Abstract
The number of patients with hematological malignancies who develop invasive fungal disease (IFD) has increased dramatically in recent decades. This increase is attributed to impairment of the host immune system due to intensive cytotoxic chemotherapies, use of corticosteroids and profound immunosuppression after hematopoietic stem cell transplantation (HSCT). Additionally, the increasing prevalence of fungal infections caused by emerging and rare pathogens, IFD of mixed etiology or of atypical localization is observed. There are also much more patients with IFD who do not belong to a well-described risk group, like patient with lymphoproliferative disorders. Within this heterogeneous group of patients, IFD epidemiology is not well defined and antifungal prophylaxis practices vary.
The aim of this paper is to present the case of a 58-year-old patient with refractory Hodgkin disease, focusing on infectious complication after subsequent lines of chemotherapy. During deep and prolonged neutropaenia the patient developed symptoms of pneumonia. Despite antifungal prophylaxis with fluconazole, IFD of mixed etiology with the presence of Candida glabrata and Aspergillus fumigatus was diagnosed. The infection showed a poor response to monotherapy with liposomal amphotericin B, but was successfully treated with therapy involving micafungin. Analysis of the presented case demonstrated the necessity of new approaches to the prevention of IFD in patients with lymphoproliferative disorders heavily pretreated with numerous chemotherapy protocols. Prolonged neutropenia and high corticosteroid exposure put these patients in high risk of IFD like patients with acute myeloid leukemia/myelodysplastic syndrome or after allogeneic HSCT.
Keywords: Candidiasis, aspergillosis, invasive fungal disease, micafungin
Introduction
Invasive fungal disease (IFD) remains an important cause of morbidity and mortality in patients with haematological malignancies, especially those undergoing intensive chemotherapy or haematopoietic stem cell transplantation (HSCT). Aspergillus and Candida remain the leading fungal pathogens, but increasing incidence of rare fungal infection, and IFD of mixed aetiology or of atypical localisation have been observed recently.
Despite the availability of new antifungal drugs, the outcome of IFD remains poor, with a mortality rate of 20-70%, depending on the risk group. The diagnosis is often established late in the course of infection, when fungal burden is high and therapy is less likely to be effective. Identification of patients at risk is crucial for improvement of antifungal treatment results. Progress in anti-neoplastic treatment, and the use of aggressive chemotherapy and new drugs affecting the immune system have rapidly expanded the patient populations predisposed to IFD development [1–4].
Herein we report a case of severe pulmonary fungal infection of mixed aetiology in a heavily pretreated patient with refractory Hodgkin lymphoma (HL).
Case report
A 58-year-old man was diagnosed in 2010 with mixed cellularity classical HL (CD30+, CD15+, CD20–, Ki67+, CD3–, EMA–, LCA–) in clinical stage II according to Ann Arbor classification with the presence of B symptoms (fever and drenching night sweats). PET-CT (positron emission tomography–computed tomography) scan revealed the presence of metabolically active cervical, supraclavicular, and left axillary lymph nodes. After the consideration of clinical risk factors, the patient was classified to intermediate stage according to the German Hodgkin Study Group (GHSG). His initial treatment consisted of six cycles of ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) followed by involved-field radiotherapy (IFRT) of axillary and supraclavicular lymph nodes, according to Centre policy. The refractory and progressive disease was diagnosed based on PET-CT reassessment and the patient underwent treatment salvage therapy according to ESHAP (etoposide, cisplatin, cytarabine, methylprednisolone), then IVE (ifosfamide, epirubicin, etoposide) and GEM-P (gemcitabine, cisplatin, methylprednisolone) protocols. ESHAP and IVE chemotherapies were well tolerated, whereas severe neutropaenia with absolute neutrophil count (ANC) < 0.5 G/l was observed after GEM-P treatment.
