C and D). In both of these studies, mice that were vaccinated subcutaneously with 106 CFU of BCG-P also showed significant reductions in organ bacterial burdens relative to those of naive controls. However, no significant differences in the protective responses were detected between the mc26020 and BCG vaccine groups.
FIG. 2.
Vaccination with mc26020 induces short-term and long-term protection in mice. Immunocompetent C57BL/6 mice were immunized subcutaneously or intravenously (i.v.) with mc26020 or BCG-P and challenged with virulent M. tuberculosis Erdman by the aerosol route at 3 (A and B) or 7 (C and D) months after the initial immunization. The CFU numbers reflect the bacterial burden at 28 days post aerosol challenge in the lungs or spleens of infected mice. The results represent means ± standard errors of five mice per group. Asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001) indicate statistically significant differences between the experimental and unvaccinated control groups.
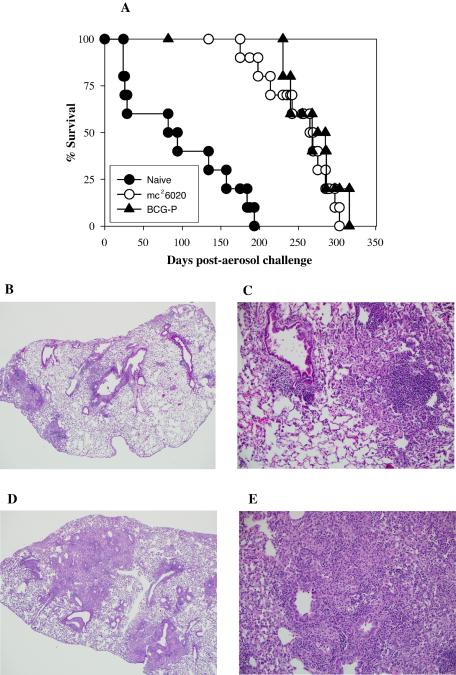
Another measure of vaccine effectiveness is the extent of pulmonary pathology seen in immunized mice in comparison to that seen in unvaccinated controls. At 28 days post-aerosol challenge, mice that had been vaccinated 7 months earlier with mc26020 displayed greatly reduced lung lesions compared to those of unvaccinated mice. Mice vaccinated with mc26020 showed multiple peribronchial lesions with limited extension to the adjacent parenchyma. These localized lesions were composed of intense lymphocytic and mononuclear inflammatory cell infiltration, with macrophages filling the alveolar spaces. They lacked the inflammatory cell composition that characterizes a typical granulomatous response (Fig. 3B and C). The severity and distribution of lesions were similar in mice vaccinated either once or twice by the subcutaneous route or intravenously. In comparison, the unvaccinated mice exhibited severe spreading and coalescing granulomatous pneumonia, with more extensive lung involvement. The areas of pneumonia were consolidated with infiltration of epithelioid cells accompanied by moderate numbers of lymphocytes causing obliteration of air spaces. There were locally intense lymphocytic infiltrations, particularly around small and larger blood vessels (Fig. 3D and E). The lung pathology of mice vaccinated with BCG was similar to that of the mc26020-immunized animals, with greatly reduced lung lesions seen in the BCG group compared to those seen in unvaccinated controls (data not shown).
FIG.3.
Vaccination with mc26020 confers long-term survival and induces milder lung pathology in immunocompetent mice. (A) Survival of immunocompetent C57BL/6 mice (n = 10) immunized subcutaneously with a single dose of mc26020 (○) or BCG-P (▴) and challenged 3 months later with virulent M. tuberculosis Erdman by the aerosol route. Unvaccinated mice served as naive controls (•). (B and C) Lungs of mice challenged 7 months after vaccination with a single dose of mc26020 by the subcutaneous route. (B) Low-power photomicrograph showing scattered areas of pneumonia adjacent to bronchioles, with limited localized spread into the adjacent parenchyma. (C) Higher-power magnification of lung lesions in these mice showing the intense localized lymphocytic perivascular accumulations and infiltration of numerous macrophages filling the adjacent alveolar spaces. (D and E) More-severe spreading lung lesions in unvaccinated mice compared with those in vaccinated mice. (D) Low-power photomicrograph showing multiple areas of extensive pneumonia. (E) Affected areas of the lung showed granulomatous pneumonia and were consolidated with loss of air spaces, caused by the extensive diffuse infiltration of epithelioid cells accompanied by large numbers of lymphocytes.
