Abstract
Cardioprotective benefits of ω-3 fatty acids such as docosahexaenoic acid (DHA) are well established, but the regulatory effect of DHA on vascular tone and pressure in pulmonary hypertension is largely unknown.
As DHA is a potent regulator of K+ channels, we hypothesised that DHA modulates the membrane potential of pulmonary artery smooth muscle cells (PASMCs) through K+ channels and thus exerts its effects on pulmonary vascular tone and pressure.
We show that DHA caused dose-dependent activation of the calcium-activated K+ (KCa) current in primary human PASMCs and endothelium-dependent relaxation of pulmonary arteries. This vasodilation was significantly diminished in KCa−/− (Kcnma1−/−) mice. In vivo, acute DHA returned the right ventricular systolic pressure in the chronic hypoxia-induced pulmonary hypertension animal model to the level of normoxic animals. Interestingly, in idiopathic pulmonary arterial hypertension the KCa channels and their subunits were upregulated. DHA activated KCa channels in these human PASMCs and hyperpolarised the membrane potential of the idiopathic pulmonary arterial hypertension PASMCs to that of the PASMCs from healthy donors.
Our findings indicate that DHA activates PASMC KCa channels leading to vasorelaxation in pulmonary hypertension. This effect might provide a molecular explanation for the previously undescribed role of DHA as an acute vasodilator in pulmonary hypertension.
Introduction
Pulmonary arterial hypertension (PAH) is a rare, life-threatening disease of the pulmonary vasculature characterised by a progressive increase in pulmonary vascular resistance and, ultimately, right ventricular failure. Loss of endogenous vasodilatory factors and the ability of the pulmonary vasculature to dilate are major contributors to the pathological features of the disease, and the therapeutic benefits of vasodilators for PAH are well established [1, 2]. Under normal physiological conditions, the pulmonary circulation is a low-pressure, low-resistance system. The low vascular tone of the pulmonary vasculature is largely maintained by the resting membrane potential of the pulmonary artery smooth muscle cells (PASMCs), which is mainly but not exclusively caused by K+ conductance [3–5]. Thus, changes in both K+ channel activity and expression have a direct impact on the resting membrane potential of PASMCs, thereby affecting the pulmonary vascular tone. There are hints from the literature that PASMCs from idiopathic PAH (IPAH; formerly primary pulmonary hypertension) patients may have a more depolarised membrane potential (they are chronically depolarised) contributing to vasoconstriction and remodelling [6], although data underlying this hypothesis are very sparse. Previous studies showed that endogenous vasoactive substances (e.g. endothelin-1 (ET-1), angiotensin II and serotonin) [7, 8], hypoxia or drugs (dasatinib and pergolide) [9–11] involved in the pathophysiology of pulmonary hypertension cause membrane depolarisation of PASMCs by inhibition of K+ channels and lead to vasoconstriction. Accordingly, agents which activate the K+ channel current (nitric oxide, iloprost or treprostinil) cause membrane hyperpolarisation of PASMCs, resulting in vasodilation [12].
Large-scale epidemiologic studies and randomised controlled trials provided evidence that ω-3 fatty acids (eicosapentaenoic acid, docosahexaenoic acid (DHA) and α-linolenic acid) significantly reduce the occurrence of cardiovascular disease events in patients with coronary artery disease [13]. Consequently, the American Heart Association (AHA) guidelines recommend the regular consumption of ω-3 fatty acids [14]. In addition, dietary fish oil decreases pulmonary hypertension and vascular remodelling during chronic hypoxia-induced pulmonary hypertension [15]. However, there is a continued need for studies investigating the molecular mechanism in order to explain the beneficial effect of fatty acids on the pulmonary circulation. DHA prevents the increase of free intracellular calcium induced by ET-1 and inhibits PASMC proliferation [16]. In addition, our previous studies provided a comprehensive analysis of the molecular events underlying these chronic inhibitory effects, and showed that DHA prompts calcium-dependent oxidative stress leading to an unfolded protein response in human primary PASMCs and thus to inhibition of proliferation [17, 18]. DHA is a potent activator of the calcium-dependent K+ (KCa) channel currents in rat coronary artery smooth muscle cells and promotes dilation of isolated small coronary arteries [19, 20]. DHA is known to reversibly activate the large-conductance KCa (BKCa) channel composed of the pore-forming Slo1 subunit (Kcnma1) and the auxiliary subunit β1 (Kcnmb1) [21]. However, the detailed mechanisms underlying the acute effects of this important fatty acid in the pulmonary circulation and pulmonary hypertension remain unclear.
In this study, we hypothesised that DHA activates K+ channels in PASMCs leading to hyperpolarisation of the resting membrane potential and reversal of pulmonary hypertension. The present study examined the acute effect of DHA in the pulmonary circulation, particularly on K+ channel function and membrane potential in primary human PASMCs. We have also addressed the impact of DHA on pulmonary arterial pressure (PAP) in a mouse model of pulmonary hypertension. With use of BKCa (Kcnma1−/−) knockout mice, we have investigated the specific contribution of KCa channels for the influence of DHA in the pulmonary circulation. Moreover, we took advantage of compartment-specific analysis via laser capture microdissection and investigated the expression of BKCa channels and subunits in pulmonary arteries obtained from IPAH patients and from healthy donors. Finally, we verified the DHA effects, observed in the in vivo and in vitro studies, on primary human PASMCs isolated from both IPAH patients and healthy donors. We found that PASMCs from IPAH were significantly depolarised and that DHA normalised this depolarisation via activation of KCa.
Materials and methods
Detailed materials and methods are available in the online supplementary material.
