Abstract
Background
Recent studies suggest that the protective effect of the current influenza vaccine could be influenced by vaccination in previous seasons. We estimated the combined effect of the previous and current influenza vaccines from the 2010–2011 season to the 2015–2016 season in Spain.
Methods
We performed a test-negative case-control study in patients ≥9 years old. We estimated the influenza vaccine effectiveness (IVE) against influenza A(H1N1)pdm09, A(H3N2), and B virus.
Results
We included 1206 influenza A(H1N1)pdm09 cases, 1358 A(H3N2) cases and 1079 B cases. IVE against A(H1N1)pdm09 virus in the pooled-season analysis was 53% (95% Confidence Interval (CI): 21% to 72%) for those vaccinated only in the current season and 50% (95%CI: 23% to 68%) for those vaccinated in the both current and previous seasons. Against the influenza A(H3N2) virus, IVE was 17% (95%CI: -43% to 52%) for those vaccinated only in the current season and 3% (95%CI: -33% to 28%) for those vaccinated in both seasons. Regarding influenza B, we obtained similar IVEs for those vaccinated only in the current and those vaccinated in both seasons: 57% (95%CI: 12% to 79%) and 56% (95%CI: 36% to 70%), respectively.
Conclusion
Our results suggested no interference between the previous and current influenza vaccines against A(H1N1)pdm09 and B viruses, but a possible negative interference against A(H3N2) virus.
Introduction
Influenza represents a public health problem, therefore it is generally recommended that population groups at risk of severe complications or death are vaccinated: the elderly (above 64 years old), those younger than 65 with chronic conditions, pregnant women or persons at risk due to their profession [1; 2].The influenza vaccine is the main preventive measure available, but as influenza viruses undergo frequent changes in their surface antigens, it needs re-formulation each year, with the aim of antigenically matching the circulating strains [3].
Since the 2008–2009 season, have estimated the influenza vaccine effectiveness (IVE) in Spain using the cycEVA study (case-control study for the influenza vaccine effectiveness in Spain), which is the Spanish component of the I-MOVE project for monitoring the IVE in Europe. Ever since, within the cycEVA study we have continuously employed a case-control test-negative design. This is a method that is used worldwide as one of the most appropriate to estimate IVE, because it minimizes the habitual biases of observational studies [4–6].
A series of factors influenced the yearly-observed IVE estimates. Apart from the antigenic match with the vaccine-circulating strain, recent studies have suggested that the protective effect of the current season´s influenza vaccinecould be also influenced by influenza vaccination in previous seasons (N.B. This article refers to “current” in the sense of the season under analysis rather than the present moment). While several papers did not detect any evidence for decreasing protection with repeated vaccination [7;8], other authors described an effect between the previous season´s vaccination and the current one. This was observed in a single season or across various seasons, with some studies suggesting a negative interference between the previous and the current season´s influenza vaccine [9–12].
We present the IVE estimates for the current season´s influenza vaccine, as well as the combined effect of the previous and current vaccine, as obtained during six post-pandemic seasons in Spain, 2010–2011 to 2015–2016, with the cycEVA study. We also interpret these results in the context of the similarities between the circulating strains and vaccine strains, both in current and previous season.
Methods
Study setting, design and data collection
We have used data obtained from the cycEVA study, from the 2010–2011 to 2015–2016 influenza seasons. The cycEVA study is an observational case-control study to monitor influenza vaccine effectiveness (IVE) in Spain, which is conducted within the framework of the Spanish Influenza Sentinel Surveillance System (SISSS)[13]. During the study period, between five and eight out of the 17 regional sentinel networks participated each season.
