Abstract
Increased perception of visceral stimuli is a key feature of Irritable Bowel Syndrome (IBS). While altered resting-state functional connectivity (rsFC) has been also reported in IBS, the relationship between visceral hypersensitivity and aberrant rsFC is unknown. We therefore assessed rsFC within the salience, sensorimotor and default mode networks in patients with and without visceral hypersensitivity and in healthy controls (HCs).
An exploratory resting-state functional magnetic resonance imaging study was performed in 41 women with IBS and 20 HCs. Group independent component analysis was used to derive intrinsic brain networks. Rectal thresholds were determined and patients were subdivided into groups with increased (hypersensitive IBS, N = 21) or normal (normosensitive IBS, N = 20) visceral sensitivity. Between-group comparisons of rsFC were carried-out using region-of-interest analyses and peak rsFC values were extracted for correlational analyses.
Relative to normosensitive IBS, hypersensitive patients showed increased positive rsFC of pregenual anterior cingulate cortex and thalamus within the salience network and of posterior insula within the sensorimotor network. When compared to both hypersensitive IBS and HCs, normosensitive IBS showed decreased positive rsFC of amygdala and decreased negative rsFC in dorsal anterior insula within the DMN. DMN and sensorimotor network rsFC were associated with rectal perception thresholds, and rsFC in posterior insula was correlated with reported symptom severity in IBS.
Our exploratory findings suggest that visceral sensitivity in IBS is related to changes in FC within resting-state networks associated with interoception, salience and sensory processing. These alterations may play an important role in hypervigilance and hyperalgesia in IBS.
Keywords: Resting-state fMRI, Visceral hypersensitivity, Irritable Bowel Syndrome, Functional connectivity, Default mode network, Salience network, Sensorimotor network
Graphical abstract
Highlights
-
•
Functional connectivity (FC) was compared between hyper- and normosensitive IBS.
-
•
Hypersensitive IBS showed enhanced salience and sensorimotor network FC.
-
•
Normosensitive IBS had decreased amygdala and anterior insula FC within the DMN.
-
•
Changes in FC were associated with visceral sensitivity and symptom severity.
-
•
Altered FC may play a key role in hypervigilance and hyperalgesia in IBS.
1. Introduction
Irritable Bowel Syndrome (IBS) is a chronic visceral pain syndrome defined by recurrent abdominal pain associated with altered bowel habits with no detectable organic causes. In the absence of a reliable biomarker (Drossman, 2016, Enck et al., 2016, Longstreth et al., 2006), current concepts support an important role of enhanced visceral perception sensitivity (“visceral hypersensitivity”) within a dysfunctional brain-gut axis (Drossman, 2016, Enck et al., 2016, Farmer and Aziz, 2013, Mayer et al., 2015b). Functional magnetic resonance imaging (fMRI) studies have made a substantial contribution to elucidating central mechanisms involved in normal and altered processing of visceral stimuli, including the perception of visceral pain. They have provided important insights into functional alterations in response to experimentally induced pain in IBS, involving brain regions of visceral afferent processing, emotional arousal and endogenous pain modulation (Tillisch et al., 2011). The investigation of spontaneous, stimulus-independent brain activation and connectivity of intrinsic brain networks by resting-state fMRI (rsfMRI) extends knowledge derived from studies involving experimental pain models (Napadow and Harris, 2014). Existing rsfMRI studies in functional gastrointestinal disorders (FGIDs) support altered functional connectivity (FC), with most consistently reported alterations in the default mode network (DMN), salience and sensorimotor networks (Lee et al., 2016, Mayer et al., 2015a).
Although visceral hypersensitivity is considered a key feature in the pathophysiology of FGIDs (Azpiroz et al., 2007, Keszthelyi et al., 2012), a significant proportion of patients have visceral pain thresholds within the normal range (Bouin et al., 2002, Lee et al., 2006, Sabate et al., 2008). Despite the absence of perceptual hypersensitivity to rectal distension, these patients exhibit chronic gastrointestinal (GI) symptoms and demonstrate alterations in brain responses to experimental pain stimuli (Elsenbruch et al., 2010a, Elsenbruch et al., 2010b, Icenhour et al., 2015). Even though there is evidence suggesting differences between hypersensitive and normosensitive patients in response to painful stimuli (Larsson et al., 2012, Van Oudenhove et al., 2010), the relation between altered FC of brain networks and visceral sensitivity remains unknown. Using a data-driven approach (independent component analysis; ICA), the current exploratory rsfMRI study aimed to address differences within the DMN, salience and sensorimotor networks, as the intrinsic brain networks most consistently exhibiting alterations in IBS, in a sample of hyper- and normosensitive patients and healthy controls (HCs). IBS subgroups were subdivided based on sensory thresholding performed subsequent to rsfMRI by means of rectal distensions with a balloon catheter placed before scanning. We hypothesized alterations in FC within these networks to be related to visceral sensitivity in IBS, as evidenced by distinct changes in both hyper- and normosensitive patients. Specifically, we tested for group differences in FC in insular and cingulate subregions, thalamus, and amygdala, as brain regions consistently reported to be activated by visceral stimulation (Tillisch et al., 2011). In addition, we addressed associations between changes in FC, visceral sensitivity, GI symptom severity and emotional disturbances in IBS.
2. Materials and methods
2.1. Participants
In total, 44 right-handed female IBS patients fulfilling Rome III diagnostic criteria were referred from primary care units and 20 age-matched, right-handed female HCs were recruited by local advertisement to participate in this fMRI study. Participants underwent a screening procedure including a standard clinical examination by a trained gastroenterologist to exclude organic GI diseases. In all patient, standard laboratory examination (minimum: hemoglobin, white blood cell count, C-reactive protein) and clinical examinations were performed before inclusion. Celiac disease was excluded by transglutaminase antibodies and f-calprotectin was used to screen for inflammatory bowel disease (IBD). Patients kept a gastrointestinal symptom diary for 2 weeks and were evaluated in terms of GI symptoms and alarm symptoms. Additional specific testing was performed when appropriate, e.g. colonoscopy when considered relevant for the exclusion of microscopic colitis. Lactose intolerance and bile acid malabsorption were excluded when appropriate. Further exclusion criteria were metabolic, neurological or severe psychiatric disorders, intake of nicotine or centrally acting medication, claustrophobia, pacemaker, large tattoos and metal implants in the brain. In HCs, a medical history of GI disturbances or complaints was exclusionary. All participants gave informed written consent and the Regional Research Committee for Ethical Issues at the Faculty of Health Sciences, Linköping, Sweden, approved the study. HCs received a monetary compensation of 1000 Swedish kronor (approx. 105 €).
