Abstract
Background
Compliance with WHO guidelines on HBV screening and treatment in HIV-coinfected patients is often challenging in resource limited countries and has been poorly assessed in sub-Saharan Africa.
Methods
Between 2015 and 2016, we assessed physician’s compliance with WHO guidelines on HIV-HBV coinfection in the largest HIV clinic in The Gambia, and the hepatic outcomes in HIV-HBV coinfected patients as compared to randomly selected HIV-monoinfected controls.
Results
870 HIV-infected patients regularly seen in this clinic agreed to participate in our study. Only 187 (21.5%, 95% CI 18.8–24.3) had previously been screened for HBsAg, 23 (12.3%, 95% CI 8.0–17.9) were positive of whom none had liver assessment and only 6 (26.1%) had received Tenofovir. Our HBV testing intervention was accepted by all participants and found 94/870 (10.8%, 95% CI 8.8–13.1) positive, 78 of whom underwent full liver assessment along with 40 HBsAg-negative controls. At the time of liver assessment, 61/78 (78.2%) HIV-HBV coinfected patients received ART with 7 (11.5%) on Tenofovir and 54 (88.5%) on Lamivudine alone. HIV-HBV coinfected patients had higher APRI score compared to controls (0.58 vs 0.42, p = 0.002). HBV DNA was detectable in 52/53 (98.1%) coinfected patients with 14/53 (26.4%) having HBV DNA >20,000 IU/L. 10/12 (83.3%) had at least one detectable 3TC-associated HBV resistance, which tended to be associated with increase in liver fibrosis after adjusting for age and sex (p = 0.05).
Conclusions
Compliance with HBV testing and treatment guidelines is poor in this Gambian HIV programme putting coinfected patients at risk of liver complications. However, the excellent uptake of HBV screening and linkage to care in our study suggests feasible improvements.
Introduction
Chronic hepatitis B virus (CHB) infection is a major public health problem and a leading cause of morbidity and mortality globally. It affects approximately 250 million persons worldwide [1] and accounts for 650,000 deaths annually [2]. Without effective interventions and treatment, CHB infection will lead to an estimated 11.8 million deaths by 2030, primarily as a result of cirrhosis and hepatocellular carcinoma (HCC) [3]. Most of these deaths will occur in low-income countries (LICs) in sub-Saharan Africa (sSA) and Asia where CHB infection is endemic.
The World Health Assembly (WHA), in response to the increasing CHB–related morbidity and mortality, adopted a resolution to improve viral hepatitis prevention, testing and treatment worldwide [4]. Subsequently, the United Nations General Assembly and the World Health Organisation (WHO) respectively incorporated viral hepatitis elimination in the 2030 Agenda for Sustainable Development [5,6] and the WHO global health sector strategy on viral hepatitis 2016–2021 [3,7]. The WHO further recommends hepatitis B surface antigen (HBsAg) testing for all HIV-infected patients, and highly active antiretroviral therapy (HAART) containing at least two drugs effective against hepatitis B virus (HBV) for all HIV-HBV coinfected patients irrespective of disease stage or CD4 count [8], or where not feasible to treat patients with severe liver disease [9].
Whilst both HIV and HBV are endemic in sSA, the prevalence of HIV-HBV coinfection is highest in West Africa [10] where as many as 1 in 10 HIV-infected patients is coinfected with HBV. Uncontrolled HIV-HBV coinfection, due either to lack of or ineffective antiretroviral therapy (ART), increases the risk of death by more than twofold compared to HIV-monoinfected patients [11], irrespective of presence of liver disease [12]. Early and effective Tenofovir Disoproxil Fumarate (TDF) based ART, on the other hand, reduces risk of death among coinfected patients [10,11] underlying the need for implementing universal HBV testing and prompt ART treatment [8].
The Prevention of Liver Fibrosis and Cancer in Africa (PROLIFICA) programme in 2016 found high HIV and HBV prevalence among Gambian communities (2% and 8.5% respectively), with poor knowledge of hepatitis B and limited access to screening and treatment for HBV infection [13]. One of Africa’s smallest and poorest nations, The Gambia has one of the highest prevalence of HCC in West Africa [14] with two-thirds of these cases attributable to HBV infection [15]. In order to comply with the recent WHO goal for HBV elimination, we assessed the coverage of hepatitis B testing and treatment, and its impact on clinical outcomes in the largest HIV treatment centre in The Gambia, West Africa.