Isolation of human PASMCs from IPAH and donor tissue
All studies on the IPAH patient and donor tissue samples involved in this study were approved by the ethics committee for the Medical University of Vienna, Austria and written informed consent obtained from all study participants. Lung tissues were obtained from IPAH patients who had undergone lung transplantation at the Division of Thoracic Surgery, Medical University of Vienna. Nontransplanted donor lungs that had been harvested for transplantation, but not used (e.g. because of size-reduced lung transplantation), served as controls. Parts of the samples were snap-frozen in liquid nitrogen or fixed in 4% (w/v) formaldehyde for laser microdissection or tissue histology, respectively. Primary PASMCs were isolated from resistance pulmonary arteries from patients and characterisation of the PASMCs was done as previously described [10]. Clinical characteristics of the donor and patients used in the study are given in table 1.
TABLE 1.
Clinical characteristics of the donors and idiopathic pulmonary arterial hypertension (IPAH) patients
| Age years | Sex | mPAP mmHg# | Tissues/cells used | |
|---|---|---|---|---|
| IPAH patient 1 | 37 | Female | 88 | LCM-PAs, PASMCs |
| IPAH patient 2 | 14 | Female | 141 | LCM-PAs |
| IPAH patient 3 | 20 | Female | 81 | LCM-PAs, PASMCs |
| IPAH patient 4 | 20 | Female | 46 | LCM-PAs |
| IPAH patient 5 | 31 | Female | 87 | LCM-PAs |
| IPAH patient 6 | 39 | Female | 31 | LCM-PAs |
| IPAH patient 7 | 32 | Female | 77 | LCM-PAs |
| IPAH patient 8 | 34 | Female | 95 | LCM-PAs |
| IPAH patient 9 | 51 | Male | 50 | PASMCs |
| IPAH patient 10 | 38 | Female | 102 | PASMCs |
| Donor 1 | 45 | Female | LCM-PAs | |
| Donor 2 | 43 | Female | LCM-PAs | |
| Donor 3 | 40 | Female | LCM-PAs | |
| Donor 4 | 16 | Male | LCM-PAs, PASMCs | |
| Donor 5 | 18 | Male | LCM-PAs | |
| Donor 6 | 62 | Male | LCM-PAs | |
| Donor 7 | 59 | Male | LCM-PAs | |
| Donor 8 | 49 | Male | LCM-PAs | |
| Donor 9 | 22 | Male | PASMCs | |
| Donor 10 | 42 | Female | PASMCs | |
| Donor 11 | 60 | Female | PASMCs |
Data are presented as n, unless otherwise stated. mPAP: mean pulmonary arterial pressure; LCM-PA: laser-captured microdissected pulmonary artery; PASMC: pulmonary artery smooth muscle cell.
: mPAP data not available for donors.
Animal studies
All experiments were approved by the local authorities according to the national regulations for animal experimentation (Austrian Ministry of Education, Science and Culture; BMWF-66.010/0076-II/3B/20116).
Statistical analysis
Numerical values are given as mean±SEM. Statistical analysis was performed using Prism 5 (GraphPad, San Diego, CA, USA). The Mann–Whitney U-test was used for nonparametric data. The paired t-test was used to assess changes in right ventricular systolic pressure (RVSP) between pre- and post-DHA infusion. In case of intergroup differences, one-way ANOVA was assessed followed by Tukey’s post hoc test. p-values <0.05 were considered statistically significant.
Results
DHA activates the KCa channel in primary human PASMCs
DHA significantly and reversibly enhanced the whole-cell K+ current recorded from a holding potential of −20 mV in human PASMCs in a dose-dependent manner (figure 1). Next, to address the source of the activated current, different K+ channel inhibitors were used. The enhancement by DHA was not affected by the voltage-activated K+ channel inhibitor 4-aminopyridine (4-AP, 5 μM; figure 2d), but was abolished by the presence of the KCa channel inhibitor Iberiotoxin (ITX, 100 nM; figure 2d). Changes in the relative K+ current amplitude (I/I0) caused by DHA are summarised in figure 2e. Clearly, the reduction by ITX was markedly greater than by 4-AP, and fractional changes did not vary significantly among the recordings with and without 4-AP. Our results indicate that the DHA-activated current is mostly derived from the KCa channels in human PASMCs.
FIGURE 1.
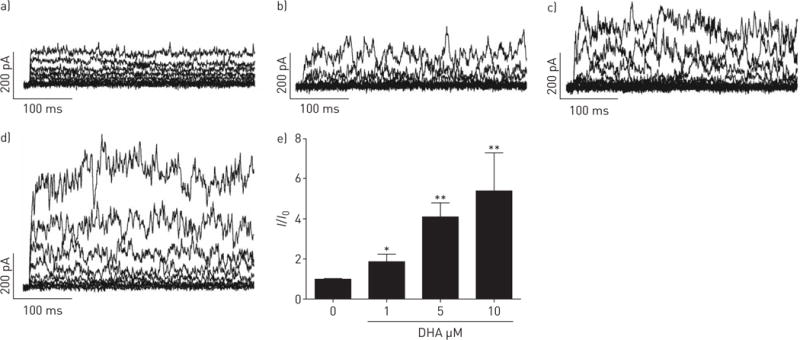
Docosahexaenoic acid (DHA) dose-dependently activates the whole-cell K+ current in human pulmonary artery smooth muscle cells (PASMCs). Original whole-cell K+ current tracing presented a) without and with increasing concentrations of b) 1, c) 5 and d) 10 μM DHA in human PASMCs. e) Summarised relative large-conductance KCa (BKCa) channel current (I/I0) upon increasing concentration of DHA in primary human PASMCs. n=4–6 in each setting. *: p<0.05; **: p<0.01.
