Abstract
Increasing evidence suggests that Cd at levels found in the human diet can cause oxidative stress and activate redox-sensitive transcription factors in inflammatory signaling. Following inflammation, tissue repair often involves activation of redox-sensitive transcription factors in fibroblasts. In lungs, epithelial barrier remodeling is required to restore gas exchange and barrier function, and aberrant myofibroblast differentiation leads to pulmonary fibrosis. Contributions of exogenous exposures, such as dietary Cd, to pulmonary fibrosis remain incompletely defined. In the current study, we tested whether Cd activates fibrotic signaling in human fetal lung fibroblasts (HFLF) at micromolar and submicromolar Cd concentrations that do not cause cell death. Exposure of HFLF to low-dose Cd (≤1.0 μM) caused an increase in stress fibers and increased protein levels of myofibroblast differentiation markers, including α-smooth muscle actin (α-SMA) and extra-domain-A-containing fibronectin (ED-A-FN). Assay of transcription factor (TF) activity using a 45-TF array showed that Cd increased activity of 12 TF, including SMAD2/3/4 (mothers against decapentaplegic homolog) signaling differentiation and fibrosis. Results were confirmed by real-time PCR and supported by increased expression of target genes of SMAD2/3/4. Immunocytochemistry of lungs of mice exposed to Cd (0.3 and 1.0 mg/L in drinking water) showed increased α-SMA staining with lung Cd accumulation similar to lung Cd in non-smoking humans. Together, the results show that relatively low Cd exposures stimulate pulmonary fibrotic signaling and myofibroblast differentiation by activating SMAD2/3/4–dependent signaling. The results indicate that dietary Cd intake could be an important variable contributing to pulmonary fibrosis in humans.
Keywords: Environmental stress, human fetal lung fibroblast, lung disease
Graphical Abstract

Introduction
Cadmium (Cd) is an environmental pollutant that causes multiple adverse health effects, including organ failure and cancer. Humans are exposed to Cd from diet at an intake level of approximately 19 – 21 μg per day (EFSA 2009), while tobacco smoking significantly adds to the exposure (2 μg per cigarette) (ATSDR 2012; Mannino et al. 2004). Cd is not effectively excreted by humans and has 10 – 30y biological half-life (ATSDR 2012). Thus chronic exposure of human to low doses of Cd mainly via food consumption results in Cd deposition in different tissues and can be a significant health hazard. Cd is classified as a carcinogen affecting multiple organ systems including lung, liver, kidney, and hematopoietic and other systems (ATSDR 2012). Cd exposure is closely associated with lung diseases including lung cancer, chronic obstructive pulmonary disease (COPD), and emphysema (Hart 2000). Previous studies also show that Cd exposure in low concentration stimulated proliferation in mouse lung cells and resulted in severe lung inflammation (Kundu et al. 2009). Despite extensive data on Cd toxicity at high doses, the roles of Cd at low dietary levels in pulmonary health remain to be fully elucidated.
Progressive and usually fatal fibrotic lung disease is characterized by fibroblast proliferation and extracellular matrix (ECM) remodeling, which results in irreversible distortion of the lung and reduction in gas exchange (Selman et al. 2001). The pathogenesis is attributed to abnormal wound healing in response to lung insult such as inflammation and environmental exposure (Taskar and Coultas 2006). During wound healing, resident fibroblasts undergo proliferation and differentiation into myofiboblasts which display exaggerated ECM production and endow a contractile apparatus allowing them to close open wounds. The de novo synthesized contractile apparatus includes expression of α-smooth muscle actin (α-SMA) and formation of stress fibers that adhere to ECM proteins (Hinz et al. 2007). Incorporation of α-SMA into stress fibers increases the cellular contractile strength and the rigidity of ECM, which further promotes the expression of α-SMA and the tissue repair process. Therefore, abnormal expression of α-SMA can affect this feedback loop during the wound healing process and contribute to pathological fibrosis.
Development of in vitro models to replicate in vivo exposures is challenging for many reasons, e.g., time frame of exposure, binding of Cd to many ligands, artificial nature of cell culture conditions. We use 1 μM as our in vitro reference dose (Go et al. 2013a, b) because this is similar to the concentration found in human lung (Chandler et al. 2016), does not cause cell death, and activates proinflammatory signaling similarly to that observed with mouse models in which oral Cd is provided to raise lung Cd to values similar to human lung Cd content (Chandler et al. 2016). Our previous study of Cd in this range showed that Cd disrupts actin cytoskeleton regulation in lung fibroblast by stimulating actin polymerization (Go et al. 2013a). Results also show that interruption of actin dynamics by Cd disrupted subcellular compartmental redox homeostasis (Cuypers et al. 2010; Go et al. 2013a) and stimulated inflammatory signaling involving NF-κB activation in HeLa cells (Go et al. 2013b). Although Cd-stimulated inflammatory signaling and elevation of oxidative stress are well known (Cuypers et al. 2010; Go et al. 2013a), the effect of comparable exposures to Cd on regulation of lung physiology specifically on the molecular mechanistic responses of lung fibroblasts remains unexplored. Therefore, in the present study we studied lung fibrosis regulation in response to 0.5 to 2 μM Cd in human lung fibroblasts. The results show that Cd activates profibrotic signaling and promotes myofibroblast differentiation by increasing expression levels of differentiation marker proteins such as α-SMA via activation of SMAD2/3/4 transcription factor. An immunocytochemical analysis of α-SMA in lungs of mice exposed to Cd in drinking water provided evidence that this process also occurs in vivo.