Materials and methods
Patients and study design
This cross-sectional study enrolled HIV infected patients aged ≥18years (range 22–63 years) registered at the Hands on Care HIV clinic in Brikama (The Gambia) who have had at least two successful clinic visits and baseline investigations. After written consent, we reviewed patient case notes to determine the coverage of hepatitis B surface antigen (HBsAg) testing for all HIV patients and use of TDF-based ART for known HIV-HBV coinfected patients. Thereafter, we screened the patients for HBsAg using the Determine® rapid point-of-care kit (Alere®, USA). All HBsAg positive patients (HIV-HBV coinfected) and randomly selected HBsAg negative controls (HIV-monoinfected) had confirmatory HBsAg serology test (Architect HBsAg, Abbott, USA) and were further invited for complete liver assessment. Demographic and clinical information such as age, gender, past medical history, duration of HIV infection and antiretroviral drug exposure were collected, and a clinical examination and bedside ultrasonography performed. Liver transaminases (Vitros 350 Analyzer, Ortho Clinical Diagnostics, USA), haemoglobin and platelet count (CELL-DYN 3700 sample loader, Abbott, USA), HIV RNA (Abbott Real Time HIV-1 assay, 40copies/mL limit of detection), CD4+ T-cell count (flow cytometry) and in-house HBV DNA viral load quantification [16] were measured for all patients.
Assessment of liver fibrosis
In accordance with WHO guidelines for setting without access a transient elastography device (Fibroscan), we assessed liver fibrosis using biochemical markers—aspartate aminotransferase (AST)-to-platelet ratio index (APRI) and FIB-4. APRI was calculated as [AST level (IU/L) / upper limit of normal for AST (IU/L) × 100/platelet count (109/L)], and FIB-4 as [age (years) x AST (IU/L) / (platelet count (109/L) x ALT (IU/L)1/2)]. Significant fibrosis defined was defined as APRI >1.5 [17] or FIB-4 >3.25 [18].
HBV molecular analysis and polymerase gene sequencing
DNA was extracted from 500μL plasma using the QIAamp DNA Blood MiniKit (QIAgen, Germany). HBV DNA was quantified was by TaqMan based quantitative PCR assay with lower limit of detection of 50IU/L [16], using probe (200nM) sequence HBVTAQPR:5'FAM-CCTCTKCATCCTGCTGCTATGCCTCATC-3’MGBNFQ with forward and reverse primer (400nM) sequences HBVTAQ1:5'-GTGTCTGCGGCGTTTTATCA-3' and HBVTAQ2:5'-GACAAACGGGCAACATACCTT-3’ respectively [19]. Hepatitis B polymerase gene sequence was assessed in all participants with HBV DNA viral load ≥20,000IU/L.
The region from nucleotide 101 to 1149 of the HBV polymerase (pol) gene containing the highly conserved tyrosine-methionine-aspartate-aspartate (YMDD) motif was amplified by standard PCR using high fidelity polymerases (Supermix High Fidelity polymerase from Invitrogen or Q5® High-Fidelity DNA Polymerase from New England BioLabs) and primers sense 5’-AYTGTCTCTTCCAYMTCRTC-3’ and antisense 5’-GGGGTAAAGGTTCAGRTAYTG-3’. Genotype of the amplified sequence was assessed using the NCBI genotyping tool [20].
Statistical analyses
Demographic, clinical and virological characteristics of the study participants were presented according to their HBsAg status, and comparison made between HIV-monoinfected and HIV-HBV coinfected patients using the chi-squared test, Fisher’s exact test, or Wilcoxon rank-sum test. Factors associated with significant fibrosis (APRI >1.5) were identified using univariable logistic regression. As the number of participants with APRI >1.5 was small, multivariable analysis was not performed.
Ethical approval
The MRC Scientific Coordinating committee and the joint Gambia Government/MRC Ethics committee approved this study (SCC1388). Consenting of participants followed group and individual sensitization, explanation of a study protocol and sufficient time for questions and discussion with spouse/family.
