Graphical abstract
Keywords: Sweet chestnut, Physicochemical, Functional, Antioxidant, Roasting
Abstract
Sweet chestnut (Castanea sativa Mill.) belongs to the family Fagaceae and sub family Castaneoideae. Bioactive components such as tannins are present in sweet chestnut in high proportion giving astringent bitter taste and reducing their palatability. Roasting reduces the anti-nutritional factors in chestnut. This study was conducted to compare the effects of pan and microwave roasting on physicochemical, functional, rheological and antioxidant properties of sweet chestnut. Antioxidant activity was determined using DPPH inhibition activity, reducing power, and total phenolic content. Structural analysis was carried out using FT-IR analysis. Protein, fat, and ash contents displayed insignificant (P > 0.05) variations. “L” value decreased from 90.66 to 81.43, whereas, “a” and “b” values increased from 0.02 to 0.90 and 11.99 to 20.5, respectively, upon roasting. Significant (P < 0.05) increase in water absorption capacity (1.32–3.39 g/g), oil absorption capacity (1.22–1.63 g/g), and antioxidant properties was observed following roasting. Flour obtained from roasted chestnuts exhibited a significant decrease in light transmittance, foaming, and pasting properties. Higher gelatinization temperatures and lower enthalpies were reported in microwave and pan roasted chestnut flours. Roasting also reduced the viscoelastic behavior of native sweet chestnut and changed the transmittance of identical functional groups as revealed by FT-IR analysis.
Introduction
Sweet chestnut (Castanea sativa Mill) belongs to the family Fagaceae and sub family Castaneoideae. Some species of this tree Castanea crenata, C. mollissima and C. dentata are distributed mainly in Asia particularly in China, Korea and Japan; in South Europe and in the U.S. [1]. Antolia in Turkey is known as the motherland and one of the oldest cultivation places of chestnut [2]. It has become a subject of increasing international interest because of enhanced consumption especially in the countries of Europe, Australia, New Zealand and the United States [3].
Sweet chestnut is nutritionally rich with high content of sugar (20–32%), starch (50–60%), dietary fibre (4–10%), high quality protein (4–7%), and low lipids (2–4%). It is rich in vitamin E, B complex vitamins and minerals such as potassium, phosphorous, and magnesium [4]. It is a good source of essential fatty acids [5], minerals [6], and vitamin C [3]. Besides it is a good source of antioxidants such as L-ascorbic acid, vitamin E, carotenoids and, phenolic compounds such as gallic and ellagic acids [7]. As oxidative stress is a common cause of chronic degenerative diseases, consumption of dietary antioxidants such as those present in chestnuts may prevent the damage of biomolecules like proteins and DNA from free radicals [8]. Recently, chestnuts have become important components of human diet due to their proven nutritional qualities and health benefits.
One of the important bioactive components present in sweet chestnut is the high proportion of tannins especially in the inner shell. Tannins are known for their astringent bitter taste that reduce the palatability of chestnuts. Roasting treatment prior to their consumption is a common practice to improve the color and flavor. The heat treatment received during roasting modifies the nutritional profile of chestnuts by increasing the antioxidant activity and reducing the anti-nutritional factors such as tannins [9]. Besides, roasting also improves the digestibility and shelf life of chestnuts. Microwave treatment is finding great applications in the field of food processing including drying, pasteurization, sterilization, thawing, tempering and baking of foods. The objective of this study was to characterize the changes produced by roasting on sweet chestnut. For this purpose, two methods were employed i.e. pan roasting and microwave roasting in order to make a comparison of the effect on nutritional, physicochemical, rheological and antioxidant properties.
Material and methods
Materials
Freshly harvested sweet chestnut sample was purchased from the local market of Srinagar, Jammu & Kashmir, India in the month of October from 2013 harvest. The sample collected was immediately put to analysis. It was divided into three lots one among which was not roasted at all (native). Rest two lots were pan roasted and microwave roasted, respectively, to compare the effect of these roasting methods on the properties of sweet chestnut. Reagents used in the present study were of analytical grade.
Sweet chestnut roasting
Pan roasting was performed using the heated pan maintained at 190 °C fitted with thermostat for automatic cut off, for about 280–300 s with constant stirring. The doneness was examined from the development of nutty aroma and changes in texture. For microwave roasting nuts (50 g) were put in a 125 mL glass beaker and placed in the center of the microwave oven (LG, Intellocook, 2450 MHz, 900 W) and roasted for 90–120 s at 900 W. Optimization was performed carefully for the pan and microwave roasting treatments. The roasted nut were cooled and dried to 10.0% moisture (dw).
Flour preparation
Chestnuts were chopped into small pieces after removing the outer shell and the inner thin papery skin. The pieces were then pulverized in a kitchen grinder (SUJATA, Mixer Grinder, New Delhi, India) and the flour was then sieved to obtain the flour of 60 mesh particle size (British Sieve Standards). The flour so prepared was then packed and sealed in polythene zip pouch bags and stored at 5 °C until further analysis.
Methods
Physicochemical properties
Proximate composition
Moisture was determined by gravimetric method (925.10), protein was determined by Kjeldhal method (920.87), fat was estimated by Soxhlet method (920.85) and ash was determined by incineration of the sample (923.03) following the standard methods of analysis AOAC [10]. Carbohydrate content was obtained by the difference (100 minus percentage moisture, protein, fat and ash content).
Color
Color of the flour was determined using Color Flex Spectrocolorimeter (Hunter lab colorimeter D-25, Hunter Associates Laboratory, Ruston, USA) after being standardized using Hunter lab color standards and their Hunter ‘L’ (lightness), ‘a’ (redness to greenness) and ‘b’ (yellowness to blueness) values were measured as described for wild arrowhead tuber starch [11].