Material and Methods
Cell culture and Cd treatment
Experiments were performed on normal human fetal lung fibroblasts (HLF1, passages ≤ 11) obtained from American Tissue Culture Collection (ATCC, Rockville, MD). HLF1 were cultured in F-12K medium (Kaighn’s Modification of Ham’s F-12 medium) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin/streptomycin, and maintained in a humidified incubator at 5% CO2 at 37 °C. For low-dose Cd treatment (0, 0.5, 1.0 and 2.0 μM as CdCl2, Sigma-Aldrich, St. Louis, MO), cells grown in cell culture plates (6-well, 96-well, cover slips) were exposed to Cd for 6 h and 24 h to examine mRNA and protein expression, respectively. Doses of 0.5 and 1.0 μM result in Cd concentrations comparable to human dietary intake while 2.0 μM represents a higher level from smoking. The condition labeled 0 μM has no Cd added and has been previously found to have negligible Cd (Go et al. 2013a, b). Human transforming growth factor-β (TGFβ, Sigma-Aldrich) was used as a positive control for myofibroblast differentiation (Thannickal et al. 2003).
Mouse lung histology and immunofluorescence imaging
C57BL/6 mice (male, 5–7 weeks old, n=4–8) were exposed to Cd in drinking water (0, 0.3 or 1.0 mg/L CdCl2) for 16 weeks, with the approval of Institutional Animal Care and Use Committee of Emory University. For these studies, the mouse food was nominally Cd-free, equivalent to intake of 0.04 mg/L in the drinking water (Chandler et al. 2016). Lung samples were harvested from the mice at the end of 16 weeks and the left lobes of lung tissues treated with 10% neutral formalin for fixation. The fixed lungs were processed and stained with anti-α-SMA and anti-vimentin purchased from Abcam (Cambridge, MA) for fluorescence microscopy following the procedures previously described (Rock et al. 2011). Immunofluorescence was visualized using an Olympus X-700 fluorescence microscope system.
Myofibroblast differentiation marker examination by fluorescence microscopy and Western blotting
To examine α-SMA and F-actin by fluorescence microscopy, HFL1 cells grown on glass coverslips were treated with Cd or TGF-β for 24 h, washed with PBS, and followed by the same procedures as described in previous studies (Go et al. 2013a, b). Briefly, cells were incubated with anti-α-SMA (Abcam) followed by Cy3. Cells were then incubated with BODIPY FL Phallacidin for F-actin and Hoechst for nuclei staining (Thermo Fisher Scientific). Fluorescence was visualized using an Olympus X-700 fluorescence microscope, and fluorescence intensity was quantified with ImageJ software (NIH). (Go et al. 2013a, b) Quantification of α-SMA was further confirmed by measuring fluorescence of cells grown in 96-well plates and treated with Cd in an independent experiment using a fluorescence plate reader (SpectraMax M2, Molecule Devices). To examine expression levels of α-SMA and ED-A FN by Western blot analysis, cells after Cd or TGF-β treatment for 24 h were lysed, proteins were prepared, and protein expression levels from Western blots were quantified as previously described (Go et al. 2013b). Primary antibodies to detect α-SMA and ED-A FN were purchased from Abcam.
Transcription factor activity array
HFL1 cells were reverse transfected with luciferase constructs pre-coated to 96-well plates (Cignal 45-Pathway Reporter Array, QIAGEN, Valencia, CA) that profile the activity of transcription factors in 45 signal transduction pathways. Each reporter contains a mixture of an inducible transcription factor responsive Firefly luciferase construct and constitutively expressing Renilla luciferase (20:1). The Firefly construct contains tandem repeats of transcription factor binding sequence (listed in Supplementary Table S1). After 24 h of transfection following the procedures provided by the manufacturer, the cells were treated with Cd for 6 h. Firefly and Renilla luciferase activity were quantified using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). All luciferase data were normalized relative to the Renilla luciferase activity. Negative and positive controls were included in all experiments to assure transfection efficiency.
