Abstract
Mucopolysaccharidosis type II (MPS II - Hunter syndrome) is an X-linked lysosomal storage disorder caused by a deficiency in the enzyme iduronate-2 sulfatase (I2S), leading to the accumulation of the glycosaminoglycans, affecting multiple organs and systems. Enzyme replacement therapy does not cross the blood brain barrier, limiting results in neurological forms of the disease. Another option of treatment for severe MPS, hematopoietic stem cell transplantation (HSCT) has become the treatment of choice for the severe form of MPS type I, since it can preserve neurocognition when performed early in the course of the disease. To date, only few studies have examined the long-term outcomes of HSCT in patients with MPS II. We describe the seven-year follow-up of a prenatally diagnosed MPS II boy with positive family history of severe MPS form, submitted to HSCT with umbilical cord blood cells at 70 days of age. Engraftment after 30 days revealed mixed chimerism with 79% donor cells; after 7 years engraftment remains at 80%. I2S activity 30 days post-transplant was low in plasma and normal in leukocytes and the same pattern is observed to date. At age 7 years growth charts are normal and he is very healthy, although mild signs of dysostosis multiplex are present, as well as hearing loss. The neuropsychological evaluation (Wechsler Intelligence Scale for Children - Fourth Edition - WISC-IV), disclosed an IQ of 47. Despite this low measured IQ, the patient continues to show improvements in cognitive, language and motor skills, being quite functional. We believe that HSCT is a therapeutic option for MPS II patients with the severe phenotype, as it could preserve neurocognition or even halt neurodegeneration, provided strict selection criteria are followed.
Keywords: Mucopolysaccharidosis, Hematopoietic stem cell transplantation, Neurocognition
1. Introduction
Mucopolysaccharidosis type II (MPS II, Hunter syndrome, OMIM 309900) is an X-linked lysosomal storage disorder caused by a deficiency in the enzyme iduronate-2 sulfatase (I2S), leading to the accumulation of the glycosaminoglycans (GAGs) dermatan sulfate and heparan sulfate [1]. The excessive storage of these GAGs both intracellularly and extracellularly leads to the clinical features of the disease.
MPS II affects multiple organs and systems with a variable age of onset of signs and symptoms. Two clinical presentations of Hunter syndrome have been reported, the mild, or attenuated, and the severe forms. The severe phenotype, with primary neural parenchymal disease is referred as neuronopathic MPS II and the mild phenotype, without neural parenchymal involvement, as nonneuronopathic MPS II. The distinguishing factor between the 2 forms is the presence, or absence, of progressive intellectual deterioration. Both phenotypes exhibit cardiorespiratory and skeletal disease [2].
Historically, the management of Hunter syndrome was directed to the specific disease symptoms and complications regardless the phenotype. In 2006, recombinant human I2S (idursulfase, Elaprase®, Shire Human Genetics Therapies, Inc., Cambridge, MA, USA) was approved for the treatment of Hunter syndrome in the United States, followed by approval in many other countries [3]. The drug showed to improve many of the somatic manifestations, but since idursulfase is not predicted to cross the blood-brain barrier, the treatment cannot alter the cognitive deterioration associated with the disease in patients with severe MPS II [4], [5].
Hematopoietic stem cell transplantation (HSCT) has become the treatment of choice for the severe form of mucopolysaccharidosis type I (Hurler syndrome) since it can preserve neurocognition when performed early in the course of the disease [6], [7], [8], [9], [10]. HSCT has also been performed in other MPS (non-MPS I), although often only evaluated as part of a larger heterogeneous cohort of transplanted patients with multiple metabolic diseases or as few case reports and with variable results [11], [12], [13]. Compared to Hurler syndrome, the overall experience in the use of HSCT for treatment of other MPSs (II, III, IV, VI and VII) is very limited. Nevertheless, the scientific basis for effectiveness of HSCT across the MPS spectrum remains the same: donor-derived cells would secrete the deficient enzyme, which would be recaptured by surrounding cells. The kinetics of cellular migration, differentiation, distribution and effective enzyme delivery, however, may differ among MPS subtypes.
