Abstract
The oral mucosa is exposed to a high density and diversity of gram-positive and gram-negative bacteria, but very little is known about how immune homeostasis is maintained in this environment, particularly in the inflammatory disease chronic periodontitis (CP). The cells of the innate immune response recognize bacterial structures via the Toll-like receptors (TLR). This activates intracellular signaling and transcription of proteins essential for the induction of an adaptive immune response; however, if unregulated, it can lead to destructive inflammatory responses. Using single-immunoenzyme labeling, we show that the human oral mucosa (gingiva) is infiltrated by large numbers of TLR2+ and TLR4+ cells and that their numbers increase significantly in CP, relative to health (P < 0.05, Student's t test). We also show that the numbers of TLR2+ but not TLR4+ cells increase linearly with inflammation (r2 = 0.33, P < 0.05). Double-immunofluorescence analysis confirms that TLR2 is coexpressed by monocytes (MC)/macrophages (mφ) in situ. Further analysis of gingival tissues by quantitative real-time PCR, however, indicates that despite a threefold increase in the expression of interleukin-1β (IL-1β) mRNA during CP, there is significant (30-fold) downregulation of TLR2 mRNA (P < 0.05, Student's t test). Also showing similar trends are the levels of TLR4 (ninefold reduction), TLR5 (twofold reduction), and MD-2 (sevenfold reduction) mRNA in CP patients compared to healthy persons, while the level of CD14 was unchanged. In vitro studies with human MC indicate that MC respond to an initial stimulus of lipopolysaccharide (LPS) from Porphyromonas gingivalis (PgLPS) or Escherichia coli (EcLPS) by upregulation of TLR2 and TLR4 mRNA and protein; moreover, IL-1β mRNA is induced and tumor necrosis factor alpha (TNF-α), IL-10, IL-6, and IL-8 proteins are secreted. However, restimulation of MC with either PgLPS or EcLPS downregulates TLR2 and TLR4 mRNA and protein and IL-1β mRNA and induces a ca. 10-fold reduction in TNF-α secretion, suggesting the induction of endotoxin tolerance by either LPS. Less susceptible to tolerance than TNF-α were IL-6, IL-10, and IL-8. These studies suggest that certain components of the innate oral mucosal immune response, most notably TLRs and inflammatory cytokines, may become tolerized during sustained exposure to bacterial structures such as LPS and that this may be one mechanism used in the oral mucosa to attempt to regulate local immune responses.
INTRODUCTION
The oral cavity harbors approximately 500 distinct bacterial species (32), including commensals and pathogens. Gut commensals play an important early role in stimulating immune responses during postnatal development. Later on, these local and systemic immune responses are downmodulated and reprogrammed, e.g., by induction of oral tolerance (34, 37). Induction of immune tolerance toward commensals combined with responsiveness to pathogens is essential to sustaining immune homeostasis while preventing life-threatening infections (37). It is unclear how the oral mucosa is able to quickly distinguish commensals from pathogens and mount an appropriate response (or lack thereof).
Toll-like receptors (TLRs) are the principle pattern recognition receptors on innate immune cells. TLRs recognize microbial structure and transmit this information into the cell, culminating in an inflammatory cytokine response and in costimulatory molecule expression involved in induction of adaptive immunity (reviewed in reference 2). TLR4, along with CD14 and other adaptor molecules, recognize pathogen-associated molecular patterns such as lipopolysaccharides (LPS) from gram-negative enteric bacteria. TLR2, along with TLR1/6, recognizes gram-positive peptodoglycans (e.g., from oral commensals) (36). One recent study indicates that both TLR2- and TLR4-positive cells infiltrate the oral mucosa (i.e., gingiva) in periodontal health and disease (28), but very little is understood about the overall expression patterns of PRRs in the human oral mucosa in health and in chronic periodontitis (CP) and how they regulate local immune responsiveness. While monocytes (MC)/macrophages (mφ) constitutively express TLRs, recent evidence indicates that TLR expression can be downregulated by repeated exposure to LPS, resulting in downmodulation of the inflammatory cytokine response (i.e., endotoxin tolerance) (11).
Porphyromonas gingivalis is a gram-negative mucosal pathogen associated with CP (12). P. gingivalis is thought to survive in and colonize the oral mucosa by evading uptake by polymorphonuclear leukocytes (8) and by invading oral epithelial cells (38) and dendritic cells (20). However, based on studies of the genetic structure of natural populations of P. gingivalis, no association has been found between specific genetic lineages of P. gingivalis and the type of disease it causes or its invasive potential (23). In short, P. gingivalis more closely resembles an opportunist (23) or commensal (27) than a pathogen. Moreover, P. gingivalis bears an LPS (PgLPS) with low endotoxin activity that primarily targets the “commensal receptor” TLR2 (15, 16, 21, 24) but also has activity for TLR4 (4, 10). PgLPS induces a predominant TH2-type immune response in vivo (33) and in vitro (18), and one report suggests that PgLPS can induce immune tolerance in vitro (5). We understand very little about the effects of PgLPS on TLR expression and on immune regulation in general.