Determination of gene expression levels by quantitative reverse transcription (qRT) PCR
Total mRNA extracted from cells after Cd treatment for 6 h was used to generate cDNAs by reverse transcription (QIAGEN). Quantification by real-time PCR was performed in triplicate on an iCycler IQ Multicolor RT-PCR Detection System (Bio-Rad Laboratories, Hercules, CA) for 40 cycles (94 °C for 30 s, 60 °C for 30 s and 72 °C for 30s) using iCycler software and normalized by 18s RNA levels. The sequences of primers purchased from Integrated DNA Technologies (Coralville, IA) were as follows (5′ to 3′): MT1/2, forward: GCACCTCCTGCAAGAAGAGCT, reverse: GCAGCCCTGGGCACACTT; Collagen1α1, forward: AGCCAGCAGATCGAGAACAT, reverse: TCTTGTCCTTGGGGTTCTTG;agr;SMactin (ACTA2), forward: GACCCTGAAGTACCCGATAGAAC, reverse: GGGCAACACGAAGCTCATTG; Fibronectin, forward: TCGAGGAGGAAATTCCAATG, reverse: ACACACGTGCACCTCATCAT; MMP2, forward: ACATCAAGGGCATTCAGGAG, reverse: GCCTCGTATACCGCATCAAT; Axin2, forward: CCTGCCACCAAGACCTACAT, reverse: CTTCATTCAAGGTGGGGAGA.
Statistics
Data from at least three independent replicates of each experimental condition were compared using one-way ANOVA followed by Tukey’s post-hoc tests. A p-value less than 0.05 was considered significant. For 45 TF activity experiments, one-way ANOVA repeated measures were used to take plate-to-plate variation into account.
Results
Cd promotes expression of differentiation markers of myofibroblasts in human fetal lung fibroblasts (HLF1)
Myofibroblasts arise de novo from multiple origins in response to tissue injury (Hinz et al. 2007). The perivascular and peribronchiolar adventitial fibroblasts are considered major precursors of lung myofibroblasts although other progenitors may be recruited at the site of injury. To examine Cd effect on formation of myofibroblasts, we treated HLF1 cells with 0, 0.5, 1.0 or 2.0 μM Cd and measured expressionα-SMA as a differentiation marker using immunocytochemistry (Fig. 1) and Western blotting (Fig 2).
Figure 1.
Low-dose Cd stimulated myofibroblast differentiation markers (α-SMA expression, stress fiber formation and myofibroblast morphology) in human lung fibroblast. Quiescent HFL1 (n=3) cells were stimulated with 0, 0.5, 1.0 and 2.0 μM Cd for 24 h in serum-free medium following previously published procedure (Thannickal et al. 2003). α-SMA and filamentous actin (F-actin) were examined by fluorescence microscopy (×300), and representative images are shown. Blue, Hoechst-labeled nuclei; red, Cy3-conjugated α-SMA; green, BODIPY FL phallacidin-labeled F-actin. Quantification of fluorescence intensity was performed by spectrometer (α-SMA, n=12), or using ImageJ software (F-actin, n=3), as shown in the bar graphs. *p<0.05, **p<0.01, ***p<0.0001 compared to control in one-way ANOVA Tukey HSD.
Figure 2.
Low-dose Cd increased protein levels of α-SMA (A) and ED-A FN (B) in human lung fibroblast. Quiescent HFL1 cells (n = 6) were stimulated with 0, 0.5, 1.0 and 2.0 μM Cd or TGF- β (2 ng/L) for 24 h in serum-free medium and then lysed for Western blotting. Intensity of bands for α-SMA and ED-A FN were normalized against cyclophilin B and vinculin, respectively. *p<0.05, **p<0.01 compared to control in one-way ANOVA Tukey HSD.
The change in abundance of α-SMA in HLF1 cells by Cd treatment was visualized by fluorescence microscopy after labeling with antibody specific to α-SMA (red fluorescence, Fig 1A, 1B top). As shown by fluorescence images, significant elevation of α-SMA expression was observed by low dose Cd treatment.α-SMA was highest at 0.5 μM Cd (0.5 μM, 1.9 ± 0.1 fold; 1.0 μM, 1.6 ± 0.1 fold, 2.0 μM, 1.5 ± 0.1 fold, Fig 1B top). In contrast, Cd-induced stress fiber formation, quantified by filamentous actin (F-actin) fluorescence (Fig 1A, 1B bottom), was elevated more strongly by Cd treatment at 1.0 and 2.0 μM (1.6 fold increase). This suggests that there could be time-dependent responses or differential sensitivity to Cd concentration in HLF1. The stimulatory effects of Cd on increased α-SMA and F-actin were also associated with morphological changes of cellular hypertrophy, another characteristic of myofibroblasts that was not observed in the control condition (Fig 1)
Cd-stimulated differentiation markers for myofibroblast were further assessed by Western blotting (Fig 2). Consistent with the cellular fluorescence data of α-SMA (Fig 1), Cd treatment significantly elevated protein levels of α-SMA in HLF1 cells (Fig 2A). Similarly, expression of fibronectin Extra-Domain-A variant (ED-A FN), a distinctive splice variant of fibronectin which is also critical for acquisition of the myofibroblast phenotype (Serini et al. 1998) and early marker for myofibroblast (Hinz et al. 2001) was also increased by Cd (2- to 3-fold by 0.5 and 1.0 μM Cd, Fig 2B). Although responses were not significant, a similar trend in dose-response was observed for α-SMA and general form of fibronectin (FN) after prolonged Cd exposure of 40 hours (Supplementary Figure S1).
