Abstract
To reduce the biothreat posed by anthrax, efforts are under way to improve the protection afforded by vaccination. This work examines the ability of immunostimulatory CpG oligodeoxynucleotides (ODN) adsorbed onto cationic polylactide-co-glycolide (PLG) microparticles (CpG ODN-PLG) to accelerate and boost the protective immunity elicited by Anthrax Vaccine Adsorbed (AVA, the licensed human anthrax vaccine). The results indicate that coadministering CpG ODN-PLG with AVA induces a stronger and faster immunoglobulin G response against the protective antigen of anthrax than AVA alone. Immunized mice were protected from lethal anthrax challenge within 1 week of vaccination with CpG ODN-PLG plus AVA, with the level of protection correlating with serum immunoglobulin G anti-protective antigen titers.
Bacillus anthracis is an aerobic gram-positive spore-forming bacterium found naturally in wild and domesticated animals (13). It is highly resistant to environmental degradation and produces a tripartite toxin that reduces the ability of the host's immune system to eliminate the pathogen (13). Human exposure to anthrax typically arises following contact with infected livestock and generally results in a mild form of cutaneous disease (8, 32). However, anthrax spores designed for aerosol delivery were intentionally released by bioterrorists in the United States in 2001. The resultant morbidity, mortality, and widespread panic underscored the potential for anthrax to be used as a bioterror agent and the need to improve the speed, magnitude, and safety of anthrax vaccination (25).
Anthrax Vaccine Adsorbed (AVA) is the only anthrax vaccine licensed for human use in the United States. It is prepared by adsorbing the culture filtrate of an attenuated toxinogenic nonencapsulated strain of B. anthracis (V770-NP1-R) onto aluminum hydroxide (16). Studies show that protective antigen (PA), the core of anthrax toxin, is the major immunogen of AVA. Antibodies against PA neutralize the toxin, inhibit spore germination, and improve the phagocytosis and killing of spores by macrophages (18, 26, 41, 42). Vaccination with AVA requires a series of six immunizations delivered over 18 months followed by yearly boosters (30, 31). This schedule has been linked to the development of adverse side effects including joint pain, gastrointestinal disorders, and pneumonia, leading many U.S. soldiers to refuse vaccination (9, 30, 33). Strategies designed to reduce the dose and number of immunizations required to achieve protection might improve compliance.
Synthetic oligodeoxynucleotides (ODN) containing immunostimulatory CpG motifs can boost the immune response to coadministered antigens, including AVA (4, 19, 20, 21, 40). CpG ODN induce the functional maturation of professional antigen-presenting cells and trigger the production of immunostimulatory cytokines and chemokines (2, 12, 20, 23, 24). Biodegradable cationic polylactide-co-glycolide (PLG) microparticles represent a different form of immune adjuvant. PLG improve the uptake and processing of adsorbed antigen by antigen-presenting cells (3, 6, 7, 28, 35, 36, 37). The current work examines whether CpG ODN adsorbed onto PLG microparticles (CpG ODN-PLG) increase the speed and magnitude of protective anti-PA immunity induced by coadministered AVA.
MATERIALS AND METHODS
Reagents.
Phosphorothioate CpG ODN 1555 (GCTAGACGTTAGCGT) and control ODN 1612 (GCTAGAGCTTAGCGT) were synthesized at the Center for Biologics Evaluation Research core facility (10). All ODN were free of endotoxin and protein contamination. ODN were adsorbed onto PLG at 1% (wt/wt) as previously described (37). Briefly, PLG microparticles with a copolymer ratio of 50/50 were emulsified with hexadecyl trimethyl ammonium bromide through a solvent evaporation process. The resultant cationic PLG microparticles were incubated with ODN overnight at 4°C with gentle shaking followed by washing and freeze-drying, and the amount of ODN adsorbed to PLG microparticles was quantitated. AVA was obtained from BioPort Corporation (East Lansing, Mich.). Recombinant PA was provided by U.S. Army Medical Research Institute of Infectious Diseases (Fort Detrick, Md.) and prepared as described (15). Recombinant lethal factor was purchased from Research Diagnostics Inc. (Flanders, N.J.). Toxinogenic (pXO1+), nonencapsulated (pXO2−) Sterne vaccine strain spores of B. anthracis (STI) were obtained from the U.S. Army Medical Research Institute of Infectious Diseases and stored at 4°C (17).