In an effort to understand innate responsiveness to LPS in oral mucosa in healthy persons and those with CP, we have analyzed the expression of TLR2/TLR4 protein and mRNA in gingiva in situ. We show here that the gingiva is increasingly infiltrated with TLR2+ and TLR4+ cells, many of which are MC/mφ, in persons with CP; however, the overall expression of TLR2 and TLR4 mRNA is negatively regulated in situ. Also showing similar trends are TLR5 and MD-2 mRNA, while CD14 was unchanged and the interleukin-1β (IL-1β) mRNA concentration was increased in situ. Study of human MC indicates that negative regulation of TLR2 and TLR4 mRNA and proteins is induced by stimulus and challenge with PgLPS or Escherichia coli LPS (EcLPS). This also results in downmodulation of tumor necrosis factor alpha (TNF-α) protein and IL-1β mRNA, while other cytokines (IL-6, IL-10) appear more resistant and IL-8 is not affected by LPS stimulus or challenge. Taken as a whole, these results suggest the intriguing possibility that decreased expression of TLR mRNA in the oral mucosa, despite infiltration with TLR+ inflammatory cells, may be a result of differential regulation induced by repeated challenge with endotoxin in situ. This may be a mechanism for the host to attempt to reestablish tissue homeostasis during chronic periodontitis.
(Work in this paper was submitted by M. Muthukuru in partial fulfillment of a Ph.D. degree at Stony Brook University.)
MATERIALS AND METHODS
Clinical diagnoses and tissue collection.
The Institutional Review Board approved our protocol. The clinical criteria for CP and health were as previously described (19). Briefly, in subjects with CP, the sextant from which tissue was harvested exhibited at least four teeth with probing depth of 5 to 10 mm, attachment loss of 5 to 10 mm, alveolar bone loss present, and bleeding on probing present. Subjects with gingival health exhibited probing depth less than 4 mm, attachment loss of 0 mm, alveolar bone loss absent, and bleeding on probing absent. Based on the clinical criterial of gingival health and CP, a total of 15 convenience samples from the interproximal papilla were collected under informed consent for immunohistochemistry and PCR analysis. Additional gingival samples totaling 12 from subjects with CP and 12 from healthy controls were obtained for PCR analysis. Whenever possible, both immunohistochemistry and PCR analysis were performed on bisected tissues from the same patients. The mean age of the subjects with CP and the healthy controls was 55 ± 14.0 and 47.3 ± 10.9 years, respectively, while the distribution of males in the subjects with CP and the controls was 57 and 36%, respectively; however, no significance differences were detected in either the age or gender distribution of subjects in the CP and control groups (P < 0.05, Student's t test).
Immunohistochemistry.
For immunohistochemistry, 7-μm-thick serial sections were stained with hematoxylin and eosin (H&E) to confirm the clinical diagnosis by histological means. Single immunoenzyme staining was performed by the biotin-streptavidin-peroxidase method (Vectastain ABC Elite kit) with the antibodies (from eBiosciences TLR2 clone TL2.1; TLR4 clone HTA125), and the specificity of the antibodies was confirmed by replacing each with the respective isotype control. To quantitate the infiltration of tissues by TLR-positive cells, light microscopy images were acquired with a Nikon Eclipse E600 microscope equipped with a color high-resolution charge-coupled device CCD camera (RT Slider; Diagnostic Instruments, Inc., Sterling Heights, Mich.) and PC running Image-Pro software (Media Cybernetics, Inc., Silver Spring, Md.) and the number of TLR+ cells was quantitated by image-enhanced histomorphometry, as described previously (17).
For double immunofluorescence staining, slides were rehydrated, blocked, and incubated for 1 h at room temperature with CD68 (immunoglobulin G2b)-Texas Red. In a subsequent secondary step, fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal antibodies to TLR2 (eBiosciences TLR2 clone TL2.1) were used. The specificity of the primary and secondary antibodies was confirmed by replacing each with the respective isotype controls. Images were sharpened using two-dimensional deconvolution.
Real-time PCR. (i) RNA extraction and cDNA synthesis.
Gingival tissues were placed in RNAlater RNA-stabilizing reagent (Qiagen) and frozen at −80°C for later use. Frozen tissues were then ground and homogenized, and total RNA was extracted (each sample weighing 75 to 100 mg) using Qiagen RNeasy midi kits as specified by the manufacturer. Avian reverse transcriptase (RT) first-strand kits (Sigma) were used to synthesize cDNA from total RNA. The concentration of total RNA was determined at via the optical density at 260 nm (OD260), and small discrepancies in the amount of gingival tissue collected were corrected by loading the same concentration of RNA for cDNA synthesis. The purity of cDNA was determined by analysis of the OD260/OD280 ratio.
(ii) Primers for PCR.
Nucleotide sequences were determined from PubMed (National Center for Biomedical Information), and the primers were custom designed using primer3 software. Table 1 shows the sequences and product sizes of the various primers used for PCR quantitation.
TABLE 1.
Primer sequences and product sizes for PCR quantitation
| Gene | Sequence (5′ to 3′) of:
|
Size of product (bp) | |
|---|---|---|---|
| Left primer | Right primer | ||
| TLR2 | GGAGGCTGCATATTCCAAGG | GCCAGGCATCCTCACAGG | 216 |
| TLR4 | CTTGACCTTCCTGGACCTCTC | ACTTGGAAAATGCTGTAGTTCC | 217 |
| TLR5 | CGGAAGGTTGTGATGAAGAGG | CTGCTGAAGCACAAATAGGC | 226 |
| CD14 | CTGTGCAACTTCTCCGAACC | CCAGTAGCTGAGCAGGAACC | 217 |
| MD2 | TCTGCAACTCATCCGATGC | GCGCTTTGGAAGATTCATGG | 200 |
| IL-1β | GGCAGAAAGGGAACAGAAAGG | AGTGAGTAGGAGAGGTGAGAGAGG | 200 |
| β-Actin | ACTCTTCCAGCCTTCCTTCC | GTTGGCGTACAGGTCTTTGC | 204 |
(iii) Real-time RT-PCR quantitation.