Cd stimulates SMAD and other TF responsible for differentiation and fibrosis signaling pathways
To study potential mechanisms stimulating differentiation signaling in fibroblasts, we examined the effects of Cd on signal transduction pathways by using a 45-TF activity array assay. This array utilizes luciferase reporter activities and includes SMAD2/3/4 and other transcription factors involved in cancer, immune signaling, development, cell differentiation, nuclear receptor and oxidative stress mechanisms. SMAD2/3/4 is a well-known TF complex regulating TGF-ββ-dependent fibroblast proliferation and differentiation. The molecular mechanism for SMAD2/3/4 TF-mediated transcription is not yet fully understood because of complicated interactions with other TF (cJun, AP-1) and coactivators [homo- or hetero-oligomers of Smad (2, 3, 4)] within the complex (Derynck et al. 1998; Shi et al. 1998; Wong et al. 1999). The nucleotide sequence for SMAD TF binding “AGCCAGACA” (Supplementary Table S1) included in the array kit is known for Smad2-, Smad3- and Smad4-consensus binding site or smad box (Shi et al. 1998; Wong et al. 1999); in the current study, we therefore used term SMAD2/3/4 to refer to this complex of these Smads. Results showed that 16 TF-mediated signaling pathways were affected by Cd treatment with dose-response characteristics. Twelve TF were upregulated by Cd and four TF were downregulated by Cd (Figure 3 and Supplementary Table S1). Three of the increased TF, including SMAD2/3/4, MEF2 (myocyte enhancer factor-2), and GR (Glucocorticoid receptor), were highly affected, showing a 3–4 fold increase in activity at 0.5 μM Cd (Fig 3A, Supplementary Table S1). The dose-response relation in Cd-induced SMAD2/3/4 stimulation was consistent with the phenotypic change in promoting differentiation of fibroblast to myofibroblast mediated by activation of the SMAD complex (Fig 3A, 3B top panel). In addition, MTF-1 (metal response element-binding transcription factor-1) was activated with 3.5-fold increase of activity at 1μM Cd (Fig 3B middle panel), while activity of ATF4/3/2 (activating transcription factors) increased along with increasing Cd dose (Fig 3B bottom panel). Effects of 0.5μM Cd on TF were validated by using IMR-90 cells, another human fetal lung fibroblast cell line [the 4th column (Cd: IMR-90 cells) of Supplemental Table S1].
Figure 3.
Low-dose Cd changed the transcription factor (TF) activity in human lung fibroblast. A, Heatmap showing 16 out of 45 TF activity were significantly changed by Cd; B, bar plots showing 3 representative TF that responded to Cd treatment. HFL1 cells (n = 3) were transfected with 45 Firefly-fused DNA constructs containing individual TF binding element. Luminescence were measured in representation of TF activity after 6 h of treatment (0, 0.5, 1.0 and 2.0 μM Cd) and reported after normalized by signal from co-transfected Renilla.
To validate TF responses by Cd, we examined gene expression levels of metallothionein (MT) and collagen-1α1, axin-2 and matrix metalloprotease-2 (MMP2), as downstream target genes for MTF-1 and SMAD. The results show that mRNA level of metallothionein (MT)-1/2 regulated by MTF-1 was strongly induced by Cd in a linear dose-response relationship (Fig 4A) supporting the result of stimulation of MTF activity by Cd. The TGF-β-SMAD pathway has about 500 responsive genes (Massague et al. 2005), many of which are co-regulated by SMAD and other transcription factors. Collagen-1α1, a gene co-regulated by SMAD and ATF4, was significantly increased by 2 μM Cd (Fig 4B). Axin-2, a target for TGF-β-SMAD and Wnt signaling (Akhmetshina et al. 2012), and MMP2, a gelatinase strongly associated with IPF (Pardo and Selman 2006) did not show significant increases at 2.0 μM Cd (Fig 4B). Together, the results show that Cd stimulates expression of key molecules for myofibroblast differentiation and fibrosis, including α-SMA, fibronectin, F-actin and collagen-1α1 by stimulation of SMAD2/3/4 and other TF such as ATF4 activity. The results also show that these relatively low levels of Cd and/or short duration of the in vitro experiments do not elicit significant changes in all markers associated with pulmonary fibrosis
Figure 4.
