Abstract
We have developed a method for obtaining pneumococcal lipoteichoic acid (LTA) with none, one, or two acyl chains. Anion-exchange chromatography at pH 9.5 yields pneumococcal LTA (labeled LTA-9.5) that has a mass spectrum identical to that of pre-ion-exchange LTA and loses 500 mass units after deacylation by alkali hydrolysis. Anion exchange at pH 10.5 produces LTA (labeled LTA-10.5) with mass peaks that are 264 mass units lower than those of pre-ion-exchange LTA, and deacylation of LTA-10.5 by alkali hydrolysis reduces the mass by only 239 mass units. This result indicates that LTA-10.5 has lost one of the two acyl chains, whereas LTA-9.5 has both acyl chains. When the biological properties of LTA-9.5 and LTA-10.5 are examined with mouse cells, only LTA-9.5 (and not LTA-10.5) is able to stimulate mouse cells to produce tumor necrosis factor alpha, interleukin-1β, and nitric oxide. In contrast, both LTA-9.5 and LTA-10.5 can stimulate human cells. LTA became inactive when both acyl chains were removed. Thus, acyl chains are critical for LTA function, and small variations in acyl chains can alter biological properties of LTA.
Lipoteichoic acid (LTA) is an important component of the cell walls of gram-positive bacteria. LTA is a polyphosphate polymer that is linked to a glycerol backbone with two acyl chains (10). The acyl chains anchor the molecule to the bacterial plasma membrane. The resulting LTA structure is amphipathic, which is also true of the lipopolysaccharide (LPS) of gram-negative bacteria. Also, like LPS, LTA is considered to be an important pathogen-associated molecular pattern capable of stimulating innate immunity and responsible for gram-positive bacterial sepsis. However, the biological properties of LTA are only beginning to be elucidated because the LTA used in previous studies may have contained biologically active contaminants (12) or may have been altered in structure (25). The biological properties of LTA have been investigated mostly with staphylococcal LTA, which stimulates platelet-activating factor receptor (24) and TLR2 (9, 30). Staphylococcal LTA has been shown to induce the production of tumor necrosis factor alpha (TNF-α) (9) and nitric oxide (NO) (7, 22). It has also been shown to induce sepsis in rats when peptidoglycan is coadministered (8, 23, 26). Thus, staphylococcal LTA may be as important in causing gram-positive bacterial sepsis as LPS is in causing gram-negative bacterial sepsis.
Along with staphylococci, pneumococci are responsible for most cases of gram-positive bacterial sepsis, which accounts for about half of all cases of bacterial sepsis (6, 35). Pneumococcal LTA has also been shown to stimulate cells via TLR2 (16, 29). Yet to date, pneumococcal LTA has not been studied as extensively as has staphylococcal LTA. Also, it is difficult to extrapolate biological properties of staphylococcal LTA to those of pneumococcal LTA, since the two LTAs have significant structural differences. For instance, the polyphosphate polymer of staphylococcal LTA is composed of 20 to 50 small and variably sized repeating units (about 130 to 320 Da) (11), whereas the polyphosphate polymer of pneumococcal LTA has 6 to 8 large and exactly sized (1,299 Da) repeating units (1, 11). To study the roles of pneumococcal LTA in sepsis, we have improved the existing method of purifying pneumococcal LTA (17) and have found that the biological properties of pneumococcal LTA depend on the number of acyl chains.
MATERIALS AND METHODS
Reagents.
Pneumococcal cell wall polysaccharide (C-PS; teichoic acid) was purchased from Statens Serum Institute (Copenhagen, Denmark). TEPC-15 (mouse immunoglobulin A [IgA], kappa) and Escherichia coli (055:B5) LPS were obtained from Sigma Chemical Co. (St. Louis, Mo.). Before use, the E. coli LPS was repurified by phenol extraction (18). Pyrogen-free water was used to prepare buffers and to perform dialysis.
Purification of pneumococcal LTA.
Pneumococcal LTA was purified according to the method used by Behr et al. (1) with an additional purification step: ion-exchange chromatography. Briefly, Streptococcus pneumoniae (strain R36A) was cultured overnight at 37°C in Todd-Hewitt broth (Difco, Detroit, Mich.) with the supplement of 0.5% yeast extract (Difco). Cells were pelleted, resuspended in 0.05 M sodium acetate buffer (pH 4.0), and disrupted by ultrasonication (SonicatorTM model W-220F from Heat Systems Ultrasonics, Inc., Plainview, N.Y.). LTA was extracted from the lysate with a chloroform-methanol-water (1.0:1.0:0.9) mixture, and the aqueous phase containing LTA was collected following phase separation. LTA was then adsorbed onto an octyl-Sepharose CL-4B column (Sigma Chemical) equilibrated in 0.05 M sodium acetate buffer (pH 4.7) containing 15% n-propanol. LTA was eluted from the column with a stepwise n-propanol gradient (20, 35, and 45%), and column fractions containing the LTA were pooled and dialyzed against water. To further purify LTA by ion-exchange chromatography, LTA was adsorbed to a Q-Sepharose Fast Flow column (Sigma Chemical) equilibrated in a 10 mM 2-amino-2-methyl-1-propanol · HCl buffer (pH 9.5 or 10.5; Sigma Chemical) containing 30% n-propanol. LTA was eluted from the column with a continuous linear salt gradient (0.0 to 0.3 M NaCl in the equilibration buffer), and the eluent was collected in 2-ml aliquots. LTA-containing fractions were pooled and dialyzed. The pool was stored at −70°C in aliquots.