Results
Study population and compliance with guidelines
Between September 2015 and February 2016, 870 HIV-infected patients regularly seen at the Hands on Care HIV clinic were invited to participate in our study and all agreed to take part. The review of patient case notes revealed that only 187 (21.5%, 95% CI 18.8–24.3) had previously been tested for HBsAg, with 23 (12.3%, 95% CI 8.0–17.9) found to be HIV-HBV coinfected. None had a liver assessment based on liver transaminases, ultrasound, measurement of liver fibrosis or liver biopsy. Whilst all 23 patients previously known to be coinfected had been receiving ART, only 6 (26.1%, 95 CI 10.2–48.4) had received a TDF-based regimen.
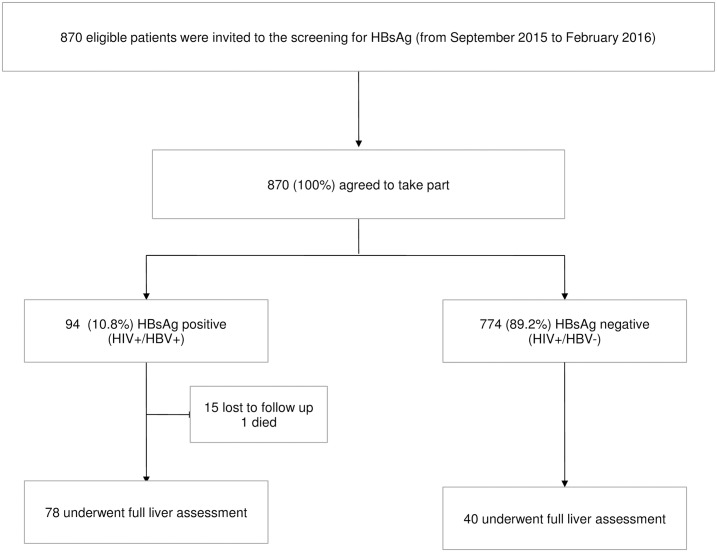
Of 870 patients accepted for the HBsAg screening, 94 tested positive giving a seroprevalence of 10.8% (95% CI 8.8–13.1). Of these, 15 (16%) were lost to follow up, and 1 died before the liver assessment. The remaining 78 HIV-HBV coinfected patients underwent full liver assessment, along with 40 randomly selected HBsAg negative controls (Fig 1).
Fig 1. Flow diagram of the study population.
Of the 78 coinfected patients who completed liver assessment as part of our study, 61 (78.2%, 95% CI 67.4–86.1) received ART, with 54/61 (88.5%, 95% CI 77.4–94.5) receiving Lamivudine (3TC) without TDF and only 7/61 (11.5%) receiving a TDF-based regimen. Median transaminase was within normal limits with only 5 patients (6.4%) having ALT above the upper limit of normal (ULN) and 1 (1.3%) with ALT >2xULN. Table 1 provides the characteristics of the study population by HBsAg status.
Table 1. Characteristics of the study population by HBV coinfection.