2.2. Study protocol
2.2.1. Resting-state fMRI data acquisition
All participants were tested between 8 a.m. and 5 p.m. and temporal overlap with menses was avoided. Participants were asked to cease medication and avoid alcohol consumption for at least 24 h and fast for at least 4 h before the experiment. After arrival, a rectal balloon catheter consisting of a noncompliant polyethylene bag (maximal volume 520 mL) attached to a polyethylene tube was placed according to a standard clinical procedure. Balloon placement before scanning was performed for patient convenience, avoiding effects of negative expectations regarding the placement procedure. Participants were given several minutes to habituate to the catheter before they were placed in the MR scanner and underwent a 5-minute adjustment phase to the scanner environment, during which no scanning was performed. Subsequent to this habituation phase, an eyes closed resting-state functional brain scan was acquired. MRI scanning was performed on a Philips Achieva 1.5 T whole-body MR scanner (Philips Healthcare, Best, The Netherlands) equipped with an 8-channel head coil, located at the Center of Medical Image Science and Visualization at the Linköping University Hospital in Linköping, Sweden. A blood oxygen level dependent (BOLD) sensitive gradient echo, echo planar imaging sequence that effectively covered the whole brain was applied with the following acquisition parameters employed: Repetition time (TR) = 3 s; Echo time (TE) = 40 ms; flip angle (FA) = 90°; voxel size 3x3x3 mm3; slice thickness = 3 mm; gap = 0.5 mm; number of slices = 35; scan time = 10 min. During scanning, participants were instructed to lie still with their eyes closed. Data from three IBS patients were excluded from further analyses due to intolerance of the MRI procedure (i.e. claustrophobia), resulting in a final sample of 41 IBS patients and 20 HCs, who reported no adverse effects of the fMRI measurement or balloon placement based on self-report, as assessed at the conclusion of rsfMRI. After the fMRI scan, participants were prompted to rate intensity and unpleasantness of currently experienced GI symptoms on scales ranging from 0 to 10 with 0 indicating no intensity/unpleasantness and 10 defined as very high intensity/unpleasantness.
2.2.2. Determination of perceptual thresholds
Following rsfMRI, visceral perceptual thresholds as measures of visceral sensitivity were determined with an electronic barostat (Dual Drive Barostat, Distender series II; G & J Electronics Inc., Toronto, ON, Canada). Specifically, intermittent phasic isobaric rectal balloon distensions of 30 seconds durations were delivered, and visceral sensitivity was assessed using an ascending method of limits with pressure increments of 5 mm Hg, as previously described (Larsson et al., 2012). Subjects were prompted to rate each sensation on a 4-point scale labeled 0 = no sensation, 1 = first/some sensation, 2 = urge to defecate and 3 = maximal tolerable distension pressure. Based on the lower range of maximal tolerable pressures in HC (mean pressure 55 mm Hg; range: 40–70 mm Hg), IBS patients were classified as either normosensitive (N = 20, mean pressure 47.75 mm Hg; range: 40–70 mm Hg) or hypersensitive (N = 21, mean pressure 29.52 mm Hg; range: 20–35 mm Hg) with no overlap in maximal tolerable distension pressures between hypersensitive IBS and HCs. This classification procedure was previously implemented in the few existing studies comparing hypersensitive and normosensitive IBS (Kuiken et al., 2005, Larsson et al., 2012). The lower range of maximal tolerable pressures in HCs is well in accordance with previously published maximal tolerable pressure volumes from visceral sensitivity testing assessed with comparable methodology in healthy women (Sloots et al., 2000).
Subsequent to rsfMRI and the thresholding procedure, a subset of participants included in the current study underwent an fMRI paradigm to investigate group-differences in cerebral responses to the expectation and presentation of standardized rectal distensions. Data from this fMRI study have previously been published (Larsson et al., 2012) and are not addressed here.
2.3. Questionnaires
2.3.1. Hospital Anxiety and Depression Scale (HADS)
In all participants, the Hospital Anxiety and Depression Scale (HADS) was used to evaluate levels of anxiety and depression (Zigmond and Snaith, 1983). HADS consists of seven items addressing states of anxiety and depression, respectively, which are scored on a 4-point scale (0–3) with each sum score ranging from 0 to 21. Cut-off scores on each subscale are defined as ≥ 8 for suspicious and ≥ 11 for definite caseness. HADS has been validated in the general population as well as well in somatic, psychiatric and primary care patients (Bjelland et al., 2002).
2.3.2. IBS Severity Scoring System (IBS-SSS)
In patients, the IBS Severity Scoring System (IBS-SSS) was used to assess GI symptom severity (Francis et al., 1997). The severity of abdominal pain, distension, stool frequency and consistency and interference of symptoms with daily life are evaluated on 0–100 mm visual analogue scales (VAS). Sum scores range from 0 to 500 with mild cases defined as scores between 75 and 175, moderate severity as scores between 175 and 300 and severe cases of IBS as scores above 300.
2.3.3. Visceral Sensitivity Index (VSI)
The Visceral Sensitivity Index (VSI) was utilized to evaluate GI symptom-specific anxiety in patients, which is considered to play a key role in the pathophysiology and in health-related outcomes in IBS (Labus et al., 2004). The 15-item questionnaire evaluates cognitive, emotional and behavioral responses to fear of GI sensations, symptoms and the context in which they are experienced. Items are scored on a reversed 6-point scale ranging from 0 to 5 with sum scores between 0 and 75 and higher scores indicating more severe GI-specific anxiety.
2.4. Resting-state fMRI data preprocessing
Functional imaging data were reconstructed on the scanner. All participants' images were separately realigned using the SPM-based toolbox INRIAlign (Freire et al., 2002, Freire and Mangin, 2001) and the translation and rotation correction parameters were individually examined to exclude significant head motion larger than 1 voxel in any direction. Spatial normalization into Montreal Neurological Institute (MNI) space was initially performed on the mean functional image volume for each participant, and these normalization parameters were then applied to each respective functional image set. The normalized images were smoothed with an 8 mm FWHM Gaussian kernel. All preprocessing steps were performed using SPM8 (Wellcome Trust Centre for Neuroimaging, UCL, London, UK) implemented in MATLAB R2015b (Mathworks, Natick, MA, USA).