Deacylation of pneumococcal LTA.
Various preparations of highly purified pneumococcal LTA were deacylated by incubation in 0.2 N NaOH at 37°C for 2 h. After hydrolysis, the samples were neutralized with HCl and extensively dialyzed against pyrogen-free water.
Structure confirmation and purity determination of LTAs.
LTA molecules were characterized by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry in the Mass Spectrometry Shared Facility at the University of Alabama at Birmingham (UAB). Briefly, 1 μl of a sample and 1 μl of matrix solution (0.5 M 2,5-dihydroxybenzoic acid and 0.1% trifluoroacetic acid in methanol) were applied to a sample plate. After drying, the sample was analyzed with a mass spectrometer (Voyager Biospectrometry DE Pro workstation) from PerSeptive Biosystems (Framingham, Mass.).
The purities of the LTAs were determined by measuring their endotoxin contents with the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.), since the assay is insensitive to LTA (25). Their DNA and RNA or protein contamination was assessed with UV absorbance at 260 or 280 nm, respectively. To assess protein contaminants, the samples were separated by polyacrylamide gel electrophoresis. Then the electrophoresis gel was stained with silver nitrate to visualize all protein bands and with the Western blot technique using TEPC-15 (5) to visualize the LTA bands.
Quantification of LTAs.
The content of the pneumococcal LTAs was determined by measuring both inorganic phosphorus (14) and the amount of LTA equivalent to C-PS. For phosphorus determinations, samples were digested with a nitric acid-sulfuric acid mixture and treated with molybdate and stannous chloride. The optical density (OD) at 600 nm of the samples was converted to phosphorus concentrations. The C-PS equivalence assay was an inhibition enzyme-linked immunosorbent assay (ELISA) conducted as follows. Immulon 2 HB flat-bottom 96-well plates (Dynex Technologies Inc., Chantilly, Va.) were coated by being incubated overnight with C-PS (1 μg/ml) in phosphate-buffered saline (PBS) and for 1 h with blocking buffer (PBS with 1% bovine serum albumin, 0.05% sodium azide, 0.05% Tween 20). Pneumococcal LTAs or C-PS that was serially diluted in the blocking buffer was added to the ELISA plates along with TEPC-15 antibody. After 1 h of incubation at 37°C, the plates were washed three times with the wash buffer (PBS-0.05% Tween 20). Then alkaline phosphatase-conjugated goat anti-mouse immunoglobulins were added to each well. After another 1 h of incubation at 37°C, the plates were washed three times with the wash buffer, and para-nitrophenyl phosphate (Sigma Chemical) solution in diethanolamine buffer (pH 9.8) was added. When the colors developed, the absorbance was measured at 405 nm, and the absorbance was converted to LTA concentrations by using the standard curve obtained with C-PS.
TNF-α production by human peripheral blood monocytes.
Peripheral blood mononuclear cells (PBMCs) were obtained from heparinized blood by isolating the buffy coat from the blood and then removing contaminating red blood cells with a Histopaque (density = 1.077) density gradient. Monocytes were isolated from the PBMCs by removing nonmonocytic cells with an indirect magnetic isolation kit (Miltenyi Biotec, Auburn, Calif.) and with monoclonal hapten-conjugated CD3, CD7, CD19, CD45RA, CD56, and IgE antibodies from Miltenyi Biotec. This procedure routinely resulted in >95% pure CD14+ cells by flow cytometry.
Human monocytes (106 cells/ml) were suspended in RPMI 1640 supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 2 mM l-glutamine, 20 mM HEPES, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The monocyte suspension was placed in 96-well plates (200 μl/well) and was stimulated with LTA (or its variants) for 20 h. The amount of TNF-α in the culture supernatant was determined with a human TNF-α ELISA Ready-SET-Go kit (eBioscience, San Diego, Calif.) following the manufacturer's protocol.
TNF-α production by mouse peritoneal cells.
Peritoneal cells from C57BL/6 mice were obtained with PBS. The cells (106 cells/ml) were suspended in RPMI 1640 supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 2 mM l-glutamine, 20 mM HEPES, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The cell suspension was placed in 96-well plates (200 μl/well) overnight and was stimulated with LTA (or its variants) for 20 h. The amount of TNF-α in the culture supernatant was determined with a mouse TNF-α ELISA Ready-SET-Go kit (eBioscience) according to the manufacturer's protocol.
Stimulation of human and mouse monocyte/macrophage cell lines for cytokine mRNA and NO production.
