Abstract
Our previous findings suggested that reversible thiol modifications of cysteine residues within the actuator (AD) and nucleotide binding domain (NBD) of the Na,K-ATPase may represent a powerful regulatory mechanism conveying redox- and oxygen-sensitivity of this multifunctional enzyme. S-glutathionylation of Cys244 in the AD and Cys 454-458-459 in the NBD inhibited the enzyme and protected cysteines’ thiol groups from irreversible oxidation under hypoxic conditions. In this study mutagenesis approach was used to assess the role these cysteines play in regulation of the Na,K-ATPase hydrolytic and signaling functions. Several constructs of mouse α1 subunit of the Na,K-ATPase were produced in which Cys244, Cys 454-458-459 or Cys 244-454-458-459 were replaced by alanine. These constructs were expressed in human HEK293 cells. Non-transfected cells and those expressing murine α1 subunit were exposed to hypoxia or treated with oxidized glutathione (GSSG). Both conditions induced inhibition of the wild type Na,K-ATPase. Enzymes containing mutated mouse α1 lacking Cys244 or all four cysteines (Cys 244-454-458-459) were insensitive to hypoxia. Inhibitory effect of GSSG was observed for wild type murine Na,K-ATPase, but was less pronounced in Cys454-458-459Ala mutant and completely absent in the Cys244Ala and Cys 244-454-458-459Ala mutants. In cells, expressing wild type enzyme, ouabain induced activation of Src and Erk kinases under normoxic conditions, whereas under hypoxic conditions this effect was inversed. Cys454-458-459Ala substitution abolished Src kinase activation in response to ouabain treatment, uncoupled Src from Erk signaling, and interfered with O2-sensitivity of Na,K-ATPase signaling function. Moreover, modeling predicted that S-glutathionylation of Cys 458 and 459 should prevent inhibitory binding of Src to NBD. Our data indicate for the first time that cysteine residues within the AD and NBD influence hydrolytic as well as receptor function of the Na,K-ATPase and alter responses of the enzyme to hypoxia or upon treatment with cardiotonic steroids.
Keywords: Na, K-ATPase, Regulatory cysteines, Glutathione, Src signaling, Mutagenesis
Graphical abstract

1. Introduction
Na,K-ATPase is a ubiquitous enzyme present in plasma membranes of almost all cells. It is formed by a catalytic α and a regulatory β subunits of which several isoforms are known (α1-α4 and β1-β3). In some tissues one more regulatory subunit belonging to the family of FXYD proteins joins the αβ dimers [7], [8]. Na,K-ATPase is a major gradient-forming enzyme moving Na+ and K+ against their electrochemical gradients. In addition Na,K-ATPase is a receptor for cardiotonic steroids and a mediator of complex signaling cascades involving kinases, Ca2+, and mitochondrial free radical burst [1], [55]. Whereas lower doses of cardiotonic steroids evoke signaling response of the Na,K-ATPase, at higher concentrations these compounds inhibit transport function of the enzyme [56].
Consuming 20–85% of ATP in cells and tissues, Na,K-ATPase is one of the major ATP sinks in all cells. It converts energy of ATP to that of transmembrane Na+ and K+ gradients. The latter is used to sustain brain function and the muscle contractility, nutrients uptake and multiple other processes. Na,K-ATPase is also known to be a sensor of redox state and O2 availability [10], [11], [55], [56]. Decrease in O2 levels compromises survival of the organism as the ion gradient maintenance may no longer be effectively preserved due to the quick (and reversible) inactivation of Na,K-ATPase [12], [14], [16], [17], [18], [19], [32], [38], [46], [52], [6], [9],41,[54], [66]. Furthermore, systemic hypoxia triggers release of low doses of cardiotonic steroids from adrenal cortex into the circulation. Binding of endogenous cardiotonic steroids to the Na,K-ATPase and activation of the receptor function of the enzyme is associated with initiation of signaling cascades involving Src-Erk kinases [18]. Tuning of the Na,K-ATPase activity to O2 availability translates into a substantial reduction in ATP consumption under hypoxic conditions, a powerful adaptive response allowing to avoid acute terminal ATP depletion. Complex mechanisms of adjustment of the Na,K-ATPase activity to the changes in environmental oxygen levels are largely coupled to the alterations in redox environment [11]. Whereas suppression of transport and hydrolytic function of the Na,K-ATPase under hypoxic conditions is well-described, little is known about the impact of hypoxia on the receptor function of the enzyme.
Oxidative thiol modifications were recently shown to play a decisive role in control of ion transport and ATP hydrolysis by the Na,K-ATPase in hypoxic cells [23], [31], [54], [66]. Onset of hypoxia is associated with a transient free radical burst from the mitochondria along with gradual ATP depletion [26]. Accumulation of oxidized glutathione (GSSG) that follows the oxidative burst joins the reaction of dithiol exchange with regulatory thiols of multiple proteins including Na,K-ATPase. In our previous study we have explored the localization of these regulatory thiols within the catalytic α1 subunit of the Na,K-ATPase [54]. Functional tests as well as mass spectrometry and in silico modeling indicated that regulatory thiols are most likely located within the ATP binding pocket of the catalytic α subunit of the Na,K-ATPase interfering thereby with ATP binding to the nucleotide binding domain (NBD) [54]. Exposure of purified Na,K-ATPase protein to GSSG in concentrations comparable with those found in hypoxic tissues resulted in complete suppression of the enzyme's hydrolytic activity [54]. In the absence of ATP inhibition of the Na,K-ATPase occurred within 5–10 min of treatment, and the activity could be restored by DTT as well as by exposure to glutaredoxin 1/NADPH mixture. Binding of ATP to the Na,K-ATPase made it insensitive to the inhibitory action of GSSG [54]. Induction of S-glutathionylation of the α-subunit and the concomitant decrease in hydrolytic activity of the Na,K-ATPase was confirmed in hypoxic myocardial tissue and several cell lines [43], [51], [54], [66].
Four cysteine residues within the actuator domain (AD) (Cys244) and NBD (Cys 454, 458 and 459) of the α1 subunit were suggested as targets of regulatory S-glutathionylation based on the data of mass-spectrometry and in silico modeling [54]. One of them, Cys454, was later identified as inaccessible to S-glutathionylation after the protein folding and could thus only become S-glutathionylated in the ER [43]. In this study point-mutagenesis approach was applied to evaluate the role of regulatory cysteines in O2-sensitivity of hydrolytic and receptor function of the Na,K-ATPase. Wild type murine catalytic α1 subunit as well as the protein in which the candidate regulatory cysteines in the AD or/and NBD were substituted by alanine were expressed in a human embryonic kidney derived cells (HEK-293). As murine α1β isozyme requires 1000-fold higher concentrations of cardiotonic steroid, ouabain, for inhibition than the human α1β isozyme, the activity of transfected mouse enzyme could be easily distinguished from the human endogenous Na,K-ATPase. Hydrolytic activity of the Na,K-ATPase as well as the signaling cascade triggered by ouabain binding to the enzyme were assessed in normoxic and hypoxic transfected HEK 293 cells. We further assessed sensitivity of wild type and mutated enzyme to GSSG.
The obtained results indicate that Cys244 is of key importance in redox-sensitivity of the Na,K-ATPase activity. Substitution of Cys458 and 459 to alanines compromises hypoxia-sensitivity and receptor function of the enzyme.
2. Materials and methods
2.1. Plasmid preparation
Wild type, murine Na,K-ATPase α1 subunit was subcloned into pcDNA3.1/myc-His (Invitrogen) from IMAGE clone 4951288, positioning the myc epitope and 6xHIS tag at the C terminus of Na,K-ATPase α1 subunit. The following site-directed mutagenesis strategy was chosen. Cysteines 244, 454-458-459 and a combination of all four cysteine residues were replaced by alanine and, as pilot experiments showed, resulted in production of a functional protein. Mutagenesis reactions were performed with the Quikchange II site-directed mutagenesis kits (Agilent). Mutagenesis primers were designed using the Agilent online primer design tool.
C244A (forward): GCCTTCTTCTCAACCAACGCTGTGGAAGGAACCGC;
CCC454/458/459AAA (forward): CTGAGTCGGCGCTCTTAAAGGCCATTGAAGTCGCCGCCGG CTCCGTGATGGAG.
All mutations were confirmed by sequencing the entire ATP1a1 cDNA. Plasmids were multiplied in E. coli and purified for transfection into HEK293 cells.
2.2. Cell culture and transfection
Human embryonic kidney-derived cell line HEK293 was used for transfection with mouse α1 or mock plasmids. Cells were plated in 25 cm2 flasks 24 h prior to the transfection at the confluence of 25–30% and maintained in DMEM supplemented with 20% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycine and 1 mM Na-pyruvate at 37 °C and 5% CO2 in gas phase. Lipofectamine® 2000 Transfection Reagent (Thermofisher Scientific) was used to transfect HEK293 cells with the plasmids (8 μg per transfection) containing the wild type and mutated mouse Na,K-ATPase α1 subunit. Standard transfection protocol of the producer was used (https://tools.thermofisher.com/content/sfs/manuals/ Lipofectamine_2000_Reag_protocol.pdf). Neomycine (G418) was added to the cells 24–48 h after the transfection at concentration of 1000 μg/ml and selection for the transfected cells performed over 72 h. Transfected cells were then directly used for experiments.