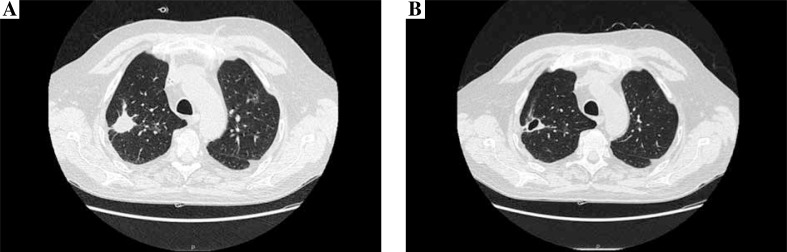
During severe neutropaenia the patient stayed in a single room, with intensified hygiene measures. The antifungal prophylaxis with fluconazole 400 mg per day was provided. He was screened with biweekly serum galactomannan test (GM). After the last course of chemotherapy, neutropaenic fever developed without improvement after administration of broad spectrum antibiotic therapy. Microbiological cultures of blood and serum GM were repetitively negative. High-resolution computer tomography (HRCT) of the lungs was performed and revealed three-centimetre well-circumscribed lesions with air-crescent sign in the right upper lung and multiple smaller low-density areas with a halo sign in both lungs. The air crescent signs were highly suggestive of infection by an angioinvasive fungus, most commonly Aspergillus. Bronchoscopy was done, and microbiological culture of bronchoalveolar lavage (BAL) specimens did not reveal any fungal pathogens, BAL-GM was also negative. The diagnostic evaluation indicated the presence of sufficient clinical evidence consistent with IFD, but without any mycological support, so according to EORTC/MSG definitions a diagnosis of a possible IFD was established. The antifungal treatment with liposomal amphotericin (AmBisome) at a dose of 3 mg/kg was initiated. After 10 days of such therapy the clinical condition of the patient did not improve substantially, as weight loss, productive cough, and continuing subfebrile temperatures were observed. There was no improvement in HRCT of the lungs. The patient underwent repeated diagnostic bronchoscopy, and microbiological evaluation of BAL specimens confirmed mixed fungal infection with the presence of Candida glabrata and Aspergillus fumigatus; BAL-GM was also positive. The treatment with micafungin was started at a dose of 100 mg per day intravenously. After four weeks of the antifungal therapy, a sufficient clinical response was observed (the patient’s cough and expectoration were improving, and his temperature dropped to 37.0˚C), and the control HRCT of the lungs showed substantial improvement: cavitation in the biggest consolidation and gradual resorption of smaller fungal infiltrations (Fig. 1). The cultures of BAL specimen and BAL-GM were negative. The patient was not monitored by beta D3 glucan and fungal PCR. Significant adverse events were not observed during the antifungal treatment. The improvement was stable, and the patient was able to continue chemotherapy.
Fig. 1.
CT scan of the chest before the initiation of micafungin treatment (A) and after 4 weeks of the fungal therapy (B)
Discussion
Patients with Hodgkin lymphoma treated with conventional chemotherapy are considered to be at low risk of IFD development. Recently published epidemiological analysis of IFD in lymphoproliferative disorders revealed that its prevalence among HL patients is 3.6% [5]. However, in patients undergoing autologous haematopoietic stem cell transplantation, who develop prolonged severe neutropaenia, invasive aspergillosis is seen in 5.5% [6]. As previously described, HL is characterised by an immune response in the involved tissues that is predominantly CD4 mediated. The link between ineffective immune response in the tissue and generalised immune deficiency in HL may consist of several components [7]. Recent advances in our understanding of HL biology and immunology show that infiltrated immune cells and cytokines in the tumoural microenvironment may play different roles that seem closely related to clinical outcomes [8]. However, the association between immune system abnormality in HL and increased risk of IFD development has not been established.
The patient presented in our report, treated with several chemotherapy regimens for lymphoma, was qualified, according to a recent recommendation, as a patient at low risk of IFD development, and anti-Candida prophylaxis was introduced [9]. He received standard doses of fluconazole, which is indicated for prevention of Candida infections in patients with neutropaenia due to cancer chemotherapy. Despite the prophylaxis, proven IFD of mixed aetiology with the presence of Candida glabrata and Aspergillus fumigatus was confirmed based on GM monitoring, early HRCT, and bronchoscopy with BAL. The study conducted by Chow et al. reported that the duration of fluconazole treatment is a significant risk factor for the development of non-albicans Candida species [10]. It is also known that drug-resistant Candida glabrata infection is common in cancer patients.