| Parameters | HIV-HBV (n = 78) | HIV (n = 40) | P value |
|---|---|---|---|
| Median age (years, IQR) | 38 (35–48) | 42 (35–46) | 0.5 |
| Male sex (n, %) | 8 (20) | 16 (21) | 0.9 |
| Median BMI (Kg/m2, IQR) | 21 (19–24) | 22 (20–25) | 0.4 |
| HIV infection type (n, %) HIV1 HIV2 HIV1+2 |
58 (78) 3 (4) 13 (18) |
34 (90) 2 (5) 2 (5) |
0.2 |
| Median duration since HIV diagnosis (months, IQR) | 34 (14–68) | 66 (20–82) | 0.04 |
| CD4+ T-cell count (n, %) <200 cells/mm3 ≥200 cells/mm3 |
24 (34) 46 (66) |
13 (34) 25 (66) |
1.0 |
| Median CD4+ T-cell count (cells/mm3, IQR) | 293.5 (147–444) | 302 (170–411) | 0.7 |
| HIV RNA (n, %) Undetectable Detectable |
30 (53) 27 (47) |
26 (70) 11 (30) |
0.09 |
| Median HIV RNA (log copies/mL, IQR) | 4.1 (3.2–5.0) | 4.2 (3.2–4.6) | 0.7 |
| HBV DNA (n, %) Undetectable 50–2,000 IU/mL 2,000–20,000 IU/mL ≥2, 000 IU/mL |
1 (1) 46 (60) 12 (16) 18 (23) |
NA | NA |
| Median ALT (IU/L, IQR) | 15 (12–25) | 17 (11–26) | 0.9 |
| Median AST (IU/L, IQR) | 28 (24–40) | 24 (19–30) | 0.004 |
| Median platelet count (x109/L, IQR) | 186 (152–220) | 198 (163–238) | 0.1 |
| APRI (n, %) <0.5 0.5–1.5 ≥1.5 |
29 (39) 38 (51) 7 (10) |
27 (69) 12 (31) 0 |
0.005 |
| Median APRI (IQR) | 0.58 (0.43–0.88) | 0.42 (0.35–0.53) | 0.002 |
| FIB-4 (n, %) <1.45 1.45–3.25 ≥3.25 |
30 (40) 39 (53) 5 (7) |
25 (64) 14 (36) 0 |
0.03 |
| Median FIB-4 (IQR) | 1.72 (1.22–2.34) | 1.35 (1.06–1.63) | 0.004 |
| ART regimen (n, %) Never treated 3TC without TDF TDF |
17 (22) 54 (69) 7 (9) |
5 (13) 29 (74) 5 (13) |
0.5 |
| Duration of 3TC exposure (n, %)*, ** <12 months 12–60 months ≥60 months |
14 (23) 32 (52) 15 (25) |
7 (21) 13 (38) 14 (41) |
0.2 |
*This analysis only includes those who have ever received ART;
**3TC without a second HBV active anti-viral drug
ALT—alanine aminotransferase; APRI—AST to platelet ratio index; ART—antiretroviral therapy; AST—aspartate aminotransferase; BMI—body mass index; HIV—Human Immunodeficiency virus; RNA—ribonucleic acid; TDF—Tenofovir Disoproxil Fumarate; 3TC—Lamivudine
Assessment of liver fibrosis
Compared to HIV-monoinfected patients, HIV-HBV coinfected patients had significantly higher median APRI (0.42 vs 0.58, p = 0.002) and FIB-4 (1.72 vs 1.35, p = 0.004), suggesting higher rate of liver fibrosis in coinfected patients. Table 2 shows the factors associated with significant liver fibrosis.
Table 2. Factors associated with significant liver fibrosis (APRI ≥1.5) in HIV-infected participants (n = 113).
| Prevalence of significant fibrosis (%) | Crude odds ratios | ||
|---|---|---|---|
| OR, 95% CI | P value | ||
| Age group <40 ≥40 |
5/58 (9) 2/55 (4) |
1.0 0.4 (0.1–2.2) |
0.3 |
| Sex Women Men |
6/90 (7) 1/23 (4) |
1.0 0.6 (0.1–5.6) |
0.7 |
| BMI <25 ≥25 |
7/86 (8) 0/27 (0) |
1.0 NA |
0.1 |
| Steatosis No Yes |
6/105 (6) 1/8 (13) |
1.0 2.4 (0.2–22.4) |
0.5 |
| HIV infection type HIV1 HIV2 HIV1+2 |
3/89 (3) 0/4 (0) 3/15 (20) |
1.0 NA 7.2 (1.3–39.6) |
0.02 |
| HIV RNA Undetectable Detectable |
0/53 (0) 6/37 (16) |
NA |
0.002 |
| CD4+ T-cell count <200 cells/mm3 ≥200 cells/mm3 |
1/36 (3) 5/68 (7) |
1.0 2.8 (0.3–24.7) |
0.4 |
| HBV co-infection No Yes |
0/39 (0) 7/74 (10) |
NA |
0.04 |
| ART Never 3TC without TDF TDF |
2/19 (11) 5/82 (6) 0/11 (0) |
1.0 0.6 (0.1–3.1) NA |
0.5 |
HBV molecular virology in 3TC exposed patients
Of 54 coinfected patients who were treated with 3TC without TDF, after a median duration of 28 months (IQR 12–60) on 3TC, HBV DNA and HIV RNA were detectable in 52/53 (98.1%, 95% CI 87.1–99.8) and 15/38 (39.5%, 95% CI 24.8–56.3) respectively. 14 of 53 (26.4%, 95% CI 16.0–40.3) had HBV DNA >20,000 IU/L. We successfully sequenced the region of the polymerase gene containing the YMDD motif in 12 of these 14 patients. 10 (83.3%) had M204V/I mutations present, with 8 (66.7%) having additional compensatory mutations at rtL180M and 3 (25.0%) at both rtL180M and rtV173L. Phylogenetic analysis of the polymerase gene sequence showed all but one patient (HBV genotype A) were infected with HBV genotype E.