The methodology of the cycEVA study has been described previously in the literature [14;15]. Briefly, following a common European protocol [16], sentinel practitioners (SPs) reported cases of influenza-like illness (ILI) on a weekly basis according to a definition that is based on the European Commission (EU) ILI case definition [17]. They systematically swabbed patients below 65 years old (the first two patients with influenza-like illness (ILI) who had consulted a sentinel physician each week) and all patients above 64 years old. For each recruited patient, the SPs collected information on demographic data (age, sex, sentinel network), previous and current vaccination status (including date of vaccination) and presence of a chronic condition (i.e., chronic cardiovascular, pulmonary, hepatic or renal diseases, congenital or acquired immunodeficiency and diabetes mellitus), as well as pregnancy status, and obesity (defined as a body mass index BMI ≥40 kg/m2).
For all six seasons, we used a case-control test-negative design. We defined cases as ILI patients who had a reverse transcription polymerase chain-reaction (RT-PCR) and/or cell culture test, positive for influenza (A(H1N1)pdm09, A(H3N2) or B); whereas controls tested negative for all influenza viruses. In each season, the study started when a sporadic circulation of influenza viruses was detected in the participant surveillance networks. We considered a patient vaccinated if he/she had received the trivalent seasonal influenza vaccine at least 14 days before the onset of ILI symptoms; patients receiving the influenza vaccine less than 14 days before symptoms’ onset were considered unvaccinated, whereas those without a vaccination date were excluded from the analysis.
Data analysis
Since the objectives of this work are different from those of previous cycEVA studies [18–21], we reanalyzed data within cycEVA using the same analysis approach across all six seasons studied. We have considered only patients of nine years and above, since below this age complete vaccination status might include vaccination in the previous season; we also restricted the analysis to the ILI patients swabbed less than eight days since the onset of symptoms. For each season, we estimated the IVE against the predominant influenza virus (≥60% of the total type/subtype of influenza virus circulation), or against viruses with a circulation of at least 25% of the total influenza viruses, during the period with continuous circulation of that specific type/subtype. We estimated the IVE for all those aged nine and above, the target groups for influenza vaccination; and, in a further sensitivity analysis, we restricted the analysis to those swabbed four days or less since onset of symptoms. We considered target groups as all those patients above 64 years old (60 in some sentinel networks), those with a chronic condition or other risk factor for influenza (obesity or pregnancy), or who met any other criteria to be vaccinated in Spain (such as healthcare workers or caregivers). We explored the distribution of the covariates between cases and controls using a χ2 test. We used logistic regression models to evaluate the odds ratios (OR) with 95% confidence intervals (95%CI), adjusting for age-groups (9–14, 15–44, 45–64, ≥65 years), sex, chronic condition, sentinel network and week of swabbing, as well as influenza season in the pooled analysis. We estimated the IVE with the formula (1-OR)x100 for vaccination.
In the main study analysis, we estimated the effect of previous and current seasons’ vaccination, against considered virus type/subtype, in pooled analysis and by each season for all study subjects. In a further refinement, we restricted the analysis to those belonging to the target groups. In the 2010–2011 influenza season, the pandemic vaccine was considered as a previous vaccination against A(H1N1)pdm09 for the purposes of the IVE. To determine current vaccination status we used the available vaccination date, while for patients vaccinated in the previous season we had only yes/no information. The effects of previous and current vaccinations were evaluated using four categories: vaccinated only in the previous season, vaccinated only in the current season, receiving both vaccines, and unvaccinated. We estimated the adjusted IVE with the 95%CI for each category, adjusting for the same variables as in the primary analysis and using the unvaccinated as a reference. Differences in the IVE between those vaccinated only in the “current” season and those receiving both vaccines were explored, using those vaccinated only in the current season as reference.
For each season we have provided the results on the genetic characterization of influenza strains, from cases notified at the national level in Spain. Strains were genetically characterized at the World Health Organization (WHO) National Influenza Centre in Madrid (Spain), by sequencing the HA1 fragment of the viral hemagglutinin gene.