Animals.
Specific-pathogen-free male A/J mice were obtained from the National Cancer Institute (Frederick, Md.). They were housed in sterile microisolator cages in a barrier environment and studied at 8 to 12 weeks of age. All animal experiments were conducted with Animal Care and Use Committee-approved protocols, and challenge studies were performed in a biosafety level 2 facility.
Immunization and challenge studies.
A/J mice were immunized intraperitoneally with AVA formulated in alum with and without CpG ODN, PLG, or CpG ODN adsorbed onto PLG (CpG ODN-PLG). The mice were bled weekly, and their serum was stored at −20°C until use. Mice were challenged intraperitoneally with 3 × 102 to 9 × 103 50% lethal doses (LD50) of STI spores suspended in 0.5 ml of sterile phosphate-buffered saline (1 LD50 = 1.1 × 103 STI spores). Survival was monitored for 21 days.
IgG anti-PA ELISA.
Immunoglobulin G (IgG) anti-PA antibody titers were monitored as described (21). Briefly, 96-well microtiter plates (Immulon 1B, Thermo Labsystems, Franklin, Mass.) were coated with 1 μg of recombinant PA per ml in phosphate-buffered saline at 4°C overnight. The plates then were blocked with 5% nonfat dry milk in phosphate-buffered saline containing 0.1% Tween 20. Plates were washed, and overlaid with serially diluted serum for 2 h at room temperature. After thorough washing, bound antibodies were detected by adding horseradish peroxidase-labeled goat anti-mouse IgG, IgG1, or IgG2a (Southern Biotechnology, Birmingham, Ala.) followed by ABTS (2,2′-azino-di-3-ethylbenzthiazoline-6-sulfonic acid) substrate (Kirkegaard & Perry, Gaithersburg, Md.). Relative antibody titers were determined by comparison to a standard curve generated with pooled sera from hyperimmunized mice and expressed as the reciprocal of the endpoint dilution which yielded an absorbance value at least three times background levels. All samples were analyzed in duplicate.
Toxin-neutralizing assay.
The toxin-neutralizing titers of individual serum samples were assessed by their ability to protect RAW264.7 cells (American Type Culture Collection, Manassas, Va.) from lethal toxin with minor modifications from previously described methods (15). RAW264.7 cells were plated at 3 × 104 cells/well in 100 μl of glutamine-free RPMI 1640 medium containing 10% fetal bovine serum and 2 mM glutamax-1 (Invitrogen Corporation). The cells were incubated at 37°C in a 5% CO2 incubator overnight. Serially diluted antiserum was 1:1 (vol/vol) mixed with lethal toxin (100 ng of recombinant PA per ml plus 100 ng of recombinant lethal factor per ml) at room temperature for 30 min to allow neutralization to occur; 100 μl of this mixture was then incubated with the cells for 6 h at 37°C. Cell viability was determined by monitoring the reduction of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, Mo.). Results were standardized against known high-titered monkey serum kindly provided by the Centers for Disease Control.
Statistics.
Differences in the kinetic development of anti-PA immune responses were determined by two-way analysis of variance. Differences in the IgG anti-PA response induced by various vaccine-adjuvant combinations were assessed by one-way analysis of variance. Differences in survival were evaluated with chi-square analysis of Kaplan-Meier curves. Correlation coefficients were determined by linear regression analysis. The predictive value of IgG anti-PA and toxin-neutralizing titers on survival was evaluated with two-parameter logistic regression (1).
RESULTS
CpG ODN-PLG boost the immunogenicity of AVA.