We assessed the joint effect of HBV co-infection and 3TC resistance mutations on significant fibrosis (APRI >1.5) in HIV-infected patients. Compared to mono-infected, the prevalence of significant fibrosis was higher in those coinfected, and especially in those with 3TC resistance mutations. While no HIV-monoinfected patients (0/39) had significant fibrosis, 6/66 (9%) of patients coinfected with wild type HBV and 1/8 (13%) coinfected with resistant HBV strain had significant fibrosis (p for trend 0.05).
Discussion
In The Gambia, a West African country highly endemic for HBV infection [13], our study provides two key messages: 1) poor compliance with the WHO hepatitis B testing and treatment guidelines [9] is poor with only 21.5% of HIV patients systematically screened for HBV infection and less than 15% on TDF-based ART, which puts HIV patients at risk of liver complications; 2) testing and linkage to care for HBV infection in HIV patients is accepted and feasible in HIV care programme in The Gambia.
To date, the compliance with the WHO guidelines [8,9] on the management of HBV and HIV coinfection in sSA—a region with high HIV-HBV coinfection prevalence [10], has been poorly documented. We found that in 2016, despite strong international recommendations and increasing global commitment to HBV elimination, HBsAg testing and effective HBV treatment remains poor in the largest Gambian HIV facility predisposing coinfected patients to severe clinical and virological outcomes, increased risk of liver-related morbidity, and diminished benefits of ART.
In our study, none of the 870 HIV-infected patients refused to be screened for HBV and almost 85% of HBsAg positive participants accepted to undergo liver assessment. This result is important since it suggests that HBV screening and linkage to care for HBV infection may be widely accepted in HIV-infected patients and feasible in HIV care facilities in sSA. This needs to be confirmed on a larger scale.
Whilst some countries in sSA successfully adopted a TDF-based first-line ART regimen in HIV reference centres [21], indication to treat and choice of ART regimen in Gambian HIV clinics was primarily determined by the type and stage of HIV infection. As a result, 3TC, a cheap and well-tolerated nucleoside analogue has been the backbone of first line ART in The Gambia since its introduction over a decade ago [22]. Although 3TC is an effective antiviral drug, it is associated with an increased risk of HBV resistance mutations following 12 to 24 months of monotherapy in patients with HBV infection [23]. Unsurprisingly, HBV DNA was detectable in almost all our study patients (98.1%) who received prolonged 3TC without TDF.
We found significantly higher prevalence (83.3%) of 3TC associated HBV resistance mutations compared to the 14.2% reported in a similar Gambian HIV-HBV cohort six year earlier who received 3TC without a second anti-HBV drug [24], as well as the 29.3% and 8.7% reported in a Ghanaian [25] and Ivorian [26] coinfected patients respectively. Our findings confirm that widespread prolonged usage of 3TC monotherapy in ART poses diagnostic and management problems to individual patients as well as a potential public health risk.
The high proportion of patients with detectable HIV viral load and low CD4 count after many months of ART, as well as the detectable HBV DNA among almost all coinfected patients receiving TDF in our study could be the result of unsatisfactory adherence to therapy, which might contribute to higher levels of HBV viremia and consequently to higher fibrosis and HBV resistance. Another explanation could be the deteriorating HIV services in The Gambia since 2007 when its former president claimed to have found herbal cure for HIV infection. This claim disrupted decades of HIV campaign and negatively impacted vital HIV services and research partnerships resulting in significant reduction in competent personnel and services in HIV facilities and suboptimal HIV care with frequent medication stock outs and several patients having their ARV regimens switched often. These underline the urgent need to improve HIV services and drug provision in Gambian HIV facilities.