Light transmittance
Light transmittance of sweet chestnut flour was determined following the method described for Indian kidney bean [12]. An aqueous flour suspension (1% dwb) was prepared by heating at 90 °C in a water bath for 30 min with constant stirring. The suspension was cooled for 1 h at 30 °C. The samples were stored for 5 days at 4 °C in a refrigerator, and transmittance was determined every 24 h by measuring absorbance at 640 nm against a water blank with a UV spectrophotometer (UV-Spectrophotometer, Model U-2900 2JI-0003, Hitachi, Japan).
Pasting properties
Pasting properties were measured using a Rapid Visco Analyser (RVA Starch Master TM, Newport Scientific, Warriewood, Australia) as described for wild arrowhead tuber starch [11]. An aqueous dispersion of flour – 14% moisture basis (10.7%, w/w; 28 g total weight) was equilibrated at 50 °C for 1 min, heated at the rate of 12.2 °C/min to 95 °C, held for 2.5 min, cooled to 50 °C at the rate of 11.8 °C/min and again held at 50 °C for 2 min. A constant paddle rotational speed (160g) was used throughout the entire analysis, except for rapid stirring at 960g for the first 10 s to disperse the sample.
Functional properties
Water absorption capacity (WAC) and oil absorption capacity (OAC)
These were determined as described earlier for wild arrowhead tuber starch [11]. A 2.5 g flour on dry weight basis (db) was mixed with 20 mL distilled water or mustard oil and then stirred for 30 min at 25 °C. The slurry was then centrifuged at 3000g for 10 min (5810R, Eppendorf, Hamburg, Germany) and the supernatant was decanted. The gain in weight was expressed as percentage of water/oil absorption capacity.
Foaming capacity (FC) and stability (FS)
Aqueous dispersions of flour (6% w/v db) were homogenized in a domestic blender SUJATA, Juicer mixer grinder) for 1 min at 10,000 rpm. Percent increase in volume of the flour dispersion was calculated as the foaming capacity [13]. The foam stability was determined by measuring the foam volume after allowing it to stand for one hour.
Thermal properties
The thermal properties were measured using a differential scanning calorimeter (DSC822, Mettler-Toledo, Switzerland) according to the method previously described for arrowhead flour [14]. A 3.5 mg sample was weighed into a 40 µL capacity aluminium pan and distilled water was added with the help of Hamilton micro syringe to make 70% slurry. Pans were hermetically sealed and allowed to stand for 1 h at room temperature before heating in DSC. The DSC was calibrated using indium and an empty aluminium pan was used as reference. Sample pans were heated at the rate of 10 °C/min from 20 to 200 °C and thermal parameters viz. Onset (To), peak (Tp), conclusion (Tc) temperature in °C and enthalpy (ΔH) in J/g were calculated from the DSC curves.
Antioxidant properties
Extraction for evaluation of antioxidant properties
Sample (2 g) was taken from each flour sample in a test tube and 20 mL of 95% methanol was added. The tubes were vortex-mixed at ambient temperature for 3 min and then transferred into beakers and covered with an aluminium foil. The mixture was allowed to stand undisturbed for 2–3 h followed by magnetic stirring for 1 h. The mixture was then centrifuged at 10,000g for 10 min at 4 °C in the centrifuge tubes. The resultant supernatant was filtered and used for determination of antioxidant properties.
DPPH (1,1-diphenyl-2-picrylhydrazyl) activity
The modified method of Brand-Williams [15] was followed for estimation of DPPH radical scavenging activity. Sample extract (80, 100, and 120 µL) was reacted with 3.9 mL of a 6 × 105 mol/L of DPPH solution. Absorbance (A) at 515 nm was read at 0 and 30 min using a methanol blank. Antioxidant activity was calculated as% discoloration.
Reducing power
It was determined from the capacity of the sample to convert Fe3+ into Fe2+ according to the method described earlier for wild edible mushrooms [16]. Sample extract (100, 150, and 200 µL) was mixed with phosphate buffer (2.5 mL) and 2.5 mL potassium ferricyanide was added followed by incubation at 50 °C. Trichloro-acetic acid solution (10 g/100 mL) was added to the mixture, which was then centrifuged at 10,000g for 10 min. The upper layer of solution (2.5 mL) was mixed with 2.5 mL deionised water and 0.5 mL ferric chloride. The absorbance of the mixture was measured at 700 nm. % reducing power was calculated from the formula:
Total phenolic content
It was determined in methanolic extracts by Folin-Ciocalteau method [17]. Extract (200 µL) was added to 1.5 mL freshly diluted (10-fold) Folin-Ciocalteu reagent. The mixture was allowed to equilibrate for 5 min and then mixed with 1.5 mL of sodium carbonate solution (60 g/L). After incubation at room temperature (25 °C) for 90 min, the absorbance of the mixture was read at 725 nm (Shimadzu, UV-1800, Tokyo, Japan). Acidified methanol was used as a blank. The results were expressed as g of gallic acid equivalents (g GAE) per gram of sample.
FT-IR spectroscopy
FTIR spectra of flour samples was measured using FTIR spectrometer system (Cary 630 FTIR, Agilent Technologies, USA) coupled to an ATR accessory as described for wild arrowhead tuber starch [11]. Analysis were carried out at room temperature, and spectra were acquired in the range of 3800–600 cm−1 at a resolution of 4 cm−1, using Resolution Pro software version 2.5.5 (Agilent Technologies, USA).
Rheological properties
Rheological properties of 6.00% w/v flour gels were determined using dynamic rheometer (MCR102, Anton Paar) following the method described for locust bean gum [18]. Flour gels were prepared in distilled water with constant stirring. Rheological measurements were performed using dynamic rheometer (MCR102, Anton Paar). Storage modulus (G′) and loss modulus (G″) were evaluated under frequency sweep of 0–100 Hz at constant strain within the linear viscoelastic range (γ = 1 Pa).