The majority of clinical experience of HSCT in MPS II patients comes from observations of case studies. To date, only few authors have examined the long-term outcomes of HSCT in groups of patients with MPS II. In these studies, HSCT was found to increase or normalize I2S activity in leukocytes, but not in serum, and was associated with decreased or normalized urinary GAG excretion. In 2009, a French report on 8 boys with Hunter syndrome treated with BMT at 3 to 16 years and followed for 7 to 17 years showed an excellent survival rate, stabilization of cardiovascular problems, resolution of hepatosplenomegaly, improvement in joint stiffness, arrested progression of perceptual hearing defect and improvement in transmission hearing defects. Improvements in neuropsychological function, however, were variable and appeared to be related to disease severity at transplant [14]. Lack of neurologic improvement was also described in 3 BMT recipients in a 1999 report with ages varying from 10 months to 5 years [15]. In a recent report of the China Children Transplant Group including 34 MPS children who underwent HSCT (12 MPS II), patients both with MPS I and II aged 2 to 6 years at transplant showed some improvements in motor and speech skills, suggesting that HSCT was also beneficial in improving mental development in MPS II patients [16].
Although benefits have been observed regarding the somatic features of the disease, the role of HSCT in MPS II remains controversial because of lack of convincing evidence of neurocognitive benefit. Despite the available reports, there are still no published data on HSCT in very young, early stage, or asymptomatic children with the neuronopathic MPS II phenotype.
2. Case report
We describe the seven years follow-up of a MPS II child submitted to HSCT with umbilical cord blood cells at 70 days of age, preceded by ERT. He was prenatally investigated due to positive family history, as there were an older brother and an uncle diagnosed with Hunter syndrome, both with the severe phenotype. Undetectable iduronate-2-sulphatase (I2S) activity and the familial p.Arg88His mutation on exon 3 (p.R88H) were detected in fibroblasts of the amniotic fluid. This is a missense mutation already reported and associated with the severe phenotype of MPS II [17], [18], [19].
The patient was born at gestational age of 37 weeks and 2 days by cesarean section due to nonprogression of vaginal delivery, weighing 3.320 g (50th centile), birth length of 51 cm (50th centile) and a head circumference of 34 cm (50th centile).
Discrete lysosomal storage in endothelial cells and in pericytes was observed in placental analysis by electronic microscopy (previously described by [20]). Enzyme I2S activity re-confirmed the diagnosis of MPS II in plasma [1,2 nmol/4 h/ml (ref = 122–463)] and leukocytes [4,3 nmol/4 h/mg/protein (ref = 31–110)].
At birth, neurological examination showed generalized hyperexcitability. Very discrete lumbar gibbus was observed and the X-ray survey demonstrated discrete irregularity on the anterior face of vertebral bodies L3, L4 and L5 (patient 1 in [21]).
Abdominal and cerebral ultrasound, echocardiogram, ophthalmology, audiology, pulmonary function, brain and spine MRI were normal, except for a discrete enlargement of CSF space posterior to the cerebellum. His neuropsychological evaluation with 1 month and 8 days of life using the Bayley Scales of Infant and Toddler Development-Third Edition (Bayley-III) demonstrated a cognitive score of 75, a language score of 74 and a motor score of 79.
At 19 days of life he presented fever and upper airways infection, leading to hospital admission and intravenous antibiotic treatment for 10 days (6 days of ampicillin, 3 days of gentamicin, 3 days of cefotaxime and 4 days of ceftriaxone).
He received 6 weekly infusions of 0,5 mg/kg idursulfase from age 10 days until the transplantation, without any adverse reaction. The response to ERT was evaluated with urinary GAG analysis. The baseline GAG level at 10 days of age was 2000 mg/g creatinine (upper limit of normal: 170 mg/g creatinine), which fell to 590 mg/g creatinine after 6infusions of ERT.
3. Transplant, engraftment and enzyme studies
HSCT with umbilical cord blood from HLA non-identical unrelated donor (match 5/6) was performed at 70 days of age. HLA typing and donor match were successful during pregnancy, and the match was reconfirmed after birth. Conditioning regimen consisted of busulphan 320 mg/m2, cyclophosphamide 200 mg/kg and thymoglobulin 5 mg/kg. He received a combination of cyclosporin and corticosteroid as graft-versus-host disease prophylaxis.
Engraftment after 30 days revealed mixed chimerism with 79% donor cells and after 7 years engraftment remains at 80%. IDS activity 30 days post-transplant was low in plasma (22 nmol/4 h/ml; ref. = 122–463) and normal in leukocytes (49 nmol/4 h/mg protein; ref. = 31–110); 270 days, 2, 3, 4, 5, 6 and 7 years after transplant the same pattern was observed.
At 75 days post-transplant, routine laboratory tests revealed positive antibodies for cytomegalovirus. He began intravenous ganciclovir 10 mg/kg/day and continued the treatment for 6 months.
Urinary glycosaminoglycans (GAG) began to decrease during ERT and reached the normal range 3 months post-transplant (135 mg/g creatinine). At the moment, GAG levels are within the normal range for age matched control (60 mg/g creatinine).