HBV co-infection increases liver fibrosis [25, 27–31] as well as both all cause and liver related mortality [11,32], often in the absence of any evidence of liver disease [12]. Although our study lacked statistical power to show definitively a positive relationship between 3TC resistance mutation and increase in liver fibrosis, we show a trend towards such relationship (p = 0.05). Prompt initiation of TDF-based ART has been shown to reduce HBV viral replication and mortality in European [33] and Africa HIV-HBV cohort [11,12], further strengthening the need for compliance with the WHO HBV testing and treatment guidelines in Africa. It is worth noting that the uptake of TDF in first-line ART regimens in sSA is poorly documented. Our study shows that until 2016, only a minority of HIV patients in The Gambia received HBV testing and an even lesser proportion (7/78) of HIV-HBV coinfected patients received TDF. None of the patients had benefitted from a liver assessment even on the basis of liver transaminases. Indeed the local laboratory at this HIV facility, and almost all other HIV facilities in The Gambia, do not have the capacity to assess liver biochemistry. The 2016 consolidated HIV guidelines [8] recommending TDF-based ART for all HIV patients irrespective of degree of liver disease are thus well adapted to resource limited African settings, and African HIV facilities need to be supported to implement these guidelines.
Our study has a few limitations: first, we analysed a limited number of HIV-HBV coinfected patients; second, we assessed liver fibrosis using APRI score as recommended by the WHO for resource-limited settings without access to Fibroscan, which is the case for most HIV facilities in Africa where Fibroscan is rarely available outside research settings. The reliability and usefulness of platelet-based biomarkers like APRI for assessing liver fibrosis in HIV patients in Africa has been questioned [34] mainly because thrombocytopenia is commonly associated with advanced HIV infection. Third, our study did not assess for hepatitis C virus (HCV) and hepatitis D virus (HDV) coinfection. However, previous studies in The Gambia confirmed very low prevalence rates of both HCV and HDV infections [13]. Finally, our study suggests that adherence to ART was not satisfactory but we did not directly assess adherence to ART.
Conclusion
Compliance with international HBV testing and treatment guidelines for HIV-infected patients is poor in this Gambian HIV programme, resulting in a low coverage of HBV testing, prolonged treatment with 3TC without TDF for coinfected patients, and poor HBV viral suppression with increased risk of HBV resistance and liver complication. However, interventions to improve HBV screening and linkage to care for HBV infection in HIV facilities are feasible.
Supporting information
(XLSX)
Acknowledgments
We thank the Medical Research Council Unit The Gambia, the Hands on Care HIV clinic and the Wellcome Trust-Imperial College Centre for Global Health Research for supporting this study. We are grateful to Professor Simon Taylor-Robinson for his continuous support all through the project, and Dr Marcus Dorner and his team for their advice and help with the molecular virology work. We thank all study participants, the PROLIFICA Gambia team, and the MRC data management unit. Lastly, we are grateful for the administrative and logistical support from Dr Peter Norsworthy, Dawn Campbell, Kimberley Trim, Susan Farrell, Mavis Foster-Nyarko and Amie Ceesay.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a Wellcome Trust-Imperial College Institutional Strategic Support Fund (ISSF) fellowship awarded to GN. Additional funding was provided by the Wellcome Trust-Imperial College Centre for Global Health Research (GN), the NIHR Imperial Biomedical Research Centre (MRT), and the EU 7th Framework Programme (FP7-AFRICA-2010, Collaborative Project PROLIFICA, Grant Nr. 265994). MLG is supported by a Wellcome Trust grant (award number 104771/Z/14/Z awarded to Dr Marcus Dorner). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of this manuscript.
References
- 1.Schweitzer A, Horn J, Mikolaiczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B infection: a systematic review of data published between 1965 and 2013. Lancet. 2015; Vol 386, Issue 10003, 1546–1555 [DOI] [PubMed] [Google Scholar]
- 2.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016; 388: 1081–88 doi: 10.1016/S0140-6736(16)30579-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. Combatting Hepatitis B and C to reach elimination by 2030. Geneva, WHO; 2016 [Google Scholar]
- 4.World Health Organisation. WHA 67.6 Hepatitis. In: Sixty-Seventh World Health Assembly. 24th May 2014, Geneva, Switzerland.
