Abstract
The data presented illustrated the in vitro anti-proliferative effect of the PSG toxin from the cuttlefish, Sepia pharaonis. The cytostatic potentials of the PSG toxin were determined by the lymphocyte migration inhibition assay. The PSG toxin (50 μg/ml) exhibited commendable inhibition of the migration of lymphocytes across the agarose gel matrix under the presence of lipopolysaccharide mitogen, with a mean migration index of 0.625. The cytotoxicity of the PSG toxin against selected cancer cell lines was determined using the MTT assay. The PSG toxin exhibited dose-dependent cytotoxicity against the MCF-7 breast cancer cells followed by KB (oral), HeLa (cervical) and A549 (lung) cancer cell lines. The PSG toxin also exhibited proportional release of LDH leakage by mitochondrial damage with an IC50 of 13.85 μM against MCF-7 breast cancer cells. The in vitro anticancer activity of the PSG toxin against the selected cell lines was evaluated by Karthik et al. (2017) [1].
Keywords: PSG toxin, Anticancer, Anti-proliferative, Breast cancer, MTT assay, LDH leakage assay
Specifications Table
| Subject area | Biology |
| More specific subject area | Breast cancer, in vitro, Anti-proliferative activity, PSG toxin |
| Type of data | Figure |
| How data was acquired | Nikon Eclipse Ti-U Inverted Microscope, USA; Bio-Rad PR4100, USA Microtiter plate reader. |
| Data format | Analyzed |
| Experimental factors | Peripheral blood mononuclear cells, MCF-7, KB, HeLa and A549 cancer cell lines were treated with PSG toxin from S. pharaonis. |
| Experimental features | Lymphocyte migration inhibition assay: Inhibition of LPS induced leucocyte migration by PSG toxin, observed in a Nikon Eclipse Ti-U Inverted Microscope, USAMTT assay: Reduction of MTT by mitochondrial succinate dehydrogenase in viable cancer cells treated with PSG toxin to a purple formazan product deteted in microplate readerLDH release assay: Reduction of the substrate lactate by LDH released into medium of cancer cells treated with PSG toxin, detected in microplate reader |
| Data source location | Faculty of Allied Health Sciences, Chettinad Academy of Research and Education, Chettinad Health City, Kelambakkam, Chennai, Tamil Nadu, India. 12.7948°N, 80.2160°E |
| Data accessibility | All data are provided with this article |
Value of the data
-
•
The provided data demonstrates the cytostatic potentials of the PSG toxin from S. pharaonis against peripheral blood mononuclear leucocytes.
-
•
The data provided illustrates the commendable anti-proliferative action of the PSG toxin from S. pharaonis against the selected cancer cell lines.
-
•
The data might be valuable to researchers interested in anti-proliferative action of toxins from marine mollusks.
-
•
The data might also be of value to researchers investigating the anti-proliferative and anticancer activity of marine bioactive compounds.
1. Data
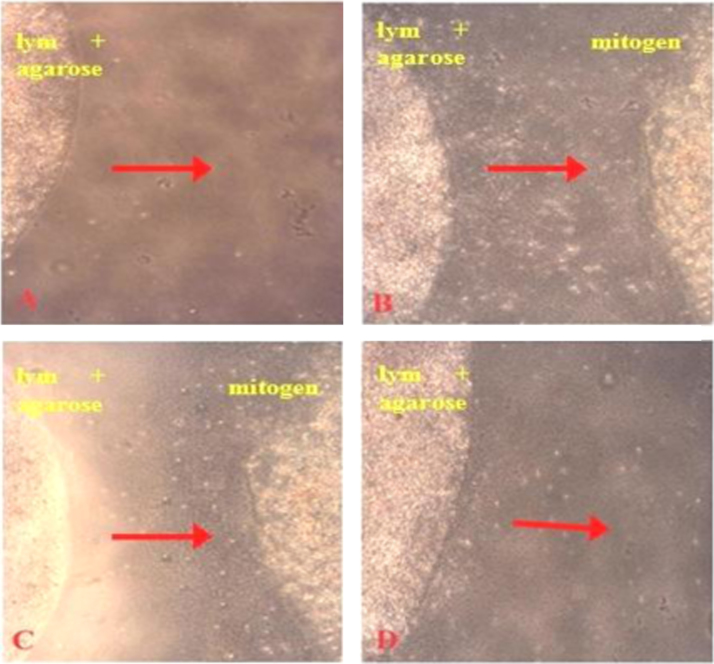
The migration of the lymphocytes was inhibited by the PSG toxin (5, 25 and 50 µg/ml) with a mean diameter of 1.65±0.55 mm and 1.42±0.64 mm respectively. The lymphocytes exhibited pronounced migration under the influence of LPS with a mean diameter of 3.45±0.83 mm (Fig. 1).