Low-dose Cd upregulated mRNA levels of metallothionein (A) and selected fibrosis marker genes (B) in human lung fibroblast. HFL1 cells (n = 5) were treated with 0, 0.5, 1.0 and 2.0 μM Cd for 6 h and assays for the mRNA levels of metallothionein (A). For the mRNA levels of collagen1α1, axin2 and MMP2, cells treated with 2.0 μM Cd were examined by RT-qPCR (B). All RT-qPCR data were normalized by levels of 18s RNA. *p<0.05, ***p<0.001 compared to control in one-way ANOVA Tukey HSD.
Cd stimulates fibroblast differentiation in mouse lung
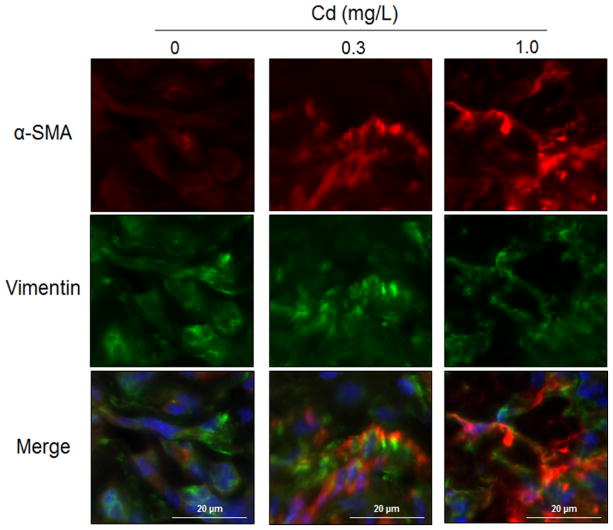
To determine whether corresponding markers of myofibroblast differentiation occur in vivo in response to low-dose Cd, lung sections were obtained from mice exposed to 0.3 and 1.0 mg Cd/L or vehicle (control, 0 mg/L Cd) in drinking water for 16 weeks as described previously (Go et al. 2015) and examined for α-SMA by fluorescence microscopy. As visualized by red fluorescence intensity of α-SMA, mouse lung exposed to Cd showed increased expression of α-SMA compared with control mouse lung (Fig 5). The location of clusters of fibroblast cells in alveolar interstitium of lung was confirmed by labeling with vimentin protein (green, Fig 5). The results extend the findings from HLF1 cells to show that low dose Cd exposure stimulates fibroblast differentiation in vivo in lung.
Figure 5.
α-SMA expression increased in interstitial fibroblastic cells in lungs from mice fed with low-level Cd. Male C57BL/6 mice were exposed to Cd in drinking water (0, 0.3 or 1.0 mg/L CdCl2) for 16 weeks. Left lung sections were processed for fluorescence staining and representative images are shown (×300). Blue, Hoechst-labeled nuclei; red, Cy3-conjugated α-SM-actin; green, Alexa488-conjugated vimentin.
Discussion
Exposures to Cd from cigarette smoking and occupational exposures are associated with multiple pulmonary diseases. These exposures are high relative to those in non-smoking individuals without occupational exposure, and little research is available on the effects of low environmental exposures as occur from dietary intake. Although precise extrapolation to human Cd internal exposures is difficult because of different absorption rates by different route of exposures, 10–30 years of long biological half-life, and free Cd amount released from Cd-bound proteins, the concentrations used in this study are within a range expected due to cumulative exposure in humans (Go et al. 2013a, b) and especially human lung tissues (Chandler et al. 2016). Additionally, the values are low relative to the LC50 of 32 μM in HeLa cells (Othumpangat et al. 2005), and no cell death or acute toxicity was observed in the present research. Our previous study with mouse fibroblasts showed that these concentrations of Cd disrupted the major cellular antioxidant thioredoxin redox system in association with perturbing actin cytoskeleton regulation (Go et al. 2013a, b). The functional consequences of such effects on lung physiology and pathophysiology are now becoming clear. Earlier studies in mice showed that Cd exposures in drinking water at 10 mg/L caused fatty liver (Go et al. 2015), and extension of these studies to lung research showed increased airway hyper-reactivity (Chandler et al. 2016) and potentiated inflammation and lethality of H1N1 influenza virus infection (Chandler et al, manuscript in preparation). The present study expands the spectrum of effects of relatively low-dose Cd to show stimulation of profibrotic responses in human lung cells and in vivo in a mouse model.