2.3. Immunoblotting for the his-tag
Cells were lysed in buffer containing 500 mM NaCl, 25 mM Tris-HCl, 10 mM imidazol (pH 7.5), 1% Nonidet-P40, 0.1% SDS, 1% sodium deoxycholate and 1 μM PMSF and incubated at 4 °C for 1 h under discontinuous stirring. The samples were then centrifuged at 13,000×g for 10 min and the supernatant was collected. Separation of His-tagged murine Na,K-ATPase was performed using high performance Ni Sepharose (GE Healthcare). Ni Sepharose was washed in buffer containing 25 mM Tris-HCl, 500 mM NaCl, 10 mM imidazole (pH 7.5). Thereafter 25 μl of Ni Sepharose was mixed with 250 μl of cell lysates and incubated at 4 °C for 1 h under constant stirring. Ni Sepharose with Na,K-ATPase bound to it was sedimented by centrifugation at 16 000×g for 1 min. Ni Sepharose-ATPase complex was collected and protein was eluted by mixing with 125 μl of buffer containing 50 mM imidazole and incubated for 5 min with continuous stirring. Subsequently Ni Sepharose was precipitated by centrifugation at 16 000×g for 1 min, and supernatant containing released Na,K-ATPase was collected, separated on SDS-PAGE and transferred to a PVDF membrane. After membrane blocking in 5% non-fat milk in PBST detection of His-tagged Na,K-ATPase was performed using anti-his-tag antibody (dilution 1:1000) and mouse monoclonal anti-Na,K-ATPase α1 antibody clone C464-6 (Upstate, Millipore, dilution 1:10000) and secondary horseradish peroxidase-conjugated antibodies along with enhance chemiluminiscence SuperSignalTM West Femto Maximum Sensitivity Substrate kit (ThermoFischer Scientific). Chemiluminiscence was detected using Bio-Rad ChemiDoc MP imaging system.
2.4. Immunoblotting for S-glutathionylated α1 subunit
S-glutathionylation of Na,K-ATPase α1 subunit was estimated using immunoblotting. Proteins from cell lysates were separated on the SDS-PAGE (without β-mercaproethanol) and transferred to PVDF membranes. After blocking, mouse monoclonal anti-glutathione antibodies (Chemicon Millipore, MAB5310) were applied. Mouse monoclonal anti-α1 antibody clone C464-6 (Upstate, Millipore) was used to detect total amount of the α1 subunit. Secondary horseradish anti-mouse antibodies were used with signal enhancement by chemiluminiscence SuperSignalTM West Femto Maximum Sensitivity Substrate kit (ThermoFisher Scientific). Chemiluminescence was detected using Bio-Rad ChemiDoc MP imaging system.
Densitometric analysis was performed using Image Lab (Bio-Rad) program and the results were presented as a ratio of S-glutathionylated fraction of α1 subunit to total amount of α1 subunit in the corresponding lane. This ratio in control non-treated sample was taken as 1.
2.5. Hypoxic exposure and detection of cell viability
Short-term hypoxic exposure was performed using pre-equilibrated hypoxic media that were applied to the cells within hypoxic bench (InVivo400 Ruskinn). Longer incubations were performed in hypoxic incubators (Hereos). Hypoxic conditions were defined as 1% O2 up to 24 h (long-term viability experiments, or 0.2% O2 for 30–60 min (acute signaling responses, for details see text and figures legends). The cells were then washed free from the culture medium with phosphate buffer or Tyrode buffer containing (in mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 glucose, and 10 HEPES-NaOH, pH 7.4, and de-attached using scrapers or by trypsinization. So obtained, cells and lysates were stored in liquid nitrogen or used immediately for cell viability assessment. ADAM-MC Automatic Cell Counter was used to test for the cell viability and cell number after the exposure of cells to hypoxia 1% O2. Decrease of viability was assessed by registration of the percent of propidium iodide-positive cells (dead cells) related to the total number of cells. Toxicity of the membrane-permeable analogue of GSSG, tetraethyl-GSSG (ttGSSG), in HEK 293 cells was assessed using MTT assay as described earlier [51]. No changes in cell viability was detected in cells treated with ttGSSG in concentrations up to 2 mM over 24 h (Suppl. Fig. 1).
2.6. Treatment of cells with oxidized glutathione
GSSG and ttGSSG were dissolved in 300 mM HEPES/NaOH buffer (pH 7.4 at room temperature) to obtain stock solution of 10 mM. Cell culture medium in 25 cm2 flasks in which HEK293 cells were growing was replaced by 1 ml Tyrode buffer. Oxidized glutathione derivatives were then added to the buffer to reach final concertation of 1 or 0.5 mM for GSSG or ttGSSG respectively. pH of Tyrode did not change after addition of GSSG or ttGSSG. Cells were incubated with ttGSSG for 1 h in the incubator in the atmosphere of air with 5% CO2 at 37 °C. Treatment with GSSG was performed immediately before the cells were de-attached using scrapers and frozen in liquid N2. Thus, the membranes of cells were in contact with GSSG upon thawing.
2.7. Detection of hydrolytic activity of Na,K-ATPase
About 1000-fold difference in affinity to ouabain is known to exist between the human and murine Na,K-ATPase [15], [48] making it possible to distinguish between the activities of human and murine isoforms. Hydrolytic activity of Na,K-ATPase in cell lysates was measured as ouabain-sensitive phosphate (Pi) production in the medium containing the following saturating levels of ions and substrates (mM): 130 NaCl, 20 KCl, 3 MgCl2, 3 ATP, 30 HEPES/NaOH (pH 7.4, 37 °C). 1 mM GSSG was additionally present in the medium for cells pre-treated with GSSG and ttGSSG during the activity measurements. Details of the protocol were described elsewhere ([54], [57]. 10 μM ouabain was used to selectively block human isozyme whereas 2 mM ouabain was effectively blocking both human and mouse Na,K-ATPase activity. Activity of murine ATPase could then be calculated by subtracting the activity of human ouabain-senisitve isozymes from the total Na,K-ATPase activity. Enzyme activity was expressed per mg protein per hour and protein content was detected using Bradford method.
2.8. Assessment of Src and Erk1/2 phopsphorylation state by immunoblotting
Phosphorylation of Src and Erk1/2 kinases was evaluated in cell lysates of non-transfected and transfected HEK293 cells that were incubated with or without 250 μM ouabain under normoxic (20% O2) or hypoxic (0.2% O2) conditions. Incubation at normal and low oxygen levels was performed in Tyrode buffer for 15 min prior to addition of 250 μM ouabain and for further 30 min in the presence of cardiotonic steroid. The cells were then de-attached by scrapers and sedimented by centrifugation for 10 min at 100×g. Cells were lysed in the RIPA buffer (25 mM tris-HCl, pH 7.6, 150 mМ NaCl, 1% Nonidet-P40, 0.1% SDS, 1% sodium deoxycholate) containing 1 μM of PMSF with stirring at 4 °C for 1 h. The probes were then centrifuged at 13,000×g for 10 min and the supernatant was collected. Proteins of cell lysates were separated on SDS-PAGE and transferred to a nitrocellulose membrane. After membrane blocking in 5% BSA (bovine serum albumin) in PBST, the detection of phospho (Tyr 416) Src or Erk1/2 and total Src or Erk1/2 was carried out by incubating the membrane in the solution of appropriate rabbit polyclonal antibodies (both from Cell Signaling Technology) in PBST. Visualization of the proteins was performed by the appropriate horseradish peroxidase-conjugated secondary antibodies followed by the enhanced luminiscent SuperSignal ™ West Femto Maximum Sensitivity Substrate kit (Thermo Scientific). Chemiluminescence was detected using Bio-Rad ChemiDoc MP instrument. Densitometric analysis was performed with Image Lab program (Bio-Rad) and the results were expressed as ratio of phospho-Src or phospho Erk1/2 to total Src or Erk1/2 band intensity (phospho-Src/Src; phospho-Erk1/2/Erk1/2).
2.9. Modeling of binding of Src kinase to the NBD of Na,K-ATPase
Modeling of the complex was performed using the structure of porcine Na,K-ATPase (PDB id 3wgu) solved at 2.8 Å resolution, and the structure of human Src kinase (PDB id 2src). Docking was performed using Na,K-ATPase NBD (377–588 residues) and kinase domain of Src kinase (residues 267–520). Global docking of these domains has been carried out with SwarmDock, GRAMM_X, ZDock and ClusPro servers. Thereafter the full-size protein structures were reconstructed. Qasdom server (http://qasdom.eimb.ru/qasdom.html) was used to identify interactions between the NBD and kinase domain of Src kinase in obtained models.