Real-time RT-PCR analysis was performed using the iCycler (Bio-Rad, Hercules, Calif.) with Sybr Green kits (Bio-Rad) and mRNA quantitation by standard curve method. For each transcript analyzed, a standard curve with predetermined concentrations and serial diluted respective PCR amplification products from 0.1 to 0.00001 ng was constructed. This approach allows the standards to be amplified in the same way as the template cDNA in the unknown samples since the product sequence and size are identical. Levels of β-actin mRNA served as an internal control to normalize samples for variations in sample volume loading, presence of inhibitors, and nucleic acid recovery during extraction and cDNA synthesis procedures (discussed in the on-line Roche PCR protocols). The normalized initial concentration of each transcript in every sample was converted to the initial copy number by using the formula Amount (copies/per microliter) = 6 × 1023 (copies/per mole) × concentration (grams per microliter)/molecular mass (grams per mole), where the average molecular weight of double-stranded DNA equals the number of base pairs × 660 Da/bp. All analyses were performed in triplicate.
Blood MC culture and LPS isolation.
MC were isolated from mononuclear fractions of peripheral blood of healthy donors by adherence to polystyrene culture plates. Briefly, whole peripheral blood was centrifuged on Ficoll and the MC fraction was pelleted and resuspended in RPMI 1640 (Invitrogen) with 10% heat-inactivated fetal calf serum (Sigma, St. Louis, Mo.). Mononuclear cells (2.7 × 108) were seeded on polystyrene culture plates and incubated for 2 h at 37°C in a CO2 incubator. After the nonadherent cells were washed off, the adherent MC were lifted off the culture dishes by using a trypsin-EDTA solution. The MC were cultured in RPMI 1640 with 10% heat-inactivated fetal calf serum. The percentage of viable MC (typically >90% after LPS stimulation) was monitored by trypan blue exclusion. LPS was isolated from either P. gingivalis 381 or E. coli (ATCC type strain 25299) by hot-phenol-water extraction followed by isopycnic density gradient centrifugation and was further purified of contaminating nucleic acids, proteins, and lipoproteins as previously described (7). In addition, some of the LPS preparations (purified identically) were provided courtesy of T. E. Van Dyke, Boston University Goldman School of Dental Medicine.
In vitro LPS stimulation.
Preliminary studies (not shown) established the dose response and kinetics of MC expression of TLR mRNA and protein when stimulated with PgLPS and EcLPS. The in vitro study design involves incubation of MC for 24 h with no LPS (no stimulus) or an initial LPS stimulus (1,000 ng of PgLPS or EcLPS per ml), followed by a challenge with the same LPS at the same dosage for 1 h to determine mRNA or 24 h for analysis of protein. The cells were then pelleted and washed with cold PBS three times to be used for PCR and flow cytometry analysis.
Flow cytometry analysis.
After LPS stimulation and challenge, MC were incubated with either TLR2 or TLR4 antibodies (or their respective isotype matched controls) for 30 min at 4°C, washed, and fixed in 1% paraformaldehyde. Analysis was performed with a FACScalibur instrument (Becton Dickinson). TLR expression was analyzed as the percentage of positive cells in the relevant population defined by forward-scatter and side-scatter characteristics. Expression levels were evaluated by assessing the mean fluorescence intensity indices calculated by relating the MFI noted with the relevant monoclonal antibody to that with the isotype control monoclonal antibody for samples labeled in parallel and acquired using the same setting.
Cytokine analysis.
MC culture supernatants were collected, and the inflammatory cytokines (TNF-α, IL-6, IL-8, and IL-10) were analyzed by flow cytometry using a cytometric bead array (CBA kit; BD Biosciences). Based on a standard curve for each cytokine, the CBA software calculates levels in picograms per milliliter. IL-1β regulation in MC was analyzed through real-time PCR, since the levels of protein were too low for detection.
RESULTS
TLR+ cells predominantly infiltrate gingival epithelium and lamina propria in health and disease, respectively; many of TLR2+ cells are MC/mφ.
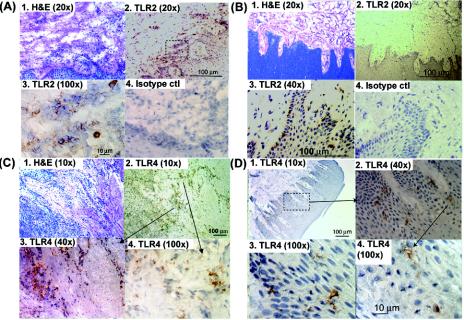
Single-immunoenzyme staining of serial sections of human gingiva indicates that the gingival epithelia and lamina propria are infiltrated with TLR2+ and TLR4+ cells in health and disease. Shown are representative staining patterns for TLR2 (Fig. 1A and 1B) and TLR4 (Fig. 1C and D) in gingival specimens from healthy controls (Fig. 1B and D) and persons with CP (Fig. 1A and C). It was observed that in the setting of gingival health most of the TLR+ cells are located in the epithelium (Fig. 1B and D) while in persons with CP most of the TLR+ cells were located in the lamina propria (Fig. 1A and C). The level of inflammation in the collected specimens from persons with CP and healthy controls were confirmed by H&E staining, as shown in panel 1 of Fig. 1A to C. Double-immunfluorescence analysis (Fig. 2) of gingiva from persons with CP revealed that MC/mφ were positive for TLR2 in situ.