Cd affects multiple signaling pathways, yet the effects are pleiotropic and dependent on the dosage and exposure time. For example, Cd can trigger both apoptosis involving mitochondria and endoplasmic reticulum (ER) stress signaling, and anti-apoptotic survival responses caused by activation of proliferation cascades (Thevenod 2009). The competing signals lead to varied cell fates that include cell death, repair and survival, or malignant transformation. The complexity of Cd toxicity is also reflected in Cd-associated human lung diseases. Lung injury induced by Cd inhalation can evolve into two divergent outcomes, interstitial lung fibrosis or emphysema. The progression depends on the synthesis of connective tissue protein in experimental animals (Niewoehner and Hoidal 1982). HFL1 cells treated with a high dose of 30 μM Cd showed characteristics depicting potential mechanisms for emphysema; yet no effects was seen at 10 μM or less (Chambers et al. 1994). By contrast, our results showed a strong profibrotic response in HLF1 at a lower Cd dose. The maximal induction of SMAD activity as well as α-SMA was caused by 0.5 μM Cd (Figure 1, 2 and 3). Upregulation of other transcriptional activities occurred at higher concentrations (Table S1), consistent with differing dose-response characteristics for different signaling pathways. For instance, the non-linear Cd dose-response relationships may result from activation of cell repair mechanisms. Similarly, to help our understanding of variable responses of myofiroblast differentiation markers to different Cd dose, studies to examine exposure time-dependent effects specifically at 0.5 μM on transcriptional and translational responses of differentiation markers are warranted. We found that dose-response of α-SMA and FN was consistent after extending Cd exposure to 40 h (Supplementary Figure S1), yet the magnitude of response was smaller compared to 24 h exposure (Figure 2). This result could be associated with an adaptive cellular response after prolonged Cd exposure (Thevenod and Lee 2013). Therefore, Cd dosage and time exposures are critical factors in lung diseases, and more research is needed on Cd doses with different time exposures that are relevant to widespread environmental agents.
MTF-1 is an extensively studied TF for which responses differ in levels of TF activity and downstream gene expression. For example, the highest induction of MTF-1 activity was 3-fold at the medium dose while the MTF-1-controlled expression of MT was elevated maximally (11 fold) by 2 μM Cd (Figure 3 and 4). Similar findings have been previously reported showing only modest increase in the MTF-1 DNA binding activity (< 2 fold) with maximal induction of MT gene expression in mouse Hepa cells (Bittel et al. 1998; Smirnova et al. 2000) suggesting the possibility of involving other TF in addition to MTF-1. Likewise, the target genes of SMAD2/3/4 are often co-regulated by other pathways such as p53 and Wnt (Massague et al. 2005), resulting in strong SMAD2/3/4 up-regulation and modest gene up-regulation in normal proliferating fibroblast (Figure 3 and 4). Suppression of activity of four other TF further emphasizes that 0.5 to 2 μM Cd broadly impacts transcriptional regulation and a complex interplay of mechanistic pathways.
Mechanisms contributing to myofibroblast emergence are critical to understanding the pathogenesis of lung fibrosis. When fibroblasts experience mechanical stress (from a wound for example), they start to assemble stress fibers and become proto-myofibroblasts (Pellegrin and Mellor 2007). Tension, TGF-β and extracellular matrix (ECM) protein dynamics promote differentiation of proto-myofibroblasts into myofibroblasts by de novo expression of α-SMA. Expression of α-SMA is negligible in undifferentiated cells (Lomas et al. 2012) and therefore, α-SMA has been used as a key marker for myofibroblast differentiation. Incorporation of α-SMA into myofibroblast stress fibers results in stronger contractile power than normal stress fibers containing only β- and γ-cytoplasmic actin (Hinz et al. 2001). In lungs of idiopathic pulmonary fibrosis (IPF) patients, α-SMA is highly expressed in fibrotic foci together with active fibroblast proliferation, differentiation and production of ECM proteins. Thus, the present finding that 0.5 μM Cd caused a 2-fold increase in α-SMA expression and formation of stress fibers that contained high levels of α-SMA (Figure 1) focuses attention to the possibility that variation in dietary intake or absorption of low levels of Cd could be a critical, undefined variable in idiopathic pulmonary fibrosis. This is supported by results showing increased expression of α-SMA in lungs of mice exposed to levels of Cd that increase mouse lung Cd to levels found in humans (Figure 5). Importantly, at higher doses, the elevation of α-SMA was diminished, but stress fibers and a proto-myofibroblast phenotype was still evident (Figure 1, F-actin staining). The possibility that these two distinct phenotypes result from relatively independent signaling networks are depicted in Figure 6 and discussed below.
Figure 6.
A proposed scheme based on the current and previous studies (Go et al. 2013a, b): chronic low dose Cd exposure induces myofibroblast differentiation by disruption of actin cytoskeleton regulation and subcellular redox organization, stimulation of nuclear translocation of Trx1 potentially affecting activity of SMAD2/3/4 TF complex, and increasing expression of α-SMA, FN and collagen1α1 to stimulate differentiation of myofibroblast and further develop lung fibrosis.
