Abstract
Chronic stress enhances risk for psychiatric disorders, and in animal models is known to evoke depression-like behavior accompanied by perturbed neurohormonal, metabolic, neuroarchitectural and transcriptional changes. Serotonergic neurotransmission, including serotonin2A (5-HT2A) receptors, have been implicated in mediating specific aspects of stress-induced responses. Here we investigated the influence of chronic unpredictable stress (CUS) on depression-like behavior, serum metabolic measures, and gene expression in stress-associated neurocircuitry of the prefrontal cortex (PFC) and hippocampus in 5-HT2A receptor knockout (5-) and wild-type mice of both sexes. While 5- male and female mice exhibited a baseline reduced anxiety-like state, this did not alter the onset or severity of behavioral despair during and at the cessation of CUS, indicating that these mice can develop stress-evoked depressive behavior. Analysis of metabolic parameters in serum revealed a CUS-evoked dyslipidemia, which was abrogated in 5- female mice with a hyperlipidemic baseline phenotype. 5- male mice in contrast did not exhibit such a baseline shift in their serum lipid profile. Specific stress-responsive genes (Crh, Crhr1, Nr3c1, and Nr3c2), trophic factors (Bdnf, Igf1) and immediate early genes (IEGs) (Arc, Fos, Fosb, Egr1-4) in the PFC and hippocampus were altered in 5- mice both under baseline and CUS conditions. Our results support a role for the 5-HT2A receptor in specific metabolic and transcriptional, but not behavioral, consequences of CUS, and highlight that the contribution of the 5-HT2A receptor to stress-evoked changes is sexually dimorphic.
Keywords: 5- mice, Prefrontal cortex, Hippocampus, Gene expression, Sexual dimorphism, Despair
1. Introduction
Stress is a major risk factor for the development of psychopathology and metabolic dysfunction (McEwen et al., 2015). Preclinical models of chronic stress, including chronic unpredictable stress (CUS) (Willner, 2016, Willner, 2005), evoke wide-ranging consequences that include, but are not restricted to, dysregulation of cognitive and mood-related behavior (Jett et al., 2017, Lupien et al., 2009), disruption of normal metabolic status (Rebuffé-Scrive et al., 1992), perturbed neurohormonal regulation (de Kloet, 2003), alterations in neuronal architecture (Watanabe et al., 1992, McEwen et al., 2016), dysregulated adult neurogenesis (Egeland et al., 2015), and changes in gene expression within key limbic brain regions (Meyer et al., 2001, Ieraci et al., 2016, Mychasiuk et al., 2016). Recent studies also indicate that stress-evoked molecular, cellular, metabolic and behavioral consequences exhibit sexual dimorphism (Dalla et al., 2005, Goldstein et al., 2010, da Silva et al., 2014, Mychasiuk et al., 2016). Several reports provide evidence that chronic stress can alter serotonergic neurotransmission (Adell et al., 1997, Chaouloff et al., 1999; Lanfumey et al., 2008, Liu and Aghajanian, 2008; Sargin et al., 2016). Despite the strong evidence linking chronic stress to disruption of serotonin signaling, the contribution of specific serotonergic receptors to stress-evoked sequelae are still not clearly understood.
5-HT2A receptors are regulated by stress and are implicated in the pathophysiology and treatment of mood-related disorders (Takao et al., 1995, Frokjaer et al., 2008; Lohoff, 2010, Ben-Efraim et al., 2013). Chronic stress enhances cortical 5-HT2A receptor expression (Ossowska et al., 2001) and binding (Fernandes et al., 1997). Pharmacological 5-HT2A receptor blockade ameliorates specific behavioral, neuroendocrine and physiological consequences in distinct stress models (Beig et al., 2009, Harvey et al., 2012, Jørgensen et al., 1998, Ootsuka et al., 2008). 5-HT2A receptor knockout mice (5-) exhibit reduced anxiety-like behavior, with no change reported in depressive-like behavior, under baseline conditions (Weisstaub et al., 2006). In response to a chronic corticosterone challenge 5- mice exhibit enhanced depressive-like behavior (Petit et al., 2014), and are reported to have a treatment-resistant phenotype following chronic antidepressant treatment (Quesseveur et al., 2013, Quesseveur et al., 2016). At present, it remains unknown whether the effects of CUS on behavior, neurohormonal and metabolic measures and gene expression within limbic brain regions are altered in 5- mice.
In the present study we examined the influence of the 5-HT2A receptor on the molecular, metabolic and behavioral consequences of CUS, in both male and female mice. Our findings highlight the importance of the 5-HT2A receptor as a key target for mediating specific aspects of the molecular and metabolic consequences of chronic stress.
2. Materials and methods
2.1. Animals
Male and female serotonin2A receptor knockout mice (5-) (Weisstaub et al., 2006) and wild-type littermate controls (WT) (7–8 month) on a 129S6/SvEv background were group housed in the Tata Institute of Fundamental Research (TIFR) animal house facility, and maintained on a 12 h light dark cycle with access to food and water ad libitum. The 5- mouse line, generated as previously described (Weisstaub et al., 2006), was maintained through heterozygous crosses and only littermate wild-type controls were used for experimental purposes. Genotypes were confirmed using PCR analysis with a KAPA Mouse genotyping kit, KAPA Biosystems (catalog no. KK7352) (Supplementary Table 2). All experimental procedures followed the guidelines of the Committee for Supervision and Care of Experimental Animals (CPCSEA), Government of India, and were approved by the TIFR Institutional Animal Ethics committee. Experiments were performed so as to minimize animal suffering, and to restrict usage of animals by optimizing experimental design.
2.2. Baseline open-field test (OFT) behavior
A cohort of naive, non-handled 5- male and female mice and age- and sex-matched wild-type littermate controls were tested for baseline anxiety behavior on the open field test (OFT) under dim light conditions (n = 6–10/group). The open field arena (40 cm × 40 cm x 40 cm), with middle area (20 cm × 20 cm) defined as center, was used to assess anxiety-like behavior. Animals were placed in one corner of the arena and allowed to explore the arena for 5 min. Automated behavioral tracking analysis was performed using Panlab SMART video tracking software (SMART 3.0) and the total distance traversed in the arena, percent distance traveled in center of arena, percent time in center and number of entries to center were assessed.
2.3. Chronic unpredictable stress (CUS) paradigm
5- male and female mice and their age and sex matched wild-type littermate controls (n = 12–15/group) were subjected to chronic unpredictable stress (CUS) (Nasca et al., 2013) (Supplementary Table 1). In brief, the CUS paradigm consisted of exposure to 1–2 stressors daily. The stressors were selected in a randomized manner and included restraint stress, cage tilting, exposure to white noise or constant tone, food water deprivation, cold exposure, an altered light-dark phase, extended dark phase, overcrowding, and wet bedding. The tail suspension test (TST) and forced swim test (FST) were incorporated as part of the CUS paradigm, and also served as interim behavioral tests to assess effects of CUS (Fig. 2A) (Supplementary Table 1). The experimental groups were as follows for both males and females: WT control, WT CUS, 5- control and 5- CUS (n = 12–15/group). All behavioral analysis was performed during the light phase. Post CUS, all experimental groups were subjected to behavioral testing and a subset of animals from this same cohort was used for both serum metabolic profiling and gene expression analysis.
2.4. Tail suspension test (TST) and forced swim test (FST)
Animals were subjected to the tail suspension test (TST) as previously described (Castagné et al., 2011) with the time spent immobile determined for the duration of 4 min not including the first minute post suspension. Animals were subjected to the Porsolt's classic forced swim test (FST) as described previously (Castagné et al., 2011) for 5 min and the time spent immobile was measured from the first to the fifth minute. Automated behavioral analysis for TST and FST was performed on video recordings where the experimenter was blind to treatment conditions using the SMART 3.0 FST/TST module.
2.5. Metabolic measurements
Body weights of all treatment groups were monitored weekly. Animals were sacrificed by rapid decapitation and trunk blood was collected for serum measurements at the end of CUS, and analyzed for metabolic parameters which included serum corticosterone, glucose and lipid profiles (Shahbazker's Diagnostic Center, Mumbai). Experimental groups were as follows for both males and females: WT control, WT CUS, 5- control and 5- CUS (n = 4–7/group). Serum corticosterone levels were measured colorimetrically using a commercially available ELISA kit (Abcam, Cat no. ab108821) as per manufacturers' instructions (n = 4–5/group).
2.6. Quantitative real time PCR (qPCR) analysis
All experimental animals were sacrificed three hours after the final stressor in the CUS regime. Experimental groups were as follows for both males and females: WT control, WT CUS, 5- control and 5- CUS (n = 5–10/group). Animals were sacrificed by decapitation and the prefrontal cortex and hippocampi were dissected out and snap-frozen in liquid nitrogen. RNA was isolated using Tri reagent (Sigma) and reverse transcribed using a complementary DNA (cDNA) synthesis kit (PrimeScript 1st strand cDNA Synthesis Kit, Takara Bio). Quantitative real time PCR (qPCR) was performed with primers for the genes of interest (Supplementary Table 2) using a Bio-Rad CFX96 real-time PCR machine. Data was quantified using the ΔΔCt method, as described previously (Bookout and Mangelsdorf, 2003), with data normalized to Hypoxanthine guanine phosphoribosyl transferase (Hprt), whose expression was unaltered across treatment groups.