2.5. Independent component analysis
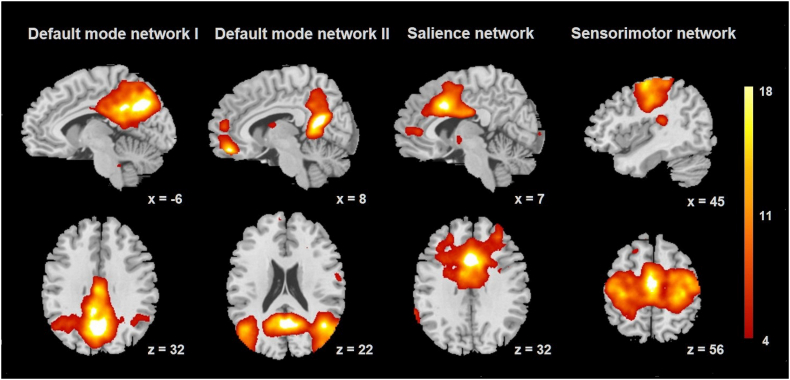
Functional connectivity was calculated using a group independent component analysis (ICA) algorithm (Calhoun et al., 2001), as implemented in the group independent component analysis of fMRI toolbox (GIFT v4.0a; http://mialab.mrn.org/software/gift/). A single ICA analysis was performed to identify spatially independent components across all 61 participants, with back reconstruction of single-subject spatial maps and time courses from the raw data (Erhardt et al., 2011). To ensure reliability of the ICA algorithm and robustness of ICA results, 500 iterations were performed using ICASSO (Himberg et al., 2004, Li et al., 2007). Twenty-four spatially independent components were estimated using the Infomax algorithm, where the number of components in the data was determined using the minimum description length (MDL) criteria adjusted to account for correlated samples (Li et al., 2007). These 24 components were individually back-reconstructed for each subject. Group-level, one-sample t-statistic images were visually inspected, and components representing the DMN, salience and sensorimotor networks were defined using spatial regression with templates provided by Smith et al. (2009) for further analyses. The results of spatial regression identified one component most strongly related to the salience network, one component representative of the sensorimotor network, and two components representing the DMN, a decomposition frequently observed (Biswal et al., 2010, Damoiseaux et al., 2006, Laird et al., 2011, Uddin et al., 2009). Within the full sample, the DMN components included precuneus, posterior cingulate cortex (PCC), medial prefrontal cortex and parahippocampus. The component identified as the salience network encompassed ACC and anterior midcingulate cortex, bilateral anterior insula and thalamus. The sensorimotor network comprised primary somatosensory and motor cortices and supplementary motor area (SMA). Results from voxel-wise one sample-t-tests representing the regional strength of functional connectivity within these components were created across the whole sample, as visualized in Fig. 1. Both the choice of preprocessing steps and group ICA analytic procedures were performed in accordance with the best practices laid out by Allen et al. (2011) for the GIFT group ICA toolbox.
Fig. 1.
Networks derived from independent component analysis. Results from group-level one sample t-tests for visualization of default mode network, salience network and sensorimotor network, defined using spatial regression with templates provided by Smith et al. (2009). Color bar indicates t-scores. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Between-group differences in functional connectivity within the networks of interest were addressed in independent sample t-tests accomplished in SPM8. Region-of-interest (ROI) analyses with small volume correction were conducted using a priori defined ROIs. ROIs were selected based on both, previous findings on neural changes in response to pain stimuli related to visceral sensitivity in FGID (Larsson et al., 2012, Van Oudenhove et al., 2010) and most consistently reported task-based and resting-state functional alterations in patients, particularly involving brain regions related to visceral afferent processing, emotional arousal and endogenous pain modulation (Mayer et al., 2015a, Tillisch et al., 2011). Of note, ROIs were chosen independent of their presence within the previously defined networks of interest in this exploratory approach, taking also negative findings into consideration. ROIs were custom-made as previously described (Larsson et al., 2012) and included amygdala, insula (dorsal and ventral anterior, mid and posterior division), anterior cingulate cortex (ACC; pregenual (pACC) and subgenual ACC (sgACC)), anterior midcingulate cortex (aMCC) and thalamus. Alpha-level for accepting statistical significance was set at pFWE < 0.05 with Family Wise Error (FWE) correction for multiple testing. No correction for number of groups, ROIs or networks tested was applied in this exploratory analysis. Results from ROI analyses are given as MNI (Montreal Neurological Institute) coordinates. For ROIs showing significant between-group differences, peak FC values for each participant were extracted and entered into correlational analyses with visceral thresholds, clinical characteristics and psychological variables as described below.
2.6. Statistical analyses of non-fMRI data
Statistical analyses of non-fMRI data were performed with IBM SPSS Statistics 23 (IBM Corporation, Armonk, NY, USA). As the Shapiro Wilk test revealed non-normality of threshold data, nonparametric tests were implemented for all analyses. Comparisons of IBS subgroups and healthy controls with respect to age and HADS anxiety and depression scores were addressed using Kruskal-Wallis test followed by post-hoc Mann-Whitney U tests with Bonferroni correction for multiple comparisons. Differences between hyper- and normosensitive IBS regarding IBS-SSS and VSI scores were accomplished with Mann-Whitney U tests. Alpha-level for all statistical tests was set at p < 0.05 and results are reported as Mean (M) ± Standard Error of the Mean (SEM), unless indicated otherwise.
2.7. Correlational analyses
Correlational analyses of non-fMRI data and peak FC values were performed using Spearman's rank correlations. Specifically, associations between visceral thresholds and symptoms of anxiety and depression were addressed in all participants and within the patient sample. Correlational analyses of self-reported symptom severity and GI symptom-specific anxiety were performed for the IBS sample. Alpha-level was set at p < 0.05.
3. Results
3.1. Differences in visceral thresholds in a priori defined IBS subgroups and HC
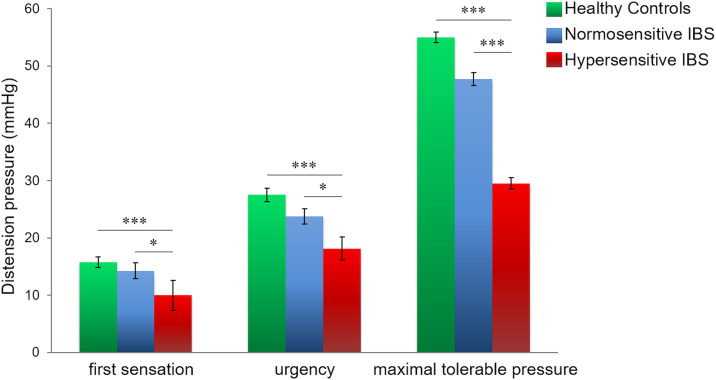
The Kruskal-Wallis tests, initially used to confirm differences in visceral thresholds, revealed a significant group effect for all thresholds assessed. Specifically, group effects were not only detected for maximal tolerable pressure (H(2) = 42.996; p < 0.001), as expected, but also for thresholds of first sensation (H(2) = 13.558; p = 0.001) and urgency (H(2) = 19.316; p < 0.001) with substantially lower pressures in hypersensitive IBS compared to both, normosensitive patients and HCs (Fig. 2).
Fig. 2.