2.10. Statistical analysis
Statistical module of SigmaPlot v.13 (SigmaPlot) was used for statistical analysis. Rank sum test or signed rank test were used for analysis whenever appropriate. p<0.05 was considered as significant difference.
3. Results
3.1. HEK293 express wild type and mutated murine α1 subunit forming functional Na,K-ATPase
Several cell lines were generated based on the HEK293 cells that expressed wild type or mutated forms of mouse α1 subunit of Na,K-ATPase along with human endogenous α1 subunit (α1 h), the only isoform expressed in this cell line [13]. Along with endogenous α1hβ1 form of Na,K-ATPase transfected cells expressed either mouse wild type enzyme (α1wtβ1) or one of the following three mutated mouse enzymes: Cys244Ala mutant (α1m-1CAβ1), Cys454-458-459Ala mutant (α1m-3CAβ1), or Cys244-454-458-459Ala mutant (α1m-4CAβ1). Expression of the transfected mouse α1 subunits was confirmed by means of immunoblotting against the histidine tags (Suppl. Fig. 2A). Cells expressing any of mouse α1 subunits showed increased levels of the α1 protein compared to the non-transfected HEK293 cells (Suppl. Fig. 2B). Functionality of the compound Na,K-ATPase molecule formed by the transfected mouse α and human β subunits was tested. Ouabain at concentration of 10 μM was used to block human α1hβ1 Na,K-ATPase. Mouse Na,K-ATPase activity was insensitive to 10 μM, but completely blocked by 2 mM ouabain. The enzymes containing mouse α1 subunit were active and contributed by 40–50% into the total hydrolytic activity of the Na,K-ATPase (Suppl. Fig. 2C).
3.2. Replacement of Cys244 by Ala makes the enzyme insensitive to GSSG
Two sets of experiments were performed to address the role of the Cys 244 in the AD and Cys 454, 458 and 459 in the NBD in sensitivity of Na,K-ATPase to GSSG. In the first set of experiments non-transfected HEK293 cells as well as the cells expressing α1wtβ1, α1m-1CAβ1, α1m-3CAβ1 and α1m-4CAβ1 Na,K-ATPase were lysed by freezing-thawing cycle in the presence of 1 mM GSSG. Activity of endogenous human Na,K-ATPase in cell lysates was suppressed after GSSG treatment by 40% (Fig. 1). For the enzyme formed by the wild type mouse α1 and human β1 subunits suppression of activity after GSSG treatment reached 60% (Fig. 2A). Mutants α1m-1CAβ1 and α1m-4CAβ1 were insensitive to the inhibitory action of GSSG, while in α1m-3CAβ1 mutant inhibitory effect of GSSG was abolished only partially. These findings were verified in the next set of experiments in which sensitivity of the Na,K-ATPase to GSSG was tested in intact cells. Plasma membrane is known to be impermeable to GSSG and loading of cells with it was performed using its membrane-permeable esterified homologue, ttGSSG. Incubation of non-transfected HEK293 cells with ttGSSG induced S-glutathionylation of the Na,K-ATPase α1 subunit (Suppl. Fig. 3A). Exposure of transfected cells to ttGSSG resulted in a decrease in α1wtβ1 Na,K-ATPase activity by more than 40% (Fig. 2B). Activity of the α1m-1CAβ1 and α1m-4CAβ1 mutants was unaffected by ttGSSG (Fig. 2B). S-glutathionylation levels of the untreated mutant-α1 subunits were similar to those in wild type murine α1 subunit (Suppl. Fig. 3B).
Fig. 1.
Sensitivity of endogenous human Na.K-ATPase to GSSG and hypoxia. Non-transfected HEK 293 cells were exposed to 1% O2 (hypoxia) for 24 h or to 1 mM GSSG during lysis and Na, K-ATPase activity measurements. Data are means ±SD for 3 experiments. * stands for the p < 0.05 compared to control.
Fig. 2.
Sensitivity of wild type and mutated murine Na,K-ATPase isozymes to GSSG. A: HEK293 cells expressing wild type, α1m-1CAβ1, α1m-3CAβ1 and α1m-4CAβ1 (passages 4 and lower) were exposed to 1 mM GSSG during harvesting and lyses as well as during measurement of Na, K-ATPase activity. Data are means of at least 3 independent experiments ±SD. B: Sensitivity of wild type, α1m-1CAβ1and α1m-4CAβ1 to cell-permeable GSSG analogue, ttGSSG. Data are means±SD of 3 in dependent experiments. * stand for p<0.05 compared to corresponding normoxic controls.
3.3. Cells expressing α1m-4CAβ1 are more susceptible to hypoxic damage
Endogenous human Na,K-ATPase in non-transfected HEK293 cells responded to hypoxia (1% O2, 24 h) with a loss of ~40% activity (Fig. 1). Similar response was observed for the α1mwt-β1 enzyme (Fig. 3A). However, α1m-3CAβ1 mutant was not inactivated by hypoxia. The α1m-1CAβ1 and α1m-4CAβ1enzymes in which Cys244 was replaced by Ala responded to deoxygenation with an increase in their hydrolytic activity (Fig. 3A). Incubation of non-transfected HEK293 cells and cells with α1mwt-β1 Na,K-ATPase at 1% O2 for 24 h resulted in decrease of cell viability by 20% (Fig. 3B). The viability of cells expressing the α1m-4CAβ1 mutant was lower than that of cells expressing α1mwt-β1 enzyme. Thus, the cells expressing the GSSG-insensitive α1m-4CAβ1 mutant of Na,K-ATPase are more susceptible to hypoxic damage.
Fig. 3.
Sensitivity of wild type and mutated murine Na,K-ATPase to hypoxia. A: Sensitivity of mouse wild type, wild type, α1m-1CAβ1, α1m-3CAβ1 and α1m-4CAβ1 isozymes to hypoxic exposure (24 h at 1% O2). Data are means ±SD B: Viability of non-transfected HEK293 cells and those transfected with wild type, α1m-1CA, α1m-3CA and α1m-4CA plasmids under hypoxic conditions. Cell viability was assessed as number of cells permeable for propidium iodide. Data are means of 3 experiments ± SD, * stands for p < 0.05 compared to normoxic controls.
3.4. Mutant α1m-3CAβ1 (Сys454-458-459Ala) is presented with alterations in signaling function
Several mammalian species respond to hypoxia by release of cardiotonic steroids from adrenal glands into the circulation. These hormones interact with the Na,K-ATPase triggering activation of Src kinase [21]. We have tested the impact of Cys-to-Ala substitution on the receptor function of the Na,K-ATPase under normoxic and hypoxic conditions. Activity of Src kinase was used as a readout of signaling initiated upon the stimulation of Na,K-ATPase with low doses of cardiotonic steroid ouabain. Selection of optimal concentrations of ouabain was performed based on the previous studies in which dose-response of Src activity was measured as a function of ouabain concentrations in mouse fibroblast cells (SC-1 cell line). Maximal activation of Src in SC-1 cells was induced by treatment with 250 μM ouabain for 30 min under normoxic conditions [31]. We have thus chosen the same conditions of ouabain treatment for HEK293 cells expressing murine Na,K-ATPase α1 subunit. We also monitored the changes in Src activity in non-transfected HEK cells in the presence of 250 µM ouabain keeping in mind that this dose of ouabain is toxic as it blocks transport function of human Na,K-ATPase. 0.2% O2 was used for acute (45 min) induction of signaling responses to hypoxia. Activation of Src kinase was assessed as the change in activating phosphorylation of Tyr416 and the activation of Erk1/2 kinases as a switch in Thr202/Tyr204 phosphorylation. As expected, activating phosphorylation of Src kinase increased upon the stimulation with ouabain under normoxic conditions in α1m-wtβ1-carrying cells (Fig. 4A). Hypoxia alone was capable of activation of Src kinase to the same extent. This stimulatory response was suppressed if ouabain was present in deoxygenated cell culture medium (Fig. 4A). Similar response was observed in cells expressing α1m-1CAβ1 mutant. However, HEK293 cells expressing α1m-3CAβ1 and α1m-4CAβ1 enzymes were completely insensitive to the stimulation with ouabain at normoxic as well as at hypoxic conditions. HEK293 cells expressing endogenous human α1 subunit alone were not responding to 250 µM ouabain with modulation of Src activity, but retained hypoxia-sensitivity (Fig. 4A).
Fig. 4.