4. Follow-up and development
Motor milestones were all normal for age. His most recent motor evaluation, with 7 years disclosed adequate muscle tone, strength and joint range of motion within the normal range.
His speech development, however, has been slower. An auditory evoked response test performed at 5 years was compatible with moderate hearing loss. Hebegan to use a hearing device and started to speak short phrases soon thereafter.
The patient always manifested increased general activity and as of 3 years of age he demonstrated aggressive behavior. Despite showing benefit after introduction of the hearing aid and decrease of aggressiveness, hyperactive behavior is still present.
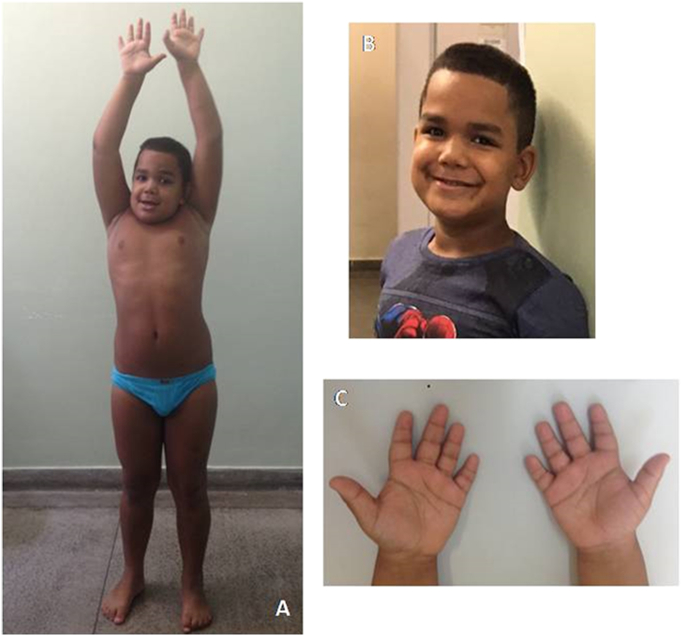
At age 7 years growth charts are normal; he weighs 39 kg (> 90th centile), standing height is 129 cm (90th centile) and head circumference is 53 cm (75th centile). Motor development is also normal, he has no skeletal problems, joint contractures or claw hands. He has never had coarse facial features, corneal clouding or hepatosplenomegaly. Physical examination discloses only short hands with stubby digits, which is in agreement with previous reports of those transplanted with the severe phenotype – Fig. 1 [22].
Fig. 1.
Transplanted MPS II patient at age 7 years. (A): no joint stiffness. (B) no MPS phenotype. (C) no claw hands.
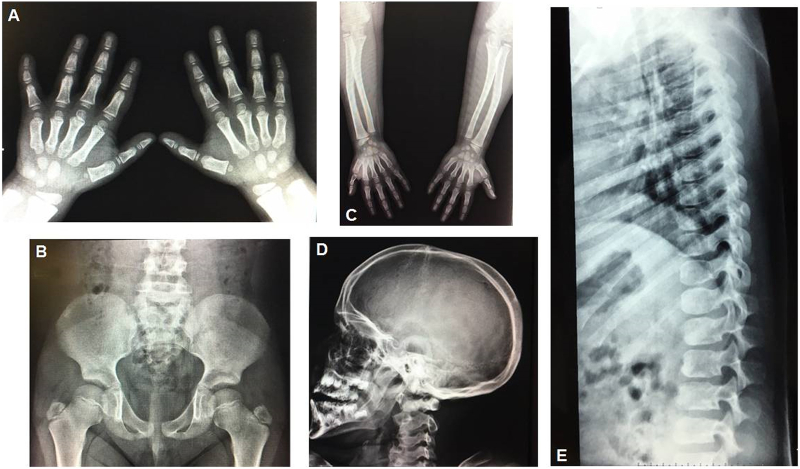
Most recent evaluations performed at age of 7 years revealed normal brain CT and normal upper airway fiberoptic findings. Echocardiogram demonstrated completely normal cardiac function with no signs of valvar dysfunction. MRI of the brain discloses mild cerebellar hypoplasia, in accordance to the findings on the first exam in the newborn period. MRI of the spine was normal and X-rays showed very mild signs of dysostosis multiplex – Fig. 2.
Fig. 2.
Mild Dysostosis Multiplex in the transplanted MPS II boy. (A) Hands: shortened and proximal pointing of metacarpals, hypoplasia of carpals. (B) Pelvis: round iliac wings and inferior iliac tapering (C) Long bones: defect of linear growth with thick shortened diaphyses. (D) Skull: thickened calvaria. (E) Spine: inferiorly beaked vertebrae and platyspondyly in lumbar spine.