Group differences in visceral thresholds. Group comparisons regarding visceral thresholds for first sensation, urgency and maximal tolerable pressure in hypersensitive IBS, normosensitive IBS and healthy volunteers. Data are given as Mean ± SEM. *p < 0.05; ***p < 0.001.
3.2. Sample characteristics and questionnaire data
Statistical details from comparisons between HC, normosensitive and hypersensitive IBS with respect to GI symptom ratings and questionnaires are given in Table 1. The groups differed significantly with respect to ratings of intensity (H(2) = 28.444; p < 0.001) and unpleasantness (H(2) = 24.616; p < 0.001) of current GI symptoms, assessed after the resting-state fMRI session. Hypersensitive IBS reported the highest levels of intensity and unpleasantness of all three groups, while normosensitive IBS reported greater intensity and unpleasantness of current GI symptoms than HCs. While group differences were further observed for anxiety (H(2) = 20.325; p < 0.001) and depression (H(2) = 16.079; p < 0.001) with significantly higher scores in both IBS groups compared to HCs, no differences were observed between hyper- and normosensitive IBS. IBS symptoms, as assessed with IBS-SSS, were moderate to severe in both IBS subgroups with higher scores in hypersensitive when compared to normosensitive patients. Both patient groups reported comparable levels of GI symptom-specific anxiety.
Table 1.
Clinical characteristics in hypersensitive and normosensitive IBS patients and in healthy controls.
| HCs (N = 20) | Normosensitive IBS (N = 20) | Hypersensitive IBS (N = 21) | pa | pb | pc | |
|---|---|---|---|---|---|---|
| Mean age (years) | 32.25 (± 2.20) | 33.25 (± 2.27) | 36.48 (± 2.71) | 0.957 | 0.334 | 0.426 |
| Symptom intensity | 0.57 (± 0.56) | 1.60 (± 0.36) | 3.57 (± 0.49) | 0.010⁎ | < 0.001⁎⁎⁎ | 0.012⁎ |
| Symptom unpleasantness | 0.22 (± 0.13) | 2.20 (± 0.57) | 3.81 (± 0.43) | 0.024⁎ | < 0.001⁎⁎⁎ | 0.038⁎ |
| HADS anxiety | 2.89 (± 0.63) | 8.10 (± 0.94) | 8.10 (± 1.08) | < 0.001⁎⁎⁎ | < 0.001⁎⁎⁎ | 0.786 |
| HADS depression | 1.17 (± 0.29) | 3.80 (± 0.59) | 4.10 (± 0.58) | < 0.001⁎⁎⁎ | < 0.001⁎⁎⁎ | 0.624 |
| IBS-SSS | – | 316.40 (± 17.13) | 365.24 (± 12.77) | – | – | 0.034⁎ |
| VSI | – | 44.85 (± 4.01) | 46.80 (± 3.40) | – | – | 0.784 |
HCs, Healthy Controls; HADS, Hospital Anxiety and Depression Scale; IBS-SSS, Irritable Bowel Syndrome Severity Scoring System; VSI, Visceral Sensitivity Index (for questionnaire references, see Materials and methods section).
Results of Mann-Whitney U tests comparing healthy controls and normosensitive IBS.
Results of Mann-Whitney U tests comparing healthy controls and hypersensitive IBS.
Results of Mann-Whitney U tests comparing hyper- and normosensitive IBS. All data are given as Mean (± SEM).
p < 0.05.
p < 0.001.
3.3. FC within DMN, salience and sensorimotor networks
Within the DMN, hypersensitive IBS patients had increased positive FC of the right amygdala and increased negative connectivity in the left dorsal anterior insula compared to normosensitive IBS patients (Fig. 3A; Table 2). In parallel, HCs also had increased positive FC of the right amygdala within the DMN along with increased negative FC of the left dorsal anterior insula compared to normosensitive IBS (Fig. 3B; Table 2). Furthermore, comparisons of hypersensitive IBS and HCs revealed increased positive FC of mid insula in patients (Table 2).
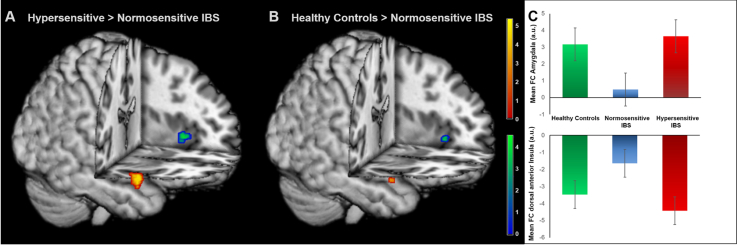
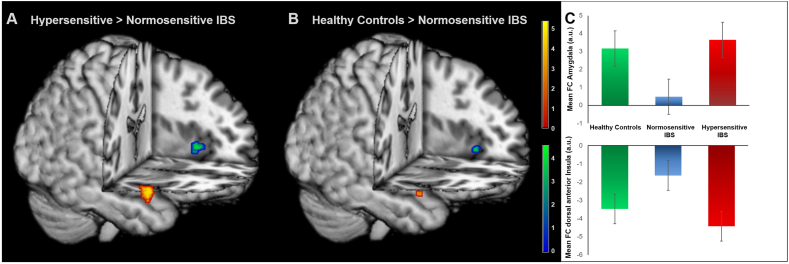
Fig. 3.
Group differences in DMN connectivity. Results from ROI analyses comparing FC within the DMN in (A) hypersensitive compared to normosensitive patients and (B) healthy controls relative to normosensitive IBS. (C) Mean FC values for each participant, extracted from one-sample t-test of DMN FC for amygdala (top) and dorsal anterior insula (bottom). Images were superimposed on a structural T1-weighted MRI used for spatial normalization and thresholded at p < 0.001 uncorrected for visualization purposes. Positive correlations are shown in red-yellow and negative correlations are depicted in blue-green. Color bars indicate t-scores. For statistical details, see Table 2. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Group differences in FC within the DMN.
| Brain region | H | Coordinates |
t | p | Volume (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Hypersensitive > normosensitive IBS | |||||||
| Amygdala | R | 28 | 2 | –22 | 5.06 | < 0.0011 | 392 |
| Dorsal anterior insula | L | − 42 | 14 | − 2 | − 3.61 | 0.029 | 40 |
| Healthy controls > normosensitive IBS | |||||||
| Amygdala | R | 28 | 0 | –22 | 3.55 | 0.023 | 16 |
| Dorsal anterior insula | L | − 32 | 22 | 2 | − 3.68 | 0.041 | 48 |
| Hypersensitive IBS > healthy controls | |||||||
| Mid insula | R | 42 | − 2 | 6 | 4.13 | 0.015 | 32 |
Results from between-group comparisons of FC within the DMN by two sample t-tests. Only results of region-of-interest analyses at pFWE-corrected < 0.05 are shown and exact unilateral p-values are given for peak voxel analyses. H = hemisphere. For visualization, see Fig. 3.