Activation of Src and Erk kinases in response to low doses of ouabain. Representative blots and densitometric analysis for these blots are presented in panel A. The changes in signals for p-Src (Tyr416) normalized to total Src are presented in panel A for the following conditions: response to 250 µM ouabain under normoxic or hypoxic (0.2% O2) conditions. Tested were non-transfected HEK cells as well as cells expressing wild type, α1m-1CAβ1, α1m-3CAβ1 and α1m-4CAβ1 isozymes. Cells were incubated at 0.2% or 20% O2 for 45 min and ouabain at final concentration of 250 μM was added to half of the samples 30 min prior to the end of hypoxic incubation. Shown is one out of 3–5 experiments each including 3 repetitive measurements. Panel B presents the representative blots and the corresponding densitometric analysis for the phospho-Erk and total Erk for the cell lysates produced from the same set of samples that was used for the Src activity assessment. Values are means ±SD for at least 3 experiments. Statistical significance was assessed using Student non-paired test. * denotes p < 0.05 compared to the corresponding ouabain-free control and # stands for p < 0.05 between the normoxic and hypoxic ouabain-free values.
Erk1/2 activating phosphorylation was assessed in the same set of cell lysates to monitor signal transfer to the downstream elements of the Src-signaling cascade. In HEK293 cells carrying α1m-wtβ1 Na,K-ATPase the changes in activating phosphorylation of Erk1/2 mirrored that of Src (Fig. 4B). In these cells hypoxia induced phosphorylation of Erk was similar to that produced by exposure of cells to 250 μM ouabain under normoxic conditions, whereas a decrease in phosphorylation of Erk kinase was observed upon stimulation of hypoxic cells with ouabain (Fig. 4B). Substitution Cys244Ala was not affecting responses to ouabain under normoxic conditions but abolished phosphorylation of Erk kinase upon deoxygenation. Substitutions Cys454-458-459Ala made Erk1/2 less sensitive to both ouabain and low oxygen (Fig. 4B). Thus, cysteine residues within the NBD are critical in defining the signaling responses of the enzyme to ouabain and hypoxia.
3.5. Modeling predicts the critical importance of Сys 458, 459 within the NBD for interaction with kinase domain of Src
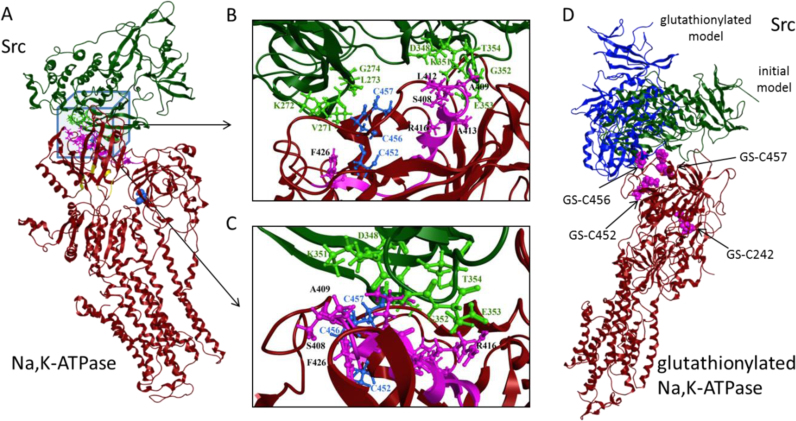
According to the reports of Li et al. [33] NBD of the Na,K-ATPase interacts with the kinase domain of Src kinase through the residues 410–429 of the α1 subunit. A short peptide NaKTide of identical sequence was effectively blocking interaction of Src kinase with Na,K-ATPase [33]. Cysteines 454, 458 and 459 within the NBD that turned to be essential for this interaction in our study (Fig. 4A) are localized out of the frame of NaKTide sequence (Fig. 5). We hence produced the model of the complex structure of kinase domain of Src kinase with the NBD of Na,K-ATPase in silico. The model was built based on the porcine Na,K-ATPase crystal structure (PDB code 3wgu) and human Src structure (PDB code 2src). The NaKTide sequence was utilized for selection of structures of Src-NBD complexes.
Fig. 5.
The model of complex of kinase domain of Src kinase with Na, K-ATPase nucleotide binding domain. A: 3D model of the complex constructed on the basis of porcine Na, K-ATPase α1β1 isozyme (PDB code 3wgu) and human Src kinase (PDB code 2src). Na, K-ATPase is represented in red, NaKTide in magenta, cysteine residues 452,456 and 457 and 242 of Na, K-ATPase in blue. Src kinase is shown in green. Residues of ATP-binding pocket 446, 475, 476, 480, 501, 544–546 are shown in yellow. Side chains of residues involved in complex formation represented as ball and sticks. Cys242 indicated as blue balls. Presented in panels B and C are two projections of the model. D. Superposition of model complexes of Src-kinase with Na, K-ATPase in initial and glutathionylated forms. Na, K-ATPase is represented in red, Src-kinase in complex with Na, K-ATPase in initial form is shown in green, Src-kinase in complex with ATPase in glutathionylated form is shown in dark blue. Glutathione molecules are shown as pink balls. According to the model, molecules of glutathione bound to Cys456(458) and 457(459) prevent interaction with NaKTide. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Out of 40 models of the complex generated by docking, the model chosen for analysis showed maximal interaction of Src kinase with NaKTide without steric difficulties in reconstruction of full-size protein structures (Fig. 5A,B). In this model the interface of interaction was formed by the NBD residues 399-QS-400, 405-DKTSA−409, 412-LA−413, R416, F426, 431-EN-432, I435, R438, 453-IELGSVKE-462, 466-RY-467, 488-NPNTAEPRHL-497, 518-GK-519, 550-HLFLPDE-556 (numbering according to 3wgu). Cysteines 456 and 457 (458 and 459 in mouse sequence, highlighted in bold) formed an interface between the NBD of the Na,K-ATPase and the kinase domain of Src kinase. Cysteine 452 (454 in mouse sequence) was inwardly-facing and interacted with the residue W411 within NaKTide. Within the NaKTide sequence 408-SATWLALSRIAGLCNRAVFQ-427 the six residues were identified as interacting with Src (highlighted in bold). Within the Src sequence the interface between its kinase domain and the NBD of the ATPase exceeded that with the NaKTide substantially and included the following amino acids: A165, 268-RLEVQ-275, E280, M283, R291, 340-YMSK-343, 347-LDFLKGETGKYL-358, Q362, M366, E396, L398, R460. Within the Src sequence the residues interacting with NaKTide are highlighted in bold, those interacting with cysteines 456 (458 in mouse sequence) and 457 (459 in mouse sequence) are underlined.
Furthermore, a model was created in which Cys 244, 454, 458, 459 were S-glutathionylated. Glutathione molecules bound to the Cys 244 and 454 were placed within the cavities proximal to these residues as discussed elsewhere [43]. S-glutathionylation of Cys 458 and Cys459 was simulated as we have described earlier [54]. Structure of S-glutathionylated protein was minimized in the Amber99 forcefield. Predictions for the interactions between the NBD of Na,K-ATPase and Src kinase were obtained with servers ClusPro, GrammX and ZDock. The resulting 30 structures were analyzed and 5 of them without steric difficulties in reconstruction of full-size protein structures were finally selected. QASDOM server was used for analysis of these models of NBD-Src kinase interaction interfaces. Interface of interaction included the following NBD residues: 392–416, 430–438, and 454–467. The best score was obtained for the model in which residues 397-ENQSGVSFDKT-407, 431-ENLPIL-436, 454-ELCC-457 of the Na,K-ATPase's NBD interact with the residues 272-KLGQGCFGEVW-282, 298-KP-299, 422-AK-423, 427 K, 467-VN-468 of the kinase domain of Src kinase (Fig. 5). In this model interface of interaction of S-glutathionylated NBD with Src kinase does not include NaKTide domain. The model predicts that glutathione bound to Cys 458 and 459 of NBD will prevent the interaction of kinase domain of Src with the NaKTide sequence within the NBD due to streric difficulties.
4. Discussion
Using mutagenesis approach we have revealed the importance of cysteine residues 244, 458 and 459 in redox- and hypoxia-sensitivity of the Na,K-ATPase as well as in its control over Src and Erk kinases. These regulatory mechanisms converge into the survival success under hypoxic conditions.
4.1. Localization of regulatory cysteines within the cytosolic domains of the catalytic α1 subunit
The possible role of cysteines within the catalytic α subunit for the Na,K-ATPase function was assessed in several studies. Substitution of all 23 cysteines in the α1 catalytic subunit by serine and alanine resulted in expression of unstable enzyme with a 2-fold reduced Km for Na+ and a 50% increased Km for K+ [28]. The enzyme containing cysteine-less α1 subunit was largely retained in the ER and Golgi apparatus and degraded 3 days after the transfection [28]. At the same time substitution of single cysteine residues by serine or alanine revealed particular importance of equivalent of Cys 244 in rat/rabbit/duck α1 subunit for folding of the α subunit and the enzyme stability in HeLa cells [60]. Substitution of analogues of Cysteines 454, 458 and 459 was associated with a reduction in maximal turnover rate to about 25% [60]. In HEK293 cells we managed to express functional protein containing α1 subunit lacking Cys244 or/and Cys 454, 458 and 459 (Suppl. Fig. 2).