FIGURE 2.
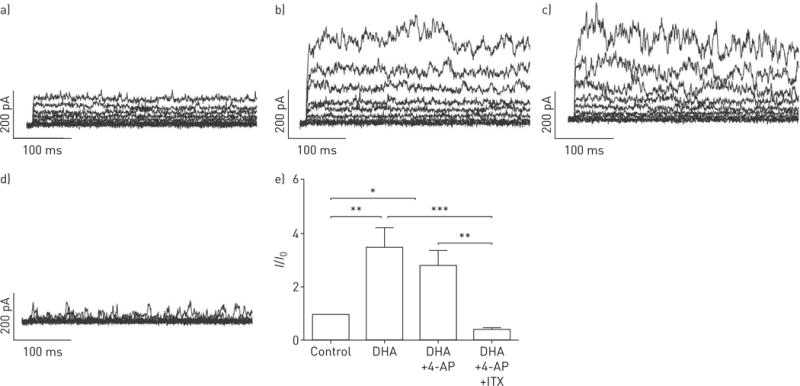
Docosahexaenoic acid (DHA) activates large-conductance KCa channels (BKCa) in human primary pulmonary artery smooth muscle cells (PASMCs). Original whole-cell K+ current tracing presented a) without and with b) DHA, c) DHA with voltage-gated K+ channel blocker 4-aminopyridine (4-AP), and d) DHA along with voltage-gated K+ channel blocker 4-AP and BKCa channel blocker Iberiotoxin (ITX) in primary human PASMCs. e) Summarised relative changes in KCa current (I/I0) elicited by DHA in primary human PASMCs. n=5–6 in each setting. *: p<0.05; **: p<0.01; ***: p<0.001.
DHA induces rapid endothelium-dependent pulmonary relaxation
To investigate the relevance of our observation in human PASMCs in vitro on the tone of pulmonary arteries, we determined the effect of DHA on the tone of isolated pre-constricted pulmonary arteries. Isometric tension measurements, performed with wire myography, showed that DHA induces a strong dose-dependent relaxation of the intrapulmonary arteries pre-constricted with U46619 (figure 3a). The relaxing effect of DHA was concentration-dependent with a half maximal effective concentration (EC50) of 3.5 μM (n=6; figure 3b). Next, we also investigated the role of endothelium in DHA-mediated pulmonary vasodilation. Employment of the nitric oxide synthase inhibitor L-NAME (L-NG-nitroarginine methyl ester, n=3; figure 3c) or endothelial denudation (n=4; figure 3d) in the rat pulmonary arteries significantly decreased vasodilatory responses to increasing concentrations of DHA in comparison with the controls (n=6) in the isometric tension measurements. Next, we investigated the role of the BKCa channel in DHA-induced relaxation. In the presence of the BKCa channel blocker ITX (n=4; figure 3e), the rat intrapulmonary arteries demonstrate a significantly reduced vasodilatory response in comparison with the controls (n=6) to increasing concentrations of DHA. Further, we investigated the regulation of the PAP by DHA in the intact lung. In the ex vivo model of isolated perfused mouse lung, administration of DHA alone did not alter the basal mean PAP (mPAP) (online supplementary figure S1A). When the lungs were subjected to U46619 prior to DHA application, DHA (10 μM) resulted in a profound drop of the mPAP (11.6±0.9, n=6) (online supplementary figure S1B). To decipher the role of the KCa channel in the functional response of the pulmonary vasodilation to DHA, mice lacking the α-subunit of the large-conductance KCa (BKCa−/−) and their wild-type counterpart (BKCa+/+) were employed in the isolated perfused mouse lung model (figure 3f). The U46619-induced pre-tone was not affected by the lack of BKCa channels. However, DHA-induced pulmonary vasodilation was significantly damped in the BKCa−/− mice compared with the BKCa+/+ mice (73±4% versus 86±2%, n=5 each group; figure 3g).
FIGURE 3.
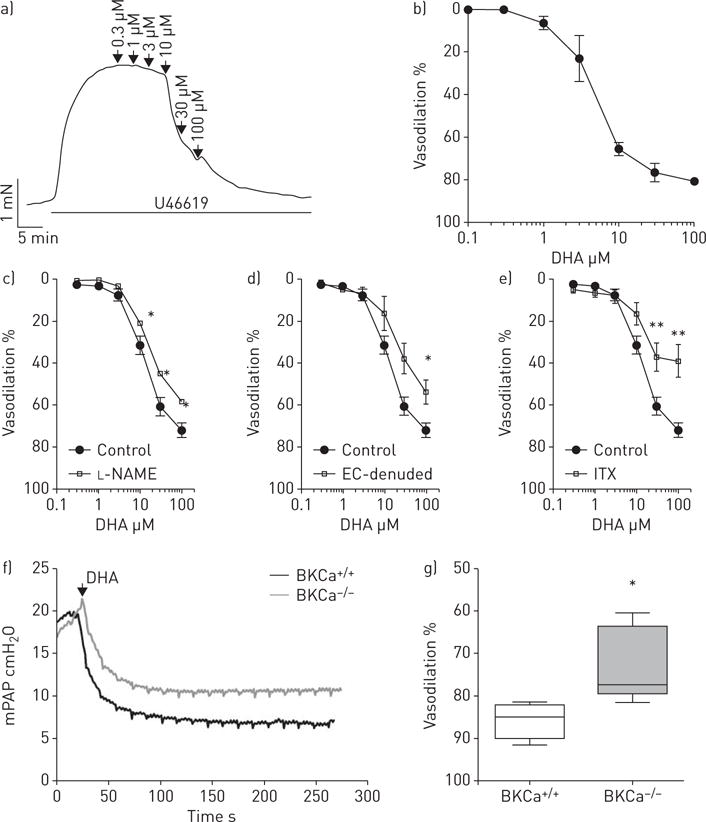
Docosahexaenoic acid (DHA) causes endothelium-dependent vasodilation in intrapulmonary arteries. a) Representative tension recording in an isolated mouse intrapulmonary artery for additive concentrations of DHA in the setting of pre-constriction with U46619. b) Cumulative concentration-dependent vasodilatory effect of DHA on intrapulmonary arteries. Relaxation of pre-constricted isolated rat intrapulmonary arteries in response to increasing concentrations of DHA in the presence/absence of c) L-NAME (n=3), d) endothelial cells (EC) (n=4) or e) Iberiotoxin (ITX) (n=4). Percentages of vasodilation are expressed relative to the maximal response of U46619. The DHA-induced vasodilatory effect is impaired in BKCa−/− mice. f) Representative mean pulmonary arterial pressure (mPAP) tracings from BKCa+/+ and BKCa−/− mice in the isolated, perfused ventilated lung model. g) Acute vasodilatory effect of DHA upon U46619 pre-tone in BKCa+/+ and BKCa−/− mice. Data are presented as median, interquartile range, and minimum and maximum values. n=5 each group. *: p<0.05; **: p<0.01.