Results
Influenza seasons and characteristics of cases and controls
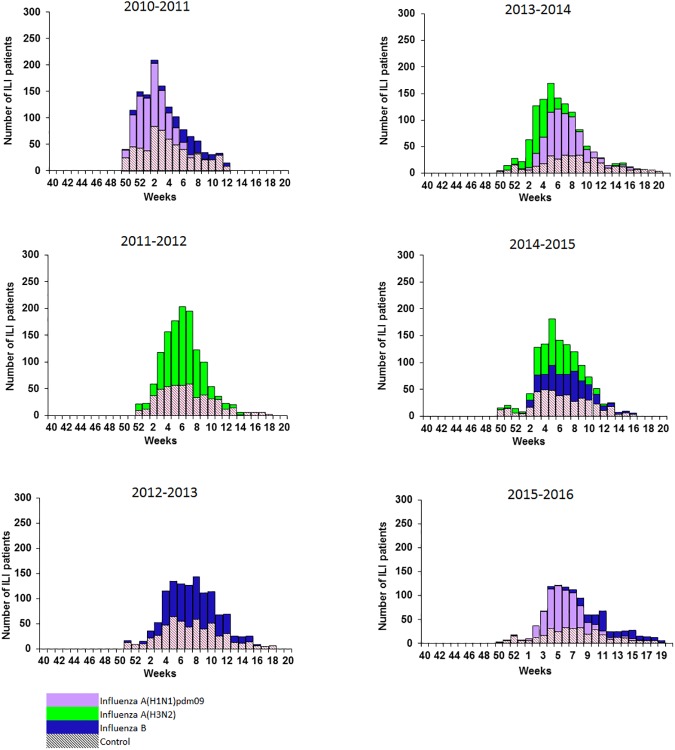
According to the seasonal distribution of recruited influenza A(H1N1)pdm09, A(H3N2) and B cases, IVE was estimated against influenza A(H1N1)pdm09 in 2010–2011, 2013–2014 and 2015–2016 seasons; against influenza A(H3N2) in the 2011–2012, 2013–2014 and 2014–2015; and against influenza B in 2010–2011, 2012–2013, 2014–2015 and 2015–2016 seasons (Fig 1).
Fig 1. Cases and controls recruitment, cycEVA study, seasons 2010–2011 to 2015–2016, Spain.
A total of 507, influenza A(H1N1)pdm09 cases were included for the 2010–2011 season, 303 for 2013–2014 and 396 for 2015–2016, respectively. There were less cases than controls belonging to target groups for vaccination in all A(H1N1)pdm09 influenza seasons. Compared to the controls, cases were less vaccinated in the current, as well as in the previous season (Table 1).
Table 1. Characteristics of influenza A(H1N1)pdm09 cases and test negative controls in Spain, seasons 2010–2011, 2013–2014 and 2015–2016.
| Influenza season | 2010–2011 | 2013–2014 | 2015–2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | Controls | Cases | Controls | Cases | ||||
| N (%) | N (%) | p | N (%) | N (%) | p | N (%) | N (%) | p | |
| Total | 443 (100) | 507 (100) | 444 (100) | 303 (100) | 265 (100) | 396 (100) | |||
| Age groups (years) | < .001 | .110 | .006 | ||||||
| 9–14 | 51 (12) | 50 (10) | 50 (11) | 33 (11) | 36 (14) | 32 (8) | |||
| 15–44 | 252 (57) | 321 (63) | 222 (50) | 165 (54) | 131 (49) | 191 (48) | |||
| 45–64 | 96 (22) | 119 (24) | 121 (27) | 86 (28) | 69 (26) | 144 (36) | |||
| ≥65 | 44 (10) | 17 (3) | 51 (11) | 19 (7) | 29 (11) | 29 (7) | |||
| Sex | .448 | .332 | .579 | ||||||
| Male | 218 (49) | 237 (47) | 221 (50) | 162 (53) | 139 (52) | 199 (50) | |||
| Female | 225 (51) | 270 (53) | 223 (50) | 141 (47) | 126 (48) | 197 (50) | |||
| Target groups | .005 | .033 | .012 | ||||||
| No | 304 (69) | 389 (77) | 302 (68) | 228 (75) | 175 (66) | 297 (75) | |||
| Yes | 139 (31) | 118 (23) | 142 (32) | 75 (25) | 90 (34) | 99 (25) | |||
| Major chronic conditions | .209 | .052 | .080 | ||||||
| No | 305 (83) | 360 (86) | 345 (78) | 253 (84) | 199 (75) | 320 (81) | |||
| Yes | 63 (17) | 58 (14) | 99 (22) | 50(16) | 66 (25) | 76 (19) | |||
| Previous influenza vaccinea | < .001 | .007 | .055 | ||||||
| No | 390 (93) | 492 (98) | 384 (86) | 281 (93) | 229 (86) | 360 (91) | |||
| Yes | 33 (7) | 9 (2) | 60 (14) | 22 (7) | 36 (14) | 35 (9) | |||