In our previous studies binding of glutathione to the Cys 244, 454, 458 and 459 was associated with the loss of Na,K-ATPase hydrolytic activity [54]. We then suggested that these residues may contribute to the enzyme's redox- and oxygen-sensitivity as sites of regulatory S-glutathionylation [54]. Further evidence that S-glutathionylation of some cysteine residues (206, 454, 601 and 700) within the α1 subunit occurs before the enzyme is folded in the ER was provided recently [43]. These cysteines cannot be de-glutathionylated unless the protein is unfolded, and thus cannot participate in acute modification of the enzyme activity, but most likely defines the enzyme folding and stability.
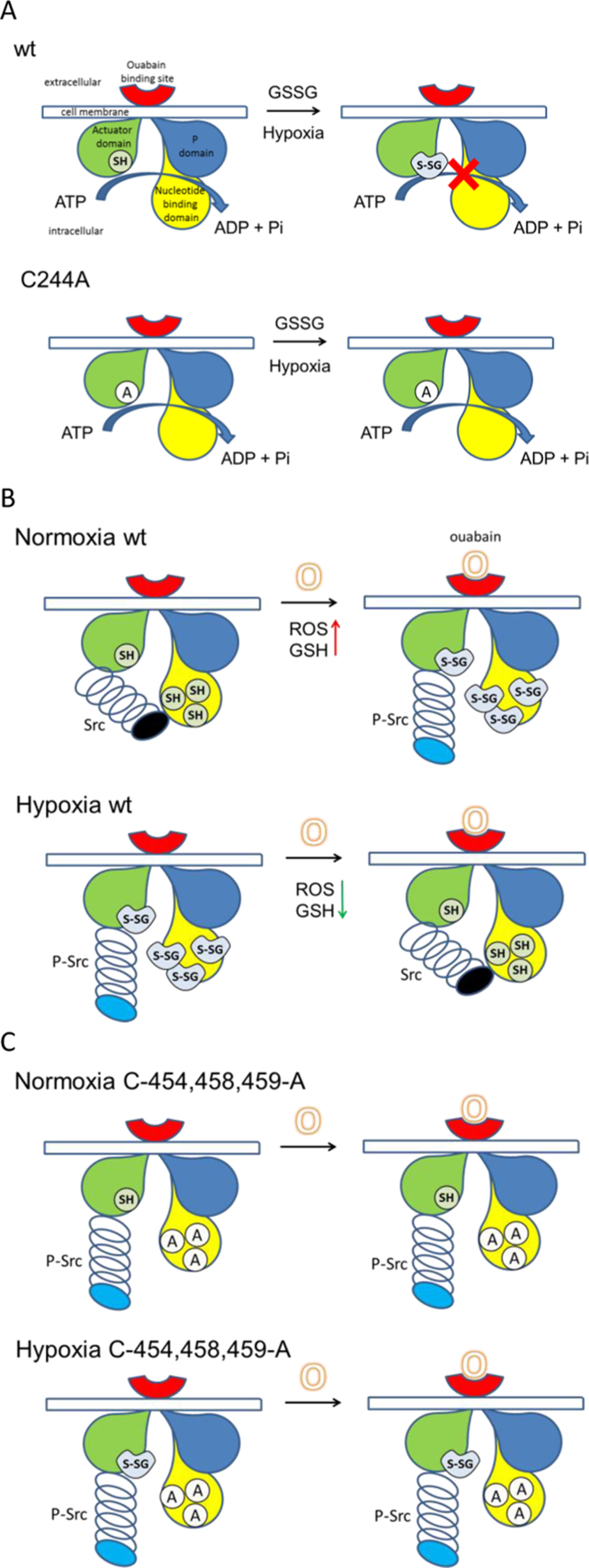
In this study we have revealed the particular importance of Cys244 for making the enzyme sensitive to GSSG. Cysteine residues 454, 458 and 459 within the NBD are less essential for Na,K-ATPase redox sensitivity (Fig. 2). This observation was somewhat unexpected. Our previous findings indicated that inhibition of the enzyme by GSSG results from the outcompeting of ATP from its binding site upon S-glutathionylation [53], [54]. Cys244 is localized within the actuator domain that does not contribute to the ATP binding site of the enzyme in E1 conformation [44]. In contrast to ADP, ATP binding to Na,K-ATPase generates a structural transition in the enzyme which causes transformation of the enzyme from the ‘‘E1-open’’ to ‘‘E1-closed’’ conformation with the following phosphorylation step [53]. Similar response was earlier described for GTP-induced conformation transitions in translational GTPases. Together with the earlier studies involving mutagenesis [59] and the observations on the sensitivity of the enzyme to oxidation [10] our present findings (Fig. 2, Fig. 3) suggest that Cys244 joins the NBD in certain conformations as schematically shown in Fig. 6A.
Fig. 6.
Schematic representation of the role of cysteines 244 in the actuator domain (green), and 454, 458 and 459 within the nucleotide binding domain (yellow) in control of redox sensitivity and signaling function of the Na,K-ATPase. Reduced and S-glutathionylated Cysteines are shown as circles with label “SH” and “S-SG”, respectively. Cys-to-Ala mutations indicated as circles with “A”. Phosphorylated (P) domain of the Na,K-ATPase is shown in blue and ouabain binding site in red. Src kinase is shown as a spring attached to the Na,K-ATPase. Kinase domain of the Src kinase is highlighted in black when inhibited and in blue when released and activated (p-Src). A: Regulation of the enzyme by exposure to GSSG. Inhibition of the enzyme upon exposure to GSSG requires the presence of Cys 244. S-glutathionylation of this regulatory thiol inhibits ATP binding to the NBD and blocks the hydrolytic activity of the enzyme. Hypoxia and GSSG treatment also triggers S-glutathionylation of the Cysteines in the NBD, but these cysteines are not essential for the inhibitory action of GSSG. The enzyme lacking Cys244 becomes GSSG-insensitive. B: Signaling function of the enzyme is initiated by binding of ouabain (indicated as “O”) to the Na,K-ATPase and the following release of kinase domain of Src kinase from its binding site within the nucleotide binding domain and its activation under normoxic conditions. This signaling event is associated with increased in free radical production and increase in S-glutathionylation of the regulatory cysteines. Hypoxia and the following free radical burst triggers S-glutathionylation in the absence of ouabain with the following activation of Src kinase. Binding of ouabain to the Na,K-ATPase in hypoxic cells decreases free radical production and inactivates Src kinase promoting docking of its kinase domain to the nucleotide binding domain of the ATPase. C: Substitution of cysteines 454, 458 and 459 within this domain with alanine blocks signal transduction upon ouabain binding to the extracellular binding site of the Na,K-ATPase to the Src kinase leaving it permanently active and O2-insensitive. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The second unexpected finding of this study is the role of the cysteine residues within the NBD in the signaling mediated upon treatment of mouse Na,K-ATPase with ouabain.