FIG. 1.
Oral mucosa infiltrated with TLR2+ and TLR4+ cells. Representative serial sections of interproximal gingival tissue from patients with CP (A and C) persons in relative health (B and D) were single-immunoenzyme stained as described in Materials and Methods. Images of TLR2+ cells (A and B) and TLR4+ cells (C and D) were obtained with a 20× objective (panels A1, A2, B1, and B2), a 40× objective (panels B3, B4, C3, and D2), a 10× objective (panels C1, C2, and D1), 100× objective (panels A3, C4, D3, and D4) and captured using image-enhanced light microscopy. The specificity of the antibodies was confirmed by negative staining with the respective isotype controls (e.g., panels A4 and B4). The sections were counterstained with hematoxylin.
FIG. 2.
TLR2+ cells in the oral mucosa include MC/mφ. A representative serial section of tissue from a person with CP was stained with monoclonal anti-CD68-Texas Red (CD68-TxR) followed by monoclonal anti-TLR2-FITC (TLR2-FITC) antibodies, as indicated in Materials and Methods. Conjugated mouse immunoglobulins of the same isotypes were used as controls (not shown). (A to C) Shown are image-enhanced double-fluorescence images, sharpened digitally with two-dimensional-deconvolution software. Evident are CD68+ MC/mφ (red) (A), TLR2+ cells (green) (B), and TLR2+ MC/mφ (merge, yellow arrows) (C). (D) A digital merge of a differential interference contrast image with panel C. (E) Final digital enlargement with a magnification of approximately ×1,500.
Increased infiltration with TLR2+ and TLR4+ cells in persons with CP.
The numbers of immunoreactive cells per 20× field in samples from healthy controls and persons with CP were determined by image-enhanced histomorphometry. We observed a statistically significant increase in the number of TLR2+ and TLR4+ cells in persons with CP (P < 0.05, Student's t test) (Fig. 3A). Moreover, linear-regression analysis of the number of TLR2+ and TLR4+ cells with the inflammatory cell infiltrate (by H&E staining) revealed a significant association of TLR2+ cells (Fig. 3B) with gingival inflammation (r2 = 0.33, p = 0.01), but not of TLR4+ cells (Fig. 3C).
FIG. 3.
Increased infiltration of gingiva with TLR2- and TLR4-positive cells in persons with CP. Interproximal gingival tissues from subjects with gingival health and those with disease (CP) were single-immunoenzyme labeled as described in Materials and Methods and analyzed by image-enhanced histomorphometry. (A) Shown are the mean number of immunoreactive cells/20× field; error bars indicate standard errors. Asterisks indicate a statistically significant increase in diseased versus healthy tissue by Student's t test (P < 0.05). (B and C) Linear-regression analysis of the number of TLR2+ (B) and TLR4+ (C) cells with the number of H&E-positive inflammatory cells.
Significant negative regulation of TLR2 mRNA in diseased human gingiva by real-time PCR.
Gingival tissues were analyzed by quantitative real-time PCR to establish the overall level of mRNA for pattern recognition receptors and for IL-1β (Fig. 4). The results indicate that, despite increased numbers of TLR2+ and TLR4+ cells infiltrating the tissues (Fig. 1 and 3A) and a threefold increase in IL-1β mRNA levels (Fig. 4F), confirming the inflammatory status in the gingival specimens (9), TLR2 mRNA levels were significantly decreased in persons with CP relative to healthy controls (30-fold [P < 0.05, Student's t test]) (Fig. 4A). Showing similar trends were TLR4 (Fig. 4B), TLR5 (Fig. 4C), and MD2 (Fig. 4E), whose levels decreased ninefold, twofold, and sevenfold, respectively. CD14 expression was unchanged (Fig. 4D) in both the groups of healthy controls and persons with CP.
FIG. 4.
Negative regulation of TLR mRNA in human gingiva from subjects with CP. Eight of the tissues used for immunohistochemistry were randomly selected and bisected for total RNA extraction as described in Materials and Methods, along with four additional samples form healthy and diseased subjects. The total RNA was normalized among all samples, and real-time RT-PCR was used to quantitate TLR2 (A), TLR4 (B), TLR5 (C), CD14 (D), and MD2 (E) mRNA expression levels, along with IL-1β as an inflammatory marker (F). For each transcript, conventional PCR-amplified products (cleaned, with concentrations determined at OD260) were used as standards (from 0.1 to 0.00001 ng) to generate a standard curve for absolute real-time quantitation. The absolute mRNA levels of all the genes were then normalized to β-actin levels of individual tissue samples. All quantitations were performed in triplicate. The normalized initial concentration of each transcript in every sample was converted to the initial copy number (see Materials and Methods). Error bars indicate standard error. The level of mRNA for TLR2 in diseased samples was statistically different from that in samples from healthy controls (P < 0.05, Student's t test).
Differential regulation of TLR2 and TLR4 expression on MC on LPS stimulus and challenge.