Statistical analysis
The data presented are mean of three observations ± standard deviation. An analysis of variance (ANOVA) at a significance level of 5% was done. Differences between means was measured using Duncan test using the commercial statistical package (SPSS, Inc, Chicago, IL, USA).
Results and discussion
Physicochemical properties of sweet chestnut
Proximate composition
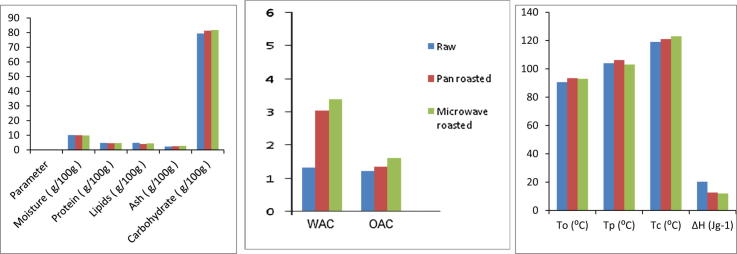
Native (unroasted) flour had a moisture content of 10.10 g/100 g and in roasted flours it was 9.97–9.83 g/100 g (Table 1). The heat treatment is likely to reduce the moisture content of roasted chestnut. Insignificant (P > 0.05) differences were observed in the proximate composition between raw and roasted samples. The protein content of 4.73 g/100 g was found in native flour and it reduced to 4.56 g/100 g in pan roasted and 4.64 g/100 g in microwave roasted sample. Lipid content of raw chestnut flour decreased insignificantly on roasting from 4.81 g/100 g to 4.00–4.40 g/100. Ash content of native flour was 2.3 g/100 g and it increased to 2.5 g/100 g (pan roasted chestnut flour) and 2.67 g/100 g (microwave roasted chestnut flour). Carbohydrate content of raw sweet chestnut flour was 79.39 g/100 g and it increased significantly (P < 0.05) on roasting. The increase was observed in the range of 81.30–81.79 g/100 g. A possible cause of the slight decrease in protein and lipid content of chestnut flours may be the loss some nitrogen containing volatile compounds during roasting. The results for proximate composition are in agreement with that of the dry roasted peanuts [19].
Table 1.
Physico-chemical properties of native and roasted sweet chestnut flours (n = 3).
| Parameter | Raw | Pan roasted | Microwave roasted |
|---|---|---|---|
| Moisture (g/100 g) | 10.10 ± 0.50a | 9.97 ± 0.58a | 9.83 ± 0.23a |
| Protein (g/100 g) | 4.73 ± 0.16a | 4.56 ± 0.05a | 4.64 ± 0.05a |
| Lipids (g/100 g) | 4.81 ± 0.27a | 4.00 ± 0.12a | 4.40 ± 0.10a |
| Ash (g/100 g) | 2.30 ± 0.30a | 2.50 ± 0.06a | 2.67 ± 0.12a |
| Carbohydrate (g/100 g) | 79.39 ± 0.50a | 81.30 ± 0.59b | 81.79 ± 0.65b |
| Color | |||
| L | 90.66 ± 0.14c | 88.14 ± 0.26b | 81.43 ± 0.71a |
| a | 0.02 ± 0.66a | 0.90 ± 0.98a | 0.83 ± 0.50a |
| b | 11.99 ± 0.08a | 14.48 ± 0.30b | 20.5 ± 0.50c |
Values expressed are mean ± standard deviation.
Means in the row with different superscript are significantly different at P ≤ 0.05
‘L’ = lightness, ‘a’ = redness to greenness and ‘b’ = yellowness to blueness.
Color
Color analysis of the flours was done to examine the effect of roasting method upon the enzymatic and non-enzymatic browning reactions that accelerate with the temperature increase while roasting. The color values of the native (unroasted) and roasted flours are given in Table 1. “L” value of native sweet chestnut flour reduced significantly (P < 0.05) on roasting from 90.66 to 88.14–81.43. The highest value of “L” in native flour (90.66) indicates lightness in comparison to roasted flours. “a” and “b” values also varied significantly (P < 0.05) between native and roasted flours. The lowest “a” value (0.02) was found in native flour which increased to 0.90 and 0.83 upon pan and microwave roasting, respectively. The higher positive “a” value of the roasted flours indicates the more redness in comparison to the native counterparts. Similarly, the “b” value of sweet chestnut flour increased with roasting from 11.99 to 20.5. This indicates that roasting significantly affected the appearance of the chestnut flours. It can be concluded that microwave roasting had a greater impact on the color attributes of the samples producing flour with least lightness and highest redness and blueness. Similar results were reported for color characteristic of roasted hazelnuts. The fall in “L”, and rise in “a” and “b” color values may be due to the browning reactions. These reactions produce brown pigment-melanoidins in advance stage of the browning reaction [20].
Light transmittance
Light transmittance of the native (unroasted) and roasted flours showed significant reduction (P < 0.05) during 96 h of storage from 0.87% to 0.65% (Table 2). It decreased from 0.60% to 0.45% and 0.70% to 0.40%, respectively, for pan and microwave roasted flours. Similar results in light transmittance were observed in Indian horse chestnut starches [21]. It has been reported that granule swelling, granule remnants, leached amylose and amylopectin and the molecular weights and chain-lengths of amylose and amylopectin ultimately lead to the development of turbidity and decreased light transmittance in starch pastes during refrigerated storage [22].
Table 2.