4.2. Cysteines and the sensitivity of the Na,K-ATPase to ouabain
High salt diet [40], psychological stress [22], intensive physical exercise [3], and reduction in O2 availability [18] trigger release of cardiotonic steroids from the adrenal gland and hypothalamus into the circulation and initiation of multiple systemic physiological response [45], [58]. Binding of ouabain to the regulatory binding site at the extracellular part of Na,K-ATPase α subunit stabilizes the enzyme in the E2-P conformation and initiates multiple events. Those include alteration of Src kinase activity, activation of inositol 3-phosphate receptors, induction of low frequency Ca2+ oscillations, and alteration in protein synthesis [56]. Src kinase is considered to be a part of the signaling complex [1], [24], [34], [63]. The existence of distinct “signaling pool” of the Na,K-ATPase controlling the activity of Src kinase along with “ion pumping pool” was recently suggested [35]. Two binding sites for Src SH2 and kinase domains are localized within the actuator and NBD domains of the α subunit of the Na,K-ATPase respectively [2], [33], [34]. Src kinase remains inactive when its kinase domain is bound to the NBD. Strength of interaction between the SH2 domain of Src and actuator domain of the Na,K-ATPase exceeds the one between the NBD and Src kinase domain, suggesting that this interaction may be maintained upon dissociation of the Src kinase domain from its binding site within NBD in response to ouabain binding to the Na,K-ATPase [2]. Our data are in line with this hypothesis and imply that cysteines within the NBD are essential for the preservation of Src activation upon ouabain treatment (Fig. 4). Downstream events following the interaction of Na,K-ATPase with ouabain are defined by the environmental O2 availability (Fig. 4). Hypoxic exposure alone is sufficient for activation of Src kinase (Figs. 4, 6B, [29], [31]. Exposure to ouabain under hypoxic conditions results in suppression of free radical production and inhibition of Src activity (Figs. 4, 6B, [31]). Replacement of cysteine residues within the NBD by alanine uncouples activity of Src from O2 availability or ouabain stimulation leaving the kinase permanently active (Figs. 4, 6C). Substitution of Cys244 with alanine does not affect ouabain- and hypoxia-driven regulation of Src activity (Fig. 4). These observations cannot be explained within the model of interaction between the kinase domain of Src kinase and NBD proposed earlier. According to Li et al. [33] Src binding site includes amino acids 410–429 of the α1 subunit. Our findings imply that Cys 454, 458 and 459 of NBD are essential for the interaction with Src kinase domain and required for Src inhibition. This suggestion is further strengthened by the outcome of in silico modeling of binding of kinase domain of Src kinase to the NBD of the Na,K-ATPase (Fig. 5). According to this model Сys 458, 459 form the interaction interface between the Na,K-ATPase and Src kinase and binding of glutathione to them compromises the interaction (Fig. 5). Further evidence confirming the importance of cysteines of the NBD for the signaling function of the Na,K-ATPase comes from the analysis of the isoform-specificity of the receptor function of the enzyme. Whereas α1- containing isozymes are well-known for being involved in the regulation of Src activity, the enzyme formed by the α2 subunit was shown to be insensitive to the stimulation with low doses of ouabain [65]. From the three cysteines within the NBD only two are preserved in the α2 isoform of the catalytic subunit, namely the 454 and 459 whereas Cys458 is not conserved. The α2 subunit also differs from the α1 in that it has one extra cysteine residue, Cys236, in the actuator domain of the ATPase [10]. It would be tempting to assume that the presence of Cys 458 is critical for the signaling activity of the Na,K-ATPase. The enzyme formed by the α3 isoform of the catalytic subunit responds to the stimulation with low doses of ouabain with activation of Erk1/2, but not Src kinases [39]. This interesting finding seems to contradict the “classical Xie model” describing Erk 1/2 activation as a downstream event following the Src activation upon ouabain binding to the enzyme [55]. In our study, uncoupling between the Src and Erk responses to ouabain and hypoxic stimulation was observed in cells lacking the three cysteines within the NBD (Fig. 6) suggesting the existence of an alternative Na,K-ATPase-driven, but Src-independent, signaling pathway involving Erk stimulation. Similar uncoupling between the Src and Erk activity has been reportered earlier in cardiac myocytes [20], [39], [64] where Erk kinases could be activated via the IP3-dependent signaling cascade that was not necessarily related to the activation of Src by ouabain. Our data suggest that this uncoupling does not occur in cells expressing wild type mouse α1 isoform, but only in cells deprived of cysteines 244 and/or 454, 458 and 459 (Fig. 4).
4.3. Cysteines, cell survival and responses of the Na,K-ATPase to hypoxia
We were able to confirm that exposure of HEK293 cells to low oxygen results in suppression of the Na,K-ATPase hydrolytic activity (Fig. 1). Substitution of any of the cysteines we have chosen as candidates for regulatory cysteines to alanine interfered with the inhibitory effect of hypoxia observed in non-transfected cells as well as in cells expressing wild type mouse α1 protein (Fig. 3). Hypoxia induces an increase in the intracellular GSSG levels (Petrushanko 2012, Yakushev 2012). Data shown in Fig. 2 indicate that this inhibitory effect of GSSG on Na,K-ATPase is due to S-glutathionylation of Cys244. Increased hydrolytic activity of the Na,K-ATPase in mutants lacking Cys244 may be related to the ATP depletion and compensatory increase in ATP affinity of the enzyme in these cells as has been observed earlier in response to GSH-depletion [50]. Further work is required for in-depth characterization of the mechanisms of the inhibitory action of hypoxia on the Na,K-ATPase that may be rather complex as recently reviewed [11].
We have revealed that hypoxic exposure of HEK293 cells as well as of HEK 293 transfected with α1wtβ1 or α1m-1CAβ1 resulted in activation of Src kinase. Signaling responses to ouabain in α1wtβ1 and α1m-1CAβ1-transfected HEK293 were inverted under hypoxic conditions (Fig. 4). Replacement of Cys 454, 458 and 459 by alanines rendered Src kinase permanently active and insensitive to ouabain and hypoxia (Fig. 4A). Data shown in Fig. 5D suggest that permanent activation of Src in hypoxic cells hypoxia is due to glutathionylation of Cys458 and Cys459. Similar to Src, Erk kinase becomes independent of O2 availability and ouabain in the absence of cysteines within the NBD domain (Fig. 4). Preconditioning triggered by brief “pulses” of treatment of isolated perfused heart or brain slices with cardiotonic steroids (ouabain, digitoxin, neriifolin) could protect the tissue from damage caused by ischemia-reperfusion [20], [4], [49], [5]. This protective action of ouabain in mouse heart relied on the of IP3 kinase-1A, and was independent of activation of Src kinases [20]. Digitoxin was identified as a potent suppressor of autophagy-related apoptosis or necrosis (autosis) of hippocampal neurons in a rat model of cerebral hypoxia-ischemia [37]. Signaling pathways causing cytoprotective effects of cardiotonic steroids are currently unknown. They are most likely associated with the changes in free radical production within the mitochondria under hypoxic conditions that in turn may be intimately linked to the calcium filling of the ER and ER stress [1], [61]. It is unclear whether activation of Src kinases under hypoxic conditions as well as in the presence of ouabain is caused or by themselves may cause the changes in free radical production. Src kinases were earlier on shown to respond with activation to the free radical burst or exposure to peroxynitrite [25], [30], [42]. Free radical burst and the related increase in peroxynitrite production and tyrosine nitration were demonstrated during the first 10–30 min of hypoxia [26], [66]. At the same time, hypoxia results in activation of Src kinase as it was shown earlier [29], [31] and in this study (Fig. 4). Bringing these two observations together the model suggests that activation of Src is caused by S-glutathionylation of the cysteines within the NBD domain and the following de-attachment of Src kinase from the Na,K-ATPase. Treatment of hypoxic HEK293 cells expressing murine α1 subunit with 250 μM of ouabain resulted in inactivation of Src and promotion of its interaction with NBD of the Na,K-ATPase (Fig. 6). Reported earlier on, inhibition of Src caused by ouabain in SC-1 cells under hypoxic conditions was associated with a decrease in production of reactive oxygen species and prevention of oxidative stress [31].
Taken together assessment of signaling responses of Na,K-ATPase (Fig. 4) and the results of modeling (Fig. 5) indicate pivotal importance of Cys 454, 458 and 459 within the NBD in interaction between the Na,K-ATPase and kinase domain of Src. Replacement of these cysteines with alanine or their S-glutathionylation prevents formation of the “inhibitory” complex between the Src kinase and the Na,K-ATPase. Src remains permanently active and insensitive to hypoxic challenge or stimulation with ouabain. Interestingly, treatment of the enzyme in cell lysates with access of GSSG or overloading of cells with it causes a different pattern of responses in which Cys 244 is of foremost importance in control of the enzyme's hydrolytic function (Fig. 2). We may hence only speculate about the possible interplay between both processes in hypoxic organism. Hypoxia triggers free radical burst originating from the mitochondria [26]. Along with that deoxygenation causes release of cardiotonic steroids from the kidney adrenal glands and hypothalamus into the circulation. Endogenous ouabain is produced from cholesterol in the mitochondria, the organelles involved in ATP production [36], [62]. This response may be adaptive and convey protection against ischemia-reperfusion injury (see above). Furthermore, suppression of the Src activity triggered by ouabain under hypoxic conditions should suppress proliferation under hypoxic conditions and preserve the cells from unnecessary high ATP consumption, the effect that powers the use of cardiotonic steroids as potential anti-cancer therapy [56]. Our findings indicate that this adaptive response may be lost in the absence of the Cys 244, 454, 458 and 459 of which Cys 458, 459 and 244 are likely to be of utmost importance (Fig. 4, Fig. 6). These cysteine residues within the catalytic α subunit are most likely involved in control of ATP expenditure under hypoxic conditions by controlling the hydrolytic activity of Na,K-ATPase and its signaling function that extends much beyond the ATP consumption by the pump alone as stated in the “channel arrest theory” [12], [19], [27], [47].
5. Conclusions
The results obtained emphasize the important role of regulatory cysteines located in the AD and NBD of the Na,K-ATPase in the ability of enzyme to respond to hypoxia tuning its hydrolytic activity and signaling function to the changes in environmental oxygen availability. For the first time the critical role of Cys244 was shown in inhibition of enzyme by regulatory S-glutathionylation. We also revealed the importance of Cys458 and Cys459 in the interaction between the Na,K-ATPase and Src kinase. This interaction is a core event in receptor function of the enzyme during exposure to cardiotonic steroids and hypoxia.