THP-1 (ATCC TIB-202) and RAW 264.7 (ATCC TIB-71) cell lines were obtained from the American Type Culture Collection (Manassas, Va.) and were grown in Dulbecco's modified Eagle's medium (Cellgro Mediatech, Herndon, Va.) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml at 37°C in a 5% CO2 humidified incubator. For cytokine mRNA studies, the cell lines were stimulated for 3 h with LTA or LPS, and their RNA was extracted by using Trizol reagent (Invitrogen, Carlsbad, Calif.). cDNA was obtained by incubating 6 μg of RNA with 6 mM deoxynucleoside triphosphate (Takara), 1 μl of random primer (Promega), 10 U of Moloney murine leukemia virus reverse transcriptase (Promega), and 6 μl of 5× buffer in a total volume of 30 μl for 1 h at 37°C and 10 min at 95°C. PCR was performed as described previously (15) using various primers. The primer sequences were 5′-ATG AGC ACA GAA AGC ATG ATC-3′ (sense) and 5′-TAC AGG CTT GTC ACT CGA ATT-3′ (antisense) for mouse TNF-α (20), 5′-AAG CTC TCA CCT CAA TGG A-3′ (sense) and 5′-CTC AGC CCT GAG AAA GGA GA-3′ (antisense) for mouse interleukin-1β (IL-1β), 5′-ATG AGC ACT GAA AGC ATG ATC-3′ (sense) and 5′-TCA CAG GGC AAT GAT CCC AAA GTA GAC CTG CCC-3′ (antisense) for human TNF-α, 5′-GCT GAG GAA GAT GCT GGT TC-3′ (sense) and 5′-TCC AGC TGT AGA GTG GGC TT-3′ (antisense) for human IL-1β, and 5′-AAG GAG AAG CTG TGC TAC GTC GC-3′ (sense) and 5′-AGA CAG CAC TGT GTT GGC GTA CA-3′ (antisense) for human β-actin. β-Actin was analyzed as a control for cDNA amounts in each sample, and the human primers were used for mouse β-actin as well. For TNF-α and IL-1β, the PCR protocol was 95°C for 10 min; 30 cycles of denaturation at 95°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min; and then 72°C for 10 min. For β-actin, PCR was performed as described above except that only 25 cycles were performed. The PCR products were separated in an agarose gel (1.5%) containing ethidium bromide, and the results were recorded with a gel documentation system (Gel Doc 2000; Life Science Research, Hercules, Calif.).
For studying NO production, RAW 264.7 cells were stimulated for 48 h before the culture supernatants were harvested, and their NO contents were determined as described previously (13). Briefly, the harvested culture media were mixed with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine hydrochloride, and 2% phosphoric acid) in a 96-well ELISA plate. The OD at 540 nm, which was determined with an ELISA plate reader, was converted to the amount of nitrite by comparing the ODs obtained with the standard amounts of NaNO2.
IL-8 production by A549 cells.
A549 (ATCC CCL185) cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 4.5 g of glucose/liter, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The A549 cells (105 cells/ml) were placed in 96-well plates (200 μl/well), and a confluent monolayer of cells was stimulated with LTA (or its variants) for 24 h. The amount of IL-8 in the culture supernatant was determined with a human IL-8 OptEIA set (BD, San Diego, Calif.) following the manufacturer's protocol.
RESULTS
Ion-exchange chromatography produces pneumococcal LTA with undetectable impurities.
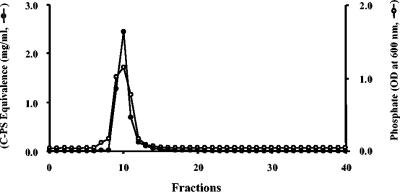
Fischer developed an efficient method for purifying pneumococcal LTA by using organic solvent partition and hydrophobic interaction chromatography (1). The LTA obtained by us in this manner was insufficiently pure for biological studies, since the biological properties of the resulting pneumococcal LTA were variable (our unpublished data). We have therefore incorporated an additional purification step: anion-exchange chromatography. During this step, pneumococcal LTA was adsorbed onto a Q-Sepharose column at pH 9.5 and was eluted from the column with an elution buffer (pH 9.5) containing increasing amounts of NaCl. A single elution peak with a front shoulder was obtained by phosphate assay (Fig. 1). The shoulder fractions did not contain significant amounts of C-PS-equivalent materials (Fig. 1). Mass spectrometry showed that the shoulder fraction contained LTA with few repeating units and that the main fraction contained a normal number of repeating units (data not shown). The fractions forming the main peak were pooled and used as LTA. A very similar elution pattern was obtained when the pH of the elution buffer was 10.5 (data not shown). To distinguish the two LTA preparations hereafter, LTA purified by the ion-exchange chromatography at pH 9.5 is referred as LTA-9.5, and LTA purified at pH 10.5 is referred as LTA-10.5.
FIG. 1.
Elution pattern of pneumococcal LTA from Q-Sepharose anion-exchange column. The phosphorus level (open circles) and C-PS equivalence (solid circles) in each fraction are shown. The column was eluted with a continuous linear salt gradient (0.0 to 0.3 M NaCl) in the equilibration buffer (pH 9.5) containing 30% n-propanol.