DHA leads to rapid pulmonary vasodilation in hypoxia-induced pulmonary hypertension
To gain insight into whether acute DHA application exhibits a vasorelaxing effect in an animal model that mimics human disease involving pulmonary vascular remodelling, we used the chronic hypoxia mouse model of pulmonary hypertension. The presence of BKCa channels in the pulmonary arteries was proved by double immunolabelling for α-smooth muscle actin (α-SMA)-positive cells and BKCa channels in control (normoxic) animals and in remodelled pulmonary arteries of hypoxia-treated mice (figure 4a). The antibody specificity was investigated with specific blocking peptide and staining performed in lung tissue of BKCa+/+ or BKCa−/− mice (online supplementary figure S3A and B). To confirm that the animals develop pulmonary hypertension, RVSP was measured by right heart catheterisation (figure 4b), while the heart rate was not different between groups (figure 4d). This was followed by measurements of right ventricular hypertrophy, where the right ventricle was completely dissected from the left ventricle and the septum. The separated ventricles were weighed to obtain the ratio of the right ventricle to the left ventricle plus septum (figure 4c). The effect of the injection of a single DHA bolus into a central vein in anaesthetised mice was detected by right heart catheterisation. Figure 4e shows the significant reduction in RVSP after DHA application in both normoxic and hypoxic groups. However, DHA had a much more pronounced effect in the hypoxic group compared with animals kept in normoxia (−9.4±1.7 versus −2.5±0.4 mmHg, n=8 versus 5; figure 4f). In hypoxia-treated mice after DHA application RVSP dropped from 37±0.9 to 27.2±2 mmHg (n=8), returning the RVSP in this chronic disease model to the level seen in mice kept in normoxia. Thus, our results suggest that DHA-induced vasodilation occurs through activation of KCa channels in a pulmonary hypertension model.
FIGURE 4.
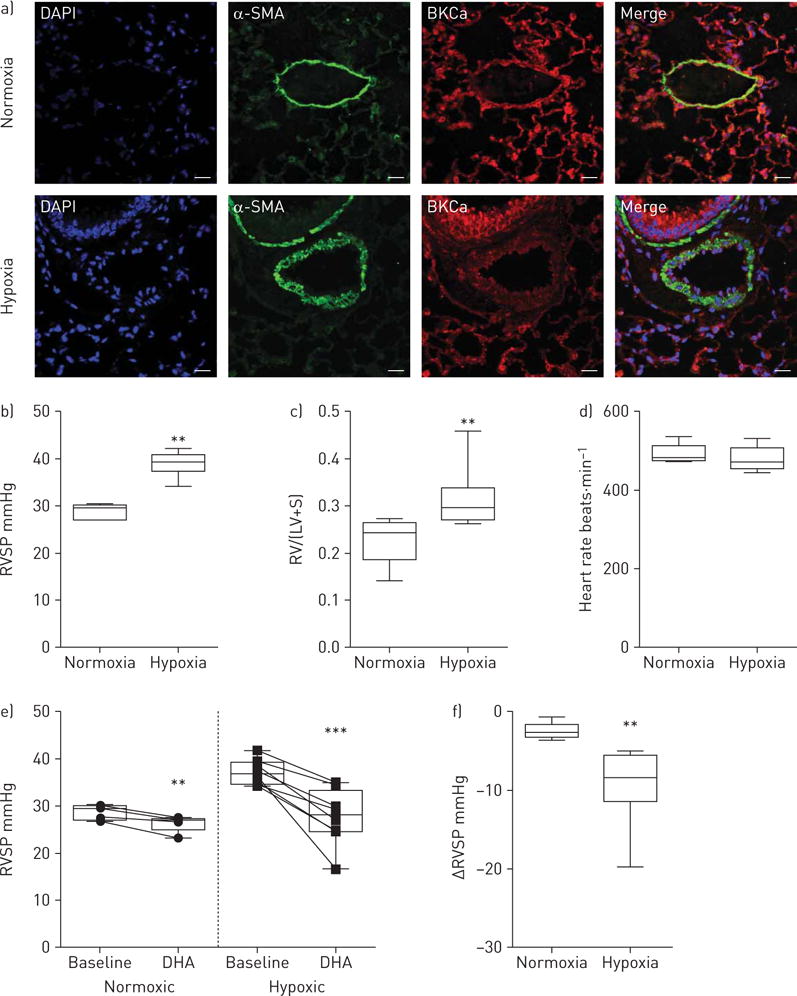
Docosahexaenoic acid (DHA) induces enhanced pulmonary vasodilation in the mouse pulmonary hypertension model. a) Double immunofluorescence staining of normoxic and chronic hypoxic mouse lungs showing the presence of large-conductance KCa (BKCa) channels (red) and α-smooth muscle actin (α-SMA; green) in pulmonary arteries. DAPI: 4′,6-diamidino-2-phenylindole. Scale bar: 20 μM. b) Right ventricular systolic pressure (RVSP), c) right ventricular hypertrophy (the right ventricle (RV) was completely dissected from the left ventricle (LV) and the septum(S)) and d) heart rate of normoxic and hypoxic mice. e) RVSP before (baseline) and after a single bolus of DHA administration in normoxic and chronic hypoxia-treated mice. f) Summarised RVSP changes in normoxic and hypoxic mice upon DHA. Data are presented as median, interquartile range, and minimum and maximum values. n=5 and 8 for normoxia and hypoxia, respectively. **: p<0.01; ***: p<0.001.