Upper limbs somatosensory evoked potentials showed N20 cortical waves with normal latencies after stimuli of ulnar nerves. Lower limbs somatosensory evoked potentials showed P37 cortical waves with prolonged latencies and prolonged central conduction time after stimuli of posterior tibial nerves at 6.5 years. The neurophysiological findings denote compromise of the sensory conduction in the posterior columns. Such finding is in accordance to somatosensory abnormalities found in MPS patients, although his findings are milder when compared to nontransplanted patients [23].
Neuropsychological evaluation with 1 year and 11 months, 20 months after HSCT transplantation using the Bayley-III scale showed cognitive score of 90, language score of 74 and motor score of 94. At 4 years and 3 months and at 6 years and 1 month, his IQ measured through Wechsler Preschool and Primary Scale of Intelligence - Third Edition (WPPSI-III Wechsler, 2002) was 57 and 50, respectively. In the last neuropsychological evaluation with 7 years and 2 months using the Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV), his measured IQ was 47, being working memory and verbal comprehension indexes his worst scores.
5. Discussion
HSCT has been performed in several lysosomal storage disorders with different degrees of success [14]. In Hunter syndrome, the results have been highly variable, with the majority of patients showing improvements of their somatic features and different responses regarding the neuropsychological outcome [14], [15], [22], [24], [25].
When indicated in MPS (consensus for severe MPS I), HSCT should be performed as early as possible as an attempt to avoid neurological deterioration. The patient described here is one of the youngest MPS II patients to have received HSCT so far; there is one patient reported to have been transplanted at 1.5 month [26]. Our described patient had the prediction of a severe phenotype proven both by the family history and the genotype [17], [18], [19].
Since ERT significantly improves patient's pre-transplant condition (improving respiratory and cardiac function), is well tolerated and does not negatively influence engraftment, we chose to start ERT prior to HSCT [9]. The initial idea was to continue ERT even after the transplant, until blood enzyme activity was normal and he had a good engraftment. Since the patient was transplanted in another Brazilian state, however, ERT was interrupted before the family travelled to the transplant site due to reimbursement issues.
Literature data show that patients with MPS I have more hearing improvement after HSCT in comparison to ERT [22], [27]. The small number of MPS II patients treated with HSCT makes it difficult to draw any meaningful conclusion regarding hearing improvement in this population. According to da Costa et al., HSCT at an early stage (< 25 months) in MPS II patients does have a significant correlation to hearing improvement [28]. Despite having been undertaken at an early stage, HSCT seems not to have prevented the sensorineural hearing loss in our patient. His poor behavior impeded a more complete audiologic assessment, besides auditory brainstem responses (ABRs), so the conductive component could not be tested. Nevertheless, he has never presented any episode of otitis media, which is known to occur in 92% of MPS II patients and is a major contributor to conductive hearing loss [2].
The CMV infection after transplantation, although only positive antigenemy was present and no clinical signs of infection were observed, as well as medications used prior and post-transplant, may have contributed to the hearing loss and confounded our data. Of note, ganciclovir and gentamicin are well known ototoxic drugs [29].
Regarding the neuropsychological evaluations, the lower cognitive score presented in patient's first evaluation at 38 days-old could have been influenced by the time he spent hospitalized (at 19 days of age due to upper airway infection), then improved in the second evaluation. It is important to mention that the Bayley scale is not predictive in regards of a child's performance as human development is complex and dynamic.
Unfortunately we had to use 3 different tests to evaluate patient's DQ/IQ due to age limitation of the scales. Moreover, these scales are only translated to Portuguese and not validated in the Brazilian population, so cultural and socioeconomic biases could have contributed to the low performance. Another weakness of the neuropsychological evaluation was the use of WISC test, a heavily language based evaluation, that may have been influenced by his hearing loss and resulted in poor performance. It is worthwhile to mention that our patient has never attended physical, occupational or language therapies, although strongly recommended, which could have improved his performance in the cognitive tests.
Literature shows that severe Hunter patients improve their cognitive function until they reach a plateau at approximately 48 to 55 months of age and thenceforth it declines rapidly [2]. Our patient is keeping a relatively stable IQ, which implies he is gaining skills at an almost normal rate. In fact, he is a very functional child who attends regular school (although a bit delayed for chronological age), interacts well with other children, practices sports, eats unaided and plays videogame. In the Pediatric Evaluation of Disability inventory (PEDI), functional skills section, he scored 156 out of 197, showing a quite good functional performance or at least much better than expected for a boy of his age with the neuronopathic MPS II phenotype. We have also assessed the activities of daily living (ADL) through ADL questionnaire for patients with Hunter syndrome designed by Tanjuakio [30]. In this instrument he obtained a score of 43 out of a total of 60, which is among the highest for patients with the severe phenotype.