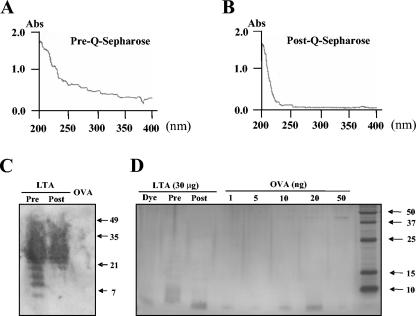
That the ion-exchange chromatography significantly increased the purity of LTA was established by several different criteria. First, the resulting LTA preparations contained less than 5 pg of endotoxin per mg of LTA, as determined by Limulus amebocyte lysate assay. Second, optical absorption was significantly reduced at UV wavelengths (Fig. 2A and B). When LTA preparations (at 1.6 mg/ml) obtained before and after the ion-exchange chromatography (at pH 9.5) were dialyzed against water and their absorbances from 200 to 400 nm were determined, the ODs at 280 nm decreased from 0.4 to undetectable (<0.05), suggesting that protein contamination had been reduced by 10-fold or more. Also, DNA and RNA contaminants may have been reduced significantly, since the ODs at 260 nm were reduced from 0.5 to undetectable (<0.05). Third, we performed sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis by immunoblotting the resulting gel with anti-phosphocholine (PC) antibody (Fig. 2C) or staining with silver nitrate (Fig. 2D). Although pneumococcal LTA has a molecular weight (MW) ranging from 7,000 to 10,000, it has been shown to migrate in SDS-PAGE at 20,000 to 30,000 protein-based MW range (36). Accordingly, the immunoblot of the materials before and after the ion exchange showed PC-containing material with a MW of 21,000 to 35,000, which was established with protein-based molecular weight standards. The immunoblot also showed PC-containing, weakly staining bands with apparent MWs of 7,000 to 20,000 that are present before but not after the ion exchange. These bands are likely contaminants, since the same MW region (5,000 to 15,000) contains silver-staining material found only in the pre- and not in the post-ion-exchange sample.
FIG. 2.
Spectrometric (A and B), Western blot (C), and silver-staining (D) characteristics of pneumococcal LTA preparations before and after Q-Sepharose anion-exchange chromatography. Optical densities from 200 to 400 nm for an LTA preparation (1.6 mg/ml) prior to anion-exchange chromatography (A) and after the anion exchange (B) are shown. In panel C, Western blotting shows the presence of LTA before (Pre) and after (Post) ion-exchange chromatography. The Western blotting was specific for LTA and did not stain the lane loaded with ovalbumin (OVA). Thirty micrograms of LTA and 20 μg of OVA were loaded on each lane. (D) Silver nitrate did not stain the lane loaded with pneumococcal LTA after ion-exchange chromatography (30 μg/lane), but it lightly stained the 7- to 15-kDa region of the lane containing pneumococcal LTA (30 μg/lane) obtained before ion-exchange chromatography. For comparison, different amounts of OVA were loaded in the five lanes. The amount of OVA per lane is shown above. A 40-kDa band was visible with 10, 20, and 50 ng of OVA.
LTA-9.5 and LTA-10.5 have different mass spectra.
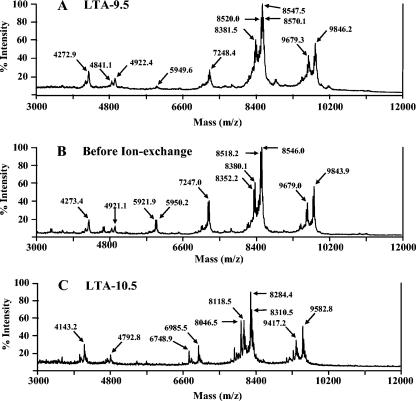
To characterize our LTA preparations, we obtained their mass spectra using MALDI-TOF. The spectra of LTA-9.5 showed three major peaks, with molecular weights of approximately 7,248.4, 8,547.5, and 9,846.2 m/z units (Fig. 3A). Several peaks surrounded each major peak, with the surrounding peaks differing from the major peaks in mass by about 28 m/z units (e.g., 8,520.0 versus 8,547.5). Since the LTA is known to have microheterogeneity in the acyl groups (1) and the mass difference corresponds to the mass difference between lipids (i.e., oleic versus palmitoleic) (10), the surrounding peaks most likely represent LTAs with different acyl chains. In addition, the three major peaks have satellite peaks that differ by about 165 to 300 m/z units. While not all of these satellite peaks have been identified, the most prominent satellite peaks are 165 m/z units lower than the major peaks (i.e., 8,381.5 versus 8,547.5 and 9,679.3 versus 9,846.2). Since phosphocholine has 166 mass units, the satellite peaks most likely represent a molecule that has lost one PC group. The peaks found with m/z values ranging from 4,272.9 to 4,922.4 correspond to the major molecular species with two (instead of one) charges. For instance, one molecular species would have produced the peaks with m/z values of 4,272.9 and 8,547.5 with two or one H+ ion, respectively.
FIG. 3.
MALDI-TOF mass spectra of pneumococcal LTA obtained after Q-Sepharose ion-exchange chromatography at pH 9.5 (A), before ion-exchange chromatography (B), and after ion-exchange chromatography at pH 10.5 (C). Panels A and B have identical patterns of mass peaks, suggesting that the LTA molecule was not altered during the ion exchange at pH 9.5. In contrast, the mass peaks in panel C have smaller m/z values (about 260 units) than the corresponding mass peaks in panel B, although the overall pattern is similar. This result suggests that the LTA molecule lost about 260 Da during the ion exchange at pH 10.5.
The major peaks differ from their neighboring peaks by about 1,300 (from 1,299 to 1,301) m/z units (e.g., 8,547.5 versus 9,846.2). This mass is exactly the size of one repeating unit, which contains four hexoses (one glucose, one 2-acetamido-4-amino-2,4,6-trideoxygalactose, and two N-acetyl-galactosamines), two phosphocholines, and one ribitol phosphate group (1). Additional studies involving acid hydrolysis suggest that the peak with 8,547.5 m/z units corresponds to a pneumococcal LTA molecule with six repeating units (data not shown).