According to a recent recommendation, mould-active prophylaxis is recommended for patients with high risk of IFD, especially for patients receiving intensive chemotherapy for acute myeloid leukaemia (AML) or myelodysplastic syndromes (MDS), or for patients with corticosteroid-requiring graft-versus-host disease following allogeneic haematopoietic stem cell transplantation (allo-HSCT). However, recently a higher incidence of IFD in patients with lymphoproliferative disorders has been observed. It may be attributed to the increasing intensity of salvage chemotherapy protocols for resistant forms of lymphoma comprising high corticosteroid exposure and prolonged periods of neutropaenia. This observation argues for new approaches to the prevention of IFD in this group of patients. There are no studies to date quantifying the burden of disease and the role of antifungal prophylaxis in HD patients. Consistent with findings in other groups of immunocompromised patients, Aspergillus and Candida are the most frequent IFD pathogens in patients with lymphoma.
Yeasts, with Candida as the absolutely dominating pathogen, are part of our normal microflora, and invasive infections arise only when barrier leakage or impaired immune function occurs [11]. Yeasts typically enter the bloodstream, causing fungaemia or deep-seated tissue infection. Candida infections of the thorax can be associated with empyaema, tracheobronchial, and mediastinal infection, as well as pneumonia. Candida pneumonia is rare and is most often found in the setting of candidaemia with dissemination to the lung in immunocompromised patients [12]. Manifestations of Candida pneumonia include cough, dyspnoea, and fever. Radiographic findings are variable and can include lobar and multifocal consolidation, as well as cavitation. Diagnosis of Candida pneumonia is complicated by a generally low specificity and low positive predictive value of isolating Candida in respiratory specimens, which is often interpreted as colonisation. Interestingly, Candida invasion of the lung was frequently reported at autopsy, despite the rare clinical occurrence of Candida pneumonia. It is not clear whether this dissemination to the lung represents true infection or is an artefact of respiratory colonisation or post-mortem seeding [4].
Candida glabrata is a more commonly seen in patients with haematological malignancies and neutropaenia [13]. The higher risk of nosocomial invasive candidiasis caused by C. glabrata is associated with prolonged use of broad spectrum antibiotics and corticosteroids, aggressive chemotherapy, prolonged use of central venous catheters [14], and previous antifungal prophylaxis with fluconazole. In the study by Farmaciotis et al., 20.5% of isolates were resistant to fluconazole, 10.3% to caspofungin, and 10 (6.8%) to multiple drugs [15]. The main mechanisms of azole resistance include alterations in the C. glabrata ERG11 gene (CgERG11), which encodes the azole target enzyme, and upregulation of the CgCDR1 and CgCDR2 genes, which encode efflux pumps [16].
Moulds, with Aspergillus as the main organism, are ubiquitous in nature and the environment, and their conidia are inhaled on a daily basis. Invasive mould infections typically arise from the airways. Aspergillosis may present as a skin/soft tissue, ocular, gastrointestinal, cardiac, sinus, central nervous system, or disseminated infection, but most commonly presents as infection limited to the lung [12].
Early diagnosis of IFD is often difficult, and sometimes the only clinical indication for its presence is isolated persistent fever in patients receiving broad-spectrum antibiotics together with subsequent non-specific pulmonary infiltrates on high-resolution computed tomography, without any microbiological documentation of its aetiology [1]. The autopsy data also revealed that 45% of patients with proven aspergillosis had repeatedly negative galactomannan test results prior to death – thus underscoring the importance of autopsy evidence for evaluating the performance of new diagnostic tests [4]. It is possible that IFD diagnoses are delayed in these patients because they lie outside traditional risk groups due to uncertainty surrounding IFD risk, the paucity of data on IFD epidemiology, and the absence of standardised antifungal prophylaxis recommendations among evolving disease treatments [5].