When comparing him to the other MPS II relatives in the family (a deceased older brother at age 12, who began ERT at the age of 6 years and a deceased 20 year old severely bedridden uncle diagnosed at 8 years, who had never been treated with ERT), his is doing much better. Table 1 and Fig. 3, Fig. 4 show differences in development and phenotype between the propositus and the other affected in the family.
Table 1.
Family comparative information about motor and neurodevelopmental milestones.
| Propositus | Older brother (deceased 12 years) | Uncle (deceased 20 years) | |
|---|---|---|---|
| Diagnosis | Pre-natal | 4 years | 8 years |
| ERT/HSCT | 6 ERT infusions followed by HSCT | Started ERT at 6 years | Never received ERT |
| Head support | 2 months | 4–5 months | 6 months |
| Sat unaided | 5 months | 8–9 months | 9 months |
| Babbled | 5–6 months | After 1 year | 1 year and 6 months |
| Crawled | 7 months | 10 months | 1 year |
| Walked unaided | 1 year | 1 year and 1 month | 1 year and 8 months |
| Toilet trained | 4 years | Never | Never |
| Self-feeding | 3 years | Never | Never |
| Shower unaided | 5 years | Never | Never |
| Reading/writing | Beginning to read and write | Never | Never |
| Hidrocephalus/shunt placement | Never | 4 years | 8 months |
| Stopped walking | – | 10 years | 10 years |
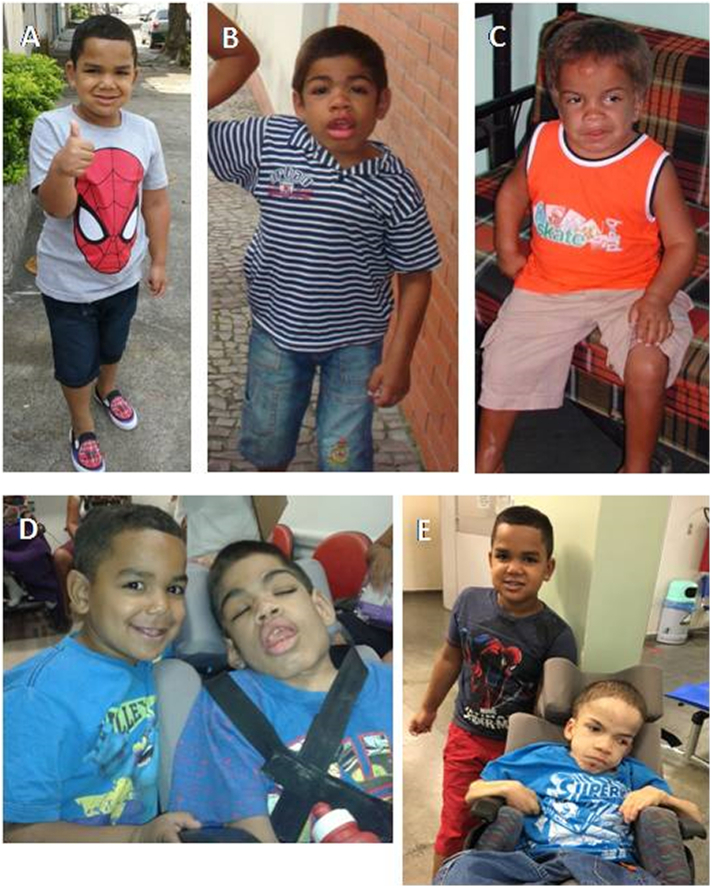
Fig. 3.
Clinical phenotype of the propositus (A), his older brother (B) and uncle (C) at age of 6 years. The propositus at age 4 years and his brother at age 12 years (D).The propositus at age 7 years and his uncle at age 20 years (E).