Light transmittance and pasting properties of native and roasted sweet chestnut (n = 3).
| Parameter | Raw | Pan roasted | Microwave roasted |
|---|---|---|---|
| Light transmittance (%) | |||
| 0 h | 0.87 ± 0.06cr | 0.60 ± 0.01cp | 0.70 ± 0.01dq |
| 24 h | 0.85 ± 0.08bcq | 0.55 ± 0.05bcp | 0.57 ± 0.06cp |
| 48 h | 0.73 ± 0.06abr | 0.57 ± 0.06bcq | 0.53 ± 0.06bcp |
| 72 h | 0.70 ± 0.01abq | 0.50 ± 0.01bcp | 0.47 ± 0.06abp |
| 96 h | 0.65 ± 0.05ar | 0.45 ± 0.05aq | 0.40 ± 0.01ap |
| Pasting properties | |||
| Peak viscosity (cP) | 1032.00 ± 2.00c | 208.00 ± 2.65a | 310.00 ± 2.00b |
| Trough viscosity (cP) | 905.33 ± 2.08c | 156.00 ± 1.00a | 258.00 ± 1.73b |
| Breakdown viscosity (cP) | 128.67 ± 1.53c | 53.33 ± 2.52a | 60.33 ± 2.08b |
| Final viscosity (cP) | 1318.00 ± 2.00c | 405.67 ± 1.53a | 491.67 ± 3.06b |
| Setback viscosity (cP) | 417.00 ± 2.65c | 246.67 ± 2.89a | 247.67 ± 2.52b |
| Pasting temperature (°C) | 71.40 ± 0.22b | 54.95 ± 1.30a | 55.10 ± 1.70a |
Values expressed are mean ± standard deviation.
Means in the row in pasting with different superscript are significantly different at P ≤ 0.05. In light transmittance means in the row and in the column with different superscript are significantly different at P ≤ 0.05
Pasting properties
The pasting properties of sweet chestnut flours are given in Table 2. Peak, trough and setback viscosity decreased significantly (P < 0.05) upon pan and microwave roasting. The highest values were observed in native (unroasted) flour which decreased upon heat treatment. Peak viscosity which is related to swelling of starch granules decreased from 1032.00 cP in native to 208.00 cP in pan roasted and 310.00 cP in microwave roasted flours. This may be attributed to the reduction in degree of polymerization of roasted starch, due to the cleavage of starch chains, thereby leading to decrease in paste viscosity [23]. Trough viscosity decreased from 905.33 cP to 156.00 cP and 258.00 cP and setback viscosities from 417.00 cP to 246.67 cP and 247.67 cP upon pan roasting and microwave roasting, respectively. Decrease in setback viscosity in roasted flours indicates that starch will retrograde slowly as it represents gelling tendency of starch. Breakdown viscosity (BDV) measures fragility of the granules, and it varied significantly (P < 0.05) from 60.33 to 128.67 cP. The highest BDV was found in native (128.67 cP) and the lowest in pan roasted (53.33 cP) flour. The lower BDV of roasted flours indicates that they are more resistant to shear thinning during cooking. Final viscosity and pasting temperature also varied significantly (P < 0.05) among native and roasted flours. The highest final viscosity was found in native flour (1318.00 cP) and the lowest in pan roasted (405.67 cP) flour. Similarly, the highest pasting temperature was found in native flour (71.40 °C) and the lowest in microwave roasted flour (55.10 °C). The low pasting temperature of roasted flours indicated their lower resistance toward swelling.
Functional properties
Water absorption capacity and oil absorption capacity
Water absorption capacity (WAC) varied significantly (P < 0.05) among the native (1.33 g/g) and roasted flours with an increase upon pan (3.04 g/g) and microwave (3.39 g/g) roasting, (Table 3). Proteins and carbohydrates contain hydrophilic groups that are responsible for water absorption of the flours. Gelatinisation of carbohydrates, partial denaturation of proteins and/or dissociation of major proteins into subunits upon heat treatment that increase the binding sites in roasted flours as compared to the native samples [24] are the possible reasons for increase in WAC. Enhanced WAC as a result of heating have also been reported for green gram, lentil and Bengal gram [25], benniseed and Bambara [26].
Table 3.
Functional and thermal properties of native and roasted sweet chestnut flours (n = 3).
| Parameter | Raw | Pan roasted | Microwave roasted |
|---|---|---|---|
| Functional properties | |||
| Water absorption capacity (g/g) | 1.32 ± 0.02a | 3.04 ± 0.02b | 3.39 ± 0.01c |
| Oil absorption capacity (g/g) | 1.22 ± 0.01a | 1.36 ± 0.01b | 1.63 ± 0.01c |
| Foaming capacity (%) | 5.00 ± 0.01b | 1.67 ± 0.58a | 1.30 ± 0.58a |
| Foaming stability (%) | 1.00 ± 0.01b | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Thermal properties | |||
| Onset temperature (To) (°C) | 90.46 ± 0.01a | 93.40 ± 0.01c | 92.98 ± 0.02b |
| Peak temperature (Tp) (°C) | 104.64 ± 0.01a | 106.19 ± 0.01b | 103.96 ± 0.01a |
| Conclusion temperature (Tc) (°C) | 119.08 ± 0.02a | 121.46 ± 0.07b | 123.94 ± 0.01c |
| ΔH (J g−1) | 20.22 ± 0.01b | 12.63 ± 0.01a | 11.94 ± 0.02a |
Values expressed are mean ± standard deviation.
Means in the row with different superscript are significantly different at P ≤ 0.05
Oil absorption capacity (OAC) was lower in native flour (1.22 g/g) as compared to pan and microwave roasted flours that absorbed 1.36 and 1.63 g of oil per gram of dry sample, respectively (Table 3). Increase in OAC may be due to the solubilization and dissociation of the proteins into subunits and subsequent increase in the number of polar as well as non-polar binding sites [25]. Variations in the type of non-polar side chains of protein subunits generated after roasting that possibly bind the hydrocarbon side chains of oil, may also explain the difference in oil binding capacity of the flours. These results agree with previous studies which reported increases in the OAC of cowpea [27].