The mass spectra of LTA-9.5 are almost identical to those of postoctyl LTA before the ion-exchange step (Fig. 3B), indicating that pneumococcal LTA was not altered or degraded during the ion-exchange step. However, to our surprise, the mass spectra of LTA-10.5 (Fig. 3C) were different from the mass spectra of the other two LTAs. LTA-10.5's mass spectra have three major peaks as above, but the peaks' m/z values were 6,985.5, 8,284.4, and 9,582.8. These values are about 264 m/z units less than the corresponding peaks of LTA-9.5. Also, there are new satellite peaks, which are about 238 m/z units lower than the major peaks (e.g., 8,046.5), and the m/z values of these new satellite peaks are identical to those of the deacylated LTA peaks shown in Fig. 4A. Since the mass differences among the major peaks of LTA-10.5 continue to be about 1,300 m/z units, the repeating units are not altered in structure and the structural alteration must have occurred in the glycolipid portion of the molecule with the loss of about 260 or 500 Da.
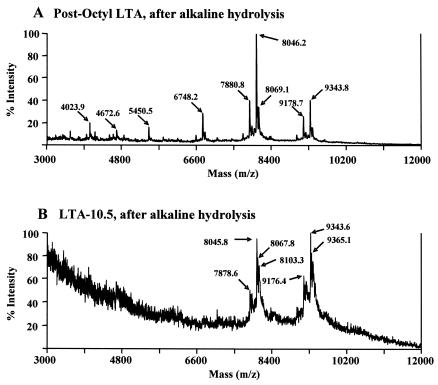
FIG. 4.
MALDI-TOF mass spectra of pneumococcal LTAs after alkaline hydrolysis. Alkaline hydrolysis selectively removes all of the acyl chains from pneumococcal LTA (3). Pneumococcal LTA used for the hydrolysis underwent either no Q-Sepharose ion-exchange chromatography (A) or ion-exchange chromatography at pH 10.5 (B).
LTA-10.5 has one acyl chain, but LTA-9.5 has two acyl chains.
Pneumococcal LTA has a glycerol group with two acyl groups: oleic acid (C18:1) at the C-2 position and palmitic acid (C16:0) at the C-1 position. Removing the oleic acid by hydrolysis should reduce the molecular weight by 264, and removing the palmitic acid should reduce it by 239. Since the mass for LTA-10.5 is about 262 Da less than that of pre-ion-exchange LTA, it is likely that oleic acid was lost from the LTA during ion-exchange chromatography at pH 10.5. Alkali hydrolysis in 0.2 N NaOH for 2 h at 37°C removes all of the acyl groups from LTA (21, 34). If LTA-10.5 had already lost oleic acid, the alkali hydrolysis would reduce the molecular weight of LTA-10.5 by only about 239 instead of 504 m/z units.
To test this prediction, both postoctyl LTA and LTA-10.5 were hydrolyzed and their mass spectra were obtained (Fig. 4). Following the hydrolysis, the main peaks of postoctyl LTA shifted from 8,546.0 to 8,046.2 m/z units (Fig. 4A), indicating that the alkali hydrolysis successfully removed both acyl chains from postoctyl LTA. In contrast, the LTA-10.5 lost only 239 m/z units (e.g., 8,284.4 [Fig. 3C] versus 8,045.8 [Fig. 4B]). These mass spectrometric data indicate that LTA-10.5 had only one acyl group of 239 Da.
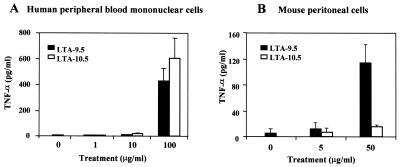
LTA-9.5 stimulates both human and mouse cells to produce TNF-α, but LTA-10.5 stimulates only human cells.
To assess the impact of acyl chains on LTA function, we examined the abilities of LTA-9.5 and LTA-10.5 to stimulate both human peripheral blood monocytes and mouse peritoneal macrophage cells to produce TNF-α. When the human cells were stimulated with LTA, both LTA preparations were indistinguishable in their stimulation of TNF-α production, and both increased the TNF-α levels about 100-fold above the background level (Fig. 5A). In contrast, completely deacylated LTA did not stimulate TNF-α production at all.
FIG. 5.
Production of TNF-α by human PBMCs (A) and mouse peritoneal macrophages (B) in response to LTA-9.5 (black bars) or LTA-10.5 (open bars) at the indicated doses (in micrograms per milliliter). Error bars indicate standard deviations for the means of three replicate data points. At 50 μg/ml, the response by mouse macrophages to LTA-9.5 was significantly greater than the response to LTA-10.5 (P < 0.01 by Student's t test).
When the mouse cells were stimulated with both LTA preparations at 50 μg/ml, the results were quite different from those obtained with human cells: LTA-9.5 induced TNF-α production about 10-fold more than did LTA-10.5 (Fig. 5B). This residual activity of LTA-10.5 may be due to a small amount of residual diacyl LTA (i.e., LTA-9.5). Completely deacylated LTA did not stimulate mouse cells (data not shown). Taken together, these data suggest that LTA-10.5 may stimulate human cells but not mouse cells.