DHA causes hyperpolarisation of PASMCs obtained from patients with IPAH
Proceeding from the beneficial effect of DHA in the animal model, next we examined the effect of DHA on primary human PASMCs obtained from IPAH patients and healthy donors. Double immunolabelling shows BKCa channels in the smooth muscle layer of the pulmonary arteries from healthy donor lungs and in the remodelled pulmonary arteries of IPAH patients (figure 5a).
FIGURE 5.
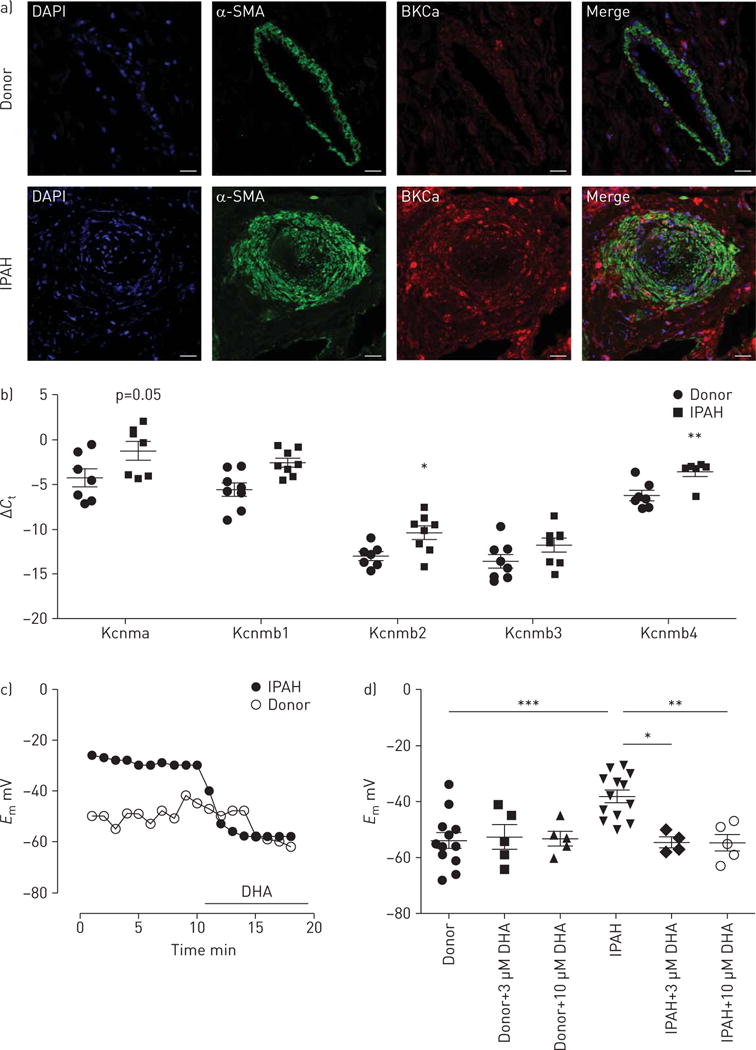
Docosahexaenoic acid (DHA) causes hyperpolarisation in pulmonary artery smooth muscle cells (PASMCs) obtained from idiopathic pulmonary arterial hypertension (IPAH) patients. a) Double immunofluorescence staining showing the localisation of large-conductance KCa (BKCa) channels (red) and α-smooth muscle actin (α-SMA; green) in the pulmonary arteries of donor and IPAH human lungs. DAPI: 4′,6-diamidino-2-phenylindole. Scale bar: 50 μM. b) Expression of BKCa channels with α-subunits (Kcnma) and their β-subunits (Kcnmb1–4) in the laser microdissected pulmonary arties of donor (n=7 or 8) and IPAH (n=6 or 8) patients. c) Representative membrane potential (Em) tracing from donor and IPAH PASMCs upon acute DHA exposure. d) Summarised membrane potential changes with increasing concentrations of DHA, and without DHA, in donor and IPAH PASMCs. Data in b, d) are presented as individual points with mean±SEM. *: p<0.05; **: p<0.01; ***: p<0.001.