Foaming capacity and stability
Foaming capacity and foaming stability of the raw and roasted sweet chestnut flours are given in Table 3. The FC of native, pan roasted and microwave roasted flour were 5.0%, 1.67% and 1.30%, respectively. It was observed that FC decreased significantly (P < 0.05) due to roasting. Similar results were reiterated in benniseed and bambara groundnut flours upon roasting [26]. Heat processing causes denaturation of several proteins in chestnut flour. It diminishes the nitrogen solubility of proteins which is expected to be the cause of lower foaming capacity of roasted samples [28]. Similar reason can be attributed to FS of chestnut flours that decreased significantly (P < 0.05) due to roasting. Results are in agreement with that of benniseed, bambara groundnut, and peanut flour [26].
Thermal properties
Thermal transition temperatures and enthalpy of gelatinisation of chestnut flours are given in Table 3. Samples revealed endothermic peaks with onset temperature (To) varying significantly (P < 0.05) among the samples from 90.46 to 93.40 °C. As To represents the melting of weakest crystallites [29], it implies the higher melting point of crystals from pan roasted flour. Peak temperature (Tp) and end-set temperature (Tc) varied from 103.96 to 106.19 °C and 119.08 to 123.94 °C, respectively. The highest peak temperature was reported in pan roasted chestnut flour which suggests the polysaccharide units to be well organised in it [30]. Comparatively higher gelatinisation temperatures in microwave and pan roasted chestnut flours may be due to the exposure to very high temperatures while roasting. Studies reported an increase in the amylose content of potato starch upon microwave treatment, and a positive correlation between amylose content and onset, peak and conclusion temperatures was shown in rice starch [31]. The gelatinization temperatures above 100 °C as reported in present study may be attributed to the formation of thermally stable orthorhombic polymorphs from monoclinic polymorphs in the sample as the temperature is raised above 90 °C while roasting. The former are metastable and require very high temperature to undergo melting [32]. Similar results were also reported in black gram flour from three different cultivars [13]. Higher gelatinization temperatures for the present study may also be attributed to the formation of amylose-lipid complexes in the chestnut flour samples. A higher profile of gelatinization temperatures in pan roasted chestnut flours is an indicative of the higher crystallinity in its starch component. This may be an implication of low temperature short time (LTST) treatment given to chestnuts during pan roasting. The melting enthalpies of the samples varied from 11.94 to 20.22 J g−1. Native chestnut flour had significantly (P < 0.05) higher enthalpy than the roasted samples. This indicates the decrease in heat flow in roasted flours which may be due to the pre-gelatinisation or the increase in the proportion of amylose content in its starch during heat treatment.
Antioxidant properties
DPPH activity
DPPH free radical scavenging activity for the native (unroasted) and roasted sweet chestnut flours was studied at the concentrations of 80 μL, 100 μL and 120 μL (Table 4). The % inhibition increased in the range of 38.95–88.71, 44.15–91.18 and 49.76–95.85 for 80 μL, 100 μL, and 120 μL, respectively on roasting. The lowest activity was found in native flour and the highest activity in microwave roasted flours. The % inhibition also increased in the range of 38.95–49.76, 59.40–79.88, and 88.71–95.85 in native, pan roasted and microwave roasted flours, respectively, with an increase in concentration from 80 to 120 μL. The enhanced antioxidant activity can be due to the formation melanoidins-non enzymatic browning products at the high temperatures [33]. Little millet also showed enhanced radical scavenging activity on roasting [34]. A significantly higher antioxidant activity was observed in the microwave roasted flours. It is assumed to be due to the high temperature short time (HTST) conditions employed during microwave roasting that supports the generation of more melanoidin pigments as compared to pan roasting.
Table 4.
Antioxidant properties of native and roasted sweet chestnut flours (n = 3).
| Concentration | Native | Pan roasted | Microwave roasted |
|---|---|---|---|
| DPPH activity (% inhibition) | |||
| 80 µL | 38.95 ± 0.54ap | 59.40 ± 1.22aq | 88.71 ± 1.05ar |
| 100 µL | 44.15 ± 1.84bq | 69.60 ± 0.99bq | 91.18 ± 0.57br |
| 120 µL | 49.76 ± 1.04cp | 79.88 ± 0.40cq | 95.85 ± 0.74cr |
| Reducing power (% reduction) | |||
| 100 µL | 64.96 ± 0.44ap | 66.99 ± 0.49aq | 70.42 ± 0.33ar |
| 150 µL | 68.02 ± 0.76bp | 73.14 ± 1.64bq | 77.50 ± 0.75br |
| 200 µL | 70.00 ± 0.77cp | 76.10 ± 0.92cq | 79.80 ± 0.65cr |
| Total phenolic content (g GAE/100g) | |||
| 500 μL | 1.60 ± 0.30a | 2.20 ± 0.05b | 3.30 ± 0.16c |
Values expressed are mean ± standard deviation.
Means in the row with different superscript are significantly different at P ≤ 0.05
Reducing power
The % reducing power for native (unroasted) and roasted flours was observed in the range of 64.96–70.42 (at the concentration of 80 μL), 68.02–77.50 (at the concentration of 100 μL) and 70.00–79.80 (at the concentration of 120 μL) (Table 4). Reducing power of the samples increased in all the chestnut (roasted and unroasted) samples upon increase in concentration (80–120 μL). The lowest reducing power was observed in native chestnuts and the values increased significantly upon roasting. Melanoidons that are produced during the roasting may lead to the increase in reducing power [33] of the roasted samples. The significantly (P < 0.05) higher reducing power of microwave roasted flours may be due to the presence of more reductones generated from the Maillard reaction. Antioxidant activity of barley flour has also been found to increase upon roasting. However, in present study it was observed that microwave roasting is a better method of chestnut processing as compared to pan roasting in terms of enhancing its bioactive potential [35].