An important parameter when estimating the persistence of vaccine-induced protective immunity is the relative survival periods for immunized mice following a challenge with virulent organisms (29). To further substantiate the protective efficacy of mc26020, mice immunized subcutaneously with a single dose of mc26020 or BCG-P were challenged with virulent M. tuberculosis Erdman by the aerosol route 3 months after the immunization and followed for survival. In these studies, the survival periods of mice vaccinated with mc26020 (MST, 252 days) or BCG-P (MST, 268 days) were significantly extended (P < 0.001) relative to those of naive mice (MST, 95 days) (Fig. 3A). There were no significant differences in survival between the BCG- and mc26020-vaccinated mice. Earlier studies have shown that robust protective memory T cells can be generated in the absence of prolonged antigenic exposure. The initial antigenic burst and effective recruitment of large numbers of antigen-specific T cells determine the strength and duration of the immune response (22, 23, 27). In this regard, the initial interaction of ΔlysA ΔpanCD mutant bacteria with appropriate antigen-presenting cells following subcutaneous vaccination results in the elicitation of a qualitatively robust protective immune response. Our data suggest that the elicitation of long-term protective memory is dependent on the quality of the initial antigenic encounter rather than the continued persistence of the antigenic stimulus. These results clearly demonstrate the vaccine potential of this highly attenuated strain in conferring long-term protection and survival against a challenge with virulent M. tuberculosis.
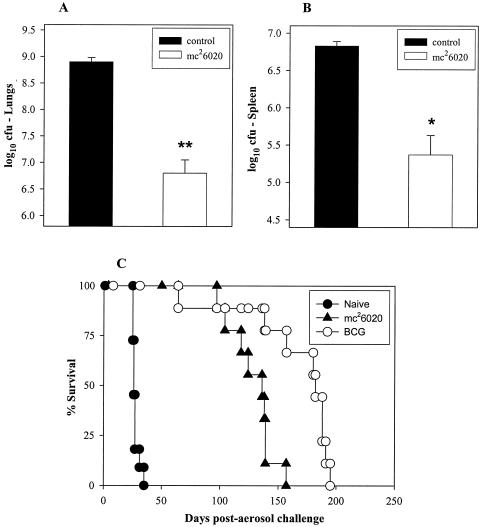
TB remains the leading cause of death in HIV-infected individuals in developing countries (41). HIV infection leads to loss of CD4+ T cells, and previous studies have demonstrated that mice deficient in CD4+ T cells are highly susceptible to M. bovis BCG (24) and M. tuberculosis (10, 28) infections. Since BCG vaccination can progress to BCG disease in HIV-infected individuals and therefore is contraindicated for this population, we wanted to determine whether mc26020, which is more attenuated, could protect CD4-deficient mice from experimental TB. For these experiments, specific-pathogen-free, CD4-deficient (B6.129S2-Cd4tm1Mak) female mice (obtained from The Jackson Laboratory) were vaccinated subcutaneously with 106 CFU of mc26020 and then aerogenically challenged with 100 to 200 CFU of M. tuberculosis Erdman 3 months later. At 28 days post aerosol challenge, the bacterial burden in the lungs (P < 0.001) and spleens (P < 0.01) of the mc26020-vaccinated mice was decreased by greater than 90% (>2 log10 CFU in the lungs and 1.5 log10 CFU in the spleen) relative to that of naive controls (Fig. 4A and B). The significant reduction of the bacterial burden exhibited by the mc26020-vaccinated mice is similar and consistent with the protection seen in BCG-vaccinated, CD4-deficient mice, as reported previously (14).