Acknowledgement
This study was supported by the Swiss National Science Foundation (SNF) (#IZK0Z3_157269) (for Fig. 1, Fig. 2, Fig. 3) and Russian Science Foundation (# 14-14-01152) (for Fig. 2, Fig. 4, Fig. 5, Fig. 6, Supplementary materials).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.05.021.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Aperia A., Akkuratov E.E., Fontana J.M., Brismar H. Na+-K+-ATPase, a new class of plasma membrane receptors. Am. J. Physiol. Cell Physiol. 2016;310:C491–C495. doi: 10.1152/ajpcell.00359.2015. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee M., Duan Q., Xie Z. SH2 ligand-Like effects of second cytosolic domain of Na/K-ATPase alpha1 subunit on Src kinase. PLoS One. 2015;10:e0142119. doi: 10.1371/journal.pone.0142119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer N., Muller-Ehmsen J., Kramer U., Hambarchian N., Zobel C., Schwinger R.H., Neu H., Kirch U., Grunbaum E.G., Schoner W. Ouabain-like compound changes rapidly on physical exercise in humans and dogs: effects of beta-blockade and angiotensin-converting enzyme inhibition. Hypertension. 2005;45:1024–1028. doi: 10.1161/01.HYP.0000165024.47728.f7. [DOI] [PubMed] [Google Scholar]
- 4.Belliard A., Gulati G.K., Duan Q., Alves R., Brewer S., Madan N., Sottejeau Y., Wang X., Kalisz J., Pierre S.V. Ischemia/reperfusion-induced alterations of enzymatic and signaling functions of the rat cardiac Na+/K+-ATPase: protection by ouabain preconditioning. Physiol. Rep. 2016:4. doi: 10.14814/phy2.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belliard A., Sottejeau Y., Duan Q., Karabin J.L., Pierre S.V. Modulation of cardiac Na+,K+-ATPase cell surface abundance by simulated ischemia-reperfusion and ouabain preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H94–H103. doi: 10.1152/ajpheart.00374.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bickler P.E., Buck L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu. Rev. Physiol. 2007;69:145–170. doi: 10.1146/annurev.physiol.69.031905.162529. [DOI] [PubMed] [Google Scholar]
- 7.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin. Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Blanco G., Mercer R.W. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanova A., Grenacher B., Nikinmaa M., Gassmann M. Hypoxic responses of Na+/K+ ATPase in trout hepatocytes. J. Exp. Biol. 2005;208:1793–1801. doi: 10.1242/jeb.01572. [DOI] [PubMed] [Google Scholar]
- 10.Bogdanova A., Petrushanko I., Boldyrev A., Gassmann M. Oxygen- and Redox-Induced Regulation of the Na/K ATPase. Curr. Enzym. Inhib. 2006;2:37–59. [Google Scholar]
- 11.Bogdanova A., Petrushanko I.Y., Hernansanz-Agustin P., Martinez-Ruiz A. "Oxygen Sensing" by Na,K-ATPase: these miraculous thiols. Front Physiol. 2016;7:314. doi: 10.3389/fphys.2016.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck L.T., Hochachka P.W. Anoxic suppression of Na(+)-K(+)-ATPase and constant membrane potential in hepatocytes: support for channel arrest. Am. J. Physiol. 1993;265:R1020–R1025. doi: 10.1152/ajpregu.1993.265.5.R1020. [DOI] [PubMed] [Google Scholar]
- 13.Carmosino M., Torretta S., Procino G., Timperio A., Zolla L., Svelto M. Na+/K+-ATPase beta1-subunit is recruited in Na-K-2Cl co-transporter isoform 2 multiprotein complexes in rat kidneys: possible role in blood pressure regulation. J. Hypertens. 2014;32:1842–1853. doi: 10.1097/HJH.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z., Krmar R.T., Dada L., Efendiev R., Leibiger I.B., Pedemonte C.H., Katz A.I., Sznajder J.I., Bertorello A.M. Phosphorylation of adaptor protein-2 mu2 is essential for Na+,K+-ATPase endocytosis in response to either G protein-coupled receptor or reactive oxygen species. Am. J. Respir. Cell Mol. Biol. 2006;35:127–132. doi: 10.1165/rcmb.2006-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes A., Cascante M., Cardenas M.L., Cornish-Bowden A. Relationships between inhibition constants, inhibitor concentrations for 50% inhibition and types of inhibition: new ways of analysing data. Biochem J. 2001;357:263–268. doi: 10.1042/0264-6021:3570263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dada L.A., Chandel N.S., Ridge K.M., Pedemonte C., Bertorello A.M., Sznajder J.I. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J. Clin. Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dada L.A., Welch L.C., Zhou G., Ben-Saadon R., Ciechanover A., Sznajder J.I. Phosphorylation and ubiquitination are necessary for Na,K-ATPase endocytosis during hypoxia. Cell Signal. 2007;19:1893–1898. doi: 10.1016/j.cellsig.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Angelis C., Haupert G.T., Jr. Hypoxia triggers release of an endogenous inhibitor of Na(+)-K(+)-ATPase from midbrain and adrenal. Am. J. Physiol. 1998;274:F182–F188. doi: 10.1152/ajprenal.1998.274.1.F182. [DOI] [PubMed] [Google Scholar]
- 19.Doll C.J., Hochachka P.W., Reiner P.B. Channel arrest: implications from membrane resistance in turtle neurons. Am. J. Physiol. 1991;261:R1321–R1324. doi: 10.1152/ajpregu.1991.261.5.R1321. [DOI] [PubMed] [Google Scholar]
- 20.Duan Q., Madan N.D., Wu J., Kalisz J., Doshi K.Y., Haldar S.M., Liu L., Pierre S.V. Role of phosphoinositide 3-kinase IA (PI3K-IA) activation in cardioprotection induced by ouabain preconditioning. J. Mol. Cell Cardiol. 2015;80:114–125. doi: 10.1016/j.yjmcc.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dvela M., Rosen H., Feldmann T., Nesher M., Lichtstein D. Diverse biological responses to different cardiotonic steroids. Pathophysiology. 2007;14:159–166. doi: 10.1016/j.pathophys.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Fedorova O.V., Anderson D.E., Lakatta E.G., Bagrov A.Y. Interaction of NaCl and behavioral stress on endogenous sodium pump ligands in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R352–R358. doi: 10.1152/ajpregu.2001.281.1.R352. [DOI] [PubMed] [Google Scholar]
- 23.Figtree G.A., Liu C.C., Bibert S., Hamilton E.J., Garcia A., White C.N., Chia K.K., Cornelius F., Geering K., Rasmussen H.H. Reversible oxidative modification: a key mechanism of Na+-K+ pump regulation. Circ. Res. 2009;105:185–193. doi: 10.1161/CIRCRESAHA.109.199547. [DOI] [PubMed] [Google Scholar]
- 24.Fontana J.M., Burlaka I., Khodus G., Brismar H., Aperia A. Calcium oscillations triggered by cardiotonic steroids. FEBS J. 2013;280:5450–5455. doi: 10.1111/febs.12448. [DOI] [PubMed] [Google Scholar]
- 25.Giannoni E., Buricchi F., Raugei G., Ramponi G., Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernansanz-Agustin P., Izquierdo-Alvarez A., Sanchez-Gomez F.J., Ramos E., Villa-Pina T., Lamas S., Bogdanova A., Martinez-Ruiz A. Acute hypoxia produces a superoxide burst in cells. Free Radic. Biol. Med. 2014;71:146–156. doi: 10.1016/j.freeradbiomed.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Hochachka P.W., Buck L.T., Doll C.J., Land S.C. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl. Acad. Sci. USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y.K., Eisses J.F., Kaplan J.H. Expression of an active Na,K-ATPase with an alpha-subunit lacking all twenty-three native cysteine residues. J. Biol. Chem. 2000;275:30734–30739. doi: 10.1074/jbc.M003737200. [DOI] [PubMed] [Google Scholar]
- 29.Knock G.A., Snetkov V.A., Shaifta Y., Drndarski S., Ward J.P., Aaronson P.I. Role of src-family kinases in hypoxic vasoconstriction of rat pulmonary artery. Cardiovasc. Res. 2008;80:453–462. doi: 10.1093/cvr/cvn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knock G.A., Ward J.P. Redox regulation of protein kinases as a modulator of vascular function. Antioxid. Redox Signal. 2011;15:1531–1547. doi: 10.1089/ars.2010.3614. [DOI] [PubMed] [Google Scholar]
- 31.Lakunina V.A., Burnysheva K.M., Mitkevich V.A., Makarov A.A., Petrushanko I. Changes in the receptor function of Na,K-ATPase during hypoxia and ischemia. Mol. Biol. (Mosk.) 2017;51:172–179. doi: 10.7868/S0026898417010104. [DOI] [PubMed] [Google Scholar]
- 32.Lecuona E., Trejo H.E., Sznajder J.I. Regulation of Na,K-ATPase during acute lung injury. J. Bioenerg. Biomembr. 2007;39:391–395. doi: 10.1007/s10863-007-9102-1. [DOI] [PubMed] [Google Scholar]