2.7. Statistical analysis
Statistical analysis was carried out using Prism 6 (GraphPad Software Inc, USA). Experiments with two groups were subjected to unpaired Student's t-test. Experiments with four groups were subjected to two-way ANOVA analysis followed by post-hoc Bonferroni comparisons. Experiments that addressed progression across time were analyzed using repeated measures two-way ANOVA analysis followed by post-hoc Bonferroni comparisons. Normality of data was tested using the Kolmogorov and Smirnov method. Significance was determined at p < 0.05. In the case of our qPCR results where we tested gene expression of multiple genes, we have applied the Benjamini-Hochberg method for False Discovery Rate (FDR) to our data analysis and have also reported FDR corrected p values (Benjamini and Hochberg, 1995, Glickman et al., 2014).
3. Results
3.1. 5-HT2A receptor knockout mice of both sexes exhibit baseline reduced anxiety-like behavior
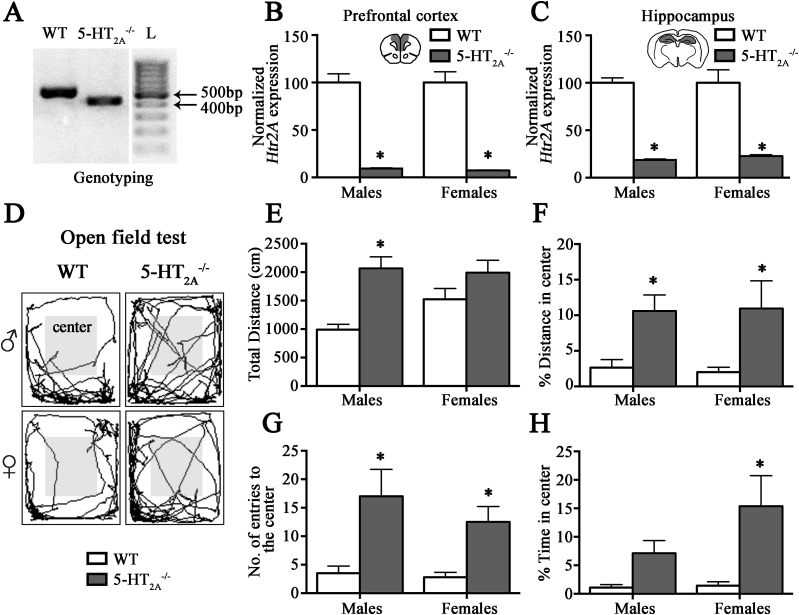
5- mice were confirmed to be deficient for 5-HT2A receptor expression using genotyping (Fig. 1A) and qPCR analysis for Htr2A mRNA expression within the prefrontal cortex (PFC) (Fig. 1B) and hippocampus (Fig. 1C). Baseline anxiety-like behavioral states of 5- male and female mice were assessed on the open-field test (OFT). Male, but not female, 5- mice exhibited significant increases in total distance traversed in the OFT (Fig. 1D, E). Both 5- male and female mice showed significant increases in the percent distance traveled in the center of the arena (Fig. 1D, F), and the number of entries (Fig. 1G) in the center of the OFT arena as compared to their sex-matched wild-type littermate controls. While female 5- mice exhibited a significant increase in percent time spent in the center of the OFT (Fig. 1H), male 5- mice showed a trend (p = 0.05) on this measure (Fig. 1H). These results indicate that naive, non-handled 5- male and female mice exhibit reduced anxiety-like behavior on the OFT as previously reported (Weisstaub et al., 2006).
Fig. 1.
5-HT2Areceptor knockout male and female mice exhibit reduced anxiety-like behavior. Animals were genotyped and were assessed for anxiety-like behavior on the open-field task (OFT). Shown in (A) is a representative photomicrograph of a gel indicating the presence of a single 500bp DNA band indicative of wild-type 5-HT2A expression, whereas a single 400bp DNA band corresponds to the mutant 5-HT2A expression in 5- mice. qPCR results further confirmed the robust reduction in Htr2A mRNA expression in the prefrontal cortex (B) and hippocampus (C) of 5- male and female mice. 5- male and female mice showed significant reduction in anxiety-like behavior on the Open field test (OFT) (D–H). Shown are representative traces from wild-type (WT) and 5- male and female mice in the OFT arena (D). 5- males, but not females showed increased total distance traveled in the OFT compared to their sex-matched WT controls (E). 5- male and female mice displayed reduced anxiety-like behavior as revealed by enhanced percent distance in the center (F) and increased number of entries to the center (G) compared to WT controls (D). The percent time spent in the center of the OFT was significantly increased in 5- female mice as compared to WT female mice (H). Results are expressed as the mean ± SEM (n = 6–10), *p < 0.05 as compared to age- and sex-, matched WT controls (unpaired Student's t-test).
3.2. Male and female 5-HT2A receptor knockout mice exhibit behavioral despair following chronic unpredictable stress
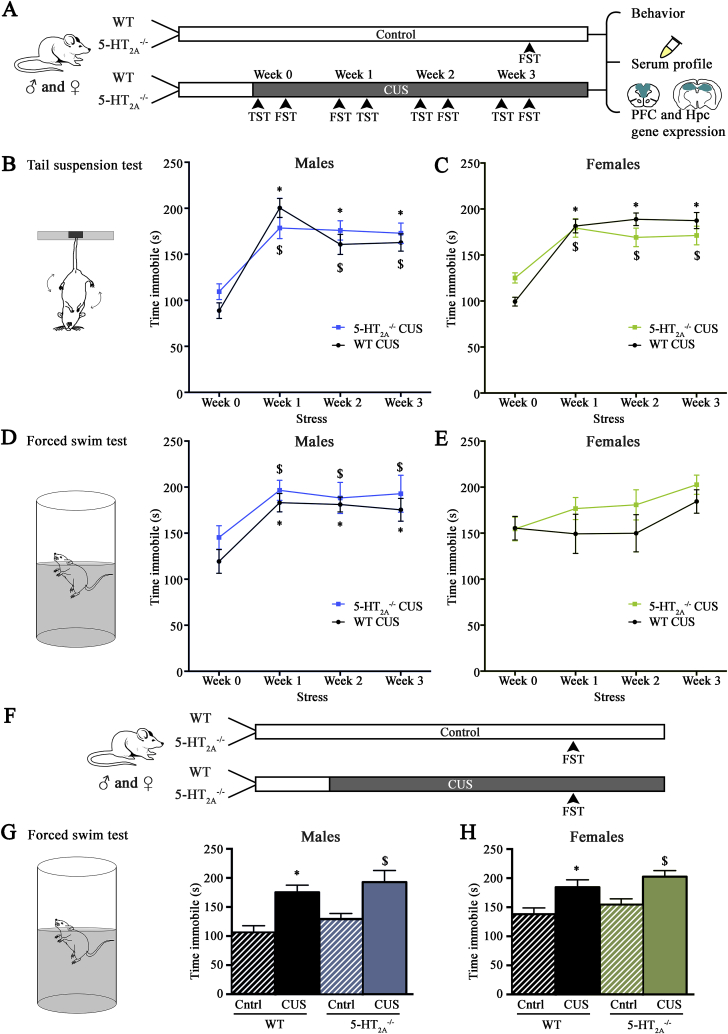
We examined whether 5-HT2A receptor deficiency influenced the behavioral consequences of CUS (Fig. 2A, Supplementary Table 1). The CUS paradigm included TST and FST on distinct days, which served as stressors while also providing a measure of behavioral despair during CUS (Fig. 2A). Time spent immobile on the TST and FST did not differ between WT and 5- male and female mice at week 0 indicating no change in behavioral despair under baseline conditions (Fig. 2B–E).
Fig. 2.
5-HT2Areceptor knockout male and female mice exhibit chronic unpredictable stress (CUS)-evoked behavioral despair on the tail suspension test (TST) and forced swim test (FST). WT and 5- male and female mice were subjected to a CUS paradigm as illustrated in the schematic (A) for 24 days, which incorporated a weekly TST (CUS days 2, 8, 12 and 19 correspond to week 0, 1, 2 and 3 respectively) and FST (CUS days 3, 7, 14 and 21 correspond to week 0, 1, 2 and 3 respectively). The non-stressed control WT and 5- male and female mice were left undisturbed, with the exception of an FST test performed at day 21 and weekly body-weight measurements. The CUS regime was completed on day 24 and the readouts consisted of behavioral analysis, serum profiling and gene expression analysis in the prefrontal cortex (PFC) and hippocampus (A). Measurements of time spent immobile revealed significant increases in immobility time on the TST and FST following CUS in both WT and 5- male (B – TST; D - FST) and female (C – TST) mice commencing from week 1 and sustained till week three as compared to respective week 0 immobility times. Measurement of immobility time on the FST in WT and 5- female mice following CUS revealed a main effect of CUS, but no significance in Bonferroni post-hoc comparisons between weeks 1–3 to week 0 (E). Results are expressed as the mean ± SEM (n = 12–15/group). (*p < 0.05 as compared to WT at week 0, $p < 0.05 as compared to 5- mice compared to week 0, Repeated measures Two-way ANOVA analysis, Bonferroni post-hoc test). Shown is a schematic (F) for the FST treatment performed on day 21 in both non-stressed and CUS administered WT and 5- male and female mice. Measurement of time spent immobile revealed a significant increase in immobility time in both male (G) and female (H) WT and 5- mice subjected to CUS as compared to their respective non-stressed WT and 5- sex-matched controls (Cntrl). Results are expressed as the mean ± SEM (n = 12–15/group). (*p < 0.05 as compared to non-stressed WT mice controls, $p < 0.05 as compared to non-stressed 5- mice controls, Two-way ANOVA analysis, Bonferroni post-hoc test).