Previous studies established that CpG ODN could act as immune adjuvants when coadministered with AVA (21). To examine whether CpG ODN adsorbed onto PLG microparticles constituted an even more effective adjuvant, CpG ODN-PLG were coadministered to A/J mice with an optimally immunogenic dose of AVA (200 μl). A/J mice were selected for study because they are susceptible to challenge by attenuated STI anthrax spores, allowing the protective activity of the resultant immune response to be examined in a biosafety level 2 facility (21).
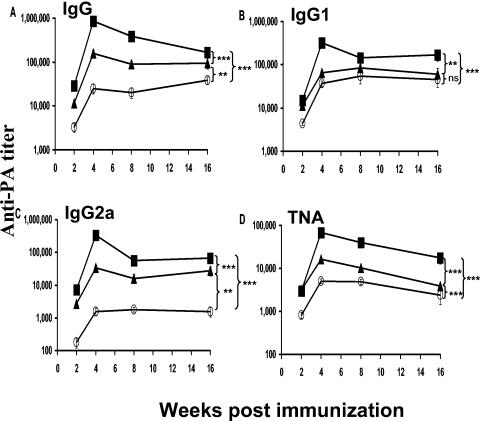
Consistent with previous studies, the magnitude of the IgG anti-PA response induced by AVA was significantly improved by coadministration of CpG ODN (P < 0.01, Fig. 1A). However, coadministering CpG ODN-PLG with AVA boosted this response by an additional 4- to 30-fold (P < 0.001, Fig. 1A). This improved humoral immune response persisted for the duration of the study (4 months). IgG1, IgG2a, and serum toxin-neutralizing antibody titers were all significantly increased by combining CpG ODN-PLG with AVA (Fig. 1B to D). IgG anti-PA antibody was undetectable in unvaccinated mice.
FIG. 1.
IgG anti-PA antibody titers in AVA-vaccinated mice. Male A/J mice were immunized intraperitoneally with 200 μl of AVA (○) with and without 20 μg of free (▴) or PLG-adsorbed CpG ODN (▪). Data represent the geometric mean ± standard error serum IgG anti-PA titer of 10 independently studied mice per group. **, P < 0.01; ***, P < 0.001; ns = not significant; determined by two-way analysis of variance. TNA, toxin-neutralizing activity.
Antigen-sparing effect of CpG ODN-PLG.
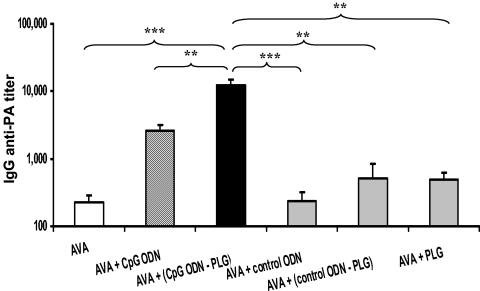
The reactogenicity of the licensed anthrax vaccine might be reduced if the amount of AVA required to induce protective immunity could be lowered. Thus, the ability of CpG ODN-PLG to reduce the dose of AVA needed to elicit protective immunity was examined. Preliminary experiments established that 8 to 25 μl of AVA induced a detectable anti-PA response in all vaccinated mice, whereas 3 μl of AVA was immunogenic in only a fraction of vaccinated animals. As seen in Fig. 2, coadministering CpG ODN-PLG with 8 to 25 μl of AVA boosted the resultant IgG anti-PA antibody response by nearly 50-fold compared to AVA alone (P < 0.001, Fig. 2). This effect required the combination of CpG ODN with PLG, since PLG microparticles (alone or in combination with control ODN) had no significant impact on the magnitude of the response induced by AVA (Fig. 2).
FIG. 2.
IgG anti-PA antibody response in A/J mice following low-dose AVA (8 to 25 μl) intraperitoneal immunization. There were no significant differences in IgG anti-PA titers at these vaccination doses, allowing data from all mice to be combined. Results represent the geometric mean ± standard error IgG anti-PA response 14 days after immunization (n = 11 to 29 independently studied mice per group). **, P < 0.01; ***, P < 0.001, determined by one-way analysis of variance.