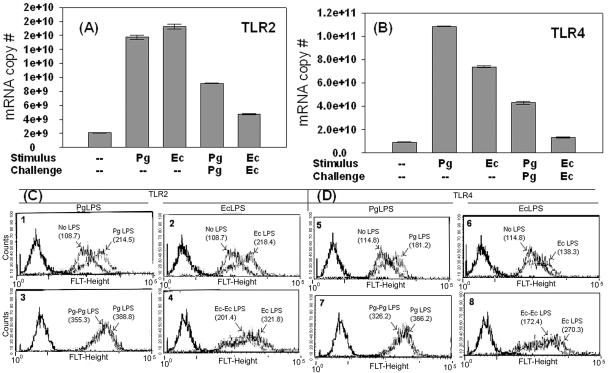
MC and mφ express TLRs constitutively (2), are found in the gingival tissues (31), and express TLRs in situ (Fig. 2) (28). We therefore used peripheral blood-derived MC as an in vitro model of TLR expression to determine if TLRs could be differentially regulated by their agonists (30). We observed that TLR2 (Fig. 5A) and TLR4 (Fig. 5B) mRNA levels increase as early as 1 h after initial stimulation with PgLPS or EcLPS. This is followed by TLR2 (Fig 5C panels 1 and 2) and TLR4 (Fig. 5D, panels 5 and 6) protein up-regulation at 24 h. To determine if TLR mRNA in MC was susceptible to negative regulation by repeated exposure to LPS, MC were challenged with a second dose of the same LPS (i.e., LPS challenge). The results (Fig. 5A and B) indicate that, indeed, LPS challenge induces negative regulation of TLR2 and TLR4 mRNA. Surface proteins of TLR2 (Fig. 5C, panels 3 and 4) and TLR4 (Fig. 5D, panels 5 and 6) were also downregulated during challenge with either LPS. Consistent with the level of mRNA, EcLPS was a stronger negative regulator of TLR2 and TLR4 surface protein (Fig. 5C, panel 4, and 5D, panel 8) than was PgLPS (Fig. 5C, panel 3, and 5D, panel 7).
FIG. 5.
Positive and negative regulation of TLR mRNA in MC by stimulus or challenge with PgLPS or EcLPS. (A and B) MC were stimulated at 37°C for 24 h with 1,000 ng of PgLPS (Pg), EcLPS (Ec), or no LPS (---), challenged with PgLPS (PgPg) or EcLPS (EcEc) for an additional 1 h, pelleted, washed, and processed for total RNA and for analysis of (A) TLR2 mRNA and (B) TLR4 mRNA by quantitative real-time PCR as described in Materials and Methods. The assay was performed three separate times, and the means and standard errors of three separate analyses are shown. (C and D) Fluorescence-activated all sorter analysis of human MC stimulated as in panels (A and B), except that the challenge occurred for 24 h to enable the proteins to be transcribed. A detailed account of the method is given in Materials and Methods. Histograms show mean fluorescence intensity, while numbers in parentheses indicate the geometric means of MC stimulated with the indicated regimen of stimulus and challenge. Results are representative of five separate experiments. FLT-Height, fluorescence intensity of TLRs.
TLR-mediated tolerance obtunds the inflammatory cytokine response.
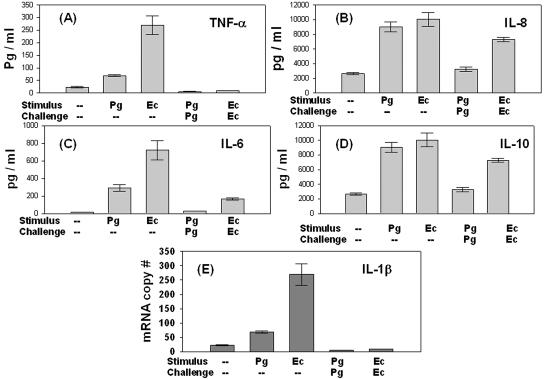
TLR activation culminates in transcription of inflammatory cytokines (2). Accordingly, the supernatants from LPS-stimulated and -challenged MCs were analyzed by flow cytometry (Fig. 6 A to D). Initial stimulation with EcLPS induced increased TNF-α and IL-6 production compared to initial stimulation with PgLPS; however, on challenge with the same LPS, a ∼10-fold reduction in TNF-α secretion (Fig. 6A) and ∼4-fold reduction in IL-6 production (Fig. 6C) was observed. IL-1β protein was undetectable, and so mRNA was analyzed by real-time PCR (Fig. 6E), revealing that IL-1β mRNA levels were downregulated by LPS challenge. However, compared to TNF-α, secretion of IL-6 (Fig. 6C), IL-10 (Fig. 6D), and IL-8 (Fig. 6B) appeared to be relatively less susceptible to endotoxin tolerance induction.
FIG. 6.
Negative regulation of cytokine response by TLR-mediated endotoxin tolerance. (A to D) Supernatants from MC treated with LPS as described in Materials and Methods were analyzed for TNF-α (A), IL-8 (B), IL-6 (C), and IL-10 (D) by flow cytometry using a CBA kit. The assay was performed in triplicate. Based on the standard curve for each cytokine, the CBA software calculates levels in picograms per milliliter. (E) Mean levels of IL-1β mRNA from triplicate samples analyzed by real-time PCR, as described in the legend to Fig. 3 and in Materials and Methods. Error bars indicate standard deviation.