Repeated measures two-way ANOVA analysis revealed that CUS resulted in an increase in the time spent immobile on both the TST (Fig. 2B) (Main effect of CUS duration - TST (F(3,81) = 38.91, p < 0.0001) and FST (Fig. 2D) (Main effect of CUS duration - FST (F(3,81) = 12.18, p < 0.0001) in WT and 5- male mice with no significant CUS x genotype interaction. Bonferroni post-hoc comparisons indicated significant increases in immobility time in week 1, 2 and 3 of the TST (Fig. 2B) and FST (Fig. 2D) in both WT and 5- male mice as compared to their respective week 0 immobility time.
Repeated measures two-way ANOVA analysis of behavioral despair on the TST for WT and 5- female mice revealed a significant CUS x genotype interaction (Fig. 2C) (F(3,69) = 4.55, p = 0.006). Statistical analysis also indicated a significant main effect of stress duration on the TST in WT and 5- female mice subjected to CUS (F(3,69) = 48.43, p < 0.0001). Bonferroni post-hoc comparisons indicated significant increases in time spent immobile on the TST (Fig. 2C) commencing from week 1 and sustained through week 3 in both WT and 5- female mice as compared to their respective week 0 immobility times. Bonferroni post-hoc group comparisons indicated no significant differences in the immobility times of 5- female mice as compared to WT females at weeks 1–3 post CUS in the TST (Fig. 2C). We observed a significant main effect of CUS duration (F(3,69) = 3.21, p = 0.028), with no significant genotype effect or CUS x genotype interaction in WT and 5- female mice on the FST (Fig. 2E). Bonferroni post-hoc group wise comparisons did not reveal any significant differences between the times spent immobile on the FST in WT and 5- female mice either when compared to their respective week 0 immobility time or in comparison with each other at individual time-points (Fig. 2E).
To determine the effects of CUS on behavioral despair as compared to non-stressed WT and 5- male and female mice, FST was performed on day 21 of the CUS regime for both non-stressed WT and 5- male and female mice, as well as WT and 5- male and female mice subjected to CUS (Fig. 2F). Two-way ANOVA analysis revealed a significant main effect of CUS (WT and 5- male mice: F(1,55) = 23.38, p < 0.0001; WT and 5- female mice: F(1,50) = 18.19, p < 0.0001), with no significant genotype effect or CUS x genotype interaction in WT and 5- male (Fig. 2G) and female (Fig. 2H) mice on the FST. Bonferroni post-hoc group comparisons revealed enhanced FST immobility times in both male and female WT and 5- mice subjected to CUS as compared to their respective non-stressed WT and 5- sex-matched controls (Fig. 2G, H). Our results reveal that WT and 5- male and female mice exhibit a similar trajectory of behavioral despair following CUS administration.
3.3. 5-HT2A receptor deficiency alters specific metabolic consequences of chronic unpredictable stress in a sexually dimorphic manner
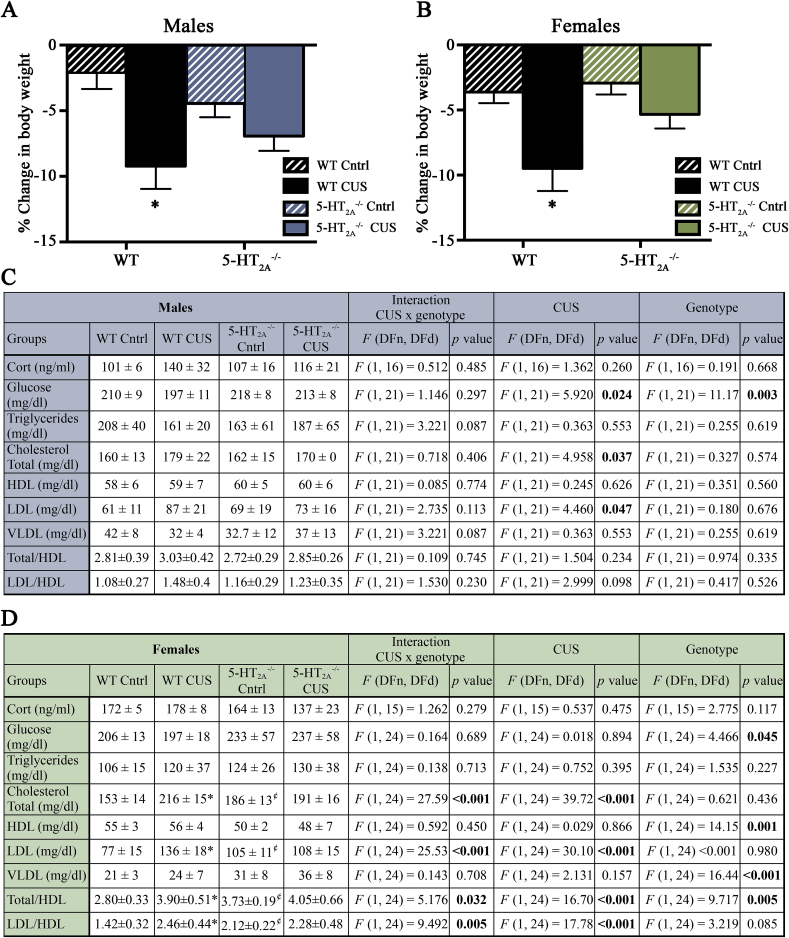
We next assessed whether the metabolic effects of CUS, including effects on body weight, corticosterone and serum glucose and lipid profiles were altered in 5- male and female mice. Body weight measurements at the beginning and post cessation of CUS were compared across non-stressed and CUS treated WT and 5- male and female mice. Two-ANOVA analysis indicated a significant main effect of stress in both males (F(1,55) = 13.08, p = 0.0006) (Fig. 3A) and females (F(1,50) = 13.11, p = 0.0007) (Fig. 3B). We did not observe any significant CUS x genotype interaction effects for either the male or female mice.
Fig. 3.
5-HT2A receptor knockout mice exhibit altered metabolic consequences of CUS in a sexually dimorphic manner. Shown are percent changes in body weight in wild-type and 5- male (A) and female (B) mice following chronic unpredictable stress (CUS). Results are expressed as the mean ± SEM percent change of body-weight (n = 14–15/group for males; n = 12–15/group for females). Two-way ANOVA analysis indicated a significant main effect of stress in both males and females, and a significant main effect for genotype in female mice. Shown is the serum profile for corticosterone (cort), glucose, triglycerides, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL) and the ratios of total cholesterol/HDL and LDL/HDL from wild-type and 5- male (C) and female (D) mice under baseline conditions and following CUS administration. Results are expressed as the mean ± SEM ng/ml for corticosterone and mg/dl for other measures of the serum profile (Corticosterone analysis: n = 5/group for males; n = 4–5/group for females; Serum glucose and lipid profile: n = 6–7/group for males; n = 7/group for females). Two-way ANOVA analysis, Bonferroni post-hoc test; *p < 0.05 WT CUS as compared to non-stressed WT controls, ¢p < 0.05 5- controls as compared to WT controls.
We next compared serum corticosterone, glucose and lipid levels across treatment groups. Corticosterone levels measured three hours post cessation of the final stressor in the CUS regime did not differ across all groups in both sexes (Fig. 3C, D). Two-way ANOVA analysis for serum glucose levels revealed a significant main effect of genotype in both males (Fig. 3C) and females (Fig. 3D). We noted a sexually dimorphic effect of CUS on circulating glucose levels with a main effect of CUS observed only in male, but not female mice (Fig. 3C, D).
The serum lipid profile in WT and 5- males appeared predominantly unaltered following CUS, with the exception of a significant main effect of CUS on total cholesterol and low density lipoprotein (LDL) (Fig. 3C). We did not observe any significant effect of genotype in the two-way ANOVA analysis for the lipid profiles in males. In contrast to the predominantly unchanged lipid profile observed in males, females exhibited significant CUS x genotype interactions, as well as significant main effects of genotype and CUS on multiple measures for lipid profile analysis (Fig. 3D). A significant CUS x genotype interaction was noted for total cholesterol, LDL, total cholesterol/high density lipoprotein (Total/HDL) ratio, and LDL/HDL ratio in females (Fig. 3D). A significant main effect of genotype was noted for HDL, VLDL and total cholesterol/HDL. A significant main CUS effect was observed for total cholesterol, LDL, total cholesterol/HDL, and LDL/HDL in females (Fig. 3D). Bonferroni post-hoc group comparisons indicated significant increases in total cholesterol, LDL, total cholesterol/HDL and LDL/HDL ratios, in non-stressed 5- female mice compared to non-stressed WT controls (Fig. 3D). In addition, while CUS evoked an increase in these measures in WT female mice, this induction was not observed in 5- female mice subjected to CUS as compared to non-stressed 5- female controls (Fig. 3D). Taken together, these results reveal a sexual dimorphism in the effects of 5-HT2A receptor deficiency on lipid metabolism, with dyslipidemia noted in 5- female mice under baseline conditions and a blunting of the CUS-evoked dyslipidemia in 5- female mice.