CpG ODN-PLG accelerate the development of AVA-mediated protection.
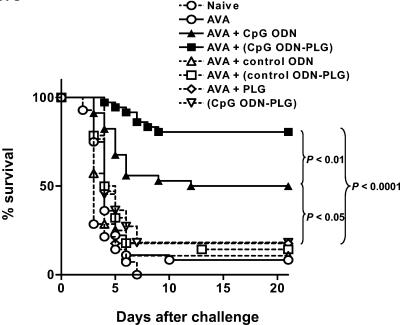
Preliminary studies demonstrated that mice immunized with 3 to 8 μl of AVA took >2 weeks to develop a protective immune response against anthrax infection (data not shown). To determine whether CpG ODN-PLG could accelerate this induction of protective immunity, A/J mice were immunized with AVA with and without adjuvant and their ability to resist anthrax challenge 1 week later was examined.
Consistent with preliminary studies, mice immunized with AVA alone were highly susceptible to infection at this early time point (>90% mortality, Fig. 3). Yet >80% of the mice immunized with CpG ODN-PLG plus AVA survived infection at this early time point (P < 0.0001, Fig. 3). Immunization with CpG ODN plus AVA in the absence of PLG yielded intermediate protection (50% survival, P < 0.01 versus CpG ODN-PLG/AVA, Fig. 3), whereas CpG ODN-PLG in the absence of AVA was not protective.
FIG. 3.
Survival of vaccinated mice. A/J mice were immunized intraperitoneally with ≤8 μl of AVA plus 20 μg of free or PLG-adsorbed ODN. The mice were challenged intraperitoneally 7 days later with 3 × 102-3 50% lethal doses of STI spores. The survival of control groups (including naive mice and mice vaccinated with AVA) was indistinguishable between experiments. Thus, data from multiple experiments were combined for 11 to 36 mice per group.
Humoral immunity as a predictor of protection.
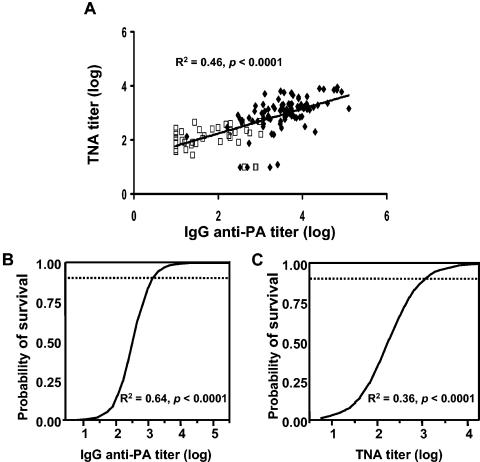
There is considerable interest in identifying a surrogate marker for protective immunity against anthrax. Towards that end, the serum toxin-neutralizing activity and IgG anti-PA titer were evaluated as predictors of survival following anthrax spore challenge. As seen in Fig. 4A, toxin-neutralizing antibody correlated significantly with IgG anti-PA titer (R2 = 0.46, P < 0.0001). Although toxin-neutralizing antibody predicted protection against anthrax, two-parameter logistic regression modeling showed that IgG anti-PA was the superior surrogate marker of survival (Fig. 4B, IgG: R2 = 0.64, P < 0.0001; Fig. 4C, toxin-neutralizing antibody: R2 = 0.36, P < 0.0001). In this context, receiver operating characteristic analysis showed that total IgG anti-PA titer was 97% accurate at predicting survival following anthrax challenge, whereas toxin-neutralizing antibody was 91% accurate. The magnitude of the IgG anti-PA response provided valuable information on an animal's resistance to anthrax infection. For example, >90% of mice are protected against 9 × 103 50% lethal doses of anthrax if their IgG anti-PA titer exceeds 1,000, while a titer of >6,000 indicates that >99% of mice are protected from such high-dose challenge.
FIG. 4.