Fig. 1.
Cytostatic activity of PSG toxin by inhibition of lymphocyte migration. Migration inhibition of PSG toxin against leucocytes. (A) Control leucocyte-agarose droplet (B) Migration of leucocytes from agarose droplet towards mitogen ().  (C) Migration inhibition in presence of PSG toxin (5 μg/ml) (D) Migration inhibition in presence of PSG toxin (50 μg/ml).
(C) Migration inhibition in presence of PSG toxin (5 μg/ml) (D) Migration inhibition in presence of PSG toxin (50 μg/ml).
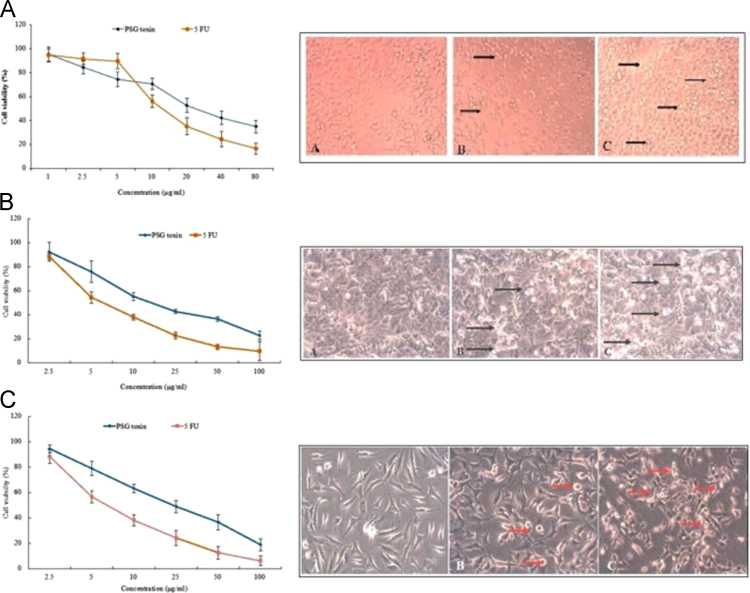
The cell viability curve of the PSG toxin against the KB oral cancer cells with an IC50 concentration of 32.5 µM. The purified PSG toxin also exhibited significant inhibition against the proliferation of HeLa cervical cancer cells and A549 lung cancer cells at an IC50 value of 16.5 µg/ml (23.2 µM) and 22.45 µg/ml (31.65 µM) (Fig. 2). The inlet figure shows the cell viability in (A) control, (B) PSG toxin (100 μg/ml) and (C) Paclitaxel treated cells showing the zones of apoptosis.
Fig. 2.
MTT cell viability curve of PSG toxin against (A) KB oral cancer cells (B) HeLa cervical cancer cells (C) A549 lung cancer cells. Microscopic images of (A) control, (B) PSG toxin treated and (C) paclitaxel treated cells indicating aggregation of apoptotic cells.
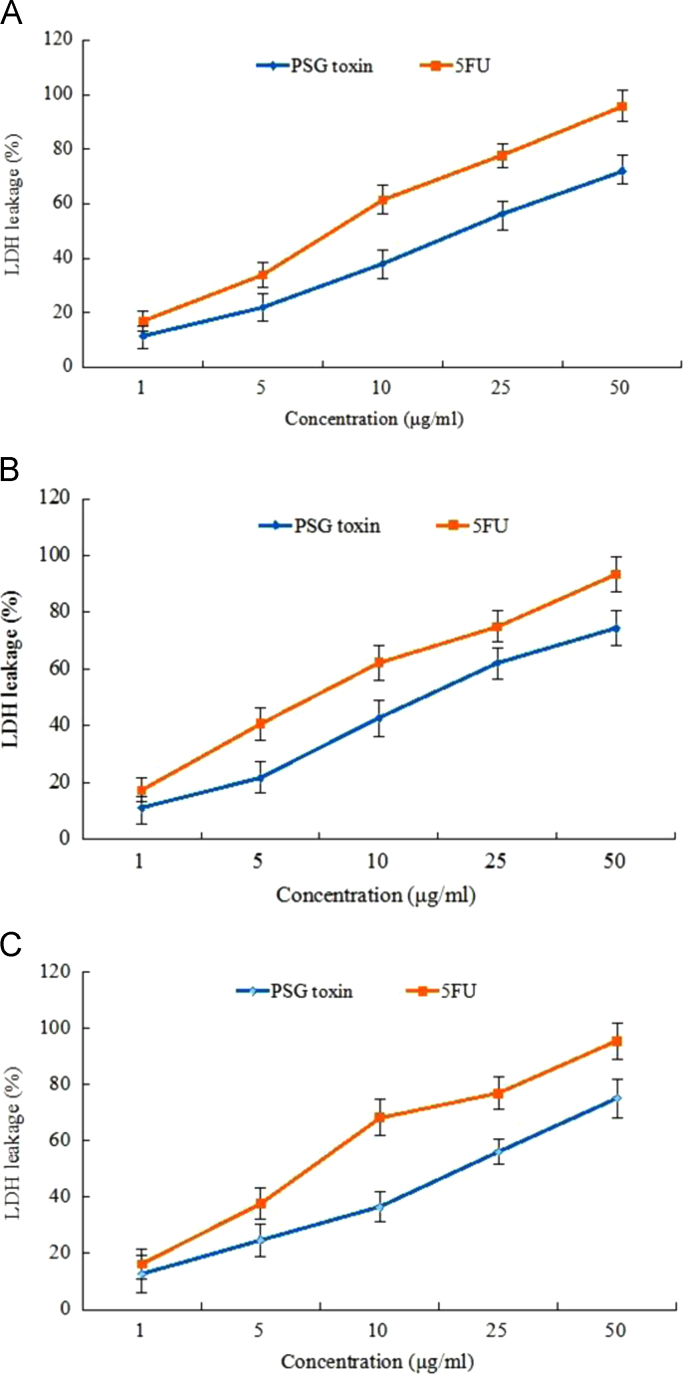
The PSG toxin also exhibited commendable LDH leakage against KB oral cancer cells, HeLa cervical cancer cells and A549 lung cancer cells with IC50 concentrations of 22.8 μg/ml, 17.45 μg/ml and 23.52 μg/ml respectively (Fig. 3).
Fig. 3.
LDH cell viability curve of PSG toxin against (A) KB oral cancer cells (B) HeLa cervical cancer cells (C) A549 lung cancer cells.
2. Materials and methods
2.1. in vitro cytotoxicity
2.1.1. Lymphocyte migration inhibition assay
The cytotoxicity of the purified PSG toxin against the primary cells peripheral blood mononuclear cells (PBMC) was determined using the lymphocyte migration inhibition assay following the method of Mousseau et al. (2007). The inhibition of leucocyte migration treated with PSG toxin (5,10,50 µg/ml) under the influence of mitogen (lipopolysaccharide) was measured using a microscope ruler [2].