DISCUSSION
We show here that TLR2+ and TLR4+ cells (Fig. 1) are present in gingival tissues from both healthy controls and subjects with CP and that MC/mφ coexpress TLR2 in situ (Fig. 2). Other cell types including polymorphonuclear leukocytes, dendritic cells, lymphocytes, epithelial cells, and endothelial cells have variably been described to express TLRs (3), but their contribution to the TLR+ populations in the human oral mucosa in situ is unknown. We further show that in inflamed tissues in persons with CP, as previously reported (9, 22), there is a significant increase in the level of IL-1β (Fig. 4F); moreover, the number of TLR2+ and TLR4+ cells increase in persons with CP (Fig. 3A), but only TLR2+ cells increase in number linearly with inflammation (Fig. 3B). A previous study, using single immunohistochemistry, also shows that TLR2+ and TLR4+ cell numbers increase in the inflamed gingival tissues (28), although levels of transcripts were not quantitated. That study further demonstrated that the lamina propria was the predominant site of accumulation of TLR2+ cells in persons with CP, as we have shown in Fig. 1.
Although the number of TLR+ cells infiltrating the gingiva increases in persons with CP, there is an overall negative regulation of mRNA for TLR2 (30-fold decrease), TLR4 (9-fold decrease), TLR5 (2-fold decrease), and MD-2 (7-fold decrease) in gingiva from persons with CP relative to healthy controls (Fig. 4). While the tissues samples used were samples of convenience and thus were not matched for sex and age (or smoking status), the distribution of these variables was not significantly different in the two groups. Moreover, in vitro mechanistic studies shown here demonstrate how TLR mRNA can be differentially regulated depending on exposure to LPS. Intestinal epithelial cells limit dysregulated LPS signaling by downregulating the expression of MD-2 and TLR4 (1). In a more recent study, intestinal epithelial cells were shown to be broadly unresponsive to commensals (i.e., TLR2 ligands), secondary to deficient expression of TLR2 and TLR6 and increased expression of Tollip (a TLR-inhibitory protein) (26). Evidence that P. gingivalis resembles a commensal clonally (23, 27) and expresses an LPS with low endotoxin activity that targets primarily TLR2 (15, 16, 21, 24) suggests the intriguing possibility that this pathogen may survive in the oral cavity by posing as a commensal, evocative of gut commensals.
We have hypothesized here that MC and other cells in the gingiva are subjected to negative regulation of TLRs as a result of repeated exposure to pathogen-associated molecular patterns from the plaque biofilm (12), inducing a local form of endotoxin tolerance. Endotoxin tolerance is a well-described in vivo phenomenon that results from sustained exposure of sublethal doses of LPS (6). Downregulation of TLRs is but one mechanism to account for endotoxin tolerance (30). Other investigators have demonstrated TLR-independent tolerance mediated by intracellular signaling pathways (11). In the present study, human peripheral blood MC were stimulated and then challenged with PgLPS or EcLPS (Fig. 5). We show that initial stimulation with either LPS results in upregulation of TLR2 and TLR4 at the level of mRNA (Fig. 5A and B) and protein (Fig. 5C, panels 1 and 2, and 5D, panels 5 and 6). PgLPS has been shown previously to upregulate TLR2 on MC and dendritic cells; moreover, exogenously added IL-10 inhibits TLR2 upregulation (5). Our previous work in vivo (33) and in vitro (18) shows that PgLPS-stimulates dendritic cells to release autocrine-acting IL-10 and IL-13 (but not IL-12 or IP-10), which inhibits T-cell proliferation and TH1 effector responses. We show here that a second stimulation (challenge) with either LPS induces downregulation of TLR2 (Fig. 5A) and TLR4 (Fig. 5B) mRNA and protein (Fig. 5C). Although PgLPS and EcLPS are TLR2 (16) and TLR4 (36) ligands, respectively, this receptor-ligand preference is not strict: they can cross-activate and cross-tolerize against other TLR agonists (10, 11).
The regulation of TLR expression on MC in vitro (Fig. 5) is consistent with the inflammatory cytokine response that follows (Fig. 5), as reported (30). We show that IL-1β, TNF-α, IL-6, IL-8, and IL-10 levels increase on initial LPS stimulus but were not equally susceptible to a decrease on LPS challenge (Fig. 6). Most notably, IL-10 and IL-8 were refractory to endotoxin tolerance. Previous studies have indicated that while IL-1β and TNF-α are suppressed in tolerized cells, cellular reprogramming results in enhanced IL-10 synthesis (34). Our cytokine data (Fig. 6) support these findings. It is tempting to speculate that preservation of IL-10, while IL-1β and TNF-α are downregulated, could play a protective role against inflammatory tissue destruction. It is important to note that IL-1β, TNF-α, and IL-6, apart from being inflammatory mediators, also facilitate soft and hard tissue destruction and favor the pathogenesis of periodontitis (31).
In addition to the TLRs, other receptors in the LPS recognition complex, as well as multiple downstream signaling events, are probably involved during endotoxin tolerance, as reported (11, 25). Moreover, TLR and IL-1β have common signaling pathways, since TLRs are part of the IL-1R family (39). There is also evidence that TNF-α acts in an autocrine fashion in inducing NF-κB through activation of TRAF2 (35) and NF-κB-inducing kinase (29). Convergence of intracellular signaling pathways by TLRs and inflammatory cytokines (IL-1β and TNF-α), along with reprogramming of signal transduction and gene transcription, may explain to a certain extent the cytokine profile during endotoxin tolerance. Interestingly, LPS-sensitized THP-1 cells are tolerized not only to further LPS stimulation but also to inflammatory cytokine (IL-1β and TNF-α) stimulation (14, 25, 40). A variable extent of cross-tolerance at the level of mitogen-activated protein kinase and NF-κB activation is exhibited when these cells are primed with these cytokines and then challenged with the same cytokines or with LPS (13). LPS- or IL-1β-sensitized THP-1 cells are resistant to TNF-α production during further IL-1β or LPS stimulation.