Correlation between serum antibody response and survival. Mice were immunized intraperitoneally with 8 to 25 μl of AVA plus free or PLG-adsorbed ODN. Two weeks postimmunization, serum IgG anti-PA and toxin-neutralizing antibody titers were determined, and the mice were challenged intraperitoneally with 9 × 103 50% lethal doses of STI spores. Results from four independent experiments involving a total of 130 mice are shown. (A) Linear regression of IgG anti-PA versus toxin-neutralizing antibody titers in mice that succumbed to (□) or survived (♦) infection. (B) Logistic regression of survival versus IgG anti-PA titer. (C) Logistic regression of survival versus toxin-neutralizing antibody titer. TNA, toxin-neutralizing activity.
DISCUSSION
Efforts are under way to increase the speed and magnitude of the protective immune response elicited by AVA. Current findings indicate that these goals may be met by coadministering CpG ODN-PLG with AVA, resulting in a more rapid and stronger anti-PA antibody response than immunization with AVA alone (or combined with CpG ODN in the absence of PLG; Fig. 1 and 2). Significant protection against anthrax challenge was present within 1 week of vaccination with CpG ODN-PLG plus AVA (Fig. 3), indicating that the combination of CpG ODN with PLG is significantly more effective than CpG ODN or PLG alone as an immune adjuvant. The quality of the resultant anti-PA response was high, as evidenced by enhanced toxin-neutralizing activity, improved in vivo protection, and high levels of IgG2a antibody (known to promote the clearance of bacterial infection by complement-mediated cytotoxicity) (29).
CpG ODN and PLG microparticles represent distinct forms of vaccine adjuvant. CpG ODN interact with immune cells expressing Toll-like receptor 9 (including B cells and plasmacytoid dendritic cells), stimulating them to secrete proinflammatory and Th1 cytokines and chemokines and to undergo functional maturation (20). CpG ODN have been shown to improve the immune response to coadministered vaccines, including AVA (5, 10, 20, 22). Clinical trials indicate that CpG ODN are safe and effective in humans, significantly improving the antibody response elicited by a coadministered hepatitis B vaccine (11).
PLG microparticles are composed of the same biodegradable and biocompatible polymers used safely in adsorbable stitches for many years (38). Antigen can be adsorbed onto (or encapsulated within) PLG microparticles. The antigen-bound particles are readily taken up and processed by professional antigen-presenting cells, promoting the induction of humoral and cytotoxic T-lymphocyte responses (7, 34, 35, 38, 39). Since the adjuvant-like properties of PLG microparticles differ from those of CpG ODN, Singh et al. examined whether the combination could improve the immunogenicity of a coadministered antigen (37). Using p55gag, a weak antigen, they showed that CpG ODN-coated PLG microparticles significantly boosted the cytotoxic T-lymphocyte response elicited compared to antigen alone or combined with free CpG ODN or PLG (37). The current results extend that finding by demonstrating that, when combined with a strong immunogen such as AVA, CpG ODN-PLG boost and accelerate the resultant immune response. This response persisted at protective levels throughout the 4-month duration of the experiment (Fig. 1 and 4). The absolute duration of protection, and whether CpG ODN-PLG can boost the secondary immune response elicited by AVA are subjects of ongoing investigation.
A goal of anthrax vaccine developers is to reduce the frequency and severity of adverse reactions. The results indicate that CpG ODN-PLG had an antigen-sparing effect, reducing the amount of AVA required to elicit protection by >20-fold. By reducing antigen load, CpG ODN-PLG might lessen the risk of adverse reactions to an AVA-based vaccine. Yet a recent report suggests that daily administration of high-dose CpG ODN for up to 3 weeks causes severe toxicity (14). Fortunately, neither local nor systemic reactions were observed in mice vaccinated with CpG ODN-PLG plus AVA in the current study. This is consistent with previous work showing that modest doses of CpG ODN can be used safely as vaccine adjuvants (4, 5, 11, 20, 21, 22, 37).