Within the salience network, hypersensitive IBS exhibited increased positive FC of pgACC and thalamus compared to normosensitive patients (Table 3). Within the sensorimotor network, we observed increased FC of posterior insula in hypersensitive relative to normosensitive patients (Table 3). When comparing all IBS as a group and HCs, increased FC of aMCC within the sensorimotor network (x = − 4; y = − 6; z = 34; t = 4.03; pFWE = 0.042) was observed in patients.
Table 3.
Group differences in FC between IBS subgroups within salience and sensorimotor networks [hypersensitive > normosensitive IBS].
| Brain region | H | Coordinates |
t | p | Volume (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Salience network | |||||||
| Pregenual ACC | L | − 2 | 38 | 6 | 4.65 | 0.010 | 176 |
| Thalamus | R | 22 | − 30 | 0 | 4.38 | 0.012 | 56 |
| Sensorimotor network | |||||||
| Posterior insula | R | 30 | − 24 | 12 | 3.61 | 0.041 | 104 |
Results from between-group analyses of FC in hypersensitive compared to normosensitive IBS patients within the salience and sensorimotor networks by two sample t-tests. Only results of region-of-interest analyses at pFWE-corrected < 0.05 are shown and exact unilateral p-values are given for peak voxel analyses. H = hemisphere; ACC = anterior cingulate cortex.
3.4. Correlations between FC, visceral thresholds and clinical characteristics
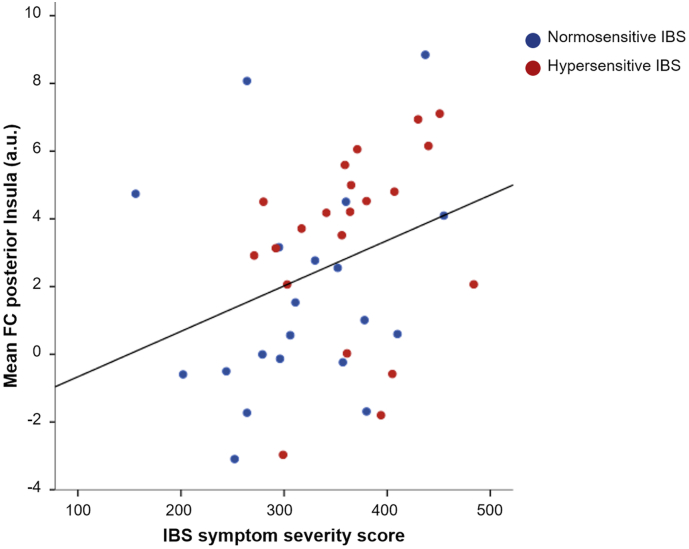
In IBS, significant negative correlations between peak connectivity of amygdala within DMN and thresholds for first sensation (rs = − 0.439; p = 0.005), urgency (rs = − 0.511; p = 0.001) and for maximal tolerable pressure (rs = − 0.580; p < 0.001) were detected. Similarly, FC values extracted from dorsal anterior insula, which decrease with higher negative FC, correlated significantly with maximal tolerable pressure (rs = 0.415; p = 0.007). Correlations with first sensation (rs = 0.297; p = 0.067) and urgency (rs = 0.302; p = 0.062) did not reach statistical significance. Also, no significant correlations with peak FC within DMN were detected in the full sample including HCs. Within the salience network, FC of pACC and thalamus yielded no significant associations with thresholds or clinical characteristics. Within the sensorimotor network, peak FC of posterior insula was significantly related to urgency (rs = − 0.382; p = 0.003) and maximal tolerable pressure (rs = − 0.277; p = 0.031) within the full sample. Posterior insula FC was further associated with symptom severity in IBS (rs = 0.341; p = 0.029; Fig. 4). No associations between FC and measures of anxiety, depression or GI symptom-specific anxiety were evident.
Fig. 4.
Correlation between posterior insula FC and IBS symptom severity. Results from Spearman's rank correlation of mean posterior insula FC within the sensorimotor network and symptom severity in normosensitive (depicted in blue) and hypersensitive (shown in red) IBS patients. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This exploratory study addressed resting-state FC in DMN, salience and sensorimotor networks in IBS patients with and without visceral hypersensitivity and healthy controls. Our study focused on region-specific alterations in connectivity within the context of canonical resting-state networks. In addition to being well-suited to identify large-scale neural networks, ICA was selected as our analytic approach, as it is less sensitive to confounding factors such as physiologic noise and head motion compared with either seed-based or graph-based approaches (Calhoun and Adalı, 2012, Power et al., 2012). ROI analyses revealed group-specific alterations within all three networks of interest.
4.1. Default mode network
The most prominent group differences were detected within the DMN, an intrinsic brain network involved in self-referential processing (Raichle, 2015) including the monitoring of the body and emotional states (Davey et al., 2016, Shulman et al., 1997). As the network most strongly affected in chronic pain (Farmer et al., 2012), altered DMN connectivity has been reported in several chronic pain conditions (Baliki et al., 2014, Farmer et al., 2012), including IBS (Qi et al., 2016a). The present analysis is the first to support specific alterations within the DMN related to visceral sensitivity in a female patient population with moderate to severe IBS. Consistent with our general hypothesis, hypersensitive and normosensitive IBS differed with respect to amygdala and dorsal anterior insula connectivity within DMN, attributable to increased FC in the hypersensitive group. This result is interesting given that both amygdala and dorsal anterior insula serve as key hubs in the integration of interoceptive signals with emotional and cognitive aspects (Craig, 2009, Etkin and Wager, 2007, Gu et al., 2013, Neugebauer, 2015). Aberrant function of these regions has consistently been reported in IBS, both in response to visceral stimulation (Mayer et al., 2015a, Tillisch et al., 2011) and at rest (Hong et al., 2014, Qi et al., 2016b), in support of their putative role in IBS pathophysiology, involving visceral processing. Evidence further suggests a role of dorsal anterior insula in attentional processes and higher cognitive function (Chang et al., 2013). Negative FC within the DMN, as observed here, is therefore well in line with the involvement of dorsal anterior insula in task-positive networks (Di and Biswal, 2014) as well as a recently described anticorrelation with regions of the DMN as a task-negative network in IBS (Hong et al., 2014).