The expression of BKCa channels was investigated in laser microdissected pulmonary arteries from healthy donors and IPAH patients. Pulmonary arteries from IPAH patients showed a marked upregulation of BKCa channel α-subunit (Kcnma1), as well as β-subunits (Kcnmb1, Kcnmb2 and Kcnmb4) (figure 5b), whereas Kcnmb3 remained unchanged. We then explored the effect of DHA on the membrane potential of primary human PASMCs obtained from both groups. A representative recording (figure 5c) demonstrates that acute perfusion of DHA hyperpolarised the resting membrane potential of IPAH PASMCs, but did not affect the PASMCs from healthy donors. Notably, the membrane potential of PASMCs from IPAH patients was significantly depolarised compared with PASMCs from healthy donors (−38.1±2.2 versus −54±2.8 mV, n=12 and 13; figure 5d). Our further analysis revealed that acute DHA administration (3 and 10 μM) did not change in the membrane potential of the control donor PASMCs. In contrast, exposure to DHA reversed the depolarisation of PASMCs from IPAH patients (−54.5±1.8 mV post 3 μM DHA versus −38.1±2.2 mV baseline, n=4 and −54.6±2.9 mV post 10 μM DHA versus −38.1±2.2 mV baseline, n=5; figure 5d), returning it to the level seen in healthy donors. These data suggest that DHA exerts a selective and direct effect on depolarised human PASMCs.
Discussion
We report the role of DHA as a KCa channel activator in the pulmonary circulation and its acute effect in a pulmonary hypertension model as well as in IPAH samples. Our study demonstrates that acute DHA administration rapidly activates the whole-cell K+ current in human primary PASMCs and shows that this activated current stems mostly from KCa channels and not from voltage-gated K+ channels. The KCa channel activation was dose-dependent, reducing pulmonary vascular tone, and causing both endothelium-dependent and -independent pulmonary vasodilation through BKCa channels in isolated pre-constricted intrapulmonary arteries. The relative contribution of endothelial versus PASMC BKCa channels in this vasodilatory response to DHA remains to be determined. Furthermore, DHA significantly decreased the PAP increased by U46619 in the isolated perfused lung; however, it did not alter baseline PAP. The impact of DHA-induced vasodilation was significantly reduced in the BKCa−/− mice, suggesting that the acute pulmonary vasodilatory effect of DHA is mediated, in part, through activation of BKCa channels. Importantly, in the chronic hypoxia pulmonary hypertension disease model in the mouse in vivo, acute DHA application returned the right ventricular systolic pressure to the level seen in control animals, demonstrating that DHA is a potent vasodilator in pulmonary hypertension. Moreover, we observed an upregulation of the BKCa channels in remodelled pulmonary arteries of IPAH patients. In line with these findings, we also demonstrated that DHA hyperpolarised the membrane potential of primary human PASMCs from IPAH patients, which were found to be depolarised at baseline, but not that from healthy donors.
Vascular tone of the pulmonary arteries is governed by the PASMCs; under physiologic (relaxed) conditions they have a relatively stable membrane potential, mainly driven by K+ permeability, although the chloride conductance should not be neglected [5, 22]. Hence, membrane potential is a vital controller of the vascular tone: the membrane potential/vascular tone slope is very steep. Therefore, even small membrane potential changes of a few millivolts cause significant changes in blood vessel diameter. Any physiological or pharmacological agent that alters membrane potential will cause a significant change in pressure. Thus, it is evident that membrane hyperpolarisation, achieved through activation of K+ channels, is a powerful mechanism to lower pressure through vasodilatation. KCa channels, built by the pore-forming α-subunit (Kcnmb1) along with auxiliary β-subunits (Kcnmb1–4), have been found in virtually every type of smooth muscle cell, and cause an impressive hyperpolarisation and vasodilatation when activated [23, 24]. In addition to the increased intracellular Ca2+ concentration and depolarisation, the most powerful mechanism to activate KCa is the phosphorylation of the channel mediated by a cAMP-or cGMP-dependent protein kinase (protein kinase A or G) [12, 25, 26]. This is of great importance for the physiologic function of KCa and has provided powerful pharmacological tools in the battle against pulmonary hypertension, as prostanoids, phosphodiesterase-5 inhibitors or soluble guanylyl cyclase activators develop their vasodilatory effects partly via activation of KCa.
BKCa channel expression and function has been shown to be elevated in chronic hypoxic mice and PASMCs [27, 28]. In concert with the pulmonary hypertension model, for the first time, we have shown that the BKCa channel and β-subunits are upregulated in remodelled pulmonary arteries obtained by laser-captured microdissection from IPAH patients. Consequently, BKCa channels in human PASMCs might provide a target for DHA in the setting of chronic vasoconstriction, and may serve as a feedback pathway to control the degree of membrane depolarisation and vasoconstriction as illustrated in the model (online supplementary figure S4).
DHA is one of the main components of ω-3 fatty acids, mostly obtained though dietary intake of marine foods. Its beneficial effects on human health emerged from epidemiological studies and clinical trials leading to the recommendation by the AHA for the regular consumption of DHA in fish or as a dietary supplement for the prevention of cardiovascular events [14, 29]. The chronic effect of DHA and its metabolic derivatives on the pulmonary circulation have been investigated on the proliferative phenotype of PASMCs and in vitro studies demonstrated the ability of DHA to counteract enhanced human PASMC proliferation, a hallmark of pulmonary arterial remodelling [30]. Chronic DHA treatment inhibited extracellular signal-regulated kinase activation and provoked an unfolded protein response causing cycle arrest and apoptosis induction [17]. Moreover, one early study showed that that DHA binds to and stimulates the G-protein coupled receptor GPR120 with an EC50 of ∼10 μM [31], indicating a potential new mechanism for the immunomodulatory effect of DHA. In addition, a study focused on DHA-induced changes in smooth muscle proposed that DHA, through the breakdown into its metabolic products, might inhibit the Rho kinase pathway and reduce calcium sensitivity [32].