Both IgG anti-PA and toxin-neutralizing activity have been proposed as surrogate markers for vaccine efficacy, although their relative merit is uncertain (27). Resolving this issue is of considerable importance, as the decision to license future anthrax vaccines will rely on surrogate markers of protection (since conventional phase III efficacy studies cannot be conducted with biothreat pathogens). While IgG anti-PA and toxin-neutralizing antibody levels both correlated with survival, two-parameter logistic regression analysis showed the former to have greater sensitivity and specificity in predicting survival (Fig. 4). From the perspective of evaluating the likely efficacy of a vaccination campaign, serum IgG anti-PA antibody titer would be useful in predicting survival following defined levels of pathogen exposure (Fig. 4).
Current findings demonstrate that CpG ODN-PLG can increase the speed and magnitude of the humoral immune response induced by AVA. This vaccine-adjuvant combination elicited protective immunity within 1 week, suggesting that a CpG ODN-PLG-based vaccine might accelerate the induction of a protective immune response in individuals potentially exposed to anthrax. Such a vaccine might also be administered to emergency personnel prior to deployment to a site of anthrax release. The current results also help clarify the relationship between serum anti-PA and toxin-neutralizing antibody titers and their utility as surrogate markers for protection. Ongoing research suggests that CpG ODN-PLG also boost the response of vaccine candidates targeting other biowarfare pathogens. While such studies need to be repeated in appropriate nonhuman primate challenge models, they suggest that CpG ODN-PLG may be useful as adjuvants for a broad range of novel vaccines.
Acknowledgments
We thank Lev Sirota for assistance in analyzing the data.
The assertions herein are the private ones of the authors and are not to be construed as official or as reflecting the views of USAMRIID or the FDA at large.
Support for this work was provided in part by Military Interdepartmental Purchase Request MM8926 and DARPA.
Editor: J. D. Clements
REFERENCES
- 1.Agresti, A., and B. A. Coull. 1996. Order-restricted tests for stratified comparisons of binomial proportions. Biometrics 52:1103-1111. [PubMed] [Google Scholar]
- 2.Ballas, Z. D., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:1840-1847. [PubMed] [Google Scholar]
- 3.Briones, M., M. Singh, M. Ugozzoli, J. Kazzaz, S. Klakamp, G. Ott, and D. O'Hagan. 2001. The preparation, characterization, and evaluation of cationic microparticles for DNA vaccine delivery. Pharm. Res 18:709-712. [DOI] [PubMed] [Google Scholar]
- 4.Davis, H. L., I. Suparto, R. Weeratna, Jumintarto, D. Iskandriati, S. Chamzah, A. Ma'ruf, C. Nente, A. M. Krieg, Heriyanto, W. Smits, and D. Sajuthi. 2000. CpG DNA overcomes hyporesponsiveness to hepatitis B vaccine in orangutans. Vaccine 18:1920-1924. [DOI] [PubMed] [Google Scholar]
- 5.Davis, H. L., R. Weeranta, T. J. Waldschmidt, L. Tygrett, J. Schorr, and A. M. Krieg. 1998. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160:870-876. [PubMed] [Google Scholar]
- 6.Denis-Mize, K. S., M. Dupuis, M. L. MacKichan, M. Singh, B. Doe, D. O'Hagan, J. B. Ulmer, J. J. Donnelly, D. M. McDonald, and G. Ott. 2000. Plasmid DNA adsorbed onto cationic microparticles mediates target gene expression and antigen presentation by dendritic cells. Gene Ther. 7:2105-2112. [DOI] [PubMed] [Google Scholar]
- 7.Denis-Mize, K. S., M. Dupuis, M. Singh, C. Woo, M. Ugozzoli, D. T. O'Hagan, J. J. Donnelly III, G. Ott, and D. M. McDonald. 2003. Mechanisms of increased immunogenicity for DNA-based vaccines adsorbed onto cationic microparticles. Cell. Immunol. 225:12-20. [DOI] [PubMed] [Google Scholar]
- 8.Friedlander, A. M., and P. S. Brachman. 1998. Anthrax, p. 729-739. In S. A. Plotkin and E. A. Mortimer (ed.), Vaccines. W. B. Saunders, Philadelphia, Pa.