- 33.Li Z., Cai T., Tian J., Xie J.X., Zhao X., Liu L., Shapiro J.I., Xie Z. NaKTide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J. Biol. Chem. 2009;284:21066–21076. doi: 10.1074/jbc.M109.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z., Xie Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflug. Arch. 2009;457:635–644. doi: 10.1007/s00424-008-0470-0. [DOI] [PubMed] [Google Scholar]
- 35.Liang M., Tian J., Liu L., Pierre S., Liu J., Shapiro J., Xie Z.J. Identification of a pool of non-pumping Na/K-ATPase. J. Biol. Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 36.Lichtstein D., Rosen H. Endogenous digitalis-like Na+, K+-ATPase inhibitors, and brain function. Neurochem. Res. 2001;26:971–978. doi: 10.1023/a:1012340702763. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Levine B. Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 2015;22:367–376. doi: 10.1038/cdd.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutz P.L., Nilsson G.E., Perez-Pinzon M.A. Anoxia tolerant animals from a neurobiological perspective. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996;113:3–13. doi: 10.1016/0305-0491(95)02046-2. [DOI] [PubMed] [Google Scholar]
- 39.Madan N., Xu Y., Duan Q., Banerjee M., Larre I., Pierre S.V., Xie Z. Src-independent ERK signaling through the alpha3 isoform of Na/K-ATPase. Am. J. Physiol. Cell Physiol. 2016;00199:02016. doi: 10.1152/ajpcell.00199.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manunta P., Messaggio E., Ballabeni C., Sciarrone M.T., Lanzani C., Ferrandi M., Hamlyn J.M., Cusi D., Galletti F., Bianchi G., Salt Sensitivity Study Group of the Italian Society Of, H. Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension. 2001;38:198–203. doi: 10.1161/01.hyp.38.2.198. [DOI] [PubMed] [Google Scholar]
- 41.Mcmullen D.C., Storey K.B. Suppression of Na+K+ -ATPase activity by reversible phosphorylation over the winter in a freeze-tolerant insect. J. Insect Physiol. 2008;54:1023–1027. doi: 10.1016/j.jinsphys.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Minetti M., Mallozzi C., Di Stasi A.M. Peroxynitrite activates kinases of the src family and upregulates tyrosine phosphorylation signaling. Free Radic. Biol. Med. 2002;33:744–754. doi: 10.1016/s0891-5849(02)00891-2. [DOI] [PubMed] [Google Scholar]
- 43.Mitkevich V.A., Petrushanko I., Poluektov Y.M., Burnysheva K.M., Lakunina V.A., Anashkina A.A., Makarov A.A. Basal glutathionylation of Na,K-ATPase alpha-subunit depends on redox status of cells during the enzyme biosynthesis. Oxid. Med. Cell Longev. 2016 doi: 10.1155/2016/9092328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morth J.P., Pedersen B.P., Toustrup-Jensen M.S., Sorensen T.L., Petersen J., Andersen J.P., Vilsen B., Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 45.Nesher M., Shpolansky U., Rosen H., Lichtstein D. The digitalis-like steroid hormones: new mechanisms of action and biological significance. Life Sci. 2007;80:2093–2107. doi: 10.1016/j.lfs.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson G.E. Surviving anoxia with the brain turned on. News Physiol. Sci. 2001;16:217–221. doi: 10.1152/physiologyonline.2001.16.5.217. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson G.E., Lutz P.L. Anoxia tolerant brains. J. Cereb. Blood Flow. Metab. 2004;24:475–486. doi: 10.1097/00004647-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Palasis M., Kuntzweiler T.A., Arguello J.M., Lingrel J.B. Ouabain interactions with the H5-H6 hairpin of the Na,K-ATPase reveal a possible inhibition mechanism via the cation binding domain. J. Biol. Chem. 1996;271:14176–14182. doi: 10.1074/jbc.271.24.14176. [DOI] [PubMed] [Google Scholar]
- 49.Pasdois P., Quinlan C.L., Rissa A., Tariosse L., Vinassa B., Costa A.D., Pierre S.V., Dos Santos P., Garlid K.D. Ouabain protects rat hearts against ischemia-reperfusion injury via pathway involving src kinase, mitoKATP, and ROS. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1470–H1478. doi: 10.1152/ajpheart.00877.2006. [DOI] [PubMed] [Google Scholar]
- 50.Petrushanko I., Bogdanov N., Bulygina E., Grenacher B., Leinsoo T., Boldyrev A., Gassmann M., Bogdanova A. Na-K-ATPase in rat cerebellar granule cells is redox sensitive. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R916–R925. doi: 10.1152/ajpregu.00038.2005. [DOI] [PubMed] [Google Scholar]
- 51.Petrushanko I., Simonenko O.V., Burnysheva K.M., Klimanova E.A., Dergousova E.A., Mitkevich V.A., Lopina O.D., Makarov A.A. The ability of cells to adapt to low-oxygen conditions is associated with glutathioylation of Na,K-ATPase. Mol. Biol. (Mosk.) 2015;49:153–160. [PubMed] [Google Scholar]
- 52.Petrushanko I.Y., Bogdanov N.B., Lapina N., Boldyrev A.A., Gassmann M., Bogdanova A.Y. Oxygen-induced regulation of Na/K ATPase in cerebellar granule cells. J. Gen. Physiol. 2007;130:389–398. doi: 10.1085/jgp.200709783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrushanko I.Y., Mitkevich V.A., Anashkina A.A., Klimanova E.A., Dergousova E.A., Lopina O.D., Makarov A.A. Critical role of gamma-phosphate in structural transition of Na,K-ATPase upon ATP binding. Sci. Rep. 2014;4:5165. doi: 10.1038/srep05165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrushanko I.Y., Yakushev S., Mitkevich V.A., Kamanina Y.V., Ziganshin R.H., Meng X., Anashkina A.A., Makhro A., Lopina O.D., Gassmann M., Makarov A.A., Bogdanova A. S-glutathionylation of the Na,K-ATPase catalytic alpha subunit is a determinant of the enzyme redox sensitivity. J. Biol. Chem. 2012;287:32195–32205. doi: 10.1074/jbc.M112.391094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pierre S.V., Xie Z. The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys. 2006;46:303–316. doi: 10.1385/cbb:46:3:303. [DOI] [PubMed] [Google Scholar]
- 56.Prassas I., Diamandis E.P. Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 2008;7:926–935. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- 57.Rathbun W.B., Betlach M.V. Estimation of enzymically produced orthophosphate in the presence of cysteine and adenosine triphosphate. Anal. Biochem. 1969;28:436–445. doi: 10.1016/0003-2697(69)90198-5. [DOI] [PubMed] [Google Scholar]
- 58.Schoner W., Scheiner-Bobis G. Role of endogenous cardiotonic steroids in sodium homeostasis. Nephrol. Dial. Transplant. 2008;23:2723–2729. doi: 10.1093/ndt/gfn325. [DOI] [PubMed] [Google Scholar]
- 59.Segall L., Lane L.K., Blostein R. Insights into the structural basis for modulation of E1<-->E2 transitions by cytoplasmic domains of the Na,K-ATPase alpha subunit. Ann. N. Y. Acad. Sci. 2003;986:58–62. doi: 10.1111/j.1749-6632.2003.tb07139.x. [DOI] [PubMed] [Google Scholar]
- 60.Shi H.G., Mikhaylova L., Zichittella A.E., Arguello J.M. Functional role of cysteine residues in the (Na,K)-ATPase alpha subunit. Biochim. Biophys. Acta. 2000;1464:177–187. doi: 10.1016/s0005-2736(99)00245-x. [DOI] [PubMed] [Google Scholar]
- 61.Soboloff J., Rothberg B.S., Madesh M., Gill D.L. STIM proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stocco D.M. StAR protein and the regulation of steroid hormone biosynthesis. Annu. Rev. Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- 63.Tian J., Cai T., Yuan Z., Wang H., Liu L., Haas M., Maksimova E., Huang X.Y., Xie Z.J. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu J., Akkuratov E.E., Bai Y., Gaskill C.M., Askari A., Liu L. Cell signaling associated with Na(+)/K(+)-ATPase: activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry. 2013;52:9059–9067. doi: 10.1021/bi4011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie J., Ye Q., Cui X., Madan N., Yi Q., Pierre S.V., Xie Z. Expression of rat Na-K-ATPase alpha2 enables ion pumping but not ouabain-induced signaling in alpha1-deficient porcine renal epithelial cells. Am. J. Physiol. Cell Physiol. 2015;309:C373–C382. doi: 10.1152/ajpcell.00103.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yakushev S., Band M., Tissot Van Patot M.C., Gassmann M., Avivi A., Bogdanova A. Cross talk between S-nitrosylation and S-glutathionylation in control of the Na,K-ATPase regulation in hypoxic heart. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H1332–H1343. doi: 10.1152/ajpheart.00145.2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material