- 5.United Nations. Transforming our World: The 2030 Agenda for Sustainable Development. United Nations, 2015
- 6.United Nations. Sustainable Development Goal 3 –Ensure healthy lives and promote well-being for all at all ages. https://sustainabledevelopment.un.org/sdg3
- 7.World Health Organisation. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Geneva, WHO; 2015 [Google Scholar]
- 8.World Health Organisation. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. 2016. update. Geneva, WHO; 2016 [PubMed] [Google Scholar]
- 9.World Health Organisation. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, WHO; 2013 [PubMed] [Google Scholar]
- 10.Stabinski L, O’Connor S, Barnhart M, Kahn RJ, Hamm TE. Prevalence of HIV and Hepatitis B Virus Co-Infection in sub-Saharan Africa and the potential impact and program feasibility of Hepatitis B surface antigen screening in resource-limited settings. J Acquir Immune Defic Syndr. 2015; 68:S274–S285 doi: 10.1097/QAI.0000000000000496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouamé MG, Moh R, Boyd A, Badjé A, Gabillard D, N’takpé JB, et al. Higher mortality in HIV-HBV co-infected persons with elevated HBV replication in the Temprano Trial. [WEAB0303]. Presented at: 21st International AIDS Conference, 18–22 July 2016; South Africa.
- 12.Japhet MM, Ndhere A, Ndwiga S, Kirwa E, Yogev R, Murphy R, et al. Early mortality risk of HIV/Hepatitis B virus coinfected patients initiating ART [Abstract Number 562]. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI), 2016; Boston, Massachusetts.
- 13.Lemoine M, Shimakawa Y, Njie R, Taal M, Ndow G, Chemin I, et al. Acceptability and feasibility of a screen-and-treat programme for hepatitis B virus infection in The Gambia: the Prevention of Liver Fibrosis and Cancer in Africa (PROLIFICA) study. The Lancet Global Health. 2016; Vol 4, Issue 8, e559–e567 [DOI] [PubMed] [Google Scholar]
- 14.Shimakawa Y, Bah E, Wild CP, Hall AJ. Evaluation of data quality at the Gambia National Cancer Registry. Int J Cancer. 2013; 132: 658–665. doi: 10.1002/ijc.27646 [DOI] [PubMed] [Google Scholar]
- 15.Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, Goedert JJ, et al. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004; 39(1):211–9 doi: 10.1002/hep.20027 [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S, Sow A, Guillot C, Jeng A, Ndow G, Njie R, et al. Implementation of an in-house quantitative real-time polymerase chain reaction method for hepatitis B virus quantification in West African countries. J Viral Hepat. 2016;23(11):897–904 doi: 10.1111/jvh.12561 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organisation. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva, WHO; 2015. [PubMed] [Google Scholar]
- 18.Bota S, Sirli R, Sporea I, Focsa M, Popescu A, Danila M, et al. A new scoring system for prediction of fibrosis in chronic hepatitis C. Hepat Mon. 2011;11:548–555. [PMC free article] [PubMed] [Google Scholar]
- 19.Garson JA, Grant PR, Ayliffe U, Ferns RB, Tedder RS. Real-time PCR quantitation of hepatitis B virus DNA using automated sample preparation and murine cytomegalovirus internal control. J Virol Meth. 2005;126(1–2):207–13 [DOI] [PubMed] [Google Scholar]
- 20.Rozanov M, Plikat U, Chappey C, Kochergin A, Tatusova T. A web-based genotyping resource for viral sequences. Nucleic Acids Res. 2004;32 (suppl_2): W654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diop SA, Fortes-Déguénonvo L, Seydi M, Dieng AB, Basse CD, Manga NM, et al. Efficacy and tolerance of tenofovir-lamivudine-efavirenz combination in HIV-1 patients in Fann Teaching Hospital in Dakar. Bull Soc Pathol Exot. 2013. February;106(1):22–6. doi: 10.1007/s13149-012-0272-7. Epub 2012 Dec 17 [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Health & Social Welfare, Republic of The Gambia. Guidelines for Antiretroviral Therapy for the Prevention and Treatment of HIV in The Gambia. Banjul, The Gambia, 2015. [Google Scholar]