In contrast, the acute effects of DHA have only recently been investigated. In the pulmonary circulation the effect of DHA was studied in the acute hypoxic setting, where the hypoxic inhibition of BKCa channels was reversed by the administration of DHA [33]. Moreover, DHA exposure provoked a rapid decrease in basal tone and thus vasodilation in the systemic circulation, suggesting a direct effect of DHA on K+ channels leading to hyperpolarisation of the smooth muscle cell membrane and consequently to vasodilation [21]. Indeed, HOSHI et al. [34] provided evidence for the direct binding of DHA to the pore-forming Slo1 and auxiliary β1-subunits of the large-conductance KCa expressed in human embryonic kidney cells. Our observations are in line with that study showing that DHA directly acts on KCa channel activity as measured by patch-clamp, as when KCa channel regulators are inhibited, the activation of KCa channels in human PASMCs remains unaltered (online supplementary figure S2A). In contrast to the previous findings in the systemic circulation, in our study DHA did not affect the basal pulmonary arterial tone in the isolated perfused mouse lung and, in the mice kept under normoxia, the acute DHA-induced reduction of the RVSP was small. However, after pre-constriction of the pulmonary arteries in the ex vivo model as well as in the in vivo mouse model of pulmonary hypertension, DHA induced an imposing drop in pulmonary pressure, suggesting a selective effect of DHA on constricted pulmonary vessels. In the pre-constricted, isolated perfused lungs of BKCa knockout mice DHA continued to cause some vasodilation, suggesting that it has additional vasodilator properties. Consequently, the fact that DHA has been previously reported as a strong vasodilator in the systemic circulation, but fails to alter systemic blood pressure in BKCa knockout mice, suggests that the DHA effect strongly depends on the specific cellular context and level of basal tone of the two vasculatures [21]. Finally, it was recently reported that lipid infusion containing ω-3 polyunsaturated fatty acids induces a potent and sustained vasodilatation in the chronically instrumented fetal lamb model of pulmonary hypertension [35]. The current study is in line with this and is thus able to further demonstrate the relevance of DHA as an acute vasodilator when the PAP is increased.
Using ex vivo and in vivo animal models of pulmonary hypertension, combined with investigations on laser microdissected remodelled pulmonary arteries and patch-clamp studies on isolated PASMCs from IPAH patients we performed detailed experimental characterisation and provided molecular insights into the DHA-induced vasodilation in pulmonary hypertension. To the best of our knowledge, this is the first study to show that DHA exhibits a rapid decrease in elevated pulmonary vascular tone and pressure. This decrease is achieved by KCa channel activation in PASMCs via directly modulating the membrane potential leading to vasorelaxation. We also demonstrated that activation of KCa channels by DHA is beneficial in the established pulmonary hypertension model and, more importantly, that DHA hyperpolarises only PASMCs obtained from IPAH patients, not control PASMCs. Furthermore, we detected the increased presence of BKCa channels in human IPAH, pointing to the utility of KCa openers for pharmacological interventions in PAH. Consequently, we propose that DHA is a potent vasodilator through KCa channel activation in pulmonary hypertension. The increased expression of KCa channels in the IPAH patient may further focus interest on DHA as a potential therapeutic option in pulmonary hypertension.
Acknowledgments
We greatly appreciate Maria Helene Schloffer, Simone Tischler and Elisabeth Blanz (Experimental Anaesthesiology, Dept of Anaesthesia and Intensive Care Medicine, Medical University of Graz, Graz, Austria) for their excellent technical support.
Support statement: A.L. Meredith is supported by National Heart, Lung and Blood Institute (grant number: HL102758). Funding information for this article has been deposited with FundRef.
Footnotes
This article has supplementary material available from erj.ersjournals.com
Conflict of interest: Disclosures can be found alongside this article at erj.ersjournals.com
References
- 1.Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31:891–901. doi: 10.1183/09031936.00097107. [DOI] [PubMed] [Google Scholar]
- 2.Badesch DB, McLaughlin VV, Delcroix M, et al. Prostanoid therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:56S–61S. doi: 10.1016/j.jacc.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 3.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 4.Nelson MT, Huang Y, Brayden JE, et al. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 5.Nelson MT, Patlak JB, Worley JF, et al. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 6.Yuan JX, Aldinger AM, Juhaszova M, et al. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 7.Tang B, Li Y, Nagaraj C, et al. Endothelin-1 inhibits background two-pore domain channel TASK-1 in primary human pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol. 2009;41:476–483. doi: 10.1165/rcmb.2008-0412OC. [DOI] [PubMed] [Google Scholar]
- 8.Varghese A, Hong Z, Weir EK. Serotonin-induced inhibition of KV current: a supporting role in pulmonary vasoconstriction? Circ Res. 2006;98:860–862. doi: 10.1161/01.RES.0000219683.65556.74. [DOI] [PubMed] [Google Scholar]
- 9.Hong Z, Smith AJ, Archer SL, et al. Pergolide is an inhibitor of voltage-gated potassium channels, including Kv1.5, and causes pulmonary vasoconstriction. Circulation. 2005;112:1494–1499. doi: 10.1161/CIRCULATIONAHA.105.556704. [DOI] [PubMed] [Google Scholar]
- 10.Nagaraj C, Tang B, Balint Z, et al. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur Respir J. 2013;41:85–95. doi: 10.1183/09031936.00211811. [DOI] [PubMed] [Google Scholar]