- 9.Geier, D. A., and M. R. Geier. 2002. Anthrax vaccination and joint related adverse reactions in light of biological warfare scenarios. Clin. Exp. Rheumatol. 20:217-220. [PubMed] [Google Scholar]
- 10.Gursel, I., M. Gursel, K. J. Ishii, and D. M. Klinman. 2001. Sterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of CpG oligonucleotides. J. Immunol. 167:3324-3328. [DOI] [PubMed] [Google Scholar]
- 11.Halperin, S. A., G. Van Nest, B. Smith, S. Abtahi, H. Whiley, and J. J. Eiden. 2003. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine 21:2461-2467. [DOI] [PubMed] [Google Scholar]
- 12.Halpern, M. D., R. J. Kurlander, and D. S. Pisetsky. 1996. Bacterial DNA induces murine interferon-gamma production by stimulation of IL-12 and tumor necrosis factor-alpha. Cell. Immunol. 167:72-78. [DOI] [PubMed] [Google Scholar]
- 13.Hanna, P. 1998. Anthrax pathogenesis and host response. Curr. Top. Microbiol. Immunol. 225:13-35. [DOI] [PubMed] [Google Scholar]
- 14.Heikenwalder, M., M. Polymenidou, T. Junt, C. Sigurdson, H. Wagner, S. Akira, R. Zinkernagel, and A. Aguzzi. 2004. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 10:187-192. [DOI] [PubMed] [Google Scholar]
- 15.Ivins, B. E., M. L. Pitt, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, Jr., P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16:1141-1148. [DOI] [PubMed] [Google Scholar]
- 16.Ivins, B. E., and S. L. Welkos. 1988. Recent advances in the development of an improved, human anthrax vaccine. Eur. J. Epidemiol. 4:12-19. [DOI] [PubMed] [Google Scholar]
- 17.Ivins, B. E., S. L. Welkos, G. B. Knudson, and S. F. Little. 1990. Immunization against anthrax with aromatic compound-dependent (Aro-) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect. Immun. 58:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivins, B. E., S. L. Welkos, S. F. Little, M. H. Crumrine, and G. O. Nelson. 1992. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect Immun. 60:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, T. R., N. Obaldia, R. A. Gramzinski, Y. Charoenvit, N. Kolodny, S. Kitov, H. L. Davis, A. M. Krieg, and S. L. Hoffman. 1999. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenic vaccine in Aotus monkeys. Vaccine 17:3065-3071. [DOI] [PubMed] [Google Scholar]
- 20.Klinman, D. M. 2004. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 4:249-258. [DOI] [PubMed] [Google Scholar]
- 21.Klinman, D. M., H. Xie, S. Little, D. Currie, and B. Ivins. 2004. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine 22:2881-2886. [DOI] [PubMed] [Google Scholar]
- 22.Klinman, D. M., K. M. Barnhart, and J. Conover. 1999. CpG motifs as immune adjuvants. Vaccine 17:19-25. [DOI] [PubMed] [Google Scholar]
- 23.Klinman, D. M., A. Yi, S. L. Beaucage, J. Conover, and A. M. Krieg. 1996. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNg. Proc. Natl. Acad. Sci. USA 93:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieg, A. M., A. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-548. [DOI] [PubMed] [Google Scholar]
- 25.Lane, H. C., J. L. Montagne, and A. S. Fauci. 2001. Bioterrorism: a clear and present danger. Nat. Med. 7:1271-1273. [DOI] [PubMed] [Google Scholar]
- 26.Little, S. F., and B. E. Ivins. 1999. Molecular pathogenesis of Bacillus anthracis infection. Microbes Infect. 1:131-139. [DOI] [PubMed] [Google Scholar]