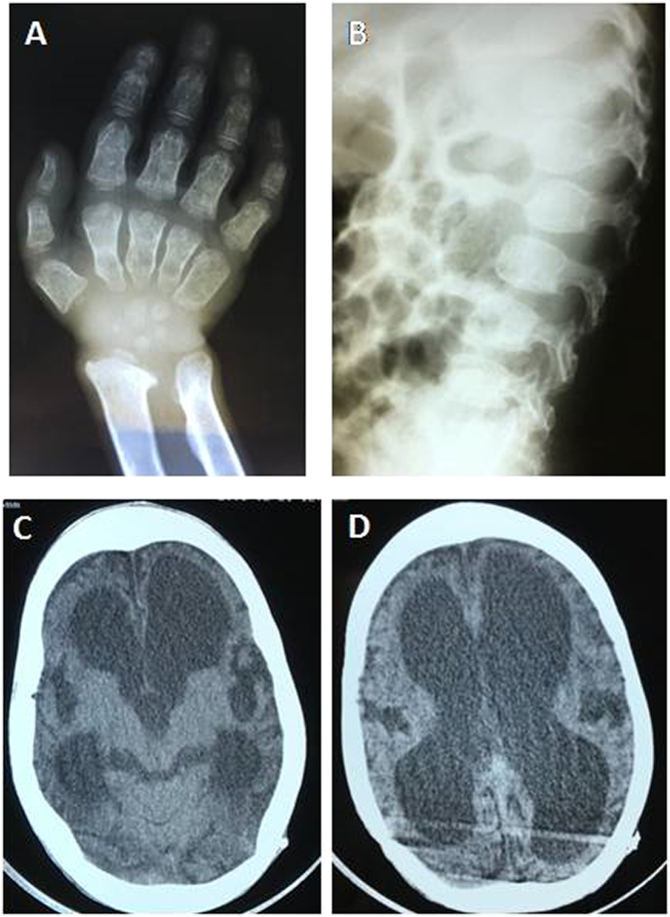
Fig. 4.
Propositus' uncle at 7 years. X-Ray images showing signs of severe dysostosis multiplex in the hand (A) - proximal pointing of metacarpals, shortened metacarpals and carpal hypoplasia - and spine (B) - abnormal modeling and inferiorly beaked vertebrae. Cranial computed tomography (C) and (D) showing enlargement of the supratentorial ventricles and cortical atrophy, even after shunting.
In regards to transplant in diseases with neurologic progression, it seems that if the procedure is performed very early in the disease process, more neurocognitive benefits would occur. For the brain, it has been observed that donor-derived macrophages cross the blood-brain barrier with subsequent differentiation into microglia, secreting the deficient enzyme for recapture by surrounding neurons. The timing of migration to and engraftment of donor-derived microglial cells after HSCT is not known, but probably, on the basis of clinical observation, it takes months after hematologic engraftment. Thus, an explanation for the failure of HSCT to prevent mental deterioration in certain patients could be the slow pace of replacement of patient tissue macrophages and microglia populations by donor hematopoietic cell progeny limiting the success for rapidly progressive neurologic diseases ([31], [32]).
It is important to point out that the majority of the available literature on HSCT in MPS II patients is outdated. There are only few case reports, showing no rigid selection criteria of the candidates, who were from different age groups and already presented signs of cerebral involvement at the time of transplantation, reasons that may have influenced the unsuccessful outcomes of transplantation. After 2006, with the approval of Elaprase®, ERT became the treatment of choice for Hunter patients. Even though the intravenous therapy is known not to cross the BBB, being unable to prevent neurodegeneration in the severe form of MPS II, for some reason it seems that HSCT was discouraged and no longer performed. We consider that HSCT for MPS II patients is still an unexplored alternative which can bring more neurological benefits than ERT.
It is also still worth mentioning that > 10 years ago HSCT in MPS patients was limited by lack of donor availability, high rates of graft failure and transplantation-related morbidity and mortality. Nowadays, the observed high engrafted survival rates with reduced toxicity and complications, due to the new transplant protocols and techniques [6], [16], along with superior metabolic correction compared with ERT [13], all together with a more careful and restricted selection of patients, may enable the extension of HSCT indication for MPS II patients.
Whether transplantation prior to the onset of neurological symptoms would prevent a severe outcome has yet to be proven in patients with MPS II. Longer follow-up and reports of further early transplanted cases are still necessary. Noteworthy, our patient, instead of losing cognitive skills as expected for a MPS II boy at his age, continues to acquire cognitive, adaptative and language skills, although at a slower rate when compared to unaffected children. This case is probably the first long-term and detailed report to demonstrate that HSCT performed very early in the course of the disease may be able to preserve cognition as our patient shows a continuous gain in skills, albeit at a slower rate than unaffected children.
6. Conclusions
We believe that HSCT is a therapeutic option for MPS II patients with the severe phenotype, as it could preserve neurocognition or even halt neurodegeneration, provided strict selection criteria are followed. The new protocols and techniques of transplantation have made the procedure more accessible and secure for the patients. HSCT could be considered a more suitable option for the patient and family, as no weekly lifelong infusions would be needed. Regarding public health policy, we dare to consider it as an interesting treatment option, both due to efficacy and to the lower overall therapy cost when compared to ERT for life.