FIG. 4.
Single-dose immunization with mc26020 protects and prolongs the survival of CD4−/− mice with TB. CD4−/− mice were immunized subcutaneously with mc26020 and challenged with virulent M. tuberculosis Erdman by the aerosol route at 3 months postimmunization. The CFU numbers reflect the bacterial burdens at 28 days post aerosol challenge in the lungs (A) and spleen (B). Asterisks (**, P < 0.001; *, P < 0.01) indicate statistically significant differences between the experimental and unvaccinated control groups. (C) Survival of CD4−/− mice (n = 9 to 11) immunized subcutaneously with a single dose of mc26020 or BCG-P and challenged 3 months later with virulent M. tuberculosis Erdman by the aerosol route. Unvaccinated mice served as naive controls.
To further evaluate the long-lived protection induced by mc26020, CD4−/− mice were vaccinated, challenged 3 months later with virulent M. tuberculosis Erdman by low-dose aerosol infection, and followed for survival. All naive mice died within 28 days (MST = 27 ± 0.9 days) after the challenge. Strikingly, the MST of the CD4−/− mice vaccinated with a single dose of mc26020 was 128 ± 6.4 days, a nearly fivefold extension of the survival period compared with that of naive mice (Fig. 4C). In contrast, the BCG-vaccinated mice survived for 164 ± 14 days. The 36-day extension of the MST of the BCG-immunized mice relative to mc26020-vaccinated animals represented a significantly improved outcome (P < 0.05). This suggests that the ability of M. bovis BCG to persist longer than the ΔlysA ΔpanCD mutant may confer better protection against TB on CD4-deficient mice.
Since BCG vaccine strains are attenuated but not avirulent, immunization with live BCG is generally contraindicated for immunocompromised persons. The results of a recent South African clinical study in which several cases of mycobacterial disease in children were attributed to BCG vaccination supports this increased risk of immunization with BCG in areas with a significant prevalence of HIV infection (19). By deleting the panCD genes, which are involved in lipid biosynthesis, in conjunction with a gene involved in lysine biosynthesis, we have created an M. tuberculosis mutant that is severely attenuated in SCID and GKO mice and thus potentially safer than the widely used BCG vaccine strains. Importantly, this attenuated mutant induced protective responses against an aerogenic M. tuberculosis challenge in both immunocompetent and immunocompromised mice. The vaccine-mediated immune mechanisms involved in the protective response in CD4-deficient mice are of particular interest because several studies have suggested that CD4+ T cells are absolutely required to generate anti-TB protective immunity (17). Since CD8+ T cells have been shown to play an important role in the control of acute TB (3, 34, 36) and persistent infection (39), it is possible that vaccine-induced stimulation of CD8+ T cells is largely responsible for the protection seen in CD4-deficient mice. Recent evidence suggests that substantial vaccine immunity can be generated in the absence of CD4+ T cells (9, 37, 42). Consistent with this premise, the secretion of tumor necrosis factor alpha and gamma interferon by CD8+ T cells has been recently shown to be the primary antimycobacterial protective immune response generated in CD4-deficient mice by a TB DNA vaccine cocktail (14). Alternatively, double-negative T cells could contribute to the anti-TB protective response induced by vaccination of CD4−/− mice with the mc26020 strain. Interestingly, Cowley and Elkins have recently demonstrated that a unique population of Thy1+ αβ+ CD4− CD8− cells strongly controlled the growth of another intracellular pathogen, Francisella tularensis (13). Clearly, further studies are required to elucidate the immune mechanisms involved in the protective immunity induced by live, attenuated M. tuberculosis vaccine strains in CD4-deficient mice.