3.4. 5-HT2A receptor deficiency alters the influence of chronic unpredictable stress on stress-related and immediate early genes in the prefrontal cortex
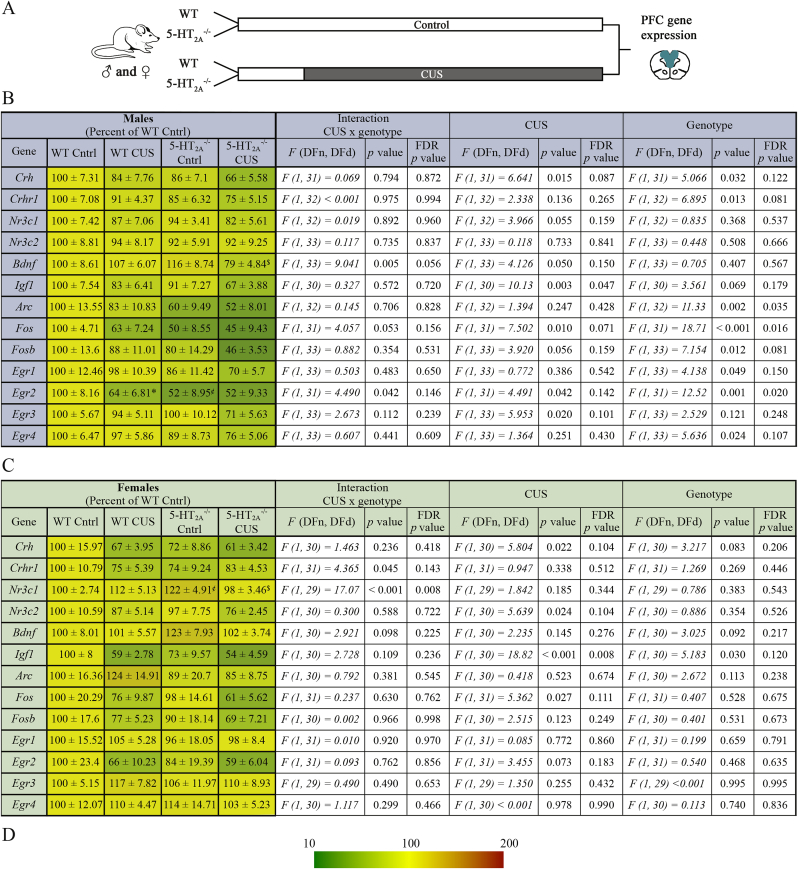
We next addressed whether the transcriptional regulation of specific stress-related and immediate early genes (IEGs) within the prefrontal cortex (PFC), which plays a critical role in top-down regulation of stress responses (Covington et al., 2010, McEwen and Morrison, 2013), is influenced by 5-HT2A receptor deficiency (Fig. 4A–D). We examined the regulation of genes involved in stress hormone regulation, namely corticotrophin releasing hormone (Crh) and it's receptor Crhr1, and the corticosterone receptors, glucocorticoid receptor (GR: Nr3c1) and the mineralocorticoid receptor (MR: Nr3c2) (Hill et al., 2012). In addition, we examined the regulation of trophic factors (Bdnf, Igf1) and IEGs (Arc, Fos, Fosb, Egr1-4) reported to be regulated by stress (Ieraci et al., 2016, Law et al., 2016, Ons et al., 2010). Since we have performed multiple comparisons for our qPCR results, we have reported both uncorrected and FDR corrected p values in Fig. 4. The results described pertain to FDR uncorrected p values, which have gone through a multiplicity adjusted correction for the two-way ANOVA on the individual gene tested, but not FDR correction. However, given we have carried out testing within multiple brain regions, across both genders and for several genes we have also provided the FDR corrected p value.
Fig. 4.
5-HT2Areceptor knockout mice exhibit altered chronic unpredictable stress-evoked regulation of stress-related and immediate early genes within the prefrontal cortex in a sexually dimorphic manner. Shown is a schematic (A) illustrating the treatment groups and CUS paradigm following which the prefrontal cortex (PFC) region of both WT and 5- male and female mice was subjected to qPCR analysis to determine gene expression of stress-related, trophic factor, and immediate early genes. Shown are normalized gene expression levels, represented as percent of wild-type controls, in the PFC following CUS administration in WT and 5- male (B) and female (C) mice. Heat maps indicate the extent of regulation represented as percent of wild-type controls (WT Cntrl), with upregulated genes shown in red and downregulated genes shown in green (key, D). The data are represented as percent of wild-type controls ± SEM (n = 7–10/group). Two-way ANOVA, Bonferroni post-hoc analysis. Our two-way ANOVA results include p values for CUS x genotype interaction, CUS and genotype effects, which are FDR uncorrected and FDR corrected. Bonferroni post-hoc analysis: *p < 0.05 (WT CUS compared to WT control), $p < 0.05 (5- CUS compared to 5- control), and ¢p < 0.05 (5- control compared to WT control). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the male PFC, we noted both a significant main effect for CUS and genotype in the regulation of Crh, with a reduction noted in prefrontal Crh mRNA levels (Fig. 4B). Further, a significant genotype effect was noted for Crhr1 expression, with reduced levels observed in 5- mice. For the trophic factors examined, we noted a significant CUS x genotype interaction for Bdnf expression, with Bonferroni post-hoc tests revealing a significant decline in Bdnf mRNA levels in the 5- mice subjected to CUS compared to non-stressed 5- controls (Fig. 4B). For Igf1 mRNA, we noted a significant main effect of CUS. IEG expression analysis in the PFC revealed a striking pattern of significant main effects of genotype noted for Arc, Fos, Fosb, Egr1, Egr2, Egr4, with a reduction noted for prefrontal IEG expression in 5- male mice. We also observed a significant main effect of CUS for Fos, Egr2 and Egr3. Amongst the IEGs examined, a significant CUS x genotype interaction was observed for only Egr2 expression, with Bonferroni post-hoc analysis revealing that the stress-evoked decline in Egr2 mRNA levels is abrogated in 5- male mice (Fig. 4B).
For the female PFC, similar to the males we noted a significant main effect for CUS in the regulation of Crh, with reduced prefrontal Crh expression observed following stress (Fig. 4C). We also observed a significant main effect for CUS for the prefrontal expression of MR (Nr3c2), with a decline noted in expression. Two-way ANOVA analysis revealed significant CUS x genotype interactions for both Crhr1 and GR (Nr3c1) expression (Fig. 4C). In the case of Crhr1, we did not observe any significance in post-hoc group comparisons. Post-hoc Bonferroni analysis for GR (Nr3c1), indicated that 5- female mice show a baseline increase in prefrontal GR expression, and CUS appears to evoke a significant reduction in expression only within 5- mice, but not in wild-type mice. For the trophic factors, we noted a significant main effect for both CUS and genotype on Igf1 mRNA expression, with a decline observed in Igf1 mRNA following CUS, and also noted in 5- female mice despite their baseline reduction in Igf1 expression. Unlike the males, analysis of IEG expression in female mice indicated no significant main effects of genotype on any of the IEGs examined (Fig. 4C). Further, in the female PFC a significant main effect of CUS was restricted to Fos expression. Collectively, our findings indicate sexually dimorphic effects of 5-HT2A receptor deficiency on IEG expression, with 5- male mice exhibiting a decline in the expression of several IEGs in the PFC, a pattern that is not recapitulated in the PFC of 5- female mice. Further, the regulation of Igf1 and GR expression indicates a sexual dimorphism with baseline expression perturbed in 5- female mice but not observed in the 5- male mice.
3.5. 5-HT2A receptor deficiency modifies the effects of chronic unpredictable stress on stress-related and immediate early genes in the hippocampus
We next addressed the influence of CUS on stress-related, trophic factor and IEG expression in the hippocampus, a brain region that is both a target for stress hormones and contributes to top-down regulation of stress-responses (Kim et al., 2015, Tasker and Herman, 2011) (Fig. 5A–D). We have reported both uncorrected and FDR corrected p values in Fig. 5. The FDR uncorrected p value has been through a multiplicity adjusted correction for the two-way ANOVA on the individual gene tested and we have used this p value for description of results. In the male hippocampus, we noted both a significant main effect for CUS and genotype in the regulation of GR (Nr3c1) and MR (Nr3c2), with a reduction noted in GR and MR expression (Fig. 5B). In the case of Crhr1 mRNA levels in the hippocampus, we noted a significant CUS x genotype interaction, with post-hoc analysis revealing a significant decline in Crhr1 expression selectively in the stressed 5- male mice as compared to their non-stressed, genotype-matched controls (Fig. 5B). For the trophic factors examined, we noted a significant main effect of CUS on Igf1 mRNA expression, with a reduction in expression noted following stress. Analysis of IEG expression in the hippocampus indicated a significant main effect of genotype for Egr1 and Egr4, with a robust induction noted in gene expression for both these genes in the hippocampi of 5- male mice under baseline conditions. We also observed a significant main effect of CUS for Arc, Fos, Egr2 and Egr3 (Fig. 5B), with the results indicative of a stress–evoked decline in gene expression for these genes. Amongst the IEGs analyzed, a significant CUS x genotype interaction was observed for only Egr4 expression with a differential effect of CUS noted in wild-type and 5- male mice. Bonferroni post-hoc comparisons revealed a baseline increase in Egr4 mRNA levels in the hippocampi of 5- male mice (Fig. 5B).