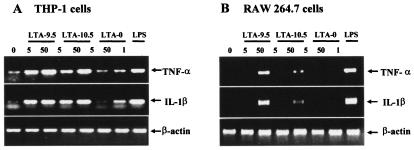
LTA-9.5 stimulates both human and mouse cells to express TNF-α and IL-1β mRNA, but LTA-10.5 stimulates only human cells.
To further investigate the above observation, human and mouse cell lines were stimulated with both LTA preparations at 5 and 50 μg/ml for 3 h, and the levels of TNF-α and IL-1β mRNA were determined by reverse transcription-PCR. For easy comparison, equivalent amounts of β-actin mRNA were investigated throughout the study. The human cell line THP-1 expresses a small amount of both IL-1β and TNF-α mRNA before and a large amount after LPS stimulation (1 μg/ml). Completely deacylated LTA (LTA-0) appears to slightly stimulate the expression of both mRNAs at 50 μg/ml. In contrast to deacylated LTA, both LTA-9.5 and LTA-10.5 strongly induced the expression of both mRNAs at 5 and 50 μg/ml (Fig. 6A). Also, the levels of mRNA expression were similar for both LTA preparations.
FIG. 6.
Effect of LTA-9.5, LTA-10.5, LTA-0, or LPS treatment on TNF-α and IL-1β mRNA levels in human THP-1 (A) and murine RAW 264.7 (B) macrophage cell lines at 3 h after the treatment. The stimulant doses (in micrograms per milliliter) are indicated below the bar indicating the stimulant. As an internal standard, the β-actin mRNA level was determined.
When the RAW 264.7 mouse cell line was studied, TNF-α and IL-1β mRNA levels were undetectable before the stimulation, but the mRNA bands became strong after LPS stimulation. Also, both mRNA levels remained undetectable after stimulation with deacylated LTA. However, in contrast to the human cell line, the mouse cell line (RAW 264.7) produced large amounts of mRNA of both TNF-α and IL-1β in response only to LTA-9.5 and produced only very small amounts of mRNA in response to LTA-10.5 (Fig. 6B). The small amount of stimulation may be due to the fact LTA-10.5 may contain small amounts of intact, diacyl LTA. These results further support the conclusion that both LTA preparations stimulate human cells but that only LTA-9.5 stimulates mouse cells.
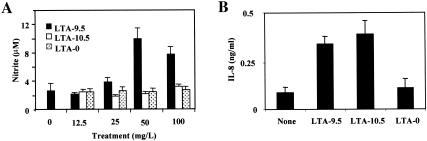
LTA-9.5, but not LTA-10.5, stimulates mouse macrophages to produce NO, but both LTA-9.5 and LTA-10.5 stimulate human cells to produce IL-8.
To further evaluate the above conclusion, we determined NO production by the mouse macrophage cell line (RAW 264.7) in response to LTA. Human cells were not tested for NO production, since human cells may not produce NO (2, 28). When RAW 264.7 cells were stimulated with less than 10 μg of LTA/ml, they produced very small amounts of NO, as do unstimulated cells. However, 100 μg of LTA-9.5/ml strongly stimulated the mouse cells to produce NO, but LTA-10.5 did not (Fig. 7A). Completely deacylated LTA did not stimulate NO production.
FIG. 7.
Production of NO by RAW 264.7 mouse macrophage cells 48 h after LTA-9.5 (black bars), LTA-10.5 (open bars), or LTA-0 (stippled bars) treatment at the indicated doses (in micrograms per milliliter) (A) and production of IL-8 by A549 cells in response to no stimulus (None), LTA-9.5, LTA-10.5, or LTA-0 (B). Error bars indicate standard deviations for the means of three replicate data points. At 50 and 100 μg/ml, the NO production in response to LTA-9.5 was significantly greater than the responses to LTA-10.5 or LTA-0 (P < 0.001 by Student's t test). IL-8 production in response to LTA-9.5 or LTA-10.5 was significantly greater (P < 0.001 by Student's t test) than to no stimulus or to LTA-0.
To further examine responsiveness of human cells to monoacyl LTA, we stimulated a type II pneumocyte cell line, A549 (31), with intact LTA (LTA-9.5), monoacyl LTA (LTA-10.5), and deacylated LTA. Both intact and monoacyl LTA induced a severalfold increase in IL-8 production (Fig. 7B). These findings further support the conclusion that only LTA-9.5 stimulates mouse cells.
DISCUSSION
To obtain pneumococcal LTA of high purity, we have added an ion-exchange step to the conventional method of purifying pneumococcal LTA (1). Our studies show that the ion-exchange step can produce LTA with only one acyl chain when the elution buffer has a high pH. The following observations support the conclusion that the lost acyl chain is not palmitic acid at the C-1 position of the glycerol backbone but is instead oleic acid at the C-2 position. First, the observed mass loss is consistent with the loss of oleic acid (264 Da) rather than palmitic acid (239 Da). Second, when we treated LTA with phospholipase A2 from pork pancreas (which removes the acyl chain at the C-2 position), its mass spectrometric pattern was the same as LTA-10.5's pattern (data not shown).