2.1.2. in vitro anticancer activity by MTT assay
The cytotoxicity of the purified PSG toxin against selected cancer cell lines was determined by the MTT cell viability assay [3]. The anti-proliferative potentials of the PSG toxin (0.5,1,5,10,25,50 µg/ml) was studied against the adherent cultures of KB (oral), HeLa (cervical) and A549 (lung) cancer cell line and the inhibitory concentrations (IC50) were determined.
2.1.3. LDH release assay
The cell viability and membrane permeability of the PSG toxin (0.5,1,5,10,25, 50 µg/ml) against the KB, HeLa and A549 cancer cells was determined using the LDH leakage assay [4]. The inhibitory effect of the PSG toxin on the mitochondrial enzymes, dehydrogenases are evaluated by the levels of LDH released into medium after action on the substrate lactate in the presence of NADH.
Acknowledgements
The authors acknowledge Prof. S. Niranjali Devaraj, Head, Department of Biochemistry, Guindy campus, University of Madras and Dr. Perumal Madan Kumar, Post Doctoral Fellow, UT South Western Medical Center, USA for helping in cell culture and MTT cytotoxicity assays.
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.dib.2017.05.010.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Karthik R., Manigandan V., Ebenezar K., Vijayashree R., Saravanan R. in vitro and in vivo anticancer activity of posterior salivary gland toxin from the cuttlefish Sepia pharaonis, Ehrenberg (1831) Chem. Biol. Int. 2017 doi: 10.1016/j.cbi.2017.04.002. Submitted for publication 272:10-20. [DOI] [PubMed] [Google Scholar]
- 2.Karthik R., Manigandan V., Sheeba R., Saravanan R., Rajesh P.R. Structural characterization and comparative biomedical properties of phloroglucinol from Indian brown seaweeds. J. Appl. Phycol. 2016;28:3561–3573. [Google Scholar]
- 3.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Met. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 4.Abdullah A.S., Mohammed A.S., Abdullah R., Mirghani M.E., Al-Qubaisi M. Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Comp. Alt. Med. 2014;14:1. doi: 10.1186/1472-6882-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material