Fig. 5.
5-HT2Areceptor knockout mice exhibit altered chronic unpredictable stress-evoked regulation of stress-related and immediate early genes within the hippocampus in a sexually dimorphic manner. Shown is a schematic (A) indicating the experimental groups and CUS paradigm following which non-stressed and CUS treated WT and 5-HT2A−/− male and female mice were subjected to gene expression analysis to determine mRNA levels of stress-related, trophic factor, and immediate early genes within the hippocampus. Shown are normalized gene expression levels, represented as percent of wild-type controls (WT Cntrls), in the hippocampus following CUS administration to WT and 5-HT2A−/− male (B) and female (C) mice. Heat maps indicate the extent of regulation represented as percent of wild-type controls (WT Cntrl), with upregulated genes shown in red and downregulated genes shown in green (key, D). The data are represented as percent of wild-type controls ± SEM (n = 6–10/group). Two-way ANOVA, Bonferroni post-hoc analysis. Our two-way ANOVA results include p values for CUS x genotype interaction, CUS and genotype effects, which are FDR uncorrected and FDR corrected. Bonferroni post-hoc analysis: *p < 0.05 (WT CUS compared to WT control), $p < 0.05 (5-HT2A−/− CUS compared to 5-HT2A−/− control), and ¢p < 0.05 (5-HT2A−/− control compared to WT control). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Gene expression analysis for stress-related, trophic factor and IEG expression in the hippocampi of wild-type and 5- female mice, under baseline and CUS conditions, revealed that the pattern of regulation in specific cases is sexually dimorphic. In female mice, we observed a significant main effect for CUS in the regulation of Crh and GR (Nr3c1), with a decline noted in both genes following stress (Fig. 5C). We observed a significant CUS x genotype interaction for MR (Nr3c2) expression in the hippocampus (Fig. 5C). Post-hoc Bonferroni analysis revealed a baseline increase in hippocampal MR expression in 5- female mice, with a CUS-evoked decline in MR expression restricted to the 5- mice and not observed in wild-type groups. This significant induction in MR hippocampal expression was observed only in the 5- control female mice (Fig. 5C) and was not observed in the 5- control male mice (Fig. 5B). In gene expression analysis for trophic factors, we observed a significant main effect of CUS and genotype on BDNF hippocampal expression in the female mice. We noted a distinct pattern of regulation of the IEGs in 5- female mice, wherein unlike the induction in expression of specific IEGs (Egr1, Egr4) in 5- male mice, the female knockout mice did not reveal such a pattern. We observed a significant main effect of CUS for Arc, Fos, Egr1, Egr2 and Egr3 (Fig. 5C), with the results indicative of a stress–evoked decline in gene expression in the hippocampi of female mice, a pattern that overlapped to a certain extent with observations in male mice. Two-way ANOVA analysis indicated significant CUS x genotype interactions for Fosb, Egr1 and Egr4 with a differential effect of CUS noted in wild-type and 5- female mice. Bonferroni post-hoc comparisons revealed a stress-evoked decline in Fosb and Egr1 in wild-type, but not 5-, female mice (Fig. 5C). Our findings highlight the sexually dimorphic effects of 5-HT2A receptor loss of function on the expression of specific IEGs in the hippocampus, with 5- male mice exhibiting an increase in specific IEGs in the hippocampus, a pattern not observed in the hippocampi of 5- female mice.
4. Discussion
The major findings of our study indicate that 5-HT2A receptor deficiency influences specific CUS-evoked consequences, with 5- mice exhibiting a perturbed pattern of both CUS-induced metabolic dysfunction, such as dyslipidemia, and CUS-evoked gene expression changes in the PFC and hippocampus. The altered metabolic and transcriptional profile following CUS noted in 5- mice exhibits sexual dimorphic features. Interestingly, the behavioral despair that emerges following CUS was not changed in either 5- male or female mice. These results highlight the sexual dimorphic role of the 5-HT2A receptor in mediating specific aspects of CUS-evoked metabolic and transcriptional, but not behavioral, responses.
4.1. 5-HT2A receptor deficiency and behavioral consequences of CUS
Our observation of reduced anxiety-like behavior in 5- mice of both sexes is in agreement with prior reports (Weisstaub et al., 2006). Despite the striking baseline reduced anxiety-like state noted in 5- mice, the commencement and severity of behavioral despair noted in the TST and FST tasks following CUS did not appear to be altered. This suggests that the baseline reduced anxiety-like behavior in 5- mice does not serve to predict any improvement in stress coping capacity, rather we find that the full spectrum of behavioral despair develops in the knockout mice. Both wild-type and 5- male and female mice subjected to CUS demonstrated similar increases in despair-like behavior on the FST and TST across the CUS paradigm. We also compared the despair-like behavior of the CUS administered wild-type and 5- mice to their non-stressed, genotype and sex-matched littermate controls and observed a significant increase in immobility time. However, one caveat to keep in mind while interpreting the comparison of CUS treated groups to their respective non-stressed controls of both genotypes is that the CUS group underwent repeated exposures to the FST and this could have an impact on their immobility behavior on this task. Interestingly, a recent report also indicates that a baseline antidepressant-like state noted in 5- mice did not prevent the emergence of depressive-like behavior following social isolation stress (Diaz et al., 2016). A previous pharmacological study indicates that 5-HT2A/C receptor blockade with ketanserin infusion into the orbitofrontal cortex, can prevent the anhedonic, increased anxiety-like and depressive-like behavior evoked by CUS, but does not influence these behavioral features under baseline conditions (Xu et al., 2016). Further, infusion of a 5-HT2A/C receptor agonist into the OFC can induce anhedonia, anxiety and behavioral despair, mimicking the effects of chronic stress (Xu et al., 2016). Our findings with the 5-HT2A receptor knockout mice differ in this regard. However, it is also important to note that ketanserin also exerts antagonistic effects at other 5-HT2 receptor subtypes (Bard et al., 1996, Herndon et al., 1992). Given that the 5-HT2B and 5-HT2C receptor are both strongly implicated in the regulation of behavioral despair and in stress-evoked responses (Mongeau et al., 2010; Quesseveur et al., 2012, Diaz et al., 2016), pharmacological evidence thus far does not allow a clear delineation of the contribution of distinct 5-HT2 receptor subtypes to the behavioral effects of chronic stress. Although previous reports do not indicate any major developmental changes in the 5- mice (Weisstaub et al., 2006), we cannot preclude the possibility that differences in studies with pharmacological blockade of the 5-HT2A receptor and the 5-HT2A receptor deficient mice may also arise due to adaptations ensuing from constitutive loss of the receptor.
Previous studies have suggested that 5- mice exhibit exacerbated depressive behavior in response to chronic corticosterone treatment (Petit et al., 2014). Our results did not reveal any worsening of the behavioral trajectory following CUS and also did not show any change in circulating corticosterone levels when observed at end of the CUS regime. It is important to note that chronic corticosterone treatment is not necessarily an equivalent of CUS treatment, although it is reported to recapitulate certain aspects of chronic stress-evoked changes (Iijima et al., 2010, Rebuffé-Scrive et al., 1992). However, these studies highlight one factor in common, namely that 5-HT2A receptor deficiency does not in any way prevent the manifestation of behavioral despair. Recent studies also indicate that both the 5-HT2A and 5-HT2B loss of function mice serve as models for treatment-resistance to pharmacological antidepressants (Diaz et al., 2016, Quesseveur et al., 2016). 5-HT2A receptor deficient mice are reported to exhibit enhanced 5-HT1A autoreceptor function, which is suggested to contribute to the treatment-resistance phenotype exhibited by these mice (Quesseveur et al., 2016, Quesseveur et al., 2013), and may also play a role in our observations that despite baseline reduced anxiety-like behavior, 5- mice are equally susceptible to the behavioral effects of adult-onset, chronic stress. It is of interest that the expression of the 5-HT2A receptor peaks during postnatal life (Murrin et al., 2007, Zhang, 2003) raising the possibility that the 5-HT2A receptor may exert a role in the effects of early life stress. Indeed, prior pharmacological studies suggest a key role for 5-HT2A receptors in contributing to the anxiety-like behavior evoked following early life stress (Benekareddy et al., 2011). Future studies are required to examine whether the trajectory of behavioral changes evoked by early stress is altered in 5-HT2A receptor deficient mice.