Total phenolic content
The total phenolic content (TPC) of native and roasted chestnut flours are shown in Table 4. The TPC (g GAE/100 g) of sweet chestnut increased significantly (P < 0.05) upon roasting from 1.6 to 2.2 and 3.3 upon pan and microwave roasting, respectively. Heat treatment is known to cause the alteration in the chemical structure of certain molecules including the proteins that are associated with phenolic compounds. The increase in their proportion may result in the high TPC in plant foods after heat treatment [36]. Black-eyed peas displayed increase in total phenolics on heating [37]. Roasting is known to reduce the total tannins that are the phenolic compounds in chestnut with anti-nutritional properties. In spite of this, the increase in the TPC of roasted chestnuts can be explained on basis of the degradation of hydrolysable tannins upon heating to smaller phenolic compounds generated as a consequence. An increase in total phenolic compounds in lentils was also found upon sprouting, when the total tannins were observed to decrease considerably [38].
FT-IR spectroscopy
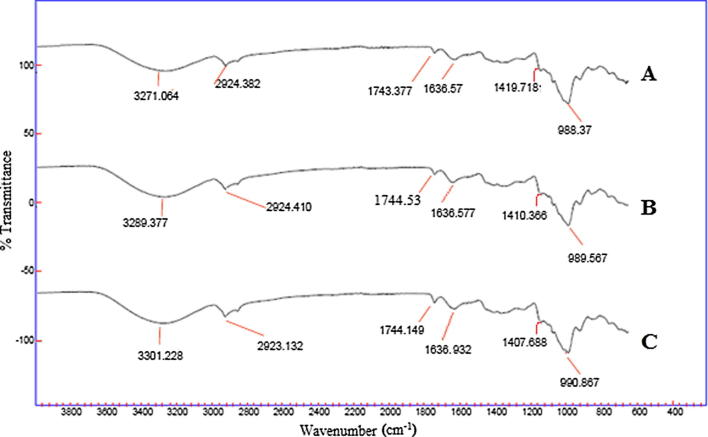
The FT-IR spectra of raw and roasted chestnut flours revealed similar band positions which indicate the presence of identical compounds in each sample (Fig. 1). The peaks were observed in the band width of 3500–3000, 1249–1731, 1429–1632, 1370–1350, and 1000–650 cm−1. These revealed the occurrence of hydroxyl (—OH), carboxylic acid (—COOH), acetyl (—COCH3), alkane (—C—H) and alkene ( C—H) functional groups, respectively. Prominent peaks were also observed close to 2924, 1637 and 1240 cm−1. These were ascribed to the presence of —C—H bonds, amine groups (—N—H) and carbonyl ( C O) bonds, respectively [39]. Raw, microwave and pan roasted samples exhibited variations in the percentage transmittance of identical functional groups. Although, negligible variations were observed between native and microwave roasted chestnut flours, yet the transmittance was very high in the pan roasted flour. In microwave roasted chestnut flour absorbance of carboxylic acids (3500–3000 cm−1) and esters (around 1744 cm−1) increased while that of amines (around 1636 cm−1) decreased slightly. This indicates the development of desirable nutty-flavor and rich aroma in microwave roasted chestnut flour. On the other hand, in pan roasted chestnut flour, an excessive decrease was observed in the absorbance of hydroxyl, alkane and alkene groups. This may be due to the reduction in the number of tannins while roasting. The decrease in amine groups of pan roasted sample is an indicative of the development of desirable flavor. However, the observed decrease in absorbance of carboxylic acid and esters can be due to the prolonged heat treatment as compared to microwave roasting which releases the volatiles and produces a blander product. Similar results were also reported in light roast almonds [39].
Fig. 1.
FT-IR spectroscopy of chestnut flours: (A) native chestnut flour; (B) microwave roasted chestnut flour; (C) pan roasted chestnut flour.
Rheological property
As shown in Fig. 2, the storage modulus (G′) and loss modulus (G″) of the chestnut flours raised as the frequency increased from 0 to 100 s−1. Storage modulus was dominant over the loss modulus throughout the frequency range applied. This indicated the flour dispersions to behave more like elastic gels. However, the flour dispersions of similar strength (6.0% w/v db) showed different oscillatory responses towards the applied stress. The rheological behaviour revealed a decreasing trend from native to pan roasted chestnut flours with intermediate values of G′ and G″ in microwave roasted flour. Thus, native flour (unroasted) exhibited the highest viscoelastic response. Roasting chestnuts using microwave and pan methods exposes them to a very high temperature for shorter and longer durations, respectively. This may cause the disruption of polymers into lower molecular weight chains that have lesser interjection points thereby decreasing the elastic behaviour of flour dispersions. It can also be due to the highly ordered structure in native flour which is responsible for increasing its viscoelastic response as reported by Idriss et al. [40] in wheat flour. Flours had the viscoelastic response resembling wheat flours and thus could be used to incorporate in the same for the purpose of generating value added products.
Fig. 2.