- 11.Orlandi EM, Rocca B, Pazzano AS, et al. Reversible pulmonary arterial hypertension likely related to long-term, low-dose dasatinib treatment for chronic myeloid leukaemia. Leuk Res. 2012;36:e4–e6. doi: 10.1016/j.leukres.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Olschewski A, Papp R, Nagaraj C, et al. Ion channels and transporters as therapeutic targets in the pulmonary circulation. Pharmacol Ther. 2014;144:349–368. doi: 10.1016/j.pharmthera.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Tur JA, Bibiloni MM, Sureda A, et al. Dietary sources of omega 3 fatty acids: public health risks and benefits. Br J Nutr. 2012;107(Suppl. 2):S23–S52. doi: 10.1017/S0007114512001456. [DOI] [PubMed] [Google Scholar]
- 14.Kris-Etherton PM, Harris WS, Appel LJ, et al. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 15.Archer SL, Johnson GJ, Gebhard RL, et al. Effect of dietary fish oil on lung lipid profile and hypoxic pulmonary hypertension. J Appl Physiol (1985) 1989;66:1662–1673. doi: 10.1152/jappl.1989.66.4.1662. [DOI] [PubMed] [Google Scholar]
- 16.Morin C, Fortin S, Rousseau E. Docosahexaenoic acid monoacylglyceride decreases endothelin-1 induced Ca2+ sensitivity and proliferation in human pulmonary arteries. Am J Hypertens. 2012;25:756–763. doi: 10.1038/ajh.2012.45. [DOI] [PubMed] [Google Scholar]
- 17.Crnkovic S, Riederer M, Lechleitner M, et al. Docosahexaenoic acid-induced unfolded protein response, cell cycle arrest, and apoptosis in vascular smooth muscle cells are triggered by Ca2+-dependent induction of oxidative stress. Free Radic Biol Med. 2012;52:1786–1795. doi: 10.1016/j.freeradbiomed.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stulnig G, Frisch MT, Crnkovic S, et al. Docosahexaenoic acid (DHA)-induced heme oxygenase-1 attenuates cytotoxic effects of DHA in vascular smooth muscle cells. Atherosclerosis. 2013;230:406–413. doi: 10.1016/j.atherosclerosis.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Lai LH, Wang RX, Jiang WP, et al. Effects of docosahexaenoic acid on large-conductance Ca2+-activated K+ channels and voltage-dependent K+ channels in rat coronary artery smooth muscle cells. Acta Pharmacol Sin. 2009;30:314–320. doi: 10.1038/aps.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang RX, Chai Q, Lu T, et al. Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc Res. 2011;90:344–352. doi: 10.1093/cvr/cvq411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshi T, Wissuwa B, Tian Y, et al. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels. Proc Natl Acad Sci USA. 2013;110:4816–4821. doi: 10.1073/pnas.1221997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaggar JH, Wellman GC, Heppner TJ, et al. Ca2+ channels, ryanodine receptors and Ca2+-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand. 1998;164:577–587. doi: 10.1046/j.1365-201X.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 23.Bentzen BH, Olesen SP, Ronn LC, et al. BK channel activators and their therapeutic perspectives. Front Physiol. 2014;5:389. doi: 10.3389/fphys.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firth AL, Remillard CV, Platoshyn O, et al. Functional ion channels in human pulmonary artery smooth muscle cells: voltage-dependent cation channels. Pulm Circ. 2011;1:48–71. doi: 10.4103/2045-8932.78103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Connolly M, Nagaraj C, et al. Peroxisome proliferator-activated receptor-beta/delta, the acute signaling factor in prostacyclin-induced pulmonary vasodilation. Am J Respir Cell Mol Biol. 2012;46:372–379. doi: 10.1165/rcmb.2010-0428OC. [DOI] [PubMed] [Google Scholar]
- 26.Archer SL, Huang JM, Hampl V, et al. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wandall-Frostholm C, Skaarup LM, Sadda V, et al. Pulmonary hypertension in wild type mice and animals with genetic deficit in KCa2.3 and KCa3.1 channels. PLoS One. 2014;9:e97687. doi: 10.1371/journal.pone.0097687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimoda LA, Sylvester JT, Sham JS. Chronic hypoxia alters effects of endothelin and angiotensin on K+ currents in pulmonary arterial myocytes. Am J Physiol. 1999;277:L431–L439. doi: 10.1152/ajplung.1999.277.3.L431. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury R, Stevens S, Gorman D, et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ. 2012;345:e6698. doi: 10.1136/bmj.e6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan J, Chen R, Liu P, et al. Docosahexaenoic acid inhibits development of hypoxic pulmonary hypertension: in vitro and in vivo studies. Int J Cardiol. 2013;168:4111–4116. doi: 10.1016/j.ijcard.2013.07.073. [DOI] [PubMed] [Google Scholar]
- 31.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morin C, Fortin S, Rousseau E. 19,20-EpDPE, a bioactive CYP450 metabolite of DHA monoacyglyceride, decreases Ca2+ sensitivity in human pulmonary arteries. Am J Physiol Heart Circ Physiol. 2011;301:H1311–H1318. doi: 10.1152/ajpheart.00380.2011. [DOI] [PubMed] [Google Scholar]
- 33.Yan J, Chen R, Liu P, et al. Docosahexaenoic acid attenuates hypoxic pulmonary vasoconstriction by activating the large conductance Ca2+-activated K+ currents in pulmonary artery smooth muscle cells. Pulm Pharmacol Ther. 2014;28:9–16. doi: 10.1016/j.pupt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Hoshi T, Tian Y, Xu R, et al. Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the omega-3 fatty acid DHA. Proc Natl Acad Sci USA. 2013;110:4822–4827. doi: 10.1073/pnas.1222003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houeijeh A, Aubry E, Coridon H, et al. Effects of n-3 polyunsaturated fatty acids in the fetal pulmonary circulation. Crit Care Med. 2011;39:1431–1438. doi: 10.1097/CCM.0b013e31821204fb. [DOI] [PubMed] [Google Scholar]