Our characterization of the lysine and pantothenate auxotroph and previous studies with a double leucine and pantothenate auxotroph (33) have demonstrated that it is possible to generate two independent unlinked deletions into M. tuberculosis to generate a safe mutant that can remain immunogenic and induce a long-term memory response against airborne infection with virulent M. tuberculosis in mice. These findings provide a paradigm for a live, attenuated vaccine strain against TB; i.e., a mutant with substantially reduced replication and persistence in the host can induce considerable and long-lasting protective immunity. Importantly, the absence of reversion and the overall excellent protection and safety data generated in vaccine studies with the lysine-pantothenate double auxotroph makes this double-deletion mutant a viable vaccine candidate for humans.
Acknowledgments
We thank Martin Pavelka for providing the ΔlysA mutant strain of M. tuberculosis. We thank Mei Chen and John Kim for help with the animal experiments.
This work was supported by NIH grant AI 26170 and funds from the Aeras Global Tuberculosis Vaccine Foundation.
Editor: A. D. O'Brien
REFERENCES
- 1.Armbruster, C., W. Junker, N. Vetter, and G. Jaksch. 1990. Disseminated bacille Calmette-Guerin infection in an AIDS patient 30 years after BCG vaccination. J. Infect. Dis. 162:1216. [DOI] [PubMed] [Google Scholar]
- 1a.Baily, G. V. 1980. Tuberculosis prevention trial, Madras. Indian J. Med. Res. 72:1-74. [PubMed] [Google Scholar]
- 2.Bardarov, S., S. Bardarov, Jr., M. S. Pavelka, Jr., V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs, Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007-3017. [DOI] [PubMed] [Google Scholar]
- 3.Behar, S. M., C. C. Dascher, M. J. Grusby, C. R. Wang, and M. B. Brenner. 1999. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189:1973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behr, M. A., and P. M. Small. 1997. Has BCG attenuated to impotence? Nature 389:133-134. [DOI] [PubMed] [Google Scholar]
- 5.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 6.Besnard, M., S. Sauvion, C. Offredo, J. Gaudelus, J. L. Gaillard, F. Veber, and S. Blanche. 1993. Bacillus Calmette-Guerin infection after vaccination of human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 12:993-997. [DOI] [PubMed] [Google Scholar]
- 7.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosch, R., S. V. Gordon, K. Eiglmeier, T. Garnier, F. Tekaia, E. Yeramian, and S. T. Cole. 2000. Genomics, biology, and evolution of the Mycobacterium tuberculosis complex. ASM Press, Washington, D.C.
- 9.Buller, R. M., K. L. Holmes, A. Hugin, T. N. Frederickson, and H. C. Morse III. 1987. Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature 328:77-79. [DOI] [PubMed] [Google Scholar]
- 10.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 11.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 13.Cowley, S. C., and K. L. Elkins. 2003. Multiple T cell subsets control Francisella tularensis LVS intracellular growth without stimulation through macrophage interferon gamma receptors. J. Exp. Med. 198:379-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derrick, S. C., C. Repique, P. Snoy, A. L. Yang, and S. Morris. 2004. Immunization with a DNA vaccine cocktail protects mice lacking CD4 cells against an aerogenic infection with Mycobacterium tuberculosis. Infect. Immun. 72:1685-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 16.Fine, P. E. M., I. A. M. Carneiro, J. B. Milstien, and C. J. Clements. 2002. Issues relating to the use of BCG in immunization programmes. [Online.] http://www.who.int/vaccines-documents/DocsPDF99/www9943.pdf.