In conclusion, negative regulation of TLRs, and the downstream events such as inflammatory cytokine production, could be regulatory measures that serve as an evolutionary advantage for the host oral mucosal immune system to help restore tissue integrity and homeostasis in the face of microbial onslaught.
Acknowledgments
These studies are supported by NIH/NIDCR grant R01 DE14328 (to C.W.C).
We thank T. E. VanDyke for contributing some of the LPS samples used here.
Editor: J. D. Clements
REFERENCES
- 1.Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. [DOI] [PubMed] [Google Scholar]
- 2.Beutler, B., K. Hoebe, X. Du, and R. J. Ulevitch. 2003. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74:479-485. [DOI] [PubMed] [Google Scholar]
- 3.Cristofaro, P., S. M. Opal. 2003. The Toll-like receptors and their role in septic shock. Expert Opin. Ther. Targets 7:603-612. [DOI] [PubMed] [Google Scholar]
- 4.Coats, S. R., R. A. Reife, B. W. Bainbridge, T. T. Pham, and R. P. Darveau. 2003. Porphyromonas gingivalis lipopolysaccharide antagonizes Escherichia coli lipopolysaccharide at Toll-like receptor 4 in human endothelial cells. Infect. Immun. 71:6799-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, N., J. Morisset, and D. Emilie. 2004. Induction of tolerance by Porphyromonas gingivalis on APCs: a mechanism implicated in periodontal infection. J. Dent. Res. 83:429-433. [DOI] [PubMed] [Google Scholar]
- 6.Cook, J. A. 1998. Molecular basis of endotoxin tolerance. Ann. N. Y. Acad. Sci. 51:426-428. [DOI] [PubMed] [Google Scholar]
- 7.Cutler, C. W., P. I. Eke, C. A. Genco, T. E. Van Dyke, and R. R. Arnold. 1996. Hemin-induced modifications of the antigenicity and hemin-binding capacity of Porphyromonas gingivalis lipopolysaccharide. Infect. Immun. 64:2282-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler, C. W., R. R. Arnold, and H. A. Schenkein. 1993. Inhibition of C3 and IgG proteolysis enhances phagocytosis of Porphyromonas gingivalis. J. Immunol. 12:7016-7029. [PubMed] [Google Scholar]
- 9.Cutler, C. W., T. W. Stanford, C. Abraham, R. A. Cederberg, T. Boardman, and C. Ross. 2000. Clinical benefits of oral irrigation for periodontitis are related to reduction of pro-inflammatory cytokine levels and plaque. J. Clin. Periodontol. 27:134-143. [DOI] [PubMed] [Google Scholar]
- 10.Darveau, R. P., S. Arbabi, I. Garcia, B. Bainbridge, and R. V. Maier. 2002. Porphyromonas gingivalis lipopolysaccharide is both agonist and antagonist for p38 mitogen-activated protein kinase activation. Infect. Immun. 70:1867-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobrovolskaia, M. A., A. E. Medvedev, K. E. Thomas, N. Cuesta, V. Toshchakov, T. Ren, M. J. Cody, S. M. Michalek, N. R. Rice, and S. N. Vogel. 2003. Induction of in vitro reprogramming by toll-like receptor (TLR) 2 and TLR4 agonists in murine macrophages: effects of TLR “homotolerance” versus “heterotolerance” on NF-kappa B signaling pathway components. J. Immunol. 170:508-519. [DOI] [PubMed] [Google Scholar]
- 12.Ezzo, P., and C. W. Cutler. 2003. Microorganisms as risk indicators for periodontal disease. Periodontology 2000 32:24-35. [DOI] [PubMed] [Google Scholar]
- 13.Ferlito, M., O. G. Romanenko, S. Ashton, F. Squadrito, P. V. Halushka, and J. A. Cook. 2001. Effect of cross-tolerance between endotoxin and TNF-α or IL-1β on cellular signaling and mediator production. J. Leukoc. Biol. 70:821-829. [PubMed] [Google Scholar]
- 14.Fraker, D. L., M. C. Stovroff, M. J. Merino, and J. A. Norton. 1988. Tolerance to tumor necrosis factor in rats and the relationship to endotoxin tolerance and toxicity J. Exp. Med. 168:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajishengallis, G., M. Martin, R. E. Schifferle, and R. J. Genco. 2002. Counteracting interactions between lipopolysaccharide molecules with differential activation of Toll-like receptors. Infect. Immun. 70:6658-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2001. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jotwani, R., A. K. Palucka, M. Al-Quotub, J. Kim, D. Bell, J. Banchereau, and C. W. Cutler. 2001. Dendritic cells infiltrate T-cell rich oral mucosa in chronic periodontitis: in situ, in vivo and in vitro studies. J. Immunol. 167:4693-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jotwani, R., B. Pulendran, S. Agrawal, and C. W. Cutler. 2003. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting TH2 effector response in vitro. Eur. J. Immunol. 33:2980-2986. [DOI] [PubMed] [Google Scholar]
- 19.Jotwani, R., and C. W. Cutler. 2003. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. J. Dental Res. 82:736-741. [DOI] [PubMed] [Google Scholar]