- 27.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422-430. [DOI] [PubMed] [Google Scholar]
- 28.O'Hagan, D., M. Singh, M. Ugozzoli, C. Wild, S. Barnett, M. Chen, M. Schaefer, B. Doe, G. R. Otten, and J. B. Ulmer. 2001. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. J. Virol. 75:9037-9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oishi, K., N. L. Koles, G. Guelde, and M. Pollack. 1992. Antibacterial and protective properties of monoclonal antibodies reactive with Escherichia coli O111:B4 lipopolysaccharide: relation to antibody isotype and complement-fixing activity. J. Infect. Dis. 165:34-45. [DOI] [PubMed] [Google Scholar]
- 30.Pittman, P. R., P. H. Gibbs, T. L. Cannon, and A. M. Friedlander. 2001. Anthrax vaccine: short-term safety experience in humans. Vaccine 20:972-978. [DOI] [PubMed] [Google Scholar]
- 31.Pittman, P. R., G. Kim-Ahn, D. Y. Pifat, K. Coonan, P. Gibbs, S. Little, J. G. Pace-Templeton, R. Myers, G. W. Parker, and A. M. Friedlander. 2002. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine 20:1412-1420. [DOI] [PubMed] [Google Scholar]
- 32.Quinn, C. P., and P. C. Turnbull. 1998. Anthrax, p. 799-818. In M. Ballow and M. Sussman (ed.), Topley and Wilson's microbiology and microbial infections Collier, London, England.
- 33.Ready, T. 2004. US soldiers refuse to fall in line with anthrax vaccination scheme. Nat. Med. 10:112. [DOI] [PubMed] [Google Scholar]
- 34.Sanceau, J., T. Kaisho, T. Hirano, and J. Wietzerbin. 1995. Triggering of the human interleukin-6 gene by interferon-gamma and tumor necrosis factor-alpha in monocytic cells involves cooperation between interferon regulatory factor-1, NF-kB, and Sp1 transcription factors. J. Biol. Chem. 270:27920-27931. [DOI] [PubMed] [Google Scholar]
- 35.Singh, M., J. Kazzaz, M. Ugozzoli, J. Chesko, and D. T. O'Hagan. 2004. Charged polylactide co-glycolide microparticles as antigen delivery systems. Expert Opin. Biol Ther 4:483-491. [DOI] [PubMed] [Google Scholar]
- 36.Singh, M., X. M. Li, J. P. McGee, T. Zamb, W. Koff, C. Y. Wang, and D. T. O'Hagan. 1997. Controlled release microparticles as a single dose hepatitis B vaccine: evaluation of immunogenicity in mice. Vaccine 15:475-481. [DOI] [PubMed] [Google Scholar]
- 37.Singh, M., G. Ott, J. Kazzaz, M. Ugozzoli, M. Briones, J. Donnelly, and D. T. O'Hagan. 2001. Cationic microparticles are an effective delivery system for immune stimulatory CpG DNA. Pharm. Res 18:1476-1479. [DOI] [PubMed] [Google Scholar]
- 38.Vajdy, M., and D. T. O'Hagan. 2001. Microparticles for intranasal immunization. Adv. Drug Deliv. Rev 51:127-141. [DOI] [PubMed] [Google Scholar]
- 39.Valiante, N. M., D. T. O'Hagan, and J. B. Ulmer. 2003. Innate immunity and biodefence vaccines. Cell. Microbiol 5:755-760. [DOI] [PubMed] [Google Scholar]
- 40.Verthelyi, D., R. T. Kenney, R. A. Seder, A. A. Gam, B. Friedag, and D. M. Klinman. 2002. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J. Immunol. 168:1659-1663. [DOI] [PubMed] [Google Scholar]
- 41.Welkos, S., S. Little, A. Friedlander, D. Fritz, and P. Fellows. 2001. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 147:1677-1685. [DOI] [PubMed] [Google Scholar]
- 42.Welkos, S. L., and A. M. Friedlander. 1988. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb. Pathog. 5:127-139. [DOI] [PubMed] [Google Scholar]