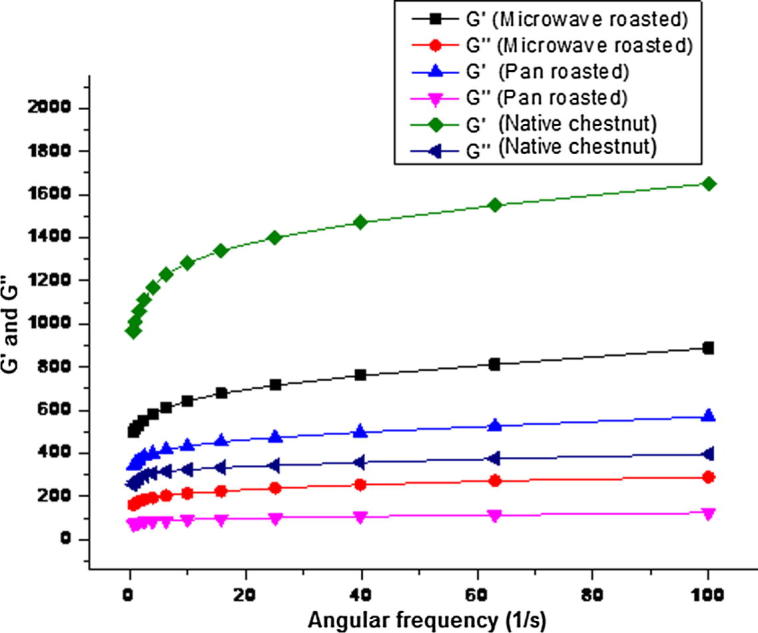
Rheological property of chestnut flours.
Conclusions
The present study revealed that roasting in general had no affect on the proximate composition. The physicochemical properties like the light transmittance and pasting properties reduced significantly upon roasting. Low setback viscosity of roasted sweet chestnut flour suggests they can be used in products where starch stability is desired at low temperature. Roasting increased the WAC and OAC of chestnut flours making them potentially useful in flavor retention and improvement of palatability. Roasted flours had higher TPC and antioxidant activity compared to the native counterparts, making them potentially beneficial health promoting foods. Microwave roasted chestnut flours were observed having superior nutritional quality in terms of their antioxidant potential. The gelatinization temperatures were higher in roasted flours while as their viscoelastic behavior was lower as compared to the native flour. Roasting also improved the flavor of chestnuts with microwave roasted chestnuts expressing better aroma as compared to the pan roasted. Pan and microwave roasting methods can be industrialized for production of chestnut fruits with better nutritional and aromatic qualities. Moreover future work is needed to assess the effect of pan and microwave roasting on the volatile components of chestnuts.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Pereira-Lorenzo S., Ramos-Cabrer A.M., Díaz-Hernández M.B., Ciordia-Ara M., Rios-Mesa D. Chemical composition of chestnut cultivars from Spain. Sci Hort. 2006;107(3):306–314. [Google Scholar]
- 2.Erturk U., Cevriye M., Arif S. Chemical composition of fruits of some important chestnut cultivars. Agric, Agrib Biotechnol. 2006;49(2):183–188. [Google Scholar]
- 3.Gold M.A., Cernusca M.M., Godsey L. University of Missouri Center for Agroforestry; 2005. Chestnut market analysis producers perspective; p. 34. [Google Scholar]
- 4.Chenlo F., Moreira R., Pereira G., Silva C.C. Evaluation of the rheological behaviour of chestnut (Castanea sativa mill) flour pastes as function of water content and temperature. Electron J Environ Agric Food Chem. 2007;6(2):1794–1802. [Google Scholar]
- 5.Borges O.B., Carvalho J.S., Corriera P.R., Silva A.P. Lipid and fatty acid profiles of Castanea sativa Mill. Chestnuts of 17 native Portuguese cultivars. J Food Compos Anal. 2007;20:80–89. [Google Scholar]
- 6.Kunsch U., Scharer H., Conedera M., Sassella A., Jermini M., Jelmini G. Quality assessment of chestnut fruits. Acta Hort. 1999;494:119–127. [Google Scholar]
- 7.Vasconcelos M.C.B.M., Bennett R.N., Rosa E.A.S., Ferreira-Cardoso J.V. Primary and secondary metabolite composition of kernels from three cultivars of Portuguese chestnut (Castanea sativa Mill.) at different stages of industrial transformation. J Agric Food Chem. 2007;55:3508–3516. doi: 10.1021/jf0629080. [DOI] [PubMed] [Google Scholar]
- 8.Naczk M., Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Chang S.K., Alasalvar C., Bolling B.W., Shahidi F. Nuts and their co-products: the impact of processing (roasting) on phenolics, bioavailability, and health benefits – a comprehensive review. J Funct Foods. 2016;26:88–122. [Google Scholar]
- 10.AOAC . 15th ed. Association of Official Analytical Chemists; 1990. Official methods of analysis. [Google Scholar]
- 11.Wani A.A., Wani I.A., Hussain P.R., Gani A., Wani T.A., Masoodi F.A. Physicochemical properties of native and γ-irradiated wild arrowhead (Sagittaria sagittifolia L.) tuber starch. Int J Biol Macromol. 2015;77:360–368. doi: 10.1016/j.ijbiomac.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Wani I.A., Sogi D.S., Gill B.S. Physicochemical properties of acetylated starches from some Indian kidney bean cultivars. Int J Food Sci Technol. 2012;47:1993–1999. doi: 10.1007/s13197-014-1480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wani I.A., Sogi D.S., Gill B.S. Physicochemical and functional properties of flours from three Black gram (Phaseolus mungo L.) cultivars. Int J Food Sci Technol. 2013;48:771–777. [Google Scholar]
- 14.Wani I.A., Gani A., Tariq A., Sharma P., Masoodi F.A., Wani H.M. Effect of roasting on physicochemical, functional and antioxidant properties of arrowhead (Sagittaria sagittifolia L.) flour. Food Chem. 2016;197(4):345–352. doi: 10.1016/j.foodchem.2015.10.125. [DOI] [PubMed] [Google Scholar]
- 15.Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical 403 method to evaluate antioxidant activity. Lebensm-Wiss Technol. 1995;28:25–30. [Google Scholar]
- 16.Barros L., Ferrira M.J., Queiros B., Ferreira I.C.F.R., Baptista P. Total phenols, ascorbic acid, β carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007;100:413–419. [Google Scholar]