- 17.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 18.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hesseling, A. C., H. S. Schaaf, W. A. Hanekom, N. Beyers, M. F. Cotton, R. P. Gie, B. J. Marais, P. van Helden, and R. M. Warren. 2003. Danish bacille Calmette-Guerin vaccine-induced disease in human immunodeficiency virus-infected children. Clin. Infect. Dis. 37:1226-1233. [DOI] [PubMed] [Google Scholar]
- 20.Hondalus, M. K., S. Bardarov, R. Russell, J. Chan, W. R. Jacobs, Jr., and B. R. Bloom. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 68:2888-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson, M., S. W. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jelley-Gibbs, D. M., N. M. Lepak, M. Yen, and S. L. Swain. 2000. Two distinct stages in the transition from naive CD4 T cells to effectors, early antigen-dependent and late cytokine-driven expansion and differentiation. J. Immunol. 165:5017-5026. [DOI] [PubMed] [Google Scholar]
- 23.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladel, C. H., S. Daugelat, and S. H. Kaufmann. 1995. Immune response to Mycobacterium bovis bacille Calmette-Guerin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur. J. Immunol. 25:377-384. [DOI] [PubMed] [Google Scholar]
- 25.Lotte, A., O. Wasz-Hockert, N. Poisson, H. Engbaek, H. Landmann, U. Quast, B. Andrasofszky, L. Lugosi, I. Vadasz, P. Mihailescu, et al. 1988. Second IUATLD study on complications induced by intradermal BCG vaccination. Bull. Int. Union Tuberc. Lung Dis. 63:47-59. [PubMed] [Google Scholar]
- 26.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 28.Mogues, T., M. E. Goodrich, L. Ryan, R. LaCourse, and R. J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North, R. J., L. Ryan, R. LaCource, T. Mogues, and M. E. Goodrich. 1999. Growth rate of mycobacteria in mice as an unreliable indicator of mycobacterial virulence. Infect. Immun. 67:5483-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavelka, M. S., Jr., B. Chen, C. L. Kelley, F. M. Collins, and W. R. Jacobs, Jr. 2003. Vaccine efficacy of a lysine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 71:4190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and S. T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9:533-539. [DOI] [PubMed] [Google Scholar]
- 32.Sambandamurthy, V. K., X. Wang, B. Chen, R. G. Russell, S. Derrick, F. M. Collins, S. L. Morris, and W. R. Jacobs, Jr. 2002. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 8:1171-1174. [DOI] [PubMed] [Google Scholar]
- 33.Sampson, S. L., C. C. Dascher, V. K. Sambandamurthy, R. G. Russell, W. R. Jacobs, Jr., B. R. Bloom, and M. K. Hondalus. 2004. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect. Immun. 72:3031-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serbina, N. V., and J. L. Flynn. 2001. CD8+ T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect. Immun. 69:4320-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, D. A., T. Parish, N. G. Stoker, and G. J. Bancroft. 2001. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 69:1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sousa, A. O., R. J. Mazzaccaro, R. G. Russell, F. K. Lee, O. C. Turner, S. Hong, L. Van Kaer, and B. R. Bloom. 2000. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 97:4204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1998. Virus-specific CD8+ T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc. Natl. Acad. Sci. USA 95:15565-15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talbot, E. A., M. D. Perkins, S. F. Silva, and R. Frothingham. 1997. Disseminated bacille Calmette-Guerin disease after vaccination: case report and review. Clin. Infect. Dis. 24:1139-1146. [DOI] [PubMed] [Google Scholar]
- 39.van Pinxteren, L. A., J. P. Cassidy, B. H. Smedegaard, E. M. Agger, and P. Andersen. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 30:3689-3698. [DOI] [PubMed] [Google Scholar]
- 40.Weber, I., C. Fritz, S. Ruttkowski, A. Kreft, and F. C. Bange. 2000. Anaerobic nitrate reductase (narGHJI) activity of Mycobacterium bovis BCG in vitro and its contribution to virulence in immunodeficient mice. Mol. Microbiol. 35:1017-1025. [DOI] [PubMed] [Google Scholar]
- 41.Whalen, C. C., P. Nsubuga, A. Okwera, J. L. Johnson, D. L. Hom, N. L. Michael, R. D. Mugerwa, and J. J. Ellner. 2000. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS 14:1219-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wuthrich, M., H. I. Filutowicz, T. Warner, G. S. Deepe, Jr., and B. S. Klein. 2003. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J. Exp. Med. 197:1405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]