4.2. 5-HT2A receptor regulation of CUS-evoked changes in serum metabolic markers
CUS is known to cause a reduction in body weight and alterations in lipid metabolism (da Silva et al., 2014, Fu et al., 2016). We did find a CUS-evoked decline in body weight with no significant CUS x genotype interaction for either males or females, although the weight decline did appear attenuated in the knockout mice. We observed mildly enhanced circulating glucose levels in the 5- mice in our serum profile studies, with a genotype effect noted in both male and female mice. Recent reports using pharmacological approaches indicate a role for peripheral 5-HT2A receptors in the liver and in adipocytes in mediating metabolic changes evoked by chronic stress, including dyslipidemia and insulin resistance (Fu et al., 2016, Hansson et al., 2016, Oh et al., 2016, Rosmond et al., 2002). Chronic stress is associated with upregulation of liver 5-HT2A and 5-HT2B receptors, and a 5-HT2A/B receptor antagonist sarpogrelate can block stress-evoked dyslipidemia (Fu et al., 2016). Our results indicate that CUS evokes dyslipidemia, with enhanced serum cholesterol and LDL, which appeared more pronounced in wild-type female mice. Further, this CUS-evoked dyslipidemia was not observed in 5- female mice, which exhibited a baseline dyslipidemic phenotype, with hypercholesterolemia as well as enhanced LDL levels. These findings suggest a possible interaction with estrogen, given that the 5- male mice did not exhibit any baseline lipid profile changes (Cavus and Duman, 2003, da Silva et al., 2014). Taken together, these findings implicate the 5-HT2A receptor in chronic stress-evoked dyslipidemia, and motivate future studies to address the relationship between 5-HT2A receptors, estrogen and chronic stress in the regulation of lipid metabolism.
4.3. 5-HT2A receptor regulation of stress-associated and trophic factor gene expression under baseline and CUS conditions
Our gene expression studies, focused on the regulation of stress associated pathways, trophic factors and IEGs in the PFC and hippocampus of 5- male and female mice following CUS. In our qPCR results (Fig. 4, Fig. 5) we report p value and FDR corrected p value for the main effects of CUS x genotype interaction, CUS and genotype for all genes in the PFC and hippocampus, of both males and females resulting in a total of 156 p values on which an FDR correction was applied. While the FDR uncorrected p value represents a multiple comparison adjusted p value for the internal two-way ANOVA run for that particular gene, given we have multiple comparison across several genes, two brain regions and both genders we have also provided the FDR corrected p value for all tests. Amongst the stress pathway associated genes, Crh, Crhr1, and the corticosterone receptors (GR: Nr3c1 and MR: Nr3c2), and the epigenetic regulation of their expression by environment has been strongly linked to development of stress susceptibility (Castro-Vale et al., 2016, Reul et al., 2015, Wan et al., 2014). Further, CRH pre-administration into the prefrontal cortex enhances 5-HT2 receptor agonist evoked anxiety-like behaviors likely through a CRHR1-induced sensitization of 5-HT2 receptor signaling, indicating an interaction between 5-HT2 receptors, CRH and CRHR1 (Magalhaes et al., 2010). In our results, we observed that the CUS evoked down-regulation of prefrontal Crh did not appear to be altered by 5-HT2A receptor deficiency in both sexes. In the hippocampus, Crh regulation by CUS was sexually dimorphic, with a decline noted only in female mice post CUS that was not influenced by 5-HT2A receptor loss. With regards to Crhr1, we noted that the CUS-induced down-regulation of prefrontal Crhr1 observed selectively in female mice was lost in the 5-HT2A receptor female nulls, and in the hippocampus the CUS-evoked decline in Crhr1 was noted selectively in 5-, but not wild-type, male mice. Similar to the pattern noted for Crhr1 expression, the 5-HT2A regulation of GR and MR receptors also exhibited sexual dimorphism, with a baseline increase in prefrontal GR (Nr3c1) and hippocampal MR (Nr3c2) expression selectively in 5- female mice. Further, this sexual dimorphism also applied to the pattern of regulation of hippocampal GR and MR by CUS, with a decline in hippocampal GR and MR noted in both wild-type and 5- male mice, with a differing pattern in their female counterparts. These observations strongly indicate a sexual dimorphic influence of 5-HT2A receptors in the baseline and CUS-mediated regulation of Crh, Crhr1, GR and MR expression in cortical brain regions.
Previous results indicate that 5-HT2A receptor agonists induce cortical Bdnf expression and reduce hippocampal Bdnf mRNA levels (Vaidya et al., 1997). Acute 5-HT2A receptor antagonist treatment does not appear to influence the baseline expression of either cortical or hippocampal Bdnf expression (Vaidya et al., 1997). Our findings revealed that baseline expression of Bdnf was significantly enhanced in the hippocampi of 5- female, but not male, mice. This is interesting given a previous report of an estrogen and 5-HT2A receptor interaction in the regulation of Bdnf expression (Cavus and Duman, 2003). Interesting, CUS regulation of prefrontal Bdnf expression was only observed in the 5-HT2A receptor null mice, with significance observed in the male knockouts and a trend in female knockouts. These findings add to the evidence of a reciprocal interaction between 5-HT2A receptors and BDNF (Homberg et al., 2014, Rios et al., 2006, Vaidya et al., 1997).
4.4. 5-HT2A receptor regulation of IEG expression under baseline and CUS conditions
Prior evidence indicates that 5-HT2A receptor agonists induce EPSPs in the cortex, likely through their expression on excitatory pyramidal neurons (Aghajanian and Marek, 1999, Martín-Ruiz et al., 2001). In contrast, 5-HT2A receptor stimulation results in enhanced IPSPs in the hippocampus due to the presence of 5-HT2A receptors on GABAergic interneurons and ensuing effects on GABA release (Piguet and Galvan, 1994, Shen and Andrade, 1998, Wyskiel and Andrade, 2016). This has been suggested to contribute to the effects of 5-HT2A receptor agonists on activity-dependent gene expression, with a robust increase noted in IEG expression in cortical brain regions and either a decline or no change in the hippocampus (Benekareddy et al., 2013, González-Maeso et al., 2007). Acute treatment with 5-HT2A receptor agonists has been reported to enhance the cortical expression of several IEGs including Arc, Fos and Egr1-4 (Benekareddy et al., 2013, González-Maeso et al., 2007, Pei et al., 2004). Our findings indicate that 5-HT2A receptor null male, but not female, mice exhibit a baseline reduction in expression of several of the IEGs tested (for eg. Arc, Fos, Egr2) in the PFC. Consistent with prior pharmacological studies of opposing effects of 5-HT2A receptor activation on cortical and hippocampal IEG expression (Santini et al., 2011), we noted a differing pattern for the baseline regulation of IEG expression in the hippocampi of 5-HT2A receptor deficient mice. 5- male, but not female, mice exhibited a robust increase in the baseline expression of specific IEGs, namely Egr1 and Egr4 in the hippocampus, with a similar pattern of upregulation also noted in other IEGs tested (Fos, Fosb). Our results provide further support to the idea that 5-HT2A receptor stimulation bidirectionally regulates the expression of IEGs within the neocortex and hippocampus. Further, these observations serve to underscore the point that the 5-HT2A receptor-mediated regulation of IEGs may exhibit sex differences, given that we did not observe any substantial baseline regulation of prefrontal or hippocampal IEG mRNA levels in 5- female mice. Overall the detailed analysis of baseline IEG expression indicates that 5-HT2A receptors exert an opposing pattern of control on several IEGs in the PFC and hippocampus primarily in male mice, suggestive of interactions between estrogen and 5-HT2A receptors in baseline IEG regulation.
Chronic stress exposure has been shown to evoke a decline in IEG expression in the PFC and the hippocampus (Ieraci et al., 2016, Law et al., 2016, Ons et al., 2010). Our findings are in agreement with reference to the regulation of Fos expression with a CUS-evoked reduction noted in the PFC and hippocampi of both male and female wild-type mice. This CUS-evoked decline in prefrontal Fos expression appeared to be attenuated in 5- male, but not female, mice, with a trend (p = 0.053) for a CUS x genotype interaction noted, likely due to the steep baseline reduction noted in 5- male mice. This pattern is similar for Egr2, with an abrogation of the CUS-evoked decline in 5- male, but not female, mice. The loss of the CUS- evoked decline in these specific IEGs (Fos, Egr2) may arise due to a floor effect due to the baseline reduction in the 5- male mice. Within the hippocampus, CUS resulted in a reduction in specific IEGs (Arc, Fos, Egr2, Egr3) irrespective of genotype or sex. Strikingly, specific IEGs (Fosb, and Egr1) showed sexual differences in their regulation by CUS, with a reduction observed only in wild-type female mice, an effect that was blunted in 5- female mice. These observations highlight that the role of the 5-HT2A receptor, both in contributing to the baseline, and CUS-mediated, regulation of specific IEGs is sexually dimorphic.
4.5. Conclusion
The results of this study provide evidence that the 5-HT2A receptor in a sexually dimorphic manner modulates the metabolic and transcriptional sequelae, but not the behavioral despair, that follow due to chronic stress exposure. Given clinical evidence that polymorphisms at the HTR2A gene locus can influence both susceptibility to major depressive disorder and treatment responsivity to antidepressants (Chang et al., 2017, Horstmann et al., 2010, McMahon et al., 2006), our findings motivate further research to examine the interactions between 5-HT2A receptors and estrogen in determining the severity of the molecular, cellular, metabolic and behavioral effects of chronic stress.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ynstr.2017.06.001.