- 17.Singleton V.L., Rossi J.A. Colorimetry of total phenolic with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic. 1965;1965(16):144–158. [Google Scholar]
- 18.Dakia P.A., Blecker C., Robert C., Wathelet B., Paquot M. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hydrocoll. 2008;22:807–818. [Google Scholar]
- 19.Abayomi P., Isaac A., Ayodele O. Effects of processing conditions and packaging materials on the quality attributes of dry roasted peanuts. J Sci Food Agric. 2002;82:1465–1471. [Google Scholar]
- 20.Sumnu G., Uysal S., Sahin S. Optimization of microwave–infrared roasting of hazelnut. J Food Eng. 2009;90:255–261. [Google Scholar]
- 21.Wani I.A., Jabeen M., Geelani H., Masoodi F.A., Saba I., Muzaffar S. Effect of gamma irradiation on physicochemical properties of Indian Horse Chestnut (Aesculus indica) starch. Food Hydrocoll. 2014;35:253–263. [Google Scholar]
- 22.Perera C., Hoover R. Influence of hydroxylpropylation on retrogradation properties of native, defatted and heat-moisture treated potato starches. Food Chem. 1999;64:361–375. [Google Scholar]
- 23.Liu T., Ying M.Y., Xue S., Shi J. Modifications of structure and physicochemical properties of maize starch by gamma irradiation treatments. LWT- Food Sci Technol. 2012;46:156–163. [Google Scholar]
- 24.Akubor P.I., Isolokwu P.C., Ugbane O., Onimawo I.A. Proximate composition and functional properties of African breadfruit kernel and flour blends. Food Res Int. 2000;33:707–712. [Google Scholar]
- 25.Ghavidel R.A., Prakash J. Effect of germination and dehulling on functional properties of legume flours. J Sci Food Agric. 2006;86:1189–1195. [Google Scholar]
- 26.Yusuf A.A., Ayedun H., Sanni L.O. Chemical composition and functional properties of raw and roasted Nigerian benniseed (Sesamum indicum) and Bambara groundnut (Vigna subterranean) Food Chem. 2008;111:277–282. doi: 10.1016/j.foodchem.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Padmashree T.S., Vijayalakshmi L., Puttaraj S. Effect of traditional processing on the functional properties of Cowpea (Vigna cajan) flour. J Food Sci Technol. 1987;24:221–226. [Google Scholar]
- 28.Yasumatsu K., Sawada K., Moritaka S., Mikasi M., Toda T., Tshi K. Whipping and emulsifying properties of soybean products. Agric Biochem. 1972;36:719–727. [Google Scholar]
- 29.Nakazawa Y., Wang Y.J. Acid hydrolysis of native and annealed starches and branched structures of their Naegeli dextrins. Carbohydr Res. 2003;338:2871–2882. doi: 10.1016/j.carres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Jindal M., Kumar V., Rana V., Tiwary A.K. Exploring potential new gum source Aegle marmelos for food and pharmaceuticals. Ind Crops Prod. 2013;45:312–318. [Google Scholar]
- 31.Varavinit S., Shobsngob S., Varanyanond W., Chinachoti P., Naivikul O. Effect of amylose content on gelatinization, retrogradation and pasting properties of flours from different cultivars of Thai Rice. Starch/Stärke. 2003;55:410–415. [Google Scholar]
- 32.Seo T.R., Kim J.Y., Lim S.T. Preparation and characterization of crystalline complexes between amylose and C18 fatty acids. LWT-Food Sci Technol. 2015;64:889–897. [Google Scholar]
- 33.Woffenden H.M., Ames J.M., Chandra S., Anese M., Nicoli C. Effect of kilning on the antioxidant and pro-oxidant activities on pale malt. J Agric Food Chem. 2002;50:4925–4933. doi: 10.1021/jf020312g. [DOI] [PubMed] [Google Scholar]
- 34.Pradeep S.R., Guha M. Effect of processing methods on the nutraceutical and antioxidant properties of little millet (Panicum sumatrense) extracts. Food Chem. 2011;126:1643–1647. doi: 10.1016/j.foodchem.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P., Gujral H.S. Effect of sand roasting and microwave cooking on antioxidant properties of barley. Food Res Int. 2011;44:235–240. [Google Scholar]
- 36.Lemos M.R.B., Siqueira E.D., Arruda S.F., Zambiazi R.C. The effect of roasting on the phenolic compounds and antioxidant potential of baru nuts. Food Res Int. 2012;48:592–597. [Google Scholar]
- 37.Boateng J., Verghese M., Walker L.T., Ogutu S. Effect of processing on antioxidant content in selected dry beans (Phaseolus spp. L.) LWT – Food Sci Technol. 2008;41:1541–1547. [Google Scholar]
- 38.Fouad A.A., Rehab F.M.A. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris medik.) sprouts. Acta Sci Pol Technol. 2015;14(3):233–246. doi: 10.17306/J.AFS.2015.3.25. [DOI] [PubMed] [Google Scholar]
- 39.Ng S., Lasekan O., Muhammad K., Sulaiman R., Hussain N. Effect of roasting conditions on color development and Fourier transform infrared spectroscopy (FTIR-ATR) analysis of Malaysian-grown tropical almond nuts (Terminalia catappa L.) Chem Cent J. 2014;8:1–11. doi: 10.1186/s13065-014-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Idriss M., Abdelrahman R.A., Senge B. Dynamic rheological properties of chickpea and wheat flour Dough’s. J Appl Sci. 2011;11(19):3405–3412. [Google Scholar]