MALDI-TOF mass spectrometry showed that the major mass peaks are separated by 1,300 mass units and that there is no evidence for a partial unit at the terminus. These observations strongly suggest that pneumococcal LTA does not have an incomplete repeating unit. We believe that this happens because the repeating unit is completely synthesized before it is added to the LTA chain. Pneumococci may synthesize each repeating unit in their cytoplasm, transport the unit out of the cell, and then attach it to the glycolipid anchor of LTA inserted into the membrane. It is possible that the synthetic steps occur in the membrane folds labeled “mesosomes,” which are found beneath the cell walls of normal pneumococci (32) and are rich in LTA (19).
We show that the monoacyl form of LTA (LTA-10.5) stimulates human cells but not mouse cells. This characteristic is similar but opposite to the action of hepta-acylated lipid A (compound 516), which stimulates mouse cells but not human cells (4). In this study, we show that the species specificity depends on the number of acyl chains. Previous studies showed that the number of acyl chains influences biological potency. The glycolipid anchor of staphylococcal LTA with two acyl chains stimulates human cells, but the anchor with just one acyl chain does not (26). Mycoplasmal lipopeptide with two acyl groups (MALP-2) is 100-fold more potent in stimulating murine macrophages to secrete nitric oxide than is MALP with one acyl group (MALP-1) (27). Our example is the first reported situation in which the number of acyl groups is associated with species-specific responses.
One can envision several possibilities to explain why the number of acyl chains influences the responsiveness in specific animal species. We used human monocytes and mouse macrophages. Thus, one possibility is that monoacyl LTA stimulates monocytes but not macrophages. However, this possibility is unlikely, as monoacyl LTA can also stimulate human type II pneumocytes. Another possibility is that pneumococcal monoacyl LTA reacts with mouse TLR2 (or CD14) less than it reacts with human TLR2, whereas normal LTA reacts with both mouse and human TLR2 equally well. Lipid IVa is an LPS antagonist for human cells but is an LPS mimic in hamster cells, and the responsiveness is associated with the species origin of TLR4 (4). The third possibility may involve the ease of LTA's entry into lipid rafts. Lipid rafts are involved in LPS action (33) and may be involved in LTA action as well. The number of acyl chains may influence the molecule's ability to enter into lipid rafts in one animal species but not affect it in another. While these possibilities are not mutually exclusive, further work is needed to test the possibilities.
Our study provides evidence that pneumococcal teichoic acid is not so inflammatory. So far, the inflammatory potential of pneumococcal teichoic acid has been uncertain, because teichoic acid cannot be easily purified without contaminations by peptidoglycan. Deacylated pneumococcal LTA is identical to teichoic acid in structure (10). Since deacylated LTA does not stimulate either human or mouse cells to produce NO or TNF-α, pneumococcal teichoic acid is not likely to be inflammatory.
Morath et al. emphasized the importance of purifying LTA without damaging it by showing that alanine present in staphylococcal LTA may be damaged (25). Although pneumococcal LTA does not contain alanine, our experience further supports the conclusion that the structural integrity of pneumococcal LTA similarly depends on its purification method. Consistent with this conclusion, we observed that pneumococcal LTA purified by precipitation with deoxycholic acid at pH 5 (U. B. S. Sorensen, unpublished data) differs in its molecular size and chemical properties from the LTA prepared by hydrophobic chromatography (data not shown). As small differences in the purification methods may yield LTA or LPS with different structures, we recommend that the structure of LTA (or LPS) must be monitored during the steps used to purify it. Further, one should consider that differences in purification methods might be responsible for some of the apparent controversies concerning the biological properties of LTA or LPS.
Acknowledgments
This work was funded by NIH grant AI-31473.
We thank W. Benjamin, Jr., M. Martin, and D. Briles for a critical reading of the manuscript. We also thank W. Robbins in the UAB fermentation facility and L. Wilson in the UAB mass spectrometry facility for their technical assistance.
Editor: A. D. O'Brien
REFERENCES
- 1.Behr, T., W. Fischer, J. Peter-Katalinic, and H. Egge. 1992. The structure of pneumococcal lipoteichoic acid. Eur. J. Biochem. 207:1063-1075. [DOI] [PubMed] [Google Scholar]
- 2.Bertholet, S., E. Tzeng, E. Felley-Bosco, and J. Mauel. 1999. Expression of the inducible NO synthase in human monocytic U937 cells allows high output nitric oxide production. J. Leukoc. Biol. 65:50-58. [DOI] [PubMed] [Google Scholar]
- 3.Bhakdi, S., T. Klonisch, P. Nuber, and W. Fischer. 1991. Stimulation of monokine production by lipoteichoic acids. Infect. Immun. 59:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caroff, M., D. Karibian, J. M. Cavaillon, and N. Haeffner-Cavaillon. 2002. Structural and functional analyses of bacterial lipopolysaccharides. Microbes Infect. 4:915-926. [DOI] [PubMed] [Google Scholar]
- 5.Claflin, J. L., and M. Cubberley. 1978. Clonal nature of the immune response to phosphocholine. VI. Molecular uniformity of a single idiotype among BALB/c mice. J. Immunol. 121:1410-1415. [PubMed] [Google Scholar]
- 6.Cohen, J., and E. Abraham. 1999. Microbiologic findings and correlations with serum tumor necrosis factor-alpha in patients with severe sepsis and septic shock. J. Infect. Dis. 180:116-121. [DOI] [PubMed] [Google Scholar]
- 7.Dalpke, A. H., M. Frey, S. Morath, T. Hartung, and K. Heeg. 2002. Interaction of lipoteichoic acid and CpG-DNA during activation of innate immune cells. Immunobiology 206:392-407. [DOI] [PubMed] [Google Scholar]
- 8.De Kimpe, S. J., M. Kengatharan, C. Thiemermann, and J. R. Vane. 1995. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92:10359-10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellingsen, E., S. Morath, T. Flo, A. Schromm, T. Hartung, C. Thiemermann, T. Espevik, D. Golenbock, D. Foster, R. Solberg, A. Aasen, and J. Wang. 2002. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med. Sci. Monit. 8:BR149-BR156. [PubMed] [Google Scholar]
- 10.Fischer, W. 2000. Pneumococcal lipoteichoic and teichoic acid, p. 155-177. In A. Tomasz (ed.), Streptococcus pneumoniae molecular biology & mechanisms of disease. Mary Ann Liebert, Inc., Larchmont, N.Y.