| Current influenza vaccine | < .001 | .012 | .001 | ||||||
| No | 388 (88) | 485 (96) | 386 (87) | 281 (93) | 223 (84) | 366 (92) | |||
| Yes | 55 (12) | 22 (4) | 58 (13) | 22 (7) | 42 (16) | 30 (8) | |||
| Onset-swabbing ≤ 4days | .057 | .541 | .736 | ||||||
| No | 18 (4) | 10 (2) | 10 (2) | 9 (3) | 5 (2) | 9 (2) | |||
| Yes | 425 (96) | 497 (98) | 434 (98) | 294 (97) | 260 (98) | 387 (98) | |||
aFor the influenza season 2010–2011, the pandemic vaccine was considered as a previous vaccine.
A total of 674 A(H3N2) cases were included for the 2011–2012 season, 322 for 2013–2014 and 362, for 2014–2015 influenza season. Cases were older than controls in all A(H3N2) seasons, and for the 2011–2012 season, cases included more patients within the target groups than negative controls (Table 2).
Table 2. Characteristics of influenza A(H3N2) cases and test negative controls in Spain, seasons 2011–2012, 2013–2014 and 2014–2015.
| Influenza season | 2011–2012 | 2013–2014 | 2014–2015 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | Controls | Cases | Controls | Cases | ||||
| N (%) | N (%) | p | N (%) | N (%) | p | N (%) | N (%) | p | |
| Total | 430 (100) | 674 (100) | 440 (100) | 322 (100) | 358 (100) | 362 (100) | |||
| Age groups (years) | .016 | .876 | .003 | ||||||
| 9–14 | 53 (12) | 83 (12) | 50 (11) | 32 (10) | 56 (16) | 84 (23) | |||
| 15–44 | 223 (52) | 301 (45) | 220 (50) | 159 (49) | 160 (45) | 158 (44) | |||
| 45–64 | 116 (27) | 191 (28) | 120 (27) | 90 (28) | 110 (31) | 76 (21) | |||
| ≥65 | 38 (9) | 99 (15) | 50 (11) | 41 (13) | 32 (9) | 44 (12) | |||
| Sex | .788 | .542 | .374 | ||||||
| Male | 209 (49) | 322 (48) | 217 (49) | 166 (52) | 178 (50) | 168 (46) | |||
| Female | 221 (51) | 352 (52) | 223 (51) | 156 (48) | 180 (50) | 194 (54) | |||
| Target groups | .023 | .817 | .866 | ||||||
| No | 320 (75) | 461 (68) | 300 (68) | 217 (67) | 267 (75) | 268 (74) | |||
| Yes | 108 (25) | 213 (32) | 140 (32) | 105 (33) | 91 (25) | 94 (26) | |||
| Major chronic conditions | .836 | .421 | .981 | ||||||
| No | 365 (85) | 569 (84) | 343 (78) | 243 (75) | 291 (81) | 294 (81) | |||
| Yes | 65 (15) | 105 (16) | 97 (22) | 79 (25) | 67 (19) | 68 (19) | |||
| Previous influenza vaccine | .805 | .706 | .208 | ||||||
| No | 374 (87) | 581 (86) | 380 (86) | 275 (85) | 324 (91) | 317 (88) | |||
| Yes | 56 (13) | 91 (14) | 60 (14) | 47 (15) | 34 (9) | 45 (12) | |||
| Current influenza vaccine | .517 | .424 | .531 | ||||||
| No | 371 (86) | 572 (85) | 382 (87) | 273 (85) | 317 (89) | 315 (87) | |||