In the patient presented in our report, infectious complications with mixed pathogens were diagnosed and treated according to ECIL and IDSA recommendations. Echinocandins are highly active against most Candida species, including C. albicans and C. glabrata. Activity of the latter is particularly important because resistance to widely used azole antifungals often complicates treatment of C. glabrata infections. Consequently, echinocandins have been elevated recently to first-line agents for treatment of invasive C. glabrata infection [17]. However, increasing echinocandin resistance among Candida spp. poses an emerging threat. According to the new definitions, rates of caspofungin non-susceptibility among C. glabrata clinical isolates range from < 10% [18] to as high as 62% [19]. True resistance (MIC of ≥ 1 μg/ml) is strictly associated with mutations in β-1,3-glucan synthase gene FKS1 or FKS2. Laboratory studies showed that mutants exhibit reduced susceptibility to caspofungin and, paradoxically, demonstrate increased susceptibility to micafungin (Mycamine, Astellas Pharma Europe B.V., Leiderdorp, Netherlands) [20]. Micafungin is approved for the treatment of candidaemia, invasive candidiasis, oesophageal candidiasis (for which intravenous therapy is appropriate), and prophylaxis of Candida infection in patients undergoing allo-HSCT, or patients who are expected to have neutropaenia for at least 10 days [21].
Invasive aspergillosis should be treated promptly and aggressively. According to recommendations of the Fifth European Conference on Infections in Leukaemia (ECIL-5), voriconazole remains the first-line treatment in invasive aspergillosis. It is followed by recommendations for LAmB (liposomal amphotericin B) and then ABLC (amphotericin B lipid complex) [22]. The options for salvage therapy include voriconazole (if not used in the first line), caspofungin, LAmB, ABLC, posaconazole, and combination therapy. In Europe micafungin is not licensed for the treatment of IA. A number of groups, mostly from Japan, have carried out clinical studies investigating the efficacy of micafungin in the treatment of Aspergillus infections in a variety of clinical settings. The results indicate that micafungin is efficacious as monotherapy and in combination therapy for the treatment of a range of deep-seated Aspergillus infections, with success rates similar to other commonly used antifungals. The major limitation is that there are currently no randomised studies comparing micafungin with standard antifungal therapy [21].
Analysis of the presented case demonstrates the need for new approaches to the prevention of IFD in patients with lymphoproliferative disorders treated with subsequent chemotherapy protocols. Prolonged neutropaenia and high corticosteroid exposure put these patients at high risk of IFD, like patients with AML/MDS or after allo-HSCT. GM monitoring, early HRCT, and bronchoscopy with BAL are the best methods for early diagnosis of IFD. Our observations based on the presented case confirm the efficacy of micafungin in the salvage treatment of invasive pulmonary candidiasis with Candida glabrata pathogen (recommendation IIA according to ECIL-5) complicated by invasive aspergillosis.
Footnotes
The authors declare no conflict of interest.