- 20.Jotwani, R., and C. W. Cutler. 2004. Fimbriated Porphyromonas gingivalis is more efficient than fimbriae-deficient P. gingivalis in entering human dendritic cells in vitro and induces an inflammatory TH1 effector response. Infect. Immun. 72:1725-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kido, J., R. Kido, Suryono, M. Kataoka, M. K. Fagerhol, and T. Nagata. 2003. Calprotectin release from human neutrophils is induced by Porphyromonas gingivalis lipopolysaccharide via the CD-14-Toll-like receptor-nuclear factor kappaB pathway. J. Periodontal Res. 38:557-563. [DOI] [PubMed] [Google Scholar]
- 22.Kornman, K. S., A. Crane, H. Y. Wang, F. S. di Giovine, M. G. Newman, F. W. Pirk, T. G. Wilson, Jr., F. L. Higginbottom, and G. W. Duff. 1997. The interleukin-1 genotype as a severity factor in adult periodontal disease. J. Clin. Periodontol. 24:72-77. [DOI] [PubMed] [Google Scholar]
- 23.Loos, B. G., D. W. Dyer, T. S. Whittam, and R. K. Selander. 1993. Genetic structure of populations of Porphyromonas gingivalis associated with periodontitis and other oral infections. Infect. Immun. 61:204-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin, M., R. E. Schifferle, N. Cuesta, S. N. Vogel, J. Katz, and S. M. Michalek. 2003. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J. Immunol. 171:717-725. [DOI] [PubMed] [Google Scholar]
- 25.Medvedev, A. E., K. M. Kopydlowski, and S. N. Vogel. 2000. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and Toll-like receptor 2 and 4 gene expression J. Immunol. 164:5564-5574. [DOI] [PubMed] [Google Scholar]
- 26.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Z. Yuehua, H. Bing, A. Moshe, and T. A. Maria. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170:1406-1415. [DOI] [PubMed] [Google Scholar]
- 27.Menard, C., and C. Mouton. 1995. Clonal diversity of the taxon Porphyromonas gingivalis assessed by random amplified polymorphic DNA fingerprinting. Infect. Immun. 63:2522-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori, Y., A. Yoshimura, T. Ukai, E. Lien, T. Espevik, and Y. Hara. 2003. Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol. Immunol. 18:54-58. [DOI] [PubMed] [Google Scholar]
- 29.Natoli, G., A. Costanzo, F. Moretti, M. Fulco, C. Balsano, and M. Levrero. 1997. Tumor necrosis factor (TNF) receptor 1 signaling downstream of TNF receptor-associated factor 2: nuclear factor kappaB (NF-κB)-inducing kinase requirement for activation of activating protein 1 and NF-κB but not of c-Jun N-terminal kinase/stress-activated protein kinase. J. Biol. Chem. 272:26079-26082. [DOI] [PubMed] [Google Scholar]
- 30.Nomura, F., S. Akashi, Y. Sakao, S. Sato, T. Kawai, M. Matsumoto, K. Nakanishi, M. Kimoto, K. Miyake, K. Takeda, and S. Akira. 2000. Endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 164:3476-3479. [DOI] [PubMed] [Google Scholar]
- 31.Ozaki, K., S. Hanazawa, A. Takeshita, Y. Chen, A. Watanabe, K. Nishida, Y. Miyata, and S. Kitano. 1996. Interleukin-1 beta and tumor necrosis factor-alpha stimulate synergistically the expression of monocyte chemoattractant protein-1 in fibroblastic cells derived from human periodontal ligament. Oral Microbiol. Immunol. 11:109-114. [DOI] [PubMed] [Google Scholar]
- 32.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulendran, B., P. Kumar, C. W. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shnyra, A., R. Brewington, A. Alipio, C. Amura, and D. C. Morrison. 1998. Reprogramming of lipopolysaccharide-primed macrophages is controlled by a counterbalanced production of IL-10 and IL-12. J. Immunol. 160:3729. [PubMed] [Google Scholar]
- 35.Song, H. Y., C. H. Regnier, C. J. Kirschning, D. V. Goeddel, and M. Rothe. 1997. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2 Proc. Natl. Acad. Sci. USA 94:9792-9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 37.Tlaskalova-Hogenova, H., L. Tuckova, R. Lodinova-Zadnikova, R. Stepankova, B. Cukrowska, D. P. Funda, I. Striz, H. Kozakova, I. Trebichavsky, D. Sokol, Z. Rehakova, J. Sinkora, P. Fundova, D. Horakova, L. Jelinkova, and D. Sanchez. 2002. Mucosal immunity: its role in defense and allergy. Int. Arch. Allergy Immunol. 128:77-90. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz, O., K. Watanabe, and R. J. Lamont. 2002. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell. Microbiol. 4:305-314. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, F. X., C. J. Kirschning, R. Mancinelli, X. P. Xu, Y. Jin, E. Faure, A. Mantovani, M. Rothe, M. Muzio, and M. Arditi. 1999. Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J. Biol. Chem. 274:7611-7614. [DOI] [PubMed] [Google Scholar]
- 40.Zingarelli, B., M. Makhlouf, P. Halushka, A. Caputi, and J. A. Cook. 1995. Altered macrophage function in tumor necrosis factor alpha- and endotoxin-induced tolerance J. Endotox. Res. 2:247-254. [Google Scholar]