- 11.Fischer, W. 1994. Teichoic acid and lipoglycans. New Compr. Biochem. 27:199-215. [Google Scholar]
- 12.Gao, J. J., Q. Xue, E. G. Zuvanich, K. R. Haghi, and D. C. Morrison. 2001. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect. Immun. 69:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg, A. E., L. S. Clesceri, and A. D. Eaton (ed.). 1995. Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, D.C.
- 15.Han, S. H., J. H. Jeon, H. R. Ju, U. Jung, K. Y. Kim, H. S. Yoo, Y. H. Lee, K. S. Song, H. M. Hwang, Y. S. Na, Y. Yang, K. N. Lee, and I. Choi. 2003. VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene 22:4035-4046. [DOI] [PubMed] [Google Scholar]
- 16.Han, S. H., J. H. Kim, M. Martin, S. M. Michalek, and M. H. Nahm. 2003. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 71:5541-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermann, C., I. Spreitzer, N. W. Schroder, S. Morath, M. D. Lehner, W. Fischer, C. Schutt, R. R. Schumann, and T. Hartung. 2002. Cytokine induction by purified lipoteichoic acids from various bacterial species—role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-gamma release. Eur. J. Immunol. 32:541-551. [DOI] [PubMed] [Google Scholar]
- 18.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 19.Horne, D., and A. Tomasz. 1985. Pneumococcal Forssman antigen: enrichment in mesosomal membranes and specific binding to the autolytic enzyme of Streptococcus pneumoniae. J. Bacteriol. 161:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, B. W., T. K. Means, K. A. Heldwein, M. A. Keen, P. J. Hill, J. T. Belisle, and M. J. Fenton. 2001. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 69:1036-1044. [PubMed] [Google Scholar]
- 21.Keller, R., W. Fischer, R. Keist, and S. Bassetti. 1992. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect. Immun. 60:3664-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kengatharan, K. M., S. De Kimpe, C. Robson, S. J. Foster, and C. Thiemermann. 1998. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 188:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kengatharan, K. M., S. J. De Kimpe, and C. Thiemermann. 1996. Role of nitric oxide in the circulatory failure and organ injury in a rodent model of gram-positive shock. Br. J. Pharmacol. 119:1411-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemjabbar, H., and C. Basbaum. 2002. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8:41-46. [DOI] [PubMed] [Google Scholar]
- 25.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morath, S., A. Stadelmaier, A. Geyer, R. R. Schmidt, and T. Hartung. 2002. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J. Exp. Med. 195:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morr, M., O. Takeuchi, S. Akira, M. M. Simon, and P. F. Muhlradt. 2002. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur. J. Immunol. 32:3337-3347. [DOI] [PubMed] [Google Scholar]
- 28.Muhl, H., and J. Pfeilschifter. 2003. Endothelial nitric oxide synthase: a determinant of TNFalpha production by human monocytes/macrophages. Biochem. Biophys. Res. Commun. 310:677-680. [DOI] [PubMed] [Google Scholar]
- 29.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 30.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 31.Talbot, U. M., A. W. Paton, and J. C. Paton. 1996. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect. Immun. 64:3772-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomasz, A. (ed.). 2000. Streptococcus pneumoniae molecular biology & mechanisms of disease, p. 9-21. Mary Ann Liebert, Inc., Larchmont, N.Y.
- 33.Triantafilou, K., M. Triantafilou, and R. L. Dedrick. 2001. A CD14-independent LPS receptor cluster. Nat. Immunol. 2:338-345. [DOI] [PubMed] [Google Scholar]
- 34.Tsutsui, O., S. Kokeguchi, T. Matsumura, and K. Kato. 1991. Relationship of the chemical structure and immunobiological activities of lipoteichoic acid from Streptococcus faecalis (Enterococcus hirae) ATCC 9790. FEMS Microbiol. Immunol. 3:211-218. [DOI] [PubMed] [Google Scholar]
- 35.Van Amersfoort, E. S., T. J. Van Berkel, and J. Kuiper. 2003. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 16:379-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, J. R., I. Idanpaan-Heikkila, W. Fischer, and E. I. Tuomanen. 1999. Pneumococcal licD2 gene is involved in phosphorylcholine metabolism. Mol. Microbiol. 31:1477-1488. [DOI] [PubMed] [Google Scholar]