When comparing the IBS groups with HCs, we observed reduced positive amygdala FC and reduced negative dorsal anterior insula FC in normosensitive IBS patients. Unexpectedly, the comparison between hypersensitive IBS and HCs revealed essentially identical FC in these regions. In other words, our results suggested that FC within the DMN is altered in normosensitive IBS compared to both hypersensitive patients as well as a healthy population. This finding is difficult to reconcile with the few existing studies in IBS that have demonstrated enhanced positive amygdala FC with corticolimbic regions (Qi et al., 2016b), and enhanced negative FC with regions of the DMN (Hong et al., 2014). However, there are several methodological differences, which may explain our findings. Specifically, subjects in previous studies were not characterized with respect to visceral sensitivity, and different analytical approaches were used (i.e., seed-based versus data-driven approaches). Most importantly, the distinctive methodological feature of balloon placement before scanning herein might have induced constant subliminal stimulation. This may have particularly affected DMN as a task-negative network, inducing the involvement of the amygdala as a brain region generally not associated with the DMN. In light of similar FC patterns in hypersensitive patients and healthy women, one may speculate that decreased FC of amygdala and dorsal anterior insula in normosensitive patients reflects a form of corticolimbic inhibition involving a downregulation of attentional and emotional processes. Modulating visceral afferent input under resting conditions could therefore be a compensatory mechanism allowing maintenance of normal visceral sensitivity despite chronic GI symptoms.
4.2. Salience network
The IBS groups also differed in salience network FC. Specifically, increased connectivity of pACC and thalamus within the salience network was observed in hypersensitive relative to normosensitive patients. These two regions, as part of a large-scale network, are involved in the integration of sensory input, including nociceptive afferent signals. The pACC, particularly, is involved in affective information processing (Vogt, 2005) and pain modulation through attentional processes (Atlas et al., 2010), as previously also demonstrated in IBS (Hong et al., 2016). Using task-based fMRI, the only two existing studies testing the role of visceral sensitivity in neural response to visceral stimuli in FGIDs revealed involvement of cingulate regions in hypersensitive when compared to normosensitive patients (Larsson et al., 2012, Van Oudenhove et al., 2010). Enhanced connectivity of pACC and thalamus may therefore reflect increased attentional and modulatory resources regarding both affective responses and visceral afferent signaling under resting conditions in patients with heightened visceral sensitivity. This may contribute to GI-related hypervigilance, especially in hypersensitive patients.
4.3. Sensorimotor network
Within the sensorimotor network, hypersensitive patients demonstrated enhanced FC of posterior insula relative to normosensitive IBS. As the primary interoceptive cortex, the posterior insula is considered a key region of interoceptive processing, providing a homeostatic representation of the physiological state of the body (Craig, 2002). It plays a crucial role in multimodal convergence of sensorimotor information, particularly involving the processing of visceral signals and pain (Chang et al., 2013). This is in line with increased distension-induced neural activation in hypersensitive patients, as recently observed (Larsson et al., 2012). Enhanced posterior insula FC within the sensorimotor network may therefore reflect increased ascending input and enhanced processing of visceral signals in patients with visceral hypersensitivity. This inference is further supported by posterior insula FC correlating with symptom severity in patients and with thresholds for urgency and maximal tolerable pressure in all participants. These findings extend a recent report demonstrating a positive association between visceral sensitivity and symptom severity in IBS (Simrén et al., 2017), by suggesting resting-state FC of posterior insula as a neural correlate of this relation.
4.4. Limitations and future directions
The current study is not without limitations. We cannot exclude that balloon placement performed prior to scanning or the empty balloon catheter, in terms of a subliminal stimulus, affected FC in the current study. We have chosen this protocol for patient convenience, particularly to eliminate negative expectations of the often fear-evoking placement procedure itself. Participants were given time outside and inside the scanner to habituate to the balloon catheter and to the reportedly stress-evoking scanner environment (Lueken et al., 2017). However, the observed group differences may reflect alterations in central processing of tonic subliminal afferent stimulation arising from the GI tract in patient subgroups. Importantly, this possibility raises a general question of crucial importance for brain imaging studies implementing distension stimuli. In task-based fMRI paradigms, the balloon catheter is present throughout all experimental phases and may induce differential neural responses not only during distension, but also in the deflated state. At the same time, it points towards a highly relevant future direction of research, addressing the impact of tonic subliminal visceral stimulation, which may for example be induced by low-grade inflammatory processes, on brain function and FC in health and FGIDs. Furthermore, in the current protocol sensory thresholding followed by an experimental paradigm involving rectal distensions (Larsson et al., 2012) subsequent to rsfMRI may have induced responses related to the anticipation of the following aversive procedure. Additionally, while a recent study revealed no group differences between IBS and controls regarding effects of the scanner environment on visceral pain perception, increases in pain intensity ratings inside the scanner observed in both groups appeared to be associated with psychological factors such as stress and anxiety (Wong et al., 2016). Future studies should therefore take effects of arousal, state anxiety and stress into account, which we cannot fully rule-out to have affected sensory testing to different extents in our sample of healthy women and IBS patients with high psychological symptom burden, and which may have profound influences on brain function and FC. Evidence supporting sex differences in functional and structural brain alterations in IBS is accumulating (Gupta et al., 2016, Hong et al., 2013, Hong et al., 2014, Jiang et al., 2013). Inferences drawn from findings obtained in women herein therefore cannot be directly translated to men suffering from IBS. Recent findings further suggest an influence of menstrual cycle phase and intake of oral contraceptives on rsFC in women (Petersen et al., 2014, Pletzer et al., 2016). While excluding time of menses, females independent of hormonal status were included in the current study. Future research should consider the putative impact of sex hormones and their natural fluctuations, which might distinctly affect not only visceral sensitivity but also brain connectivity in patients. Finally, our results from FWE-corrected ROI analyses were not additionally corrected for multiple comparisons regarding number of groups, networks or ROIs, yielding them exploratory in nature due to the risk of false-positive results.
4.5. Conclusion
While warranting cautious interpretation, our findings provide new evidence connecting visceral hypersensitivity, as defined by decreased thresholds to experimental visceral stimulation (Farmer and Aziz, 2013), and brain mechanisms in IBS. Under resting conditions, group differences related to visceral sensitivity may be induced by enhanced sensory input from the gut, or centrally-mediated modulatory processes. Our findings are consistent with both mechanisms contributing to central alterations related to visceral sensitivity in IBS. Group differences in salience and sensorimotor networks may reflect enhanced ascending input as well as increased attentional resources in hypersensitive patients, calling for future research to delineate top-down and bottom-up processes and their possible interactions in visceral hypersensitivity. Although speculative, distinct connectivity patterns within the DMN suggest that normosensitive patients maintain visceral sensitivity within a normal range via central pathways, potentially involving a regulation of attentional and emotional processes. Hyper- and normosensitive patients may therefore differ with respect to both origin of symptoms and regulatory resources, which may have profound consequences when investigating mechanisms contributing to IBS pathophysiology. Further research should therefore address the putative role of corticolimbic inhibition (Berman et al., 2008) and psychological factors (Grinsvall et al., 2015), including coping skills or other resilience mechanisms (Alschuler et al., 2016) in the regulation of visceral sensitivity, which may be of crucial relevance to visceral hyperalgesia and hypervigilance in IBS.