Acknowledgements
We acknowledge Dr. Shital Suryavanshi for assistance with animal breeding.
Funding sources
This research was supported by a TIFR intramural grant (VV).
Statement of interest
The authors report no conflicting financial or non-financial interests.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1.
Fig. S2.
References
- Adell A., Casanovas J.M., Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Aghajanian G.K., Marek G.J. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Bard J.A., Kucharewicz S.A., Zgombick J.M., Weinshank R.L., Branchek T.A., Cohen M.L. Differences in ligand binding profiles between cloned rabbit and human 5-HT1D alpha and 5-HT1D beta receptors: ketanserin and methiothepin distinguish rabbit 5-HT1D receptor subtypes. Naunyn. Schmiedeb. Arch. Pharmacol. 1996;354:237–244. doi: 10.1007/BF00171053. [DOI] [PubMed] [Google Scholar]
- Beig M.I., Baumert M., Walker F.R., Day T.A., Nalivaiko E. Blockade of 5-HT2A receptors suppresses hyperthermic but not cardiovascular responses to psychosocial stress in rats. Neuroscience. 2009;159:1185–1191. doi: 10.1016/j.neuroscience.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Ben-Efraim Y.J., Wasserman D., Wasserman J., Sokolowski M. Family-based study of HTR2A in suicide attempts: observed gene, gene × environment and parent-of-origin associations. Mol. Psychiatry. 2013;18:758–766. doi: 10.1038/mp.2012.86. [DOI] [PubMed] [Google Scholar]
- Benekareddy M., Nair A.R., Dias B.G., Suri D., Autry A.E., Monteggia L.M., Vaidya V.A. Induction of the plasticity-associated immediate early gene Arc by stress and hallucinogens: role of brain-derived neurotrophic factor. Int. J. Neuropsychopharmacol. 2013;16:405–415. doi: 10.1017/S1461145712000168. [DOI] [PubMed] [Google Scholar]
- Benekareddy M., Vadodaria K.C., Nair A.R., Vaidya V.A. Postnatal serotonin type 2 receptor blockade prevents the emergence of anxiety behavior, dysregulated stress-induced immediate early gene responses, and specific transcriptional changes that arise following early life stress. Biol. Psychiatry. 2011;70:1024–1032. doi: 10.1016/j.biopsych.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the False discovery rate: a practical and powerful approach to multiple testing on JSTOR. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- Bookout A.L., Mangelsdorf D.J. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recept. Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné V., Moser P., Roux S., Porsolt R.D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011 doi: 10.1002/0471142301.ns0810as55. (Chapter 8), Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Castro-Vale I., van Rossum E.F.C., Machado J.C., Mota-Cardoso R., Carvalho D. Genetics of glucocorticoid regulation and posttraumatic stress disorder–What do we know? Neurosci. Biobehav. Rev. 2016;63:143–157. doi: 10.1016/j.neubiorev.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Cavus I., Duman R.S. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biol. Psychiatry. 2003;54:59–69. doi: 10.1016/s0006-3223(03)00236-1. [DOI] [PubMed] [Google Scholar]
- Chang C.-C., Fang W.-H., Chang H.-A., Chang T.-C., Shyu J.-F., Huang S.-Y. Serotonin 2A receptor (5-HT2A) gene promoter variant interacts with chronic perceived stress to modulate resting parasympathetic activity in humans. Psychoneuroendocrinology. 2017;76:119–126. doi: 10.1016/j.psyneuen.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Chaouloff F., Berton O., Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Covington H.E., Lobo M.K., Maze I., Vialou V., Hyman J.M., Zaman S., LaPlant Q., Mouzon E., Ghose S., Tamminga C.A., Neve R.L., Deisseroth K., Nestler E.J. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva C.C., Lazzaretti C., Fontanive T., Dartora D.R., Bauereis B., Gamaro G.D. Estrogen-dependent effects on behavior, lipid-profile, and glycemic index of ovariectomized rats subjected to chronic restraint stress. Behav. Process. 2014;103:327–333. doi: 10.1016/j.beproc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Dalla C., Antoniou K., Drossopoulou G., Xagoraris M., Kokras N., Sfikakis A., Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R. Hormones, brain and stress. Endocr. Regul. 2003;37:51–68. [PubMed] [Google Scholar]
- Diaz S.L., Narboux-Nême N., Boutourlinsky K., Doly S., Maroteaux L. Mice lacking the serotonin 5-HT2B receptor as an animal model of resistance to selective serotonin reuptake inhibitors antidepressants. Eur. Neuropsychopharmacol. 2016;26:265–279. doi: 10.1016/j.euroneuro.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Egeland M., Zunszain P.A., Pariante C.M. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat. Rev. Neurosci. 2015;16:189–200. doi: 10.1038/nrn3855. [DOI] [PubMed] [Google Scholar]
- Fernandes C., McKittrick C.R., File S.E., McEwen B.S. Decreased 5-HT1A and increased 5-HT2A receptor binding after chronic corticosterone associated with a behavioural indication of depression but not anxiety. Psychoneuroendocrinology. 1997;22:477–491. doi: 10.1016/s0306-4530(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Frokjaer V.G., Mortensen E.L., Nielsen F.A., Haugbol S., Pinborg L.H., Adams K.H., Svarer C., Hasselbalch S.G., Holm S., Paulson O.B., Knudsen G.M. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol. Psychiatry. 2008;63:569–576. doi: 10.1016/j.biopsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fu J., Ma S., Li X., An S., Li T., Guo K., Lin M., Qu W., Wang S., Dong X., Han X., Fu T., Huang X., Wang T., He S. Long-term stress with hyperglucocorticoidemia-induced hepatic steatosis with VLDL overproduction is dependent on both 5-HT2 receptor and 5-HT synthesis in liver. Int. J. Biol. Sci. 2016;12:219–234. doi: 10.7150/ijbs.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman M.E., Rao S.R., Schultz M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 2014;67:850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Goldstein J.M., Jerram M., Abbs B., Whitfield-Gabrieli S., Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J., Weisstaub N.V., Zhou M., Chan P., Ivic L., Ang R., Lira A., Bradley-Moore M., Ge Y., Zhou Q., Sealfon S.C., Gingrich J.A. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Hansson B., Medina A., Fryklund C., Fex M., Stenkula K.G. Serotonin (5-HT) and 5-HT2A receptor agonists suppress lipolysis in primary rat adipose cells. Biochem. Biophys. Res. Commun. 2016;474:357–363. doi: 10.1016/j.bbrc.2016.04.110. [DOI] [PubMed] [Google Scholar]
- Harvey M.L., Swallows C.L., Cooper M.A. A double dissociation in the effects of 5-HT2A and 5-HT2C receptors on the acquisition and expression of conditioned defeat in Syrian hamsters. Behav. Neurosci. 2012;126:530–537. doi: 10.1037/a0029047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon J.L., Ismaiel A., Ingher S.P., Teitler M., Glennon R.A. Ketanserin analogues: structure-affinity relationships for 5-HT2 and 5-HT1C serotonin receptor binding. J. Med. Chem. 1992;35:4903–4910. doi: 10.1021/jm00104a017. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Hellemans K.G.C., Verma P., Gorzalka B.B., Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci. Biobehav. Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg J.R., Molteni R., Calabrese F., Riva M.a. The serotonin-BDNF duo: developmental implications for the vulnerability to psychopathology. Neurosci. Biobehav. Rev. 2014;43:35–47. doi: 10.1016/j.neubiorev.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Horstmann S., Lucae S., Menke A., Hennings J.M., Ising M., Roeske D., Müller-Myhsok B., Holsboer F., Binder E.B. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology. 2010;35:727–740. doi: 10.1038/npp.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A., Mallei A., Popoli M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016;2016:6212983. doi: 10.1155/2016/6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M., Ito A., Kurosu S., Chaki S. Pharmacological characterization of repeated corticosterone injection-induced depression model in rats. Brain Res. 2010;1359:75–80. doi: 10.1016/j.brainres.2010.08.078. [DOI] [PubMed] [Google Scholar]