Competing interests
AL Barth has received educational travel grants and/or speaker honoraria from Biomarin, Shire and Sanofi Genzyme; TSPC Magalhães is currently an employee of Biomarin; R Giugliani has received investigator fees, speaker honoraria, and travel grants from Actelion, Amicus, Armagen, Biomarin, Sanofi Genzyme, Shire and Ultragenyx; DA Torres has received educational travel grants and/or speaker honoraria from Biomarin and Shire; JC Llerena Jr. has received educational travel grants and/or speaker honoraria from Biomarin, Shire and Sanofi Genzyme, DDG Horovitz has received educational travel grants and/or speaker honoraria from Biomarin, Shire, Sanofi Genzyme and Ultragenyx; ABR Reis; MLC Oliveira; FB Scalco; NC Cavalcanti; DS Silva; AAP Costa and C Bonfim have no competing interests.
Acknowledgments
The authors thank Drs Maira Burin, Ida Schwartz, Sandra Leistner-Segal, Guilherme Baldo, Rejane Gus Kessler and the Brazilian MPS Network, all based at MGS/HCPA/UFRGS – Brazil, for the prenatal diagnosis support, enzyme and molecular studies; Lisandro Lima Ribeiro and Gisele Loth from BMT Unit/UFPR – Brazil, for the medical support during HSCT.
References
- 1.Neufeld E.F., Muenzer J. The Mucopolysaccharidoses. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. McGrow-Hill; New York: 2001. pp. 3421–3452. [Google Scholar]
- 2.Holt J.B., Poe M.D., Escolar M.L. Natural progression of neurological disease in mucopolisaccharidosis type II. Pediatrics. 2011;127:e1258–e1265. doi: 10.1542/peds.2010-1274. [DOI] [PubMed] [Google Scholar]
- 3.Muenzer J., Bodamer O., Burton B., Clarke L. The role of enzyme replacement therapy in severe Hunter syndrome – an expert panel consensus. Eur. J. Pediatr. 2012;171:181–188. doi: 10.1007/s00431-011-1606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boado R.J., Hui E.K., Lu J.Z., Sumbria R.K., Pardridge W.M. Blood-brain barrier molecular Trojan horse enables imaging of brain uptake of radioiodinated recombinant protein in the rhesus monkey. Bioconjug. Chem. 2013;24:1741–1749. doi: 10.1021/bc400319d. [DOI] [PubMed] [Google Scholar]
- 5.Lampe C., Bosserhoff A.K., Burton B.K., Giugliani R. Long-term experience with enzyme replacement therapy (ERT) in MPS II patients with a severe phenotype: an international case. J. Inherit. Metab. Dis. 2014;37:823–829. doi: 10.1007/s10545-014-9686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldenhoven M., Wynn R.F., Orchard P.J., O'Meara A. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: an international multicenter study. Blood. 2015;125:2164–2172. doi: 10.1182/blood-2014-11-608075. [DOI] [PubMed] [Google Scholar]
- 7.Gosh A., Miller W., Orchard P.J., Jones S.A. Enzyme replacement therapy prior to hematopoietic stem cell transplantation in mucopolysaccharidosis type I: 10 year combined experience of 2 centers. Mol. Genet. Metab. 2016;117:373–377. doi: 10.1016/j.ymgme.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poe M.D., Chagnon S.L., Escolar M.L. Early treatment is associated with improved cognition in Hurler syndrome. Ann. Neurol. 2014;76:747–753. doi: 10.1002/ana.24246. [DOI] [PubMed] [Google Scholar]
- 9.de Ru M.H., Boelens J.J., Das A.M., Jones S.A. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J. Rare Dis. 2011;6:55. doi: 10.1186/1750-1172-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wraith J.E., Beck M., Lane R., van der Ploeg A. Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: results of a multinational study of recombinant human alpha-L-iduronidase (laronidase) Pediatrics. 2007;120:e37–e46. doi: 10.1542/peds.2006-2156. [DOI] [PubMed] [Google Scholar]
- 11.Peters C., Sterward C.G. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003;31:229–239. doi: 10.1038/sj.bmt.1703839. [DOI] [PubMed] [Google Scholar]