References
- 1.Racil Z, Toskova M, Kocmanova I, et al. Micafungin as empirical antifungal therapy in hematological patients: a retrospective, multicenter study in the Czech and Slovak Republics. Leuk Lymphoma. 2013;54:1042–1047. doi: 10.3109/10428194.2012.729057. [DOI] [PubMed] [Google Scholar]
- 2.Chen SC, Sorrell TC, Chang CC, et al. Consensus guidelines for the treatment of yeast infections in the haematology, oncology and intensive care setting, 2014. Intern Med J. 2014;44:1315–1332. doi: 10.1111/imj.12597. [DOI] [PubMed] [Google Scholar]
- 3.Blyth CC, Gilroy NM, Guy SD, et al. Consensus guidelines for the treatment of invasive mould infections in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern Med J. 2014;44:1333–1349. doi: 10.1111/imj.12598. [DOI] [PubMed] [Google Scholar]
- 4.Lewis RE, Cahyame-Zuniga L, Leventakos K, et al. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses. 2013;56:638–645. doi: 10.1111/myc.12081. [DOI] [PubMed] [Google Scholar]
- 5.Teng JC, Slavin MA, Teh BW, et al. Epidemiology of invasive fungal disease in lymphoproliferative disorders. Haematologica. 2015;100:e462–466. doi: 10.3324/haematol.2015.126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gil L, Kozlowska-Skrzypczak M, Mol A, et al. Increased risk for invasive aspergillosis in patients with lymphoproliferative diseases after autologous hematopoietic SCT. Bone Marrow Transplant. 2009;43:121–126. doi: 10.1038/bmt.2008.303. [DOI] [PubMed] [Google Scholar]
- 7.Poppema S. Immunology of Hodgkin’s disease. Baillieres Clin Haematol. 1996;9:447–457. doi: 10.1016/s0950-3536(96)80020-5. [DOI] [PubMed] [Google Scholar]
- 8.de la Cruz-Merino L, Lejeune M, Nogales Fernàndez E, et al. Role of immune escape mechanisms in Hodgkin’s lymphoma development and progression: a whole new world with therapeutic implications. Clin Dev Immunol. 2012;2012:756353. doi: 10.1155/2012/756353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming S, Yannakou CK, Haeusler GM, et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern Med J. 2014;44:1283–1297. doi: 10.1111/imj.12595. [DOI] [PubMed] [Google Scholar]
- 10.Chow JK, Golan Y, Ruthazer R, et al. Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin Infect Dis. 2008;46:1206–1213. doi: 10.1086/529435. [DOI] [PubMed] [Google Scholar]
- 11.Arendrup MC. Epidemiology of invasive candidiasis. Curr Opin Crit Care. 2010;16:445–452. doi: 10.1097/MCC.0b013e32833e84d2. [DOI] [PubMed] [Google Scholar]
- 12.Broaddus VC, Mason RC, Ernst JD, et al. Murray and Nadel’s Textbook of Respiratory Medicine. Elsevier Health Sciences. 2015 [Google Scholar]
- 13.Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag. 2014;10:95–105. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima R, Shams W, Kalra S, Borthwick T. Candida glabrata liver abscess and fungemia complicating severe calculus cholecystitis in an immunocompetent nondiabetic host. South Med J. 2010;103:245–247. doi: 10.1097/SMJ.0b013e3181c9803b. [DOI] [PubMed] [Google Scholar]
- 15.Farmakiotis D, Tarrand JJ, Kontoyiannis DP. Drug-resistant Candida glabrata infection in cancer patients. Emerg Infect Dis. 2014;20:1833–1840. doi: 10.3201/eid2011.140685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanguinetti M, Posteraro B, Fiori B, et al. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob Agents Chemother. 2005;49:668–679. doi: 10.1128/AAC.49.2.668-679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fothergill AW, Sutton DA, McCarthy DI, Wiederhold NP. Impact of new antifungal breakpoints on antifungal resistance in Candida species. J Clin Microbiol. 2014;52:994–997. doi: 10.1128/JCM.03044-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wisplinghoff H, Ebbers J, Geurtz L, et al. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int J Antimicrob Agents. 2014;43:78–81. doi: 10.1016/j.ijantimicag.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Healey KR, Katiyar SK, Castanheira M, et al. Candida glabrata mutants demonstrating paradoxical reduced caspofungin susceptibility but increased micafungin susceptibility. Antimicrob Agents Chemother. 2011;55:3947–3949. doi: 10.1128/AAC.00044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enoch DA, Idris SF, Aliyu SH, et al. Micafungin for the treatment of invasive aspergillosis. J Infect. 2014;68:507–526. doi: 10.1016/j.jinf.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Pagano L, Lyon S. Celebrating 40 years of progress in bone marrow transplantation: a report from the 40th Annual Meeting of the European Society for Blood and Marrow Transplantation. Future Microbiol. 2014;9:1117–1121. [Google Scholar]