- Jett J.D., Bulin S.E., Hatherall L.C., McCartney C.M., Morilak D.A. Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen H., Knigge U., Kjaer A., Vadsholt T., Warberg J. Serotonergic involvement in stress-induced ACTH release. Brain Res. 1998;811:10–20. doi: 10.1016/s0006-8993(98)00901-9. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Pellman B., Kim J.J. Stress effects on the hippocampus: a critical review. Learn. Mem. 2015;22:411–416. doi: 10.1101/lm.037291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfumey L., Mongeau R., Cohen-Salmon C., Hamon M. Corticosteroid–serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci. Biobehav. Rev. 2008;32:1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Law J., Ibarguen-Vargas Y., Belzung C., Surget A. Decline of hippocampal stress reactivity and neuronal ensemble coherence in a mouse model of depression. Psychoneuroendocrinology. 2016;67:113–123. doi: 10.1016/j.psyneuen.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Liu R.-J., Aghajanian G.K. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff F.W. Overview of the genetics of major depressive disorder. Curr. Psychiatry Rep. 2010;12:539–546. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magalhaes A.C., Holmes K.D., Dale L.B., Comps-Agrar L., Lee D., Yadav P.N., Drysdale L., Poulter M.O., Roth B.L., Pin J.-P., Anisman H., Ferguson S.S.G. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat. Neurosci. 2010;13:622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Ruiz R., Puig M.V., Celada P., Shapiro D.A., Roth B.L., Mengod G., Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J. Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B., Morrison J. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Gray J.D., Nasca C. 60 Years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J. Endocrinol. 2015;226:T67–T83. doi: 10.1530/JOE-15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon F.J., Buervenich S., Charney D., Lipsky R., Rush A.J., Wilson A.F., Sorant A.J.M., Papanicolaou G.J., Laje G., Fava M., Trivedi M.H., Wisniewski S.R., Manji H. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am. J. Hum. Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U., van Kampen M., Isovich E., Flügge G., Fuchs E. Chronic psychosocial stress regulates the expression of both GR and MR mRNA in the hippocampal formation of tree shrews. Hippocampus. 2001;11:329–336. doi: 10.1002/hipo.1047. [DOI] [PubMed] [Google Scholar]
- Mongeau R., Martin C.B.P., Chevarin C., Maldonado R., Hamon M., Robledo P., Lanfumey L. 5-HT2C receptor activation prevents stress-induced enhancement of brain 5-HT turnover and extracellular levels in the mouse brain: modulation by chronic paroxetine treatment. J. Neurochem. 2010;115:438–449. doi: 10.1111/j.1471-4159.2010.06932.x. [DOI] [PubMed] [Google Scholar]
- Murrin L.C., Sanders J.D., Bylund D.B. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem. Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R., Muhammad A., Kolb B. Chronic stress induces persistent changes in global DNA methylation and gene expression in the medial prefrontal cortex, orbitofrontal cortex, and hippocampus. Neuroscience. 2016;322:489–499. doi: 10.1016/j.neuroscience.2016.02.053. [DOI] [PubMed] [Google Scholar]
- Nasca C., Xenos D., Barone Y., Caruso A., Scaccianoce S., Matrisciano F., Battaglia G., Mathé A.A., Pittaluga A., Lionetto L., Simmaco M., Nicoletti F. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4804–4809. doi: 10.1073/pnas.1216100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C.M., Park S., Kim H. Serotonin as a new Therapeutic target for diabetes mellitus and obesity. Diabetes Metab. J. 2016;40:89–98. doi: 10.4093/dmj.2016.40.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ons S., Rotllant D., Marín-Blasco I.J., Armario A. Immediate-early gene response to repeated immobilization: fos protein and arc mRNA levels appear to be less sensitive than c-fos mRNA to adaptation. Eur. J. Neurosci. 2010;31:2043–2052. doi: 10.1111/j.1460-9568.2010.07242.x. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y., Blessing W.W., Nalivaiko E. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress. 2008;11:125–133. doi: 10.1080/10253890701638303. [DOI] [PubMed] [Google Scholar]
- Ossowska G., Nowak G., Kata R., Klenk-Majewska B., Danilczuk Z., Żebrowska-Łupina I. Brain monoamine receptors in a chronic unpredictable stress model in rats. J. Neural Transm. 2001;108:311–319. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- Pei Q., Tordera R., Sprakes M., Sharp T. Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacology. 2004;46:331–339. doi: 10.1016/j.neuropharm.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Petit A.C., Quesseveur G., Gressier F., Colle R., David D.J., Gardier A.M., Ferreri F., Lépine J.P., Falissard B., Verstuyft C., Guiard B.P., Corruble E. Converging translational evidence for the involvement of the serotonin 2A receptor gene in major depressive disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry. 2014;54:76–82. doi: 10.1016/j.pnpbp.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Piguet P., Galvan M. Transient and long-lasting actions of 5-HT on rat dentate gyrus neurones in vitro. J. Physiol. 1994;481(Pt 3):629–639. doi: 10.1113/jphysiol.1994.sp020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesseveur G., Nguyen H.T., Gardier A.M., Guiard B.P. 5-HT2 ligands in the treatment of anxiety and depression. Expert Opin. Investig. Drugs. 2012;21:1701–1725. doi: 10.1517/13543784.2012.719872. [DOI] [PubMed] [Google Scholar]
- Quesseveur G., Petit A.C., Nguyen H.T., Dahan L., Colle R., Rotenberg S., Seif I., Robert P., David D., Guilloux J.-P., Gardier A.M., Verstuyft C., Becquemont L., Corruble E., Guiard B.P. Genetic dysfunction of serotonin 2A receptor hampers response to antidepressant drugs: a translational approach. Neuropharmacology. 2016;105:142–153. doi: 10.1016/j.neuropharm.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Quesseveur G., Repérant C., David D.J., Gardier A.M., Sanchez C., Guiard B.P. 5-HT₂A receptor inactivation potentiates the acute antidepressant-like activity of escitalopram: involvement of the noradrenergic system. Exp. Brain Res. 2013;226:285–295. doi: 10.1007/s00221-013-3434-3. [DOI] [PubMed] [Google Scholar]
- Rebuffé-Scrive M., Walsh U.A., McEwen B., Rodin J. Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Physiol. Behav. 1992;52:583–590. doi: 10.1016/0031-9384(92)90351-2. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., Collins A., Saliba R.S., Mifsud K.R., Carter S.D., Gutierrez-Mecinas M., Qian X., Linthorst A.C.E. Glucocorticoids, epigenetic control and stress resilience. Neurobiol. Stress. 2015;1:44–59. doi: 10.1016/j.ynstr.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M., Lambe E.K., Liu R., Teillon S., Liu J., Akbarian S., Roffler-Tarlov S., Jaenisch R., Aghajanian G.K. Severe deficits in 5-HT2A -mediated neurotransmission in BDNF conditional mutant mice. J. Neurobiol. 2006;66:408–420. doi: 10.1002/neu.20233. [DOI] [PubMed] [Google Scholar]
- Rosmond R., Bouchard C., Björntorp P. 5-HT2A receptor gene promoter polymorphism in relation to abdominal obesity and cortisol. Obes. Res. 2002;10:585–589. doi: 10.1038/oby.2002.79. [DOI] [PubMed] [Google Scholar]
- Santini M.A., Klein A.B., El-Sayed M., Ratner C., Knudsen G.M., Mikkelsen J.D., Aznar S. Novelty-induced activity-regulated cytoskeletal-associated protein (Arc) expression in frontal cortex requires serotonin 2A receptor activation. Neuroscience. 2011;190:251–257. doi: 10.1016/j.neuroscience.2011.05.048. [DOI] [PubMed] [Google Scholar]
- Sargin D., Oliver D.K., Lambe E.K. Chronic social isolation reduces 5-HT neuronal activity via upregulated SK3 calcium-activated potassium channels. Elife. 2016;5 doi: 10.7554/eLife.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R.Y., Andrade R. 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J. Pharmacol. Exp. Ther. 1998;285:805–812. [PubMed] [Google Scholar]
- Takao K., Nagatani T., Kitamura Y., Kawasaki K., Hayakawa H., Yamawaki S. Chronic forced swim stress of rats increases frontal cortical 5-HT2 receptors and the wet-dog shakes they mediate, but not frontal cortical beta-adrenoceptors. Eur. J. Pharmacol. 1995;294:721–726. doi: 10.1016/0014-2999(95)00620-6. [DOI] [PubMed] [Google Scholar]
- Tasker J.G., Herman J.P. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14:398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya V.a, Marek G.J., Aghajanian G.K., Duman R.S. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J. Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q., Gao K., Rong H., Wu M., Wang H., Wang X., Wang G., Liu Z. Histone modifications of the Crhr1 gene in a rat model of depression following chronic stress. Behav. Brain Res. 2014;271:1–6. doi: 10.1016/j.bbr.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Gould E., McEwen B.S. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weisstaub N.V., Zhou M., Lira A., Lambe E., González-Maeso J., Hornung J.-P., Sibille E., Underwood M., Itohara S., Dauer W.T., Ansorge M.S., Morelli E., Mann J.J., Toth M., Aghajanian G., Sealfon S.C., Hen R., Gingrich J.A. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Willner P. Reliability of the chronic mild stress model of depression: a user survey. Neurobiol. Stress. 2016 doi: 10.1016/j.ynstr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Wyskiel D.R., Andrade R. Serotonin excites hippocampal CA1 GABAergic interneurons at the stratum radiatum-stratum lacunosum moleculare border. Hippocampus. 2016;26:1107–1114. doi: 10.1002/hipo.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Ma X.M., Chen H. Bin, Zhou M.H., Qiao H., An S.C. Orbitofrontal cortex 5-HT2A receptor mediates chronic stress-induced depressive-like behaviors and alterations of spine density and Kalirin7. Neuropharmacology. 2016;109:7–17. doi: 10.1016/j.neuropharm.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Zhang Z.-W. Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J. Neurosci. 2003;23:3373–3384. doi: 10.1523/JNEUROSCI.23-08-03373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]