- 12.Prasad V.K., Mendizabal A., Parikh S.H., Szabolcs P. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–2989. doi: 10.1182/blood-2008-03-140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn R.F., Wraith J.E., Mercer J. Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy. J. Pediatr. 2009;154:609–611. doi: 10.1016/j.jpeds.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Guffon N., Bertrand Y., Forest I., Fouilhoux A., Froissart R. Bone marrow transplantation in children with Hunter syndrome: outcome after 7 to 17 years. J. Pediatr. 2009;154:733–737. doi: 10.1016/j.jpeds.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Vellodi A., Young E., Cooper A., Lidchi V. Long-term follow-up following bone marrow transplantation for Hunter disease. J. Inherit. Metab. Dis. 1999;22:638–648. doi: 10.1023/a:1005525931994. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Luan Z., Jiang H., Fang J. Allogeneic hematopoietic stem cell transplantation in 34 pediatric cases of mucopolysaccharidosis – a 10-year report from China children transplant group. Biol. Blood Marrow Transplant. 2016;22:2104–2108. doi: 10.1016/j.bbmt.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Brusius-Facchin A.C., Schwartz I.V.D., Zimmer C., Ribeiro M.G. Muchopolysaccharidosis type II: identification of 30 novel mutation among Latin American patients. Mol. Genet. Metab. 2014;111:133–138. doi: 10.1016/j.ymgme.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Chiong M.A.D., Canson D.M., Abacan M.A.R., Baluyot M.M.P., Cordero C.P., Silao C.L.T. Clinical, biochemical and molecular characteristics of Filipino patients with mucopolysaccharidosis type II – Hunter syndrome. Orphanet J. Rare Dis. 2017;12:7. doi: 10.1186/s13023-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froissart R., da Silva I.M., Maire I. Mucopolysaccharidosis type II: an update on mutation spectrum. Acta Paediatr. 2007;96:71–77. doi: 10.1111/j.1651-2227.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 20.Baldo G., Matte U., Artigalas O., Schwartz I.V. Placenta analysis of prenatally diagnosed patients reveals early GAG storage in mucopolysaccharidoses II and VI. Mol. Genet. Metab. 2011;103:197–198. doi: 10.1016/j.ymgme.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Lampe C., Atherton A., Burton B.K., Descartes M. Enzyme replacement therapy in mucopolysaccharidosis II patients under 1 year of age. JIMD Rep. 2014;14:99–113. doi: 10.1007/8904_2013_289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souillet G., Guffon N., Maire I., Pujol M. Outcome of 27 patients with Hurler's syndrome transplanted either from related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- 23.Silva D.S., Barth A.L., Esposito A.C., Costa A.A.P. Neurophysiological evaluation in cervical myelopathy in patients with mucopolysaccharidosis: the somatosensory and motor-evoked potentials. JIEMS. 2014;2:S25. [Google Scholar]
- 24.McKinnis E.J.R., Sulzbacher S., Rutledge J.C., Sanders J., Scott C.R. Bone marrow transplantation in Hunter syndrome. J. Pediatr. 1996;129:145–148. doi: 10.1016/s0022-3476(96)70202-0. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka A., Okuyama T., Suzuki Y., Sakai N. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol. Genet. Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Escolar M., Poe M., Rajan D., Szabolcs P. Longterm outcomes of patients receiving umbilical blood stem cell transplantation for MPS II. Mol. Genet. Metab. 2012;108:S37–S38. [Google Scholar]
- 27.Papsin B.C., Vellodi A., Bailey C.M., Ratcliffe P.C., Leighton S.E. Otologic and laryngologic manifestations of mucopolysaccharidosis after bone marrow transplantation. Otolaryngol. Head Neck Surg. 1998;118:30–36. doi: 10.1016/S0194-5998(98)70371-7. [DOI] [PubMed] [Google Scholar]
- 28.da Costa V., O'Grady G., Jackson L., Kaylie D., Raynor E. Improvements in sensorineural hearing loss after cord blood transplant in patients with mucopolysaccharidosis. Arch. Otolaryngol. Head Neck Surg. 2012;138:1071–1076. doi: 10.1001/jamaoto.2013.597. [DOI] [PubMed] [Google Scholar]
- 29.Cianfrone G., Pentangelo D., CianfroneF Mazzei F. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur. Rev. Med. Pharmacol. Sci. 2011;15:601–636. [PubMed] [Google Scholar]
- 30.Tanjuakio J., Suzuki Y., Patel P., Yasuda E. Activities of daily living in patients with Hunter syndrome: impact of enzyme replacement therapy and hematopoietic stem cell transplantation. Mol. Genet. Metab. 2015;114:161–169. doi: 10.1016/j.ymgme.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldenhoven M., Kurtzberger J. Cord blood is the optimal graft source for the treatment of pediatric patients with lysosomal storage diseases: clinical outcomes and future directions. Cytotherapy. 2015;17:765–774. doi: 10.1016/j.jcyt.2015.03.609. [DOI] [PubMed] [Google Scholar]
- 32.Wynn R. Stem cell transplantation in inherited metabolic disorders. Hematology Am. Soc. Hematol. Educ. Program. 2011;2011:285–291. doi: 10.1182/asheducation-2011.1.285. [DOI] [PubMed] [Google Scholar]