Regardless of etiology, profibrotic signaling pathways are associated with oxidative stress (Jiang et al. 2014; Ramirez et al. 2007; Sueblinvong et al. 2016; Yang et al. 2016; Zhang et al. 2015). Stress fiber formation is regulated by pathways involving GTPase signaling and actin depolymerization (Pellegrin and Mellor 2007) which is affected by Cd-induced oxidative stress in mouse lung fibroblast (Go et al. 2013a) (Figure 6). Notably, oxidative stress disrupts actin dynamics by inactivating actin depolymerization factors, leading to stiff cytoskeleton (Samstag et al. 2013) and cytoskeletal reorganization (Fiaschi et al. 2006). In our previous study, we found that nuclear translocation of Trx1 by low dose Cd is dependent on actin cytoskeleton reorganization and potentiates NF-κB activation (Go et al. 2013a). Similarly, we observed an increased nuclear translocation of Trx1 from cytoplasm in response to Cd in HLF1 cells in the current study (Supplemental Fig S2). Although more studies are needed to determine the role of Trx1 in signaling for Cd-stimulated myofibroblast differentiation, e.g., manipulating expression level of Trx1 in HLF1 cells by knocking down or increasing expression, based on the data from the current study and previous studies, we have proposed a scheme for Cd-stimulated fibrosis showing that disruption of subcellular redox organization could be a critical factor for fibrosis involving alteration of subcellular localization of Trx1 by Cd exposure (Supplemental Fig S2, Figure 6).
Subcellular redox disruption possibly impacts transcriptional activity of SMAD2/3/4 complex. A previous study by Chen et al. showed that Trx1 directly interacts with SMAD3, and promotes phosphorylation and nuclear translocation of SMAD3 (Chen et al. 2013). In addition, Jiang et al showed that fibrosis mediated by TGF-β/SMAD activation involves NADPH oxidase-dependent redox signaling (Jiang et al. 2014). Accordingly, based on available data from previous studies and the results from the current study, we propose a potential scheme for lung fibrosis stimulation by chronic exposure to low-dose Cd as follows: Cd stimulates stress fiber formation, disrupts subcellular redox organization including an increased nuclear translocation of Trx1, and affects transcriptional activity of SMAD complex and subsequent gene expression to promote later fibrotic response such as differentiation of proto-myofibroblast to myofibroblast (Fig 6).
In summary, the present study of human lung fibroblasts and mouse lung in vivo shows that relatively low levels of Cd activate SMAD2/3/4 and stimulate profibrotic responses including increased α-SMA and ED-A FN, and F-actin. These characteristics of myofibroblast differentiation show that low level Cd has the potential to contribute to etiologies of chronic fibrotic conditions of the human lung. The result of an increased level of nuclear translocation of Trx1 by Cd exposure suggests that Trx1 could have a role in regulating SMAD2/3/4 TF activity and subsequent gene expression responses. Given the complex mechanisms, further studies of Cd exposures are needed in humans in the context of other risk factors.
Supplementary Material
Highlights.
Low-dose Cd stimulates differentiation of human lung fibroblast to myofibroblast
Cd-stimulated fibrosis signaling involves activation of SMAD transcription factor
Low-dose Cd intake in mice activates myofibroblast differentiation
Acknowledgments
Drs. Young-Mi Go and Dean P. Jones share equal senior authorship in this collaborative research.
Funding
This study was supported by NIEHS Grant R01 ES023485 (DPJ and YMG) and R21 ES025632 (DPJ and YMG).
Abbreviations
- αSMA
α-smooth muscle-actin
- Cd
cadmium
- ECM
extracellular matrix
- ED-A FN
Extra-Domain-A fibronection
- HFLF
human fetal lung fibroblast
- MT
metallothionein
- MTF-1
metal response element-binding transcription factor-1
- TF
transcription factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. Toxicological profile fore cadmium 2012 [Google Scholar]
- Bittel D, Dalton T, Samson SL, Gedamu L, Andrews GK. The DNA binding activity of metal response element-binding transcription factor-1 is activated in vivo and in vitro by zinc, but not by other transition metals. J Biol Chem. 1998;273:7127–7133. doi: 10.1074/jbc.273.12.7127. [DOI] [PubMed] [Google Scholar]
- Chambers RC, McAnulty RJ, Shock A, Campa JS, Newman Taylor AJ, Laurent GJ. Cadmium selectively inhibits fibroblast procollagen production and proliferation. Am J Physiol. 1994;267:L300–308. doi: 10.1152/ajplung.1994.267.3.L300. [DOI] [PubMed] [Google Scholar]
- Chandler JD, Wongtrakool C, Banton SA, Li S, Orr ML, Barr DB, Neujahr DC, Sutliff RL, Go YM, Jones DP. Low-dose oral cadmium increases airway reactivity and lung neuronal gene expression in mice. Physiol Rep. 2016:4. doi: 10.14814/phy2.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang W, Shen T, Qi R. Thioredoxin1 downregulates oxidized low-density lipoprotein-induced adhesion molecule expression via Smad3 protein. PLoS One. 2013;8:e76226. doi: 10.1371/journal.pone.0076226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- EFSA. Cadmium in food. The EFSA Journal. 2009;980:1–139. [Google Scholar]
- Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem. 2006;281:22983–22991. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- Go YM, Orr M, Jones DP. Actin cytoskeleton redox proteome oxidation by cadmium. Am J Physiol Lung Cell Mol Physiol. 2013a;305:L831–843. doi: 10.1152/ajplung.00203.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Orr M, Jones DP. Increased nuclear thioredoxin-1 potentiates cadmium-induced cytotoxicity. Toxicological sciences. 2013b;131:84–94. doi: 10.1093/toxsci/kfs271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Sutliff RL, Chandler JD, Khalidur R, Kang BY, Anania FA, Orr M, Hao L, Fowler BA, Jones DP. Low-Dose Cadmium Causes Metabolic and Genetic Dysregulation Associated With Fatty Liver Disease in Mice. Toxicol Sci. 2015;147:524–534. doi: 10.1093/toxsci/kfv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B. Response of the respiratory tract to cadmium 2000 [Google Scholar]
- Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Liu GS, Dusting GJ, Chan EC. NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox Biol. 2014;2:267–272. doi: 10.1016/j.redox.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Sengupta S, Chatterjee S, Mitra S, Bhattacharyya A. Cadmium induces lung inflammation independent of lung cell proliferation: a molecular approach. J Inflamm (Lond) 2009;6:19. doi: 10.1186/1476-9255-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas NJ, Watts KL, Akram KM, Forsyth NR, Spiteri MA. Idiopathic pulmonary fibrosis: immunohistochemical analysis provides fresh insights into lung tissue remodelling with implications for novel prognostic markers. Int J Clin Exp Pathol. 2012;5:58–71. [PMC free article] [PubMed] [Google Scholar]
- Mannino DM, Holguin F, Greves HM, Savage-Brown A, Stock AL, Jones RL. Urinary cadmium levels predict lower lung function in current and former smokers: data from the Third National Health and Nutrition Examination Survey. Thorax. 2004;59:194–198. doi: 10.1136/thorax.2003.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Niewoehner DE, Hoidal JR. Lung fibrosis and emphysema: divergent responses to a common injury? Science. 1982;217:359–360. doi: 10.1126/science.7089570. [DOI] [PubMed] [Google Scholar]
- Othumpangat S, Kashon M, Joseph P. Eukaryotic translation initiation factor 4E is a cellular target for toxicity and death due to exposure to cadmium chloride. J Biol Chem. 2005;280:25162–25169. doi: 10.1074/jbc.M414303200. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc Am Thorac Soc. 2006;3:383–388. doi: 10.1513/pats.200601-012TK. [DOI] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Ramadan B, Ritzenthaler JD, Rivera HN, Jones DP, Roman J. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L972–981. doi: 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstag Y, John I, Wabnitz GH. Cofilin: a redox sensitive mediator of actin dynamics during T-cell activation and migration. Immunol Rev. 2013;256:30–47. doi: 10.1111/imr.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Wang YF, Jayaraman L, Yang H, Massague J, Pavletich NP. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell. 1998;94:585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- Smirnova IV, Bittel DC, Ravindra R, Jiang H, Andrews GK. Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J Biol Chem. 2000;275:9377–9384. doi: 10.1074/jbc.275.13.9377. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Mills ST, Neujahr DC, Go YM, Jones DP, Guidot DM. Nuclear Thioredoxin-1 Overexpression Attenuates Alcohol-Mediated Nrf2 Signaling and Lung Fibrosis. Alcohol Clin Exp Res. 2016;40:1846–1856. doi: 10.1111/acer.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 2006;3:293–298. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- Thevenod F. Cadmium and cellular signaling cascades: to be or not to be? Toxicol Appl Pharmacol. 2009;238:221–239. doi: 10.1016/j.taap.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Thevenod F, Lee WK. Cadmium and cellular signaling cascades: interactions between cell death and survival pathways. Arch Toxicol. 2013;87:1743–1786. doi: 10.1007/s00204-013-1110-9. [DOI] [PubMed] [Google Scholar]
- Wong C, Rougier-Chapman EM, Frederick JP, Datto MB, Liberati NT, Li JM, Wang XF. Smad3-Smad4 and AP-1 complexes synergize in transcriptional activation of the c-Jun promoter by transforming growth factor beta. Mol Cell Biol. 1999;19:1821–1830. doi: 10.1128/mcb.19.3.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Kim SC, Kim KM, Jang CH, Cho SS, Kim SJ, Ku SK, Cho IJ, Ki SH. Isorhamnetin attenuates liver fibrosis by inhibiting TGF-beta/Smad signaling and relieving oxidative stress. Eur J Pharmacol. 2016;783:92–102. doi: 10.1016/j.ejphar.2016.04.042. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Liu YJ, Mao YF, Dong WW, Zhu XY, Jiang L. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-beta1 signaling. Clin Nutr. 2015;34:752–760. doi: 10.1016/j.clnu.2014.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.