- 23.Lok ASF, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology, Volume 125, Issue 6, 1714–1722. [DOI] [PubMed] [Google Scholar]
- 24.Stewart B, Jobarteh ML, Sarge-Njie R, Alabi A, de Silva T, Peterson K, et al. Emergence of HBV resistance to lamivudine (3TC) in HIV/HBV co-infected patients in The Gambia, West Africa. BMC Research Notes. 2011; 4:561 doi: 10.1186/1756-0500-4-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockdale AJ, Phillips RO, Beloukas A, Appiah LT, Chadwick D, Bhagani S, et al. Liver Fibrosis by Transient Elastography and Virologic Outcomes After Introduction of Tenofovir in Lamivudine-Experienced Adults With HIV and Hepatitis B Virus Coinfection in Ghana. Clin Infect Dis. 2015. September 15:61(6):883–91. doi: 10.1093/cid/civ421. Epub 2015 May 28. [DOI] [PubMed] [Google Scholar]
- 26.Boyd A, Moh R, Gabillard D, le Carrou J, Danel C, Anglaret X, et al. Low risk of lamivudine-resistant HBV and hepatic flares in treated HIV-HBV-coinfected patients from Côte d'Ivoire. Antivir Ther. 2015;20(6):643–54. doi: 10.3851/IMP2959. Epub 2015 Apr 8. [DOI] [PubMed] [Google Scholar]
- 27.Stabinski L, Reynolds SJ, Ocama P, Laeyendecker O, Ndyanabo A, Kiggundu V, et al. High prevalence of liver fibrosis associated with HIV infection: a study in rural Rakai, Uganda. Antivir Ther. 2011; 16(3):405–11. doi: 10.3851/IMP1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawkins C, Agbaji O, Ugoagwu P, Thio CL, Auwal MM, Ani C, et al. Assessment of liver fibrosis by transient elastography in patients with HIV and hepatitis B virus coinfection in Nigeria. Clin Infect Dis. 2013;57(12):e189–92 doi: 10.1093/cid/cit564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinikoor MJ, Mulenga L, Siyunda A, Musukuma K, Chilengi R, Moore CB, et al. Association between hepatitis B co-infection and elevated liver stiffness among HIV-infected adults in Lusaka, Zambia. Trop Med Int Health. 2016; 21: 1435–1441. doi: 10.1111/tmi.12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramírez-Mena A, Glass TR, Winter A, Kimera N, Ntamatungiro A, Hatz C, et al. Prevalence and Outcomes of Hepatitis B Coinfection and Associated Liver Disease Among Antiretroviral Therapy-Naive Individuals in a Rural Tanzanian Human Immunodeficiency Virus Cohort. Open Forum Infect Dis. 2016. July 29:3(3):ofw162 eCollection 2016 doi: 10.1093/ofid/ofw162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gitau SN, Vinayak S, Silaba M, Adam R, Shah R. High Prevalence of Liver Fibrosis in Patients with Human Immunodeficiency Virus Monoinfection and Human Immunodeficiency Virus Hepatitis-B Co-infection as Assessed by Shear Wave Elastography: Study at a Teaching Hospital in Kenya. J Clin Imaging Sci. 2016;6:22 doi: 10.4103/2156-7514.183582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinikoor MJ, Sinkala E, Mweemba A, Zanolini A, Mulenga L, Sikazwe I, et al. Elevated AST-to-platelet ratio index is associated with increased all-cause mortality among HIV-infected adults in Zambia. Liver Int. 2015; 35: 1886–1892 doi: 10.1111/liv.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd A, Bottero J, Miailhes P, Lascoux-Combe C, Rougier H, Girard PM, et al. Liver fibrosis regression and progression during controlled hepatitis B virus infection among HIV-HBV patients treated with Tenofovir Disoproxil Fumarate in France: a prospective cohort study. Journal of International AIDS Society. 2017; 20:21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johannessen A, Lemoine M. Is aspartate aminotransferase-to-platelet ratio index a reliable tool in human immunodeficiency virus patients in Africa? Liver Int. 2015; 35: 2059 doi: 10.1111/liv.12793 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.