Acknowledgements
Funding: This work was supported by the County Council of Östergötland, Sweden [grant number LIO-123451], the Bengt Ihresfond [grant number SLS-178511], the Svenska Läkaresällskapet [grant number SLS-178471] and the National Institute of Health (NIH) grant R01 DK048351.
Conflict of interest: The authors disclose no conflict.
Author contributions: S. Walter, M. Engström, K. Tillisch and E. Mayer designed the study; M. Lowén acquired the data; A. Icenhour and S.T. Witt analyzed the data; A. Icenhour, S.T. Witt, S. Elsenbruch and S. Walter drafted the manuscript; all authors contributed to the interpretation of data and critical revision of the manuscript for important intellectual content.
References
- Allen E., Erhardt E., Damaraju E., Gruner W., Segall J., Silva R., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A., Caprihan A., Turner J., Eichele T., Adelsheim S., Bryan A., Bustillo J., Clark V., Feldstein Ewing S., Filbey F., Ford C., Hutchison K., Jung R., Kiehl K., Kodituwakku P., Komesu Y., Mayer A., Pearlson G., Phillips J., Sadek J., Stevens M., Teuscher U., Thoma R., Calhoun V. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011 doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alschuler K.N., Kratz A.L., Ehde D.M. Resilience and vulnerability in individuals with chronic pain and physical disability. Rehabil. Psychol. 2016 doi: 10.1037/rep0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas L.Y., Bolger N., Lindquist M.A., Wager T.D. Brain mediators of predictive cue effects on perceived pain. J. Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz F., Bouin M., Camilleri M., Mayer E.A., Poitras P., Serra J., Spiller R.C. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol. Motil. 2007;19:62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- Baliki M.N., Mansour A.R., Baria A.T., Apkarian A.V. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S.M., Naliboff B.D., Suyenobu B., Labus J.S., Stains J., Ohning G., Kilpatrick L., Bueller J.A., Ruby K., Jarcho J., Mayer E.A. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J. Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.-N., Gohel S., Kelly C., Smith S.M., Beckmann C.F., Adelstein J.S., Buckner R.L., Colcombe S., Dogonowski A.-M., Ernst M., Fair D., Hampson M., Hoptman M.J., Hyde J.S., Kiviniemi V.J., Kötter R., Li S.-J., Lin C.-P., Lowe M.J., Mackay C., Madden D.J., Madsen K.H., Margulies D.S., Mayberg H.S., McMahon K., Monk C.S., Mostofsky S.H., Nagel B.J., Pekar J.J., Peltier S.J., Petersen S.E., Riedl V., Rombouts S.A.R.B., Rypma B., Schlaggar B.L., Schmidt S., Seidler R.D., Siegle G.J., Sorg C., Teng G.-J., Veijola J., Villringer A., Walter M., Wang L., Weng X.-C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.-F., Zhang H.-Y., Castellanos F.X., Milham M.P. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. J. Psychosom. Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Bouin M., Plourde V., Boivin M., Riberdy M., Lupien F., Laganière M., Verrier P., Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adalı T. Multi-subject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev. Biomed. Eng. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L.J., Yarkoni T., Khaw M.W., Sanfey A.G. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb. Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.G., Pujol J., Harrison B.J. Mapping the self in the brain's default mode network. NeuroImage. 2016;132:390–397. doi: 10.1016/j.neuroimage.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Di X., Biswal B.B. Modulatory interactions between the default mode network and task positive networks in resting-state. PeerJ. 2014;2 doi: 10.7717/peerj.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman D.A. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology. 2016;150:1262–1279.e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S., Rosenberger C., Bingel U., Forsting M., Schedlowski M., Gizewski E.R. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S., Rosenberger C., Enck P., Forsting M., Schedlowski M., Gizewski E.R. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59:489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- Enck P., Aziz Q., Barbara G., Farmer A.D., Fukudo S., Mayer E.A., Niesler B., Quigley E.M.M., Rajilić-Stojanović M., Schemann M., Schwille-Kiuntke J., Simren M., Zipfel S., Spiller R.C. Irritable bowel syndrome. Nat. Rev. Dis, Prim. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt E.B., Rachakonda S., Bedrick E.J., Allen E.A., Adali T., Calhoun V.D. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum. Brain Mapp. 2011;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer A.D., Aziz Q. Gut pain & visceral hypersensitivity. Br. J. Pain. 2013;7:39–47. doi: 10.1177/2049463713479229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer M.A., Baliki M.N., Apkarian A.V. A dynamic network perspective of chronic pain. Neurosci. Lett. 2012;520:197–203. doi: 10.1016/j.neulet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- Freire L., Mangin J.-F. Motion correction algorithms may create spurious brain activations in the absence of subject motion. NeuroImage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L., Roche A., Mangin J.F. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans. Med. Imaging. 2002 doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Grinsvall C., Törnblom H., Tack J., Van Oudenhove L., Simrén M. Psychological factors selectively upregulate rectal pain perception in hypersensitive patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2015;27:1772–1782. doi: 10.1111/nmo.12689. [DOI] [PubMed] [Google Scholar]
- Gu X., Hof P.R., Friston K.J., Fan J. Anterior insular cortex and emotional awareness. J. Comp. Neurol. 2013;521:3371–3388. doi: 10.1002/cne.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Labus J., Kilpatrick L.A., Bonyadi M., Ashe-McNalley C., Heendeniya N., Bradesi S., Chang L., Mayer E.A. Interactions of early adversity with stress-related gene polymorphisms impact regional brain structure in females. Brain Struct. Funct. 2016;221:1667–1679. doi: 10.1007/s00429-015-0996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J., Hyvärinen A., Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hong J.-Y., Kilpatrick L.A., Labus J., Gupta A., Jiang Z., Ashe-McNalley C., Stains J., Heendeniya N., Ebrat B., Smith S., Tillisch K., Naliboff B., Mayer E.A. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J. Neurosci. 2013;33:11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J.-Y., Kilpatrick L.A., Labus J.S., Gupta A., Katibian D., Cody Ashe-Mcnalley X., Stains J., Heendeniya N., Smith S.R., Tillisch K., Naliboff B., Emeran X., Mayer A., Ashe-McNalley C., Stains J., Heendeniya N., Smith S.R., Tillisch K., Naliboff B., Mayer E.A., Cody Ashe-Mcnalley X., Stains J., Heendeniya N., Smith S.R., Tillisch K., Naliboff B., Emeran X., Mayer A. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J. Neurosci. 2014;34:14252–14259. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J.-Y., Naliboff B., Labus J.S., Gupta A., Kilpatrick L.A., Ashe-McNalley C., Stains J., Heendeniya N., Smith S.R., Tillisch K., Mayer E.A. Altered brain responses in subjects with irritable bowel syndrome during cued and uncued pain expectation. Neurogastroenterol. Motil. 2016;28:127–138. doi: 10.1111/nmo.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenhour A., Langhorst J., Benson S., Schlamann M., Hampel S., Engler H., Forsting M., Elsenbruch S. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol. Motil. 2015;27:114–127. doi: 10.1111/nmo.12489. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Dinov I.D., Labus J., Shi Y., Zamanyan A., Gupta A., Ashe-McNalley C., Hong J.Y., Tillisch K., Toga A.W., Mayer E.A. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keszthelyi D., Troost F.J., Masclee A.A. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. AJP Gastrointest. Liver Physiol. 2012;303:G141–G154. doi: 10.1152/ajpgi.00060.2012. [DOI] [PubMed] [Google Scholar]
- Kuiken S.D., Lindeboom R., Tytgat G.N., Boeckxstaens G.E. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2005;22:157–164. doi: 10.1111/j.1365-2036.2005.02524.x. [DOI] [PubMed] [Google Scholar]
- Labus J.S., Bolus R., Chang L., Wiklund I., Naesdal J., Mayer E.A., Naliboff B.D. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment. Pharmacol. Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- Laird A.R., Fox P.T.M., Eickhoff S.B., Turner J.A., Ray K.L., McKay D.R., Glahn D.C., Beckmann C.F., Smith S.M., Fox P.T.M. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M.B.O., Tillisch K., Craig A.D., Engström M., Labus J., Naliboff B., Lundberg P., Ström M., Mayer E.A., Walter S.A. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142:463–472. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.J., Kim J.H., Cho S.W. Relationship of underlying abnormalities in rectal sensitivity and compliance to distension with symptoms in irritable bowel syndrome. Digestion. 2006;73:133–141. doi: 10.1159/000094099. [DOI] [PubMed] [Google Scholar]
- Lee I.-S., Wang H., Chae Y., Preissl H., Enck P. Functional neuroimaging studies in functional dyspepsia patients: a systematic review. Neurogastroenterol. Motil. 2016;28:793–805. doi: 10.1111/nmo.12793. [DOI] [PubMed] [Google Scholar]
- Li Y.-O., Adalı T., Calhoun V.D. Estimating the number of independent components for functional magnetic resonance imaging data. Hum. Brain Mapp. 2007;28:1251–1266. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth G.F., Thompson W.G., Chey W.D., Houghton L.A., Mearin F., Spiller R.C. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Lueken U., Muehlhan M., Evens R., Wittchen H.-U., Kirschbaum C. Within and between session changes in subjective and neuroendocrine stress parameters during magnetic resonance imaging: a controlled scanner training study. Psychoneuroendocrinology. 2017;37:1299–1308. doi: 10.1016/j.psyneuen.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Mayer E.A., Gupta A., Kilpatrick L.A., Hong J.-Y. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156(Suppl):S50–S63. doi: 10.1097/j.pain.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A., Labus J.S., Tillisch K., Cole S.W., Baldi P. Towards a systems view of IBS. Nat. Rev. Gastroenterol. Hepatol. 2015;12:592–605. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., Harris R.E. What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management of “centralized” pain? Arthritis Res. Ther. 2014;16:425. doi: 10.1186/s13075-014-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V. Amygdala pain mechanisms. Handb. Exp. Pharmacol. 2015 doi: 10.1007/978-3-662-46450-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N., Kilpatrick L.A., Goharzad A., Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. NeuroImage. 2014;90:24–32. doi: 10.1016/j.neuroimage.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B., Crone J.S., Kronbichler M., Kerschbaum H. Menstrual cycle and hormonal contraceptive-dependent changes in intrinsic connectivity of resting-state brain networks correspond to behavioral changes due to hormonal status. Brain Connect. 2016;6:572–585. doi: 10.1089/brain.2015.0407. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R., Ke J., Schoepf U.J., Varga-Szemes A., Milliken C.M., Liu C., Xu Q., Wang F., Zhang L.J., Lu G.M. Topological reorganization of the default mode network in irritable bowel syndrome. Mol. Neurobiol. 2016;53:6585–6593. doi: 10.1007/s12035-015-9558-7. [DOI] [PubMed] [Google Scholar]
- Qi R., Liu C., Ke J., Xu Q., Ye Y., Jia L., Wang F., Zhang L.J., Lu G.M. Abnormal amygdala resting-state functional connectivity in irritable bowel syndrome. Am. J. Neuroradiol. 2016;37:1139–1145. doi: 10.3174/ajnr.A4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Sabate J.-M., Veyrac M., Mion F., Siproudhis L., Ducrotte P., Zerbib F., Grimaud J.-C., Dapoigny M., Dyard F., Coffin B. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008;28:484–490. doi: 10.1111/j.1365-2036.2008.03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.L., Fiez J.A., Corbetta M., Buckner R.L., Miezin F.M., Raichle M.E., Petersen S.E. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simrén M., Törnblom H., Palsson O.S., van Tilburg M.A.L., Van Oudenhove L., Tack J., Whitehead W.E. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2017 doi: 10.1136/gutjnl-2016-312361. (gutjnl-2016-312361) [DOI] [PubMed] [Google Scholar]
- Sloots C.E.J., Felt-Bersma R.J.F., Cuesta M.A., Meuwissen S.G.M. Rectal visceral sensitivity in healthy volunteers: influences of gender, age and methods. Neurogastroenterol. Motil. 2000;12:361–368. doi: 10.1046/j.1365-2982.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T.M., Miller K.L., Glahn D.C., Fox P.T.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K., Mayer E.A., Labus J.S. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Kelly A.M., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oudenhove L., Vandenberghe J., Dupont P., Geeraerts B., Vos R., Dirix S., Van Laere K., Bormans G., Vanderghinste D., Demyttenaere K., Fischler B., Tack J. Regional brain activity in functional dyspepsia: a H(2)(15)O-PET study on the role of gastric sensitivity and abuse history. Gastroenterology. 2010;139:36–47. doi: 10.1053/j.gastro.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Vogt B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.K., Van Oudenhove L., Li X., Cao Y., Ho K.Y., Wilder-Smith C.H. Visceral pain perception in patients with irritable bowel syndrome and healthy volunteers is affected by the MRI scanner environment. United Eur. Gastroenterol. J. 2016;4:132–141. doi: 10.1177/2050640615580888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]