| Yes | 59 (14) | 102 (15) | 58 (13) | 49 (159 | 41 (11) | 47 (13) | |||
| Onset-swabbing ≤ 4days | .062 | .697 | .197 | ||||||
| No | 18 (4) | 15 (2) | 10 (2) | 6 (2) | 7 (2) | 3 (1) | |||
| Yes | 412 (96) | 659 (98) | 430 (98) | 316 (98) | 351 (98) | 359 (99) | |||
Regarding influenza B, in the 2010–2011 a total of 127 cases were included in the analysis, 512 in 2012–2013, 301 in 2014–2015 and 139 in 2015–2016 seasons, , and, respectively. In the 9–14 years age-group there were more cases than controls in the 2010–2011, 2012–2013 and 2015–2016 seasons. Overall, in comparison with the controls, cases were less vaccinated in the seasons 2012–2013 and 2015–2016, whereas previous vaccination showed a statistically significant difference only for the 2012–2013 season (Table 3).
Table 3. Characteristics of influenza B cases and test negative controls in Spain, seasons 2010–2011 to 2015–2016.
| Influenza season | 2010–2011 | 2012–2013 | 2014–2015 | 2015–2016 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | |||||
| N (%) | N (%) | p | N (%) | N (%) | p | N (%) | N (%) | p | N (%) | N (%) | p | |
| Total | 489 (100) | 127 (100) | 435 (100) | 512 (100) | 345 (100) | 301 (100) | 283 (100) | 139 (100) | ||||
| Age groups (years) | < .001 | .014 | .806 | < .001 | ||||||||
| 9–14 | 57 (12) | 43 (34) | 64 (15) | 104 (20) | 54 (16) | 44 (15) | 40 (14) | 45 (32) | ||||
| 15–44 | 272 (56) | 58 (46) | 221 (51) | 219 (43) | 157 (46) | 129 (43) | 136 (48) | 63 (45) | ||||
| 45–64 | 113 (23) | 14 (11) | 110 (25) | 153 (30) | 106 (31) | 103 (34) | 75 (27) | 21 (15) | ||||
| ≥65 | 47 (10) | 12 (9) | 40 (9) | 36 (7) | 28 (8) | 25 (8) | 32 (11) | 10 (7) | ||||
| Sex | .138 | .512 | .518 | .430 | ||||||||
| Male | 244 (50) | 73 (58) | 220 (51) | 248 (48) | 175 (51) | 145 (48) | 150 (53) | 68 (49) | ||||
| Female | 245 (50) | 54 (42) | 215 (49) | 264 (52) | 170 (49) | 156 (52) | 133 (47) | 71 (51) | ||||
| Target groups | .577 | .515 | .582 | .014 | ||||||||
| No | 330 (67) | 89 (70) | 306 (70) | 370 (72) | 256 (74) | 229 (76) | 187 (66) | 108 (78) | ||||
| Yes | 159 (33) | 38 (30) | 129 (30) | 142 (28) | 89 (26) | 72 (24) | 96 (34) | 31 (22) | ||||
| Major chronic conditions | .389 | .203 | .189 | .067 | ||||||||
| No | 338 (83) | 84 (79) | 338 (78) | 415 (81) | 280 (81) | 256 (85) | 214 (76) | 116 (83) | ||||
| Yes | 70 (17) | 22 (21) | 97 (22) | 97 (19) | 65 (19) | 45 (15) | 69 (24) | 23 (17) | ||||
| Previous season influenza vaccinationa | .806 | .001 | .386 | .088 | ||||||||
| No | 433 (93) | 116 (94) | 379 (87) | 487 (94) | 312 (90) | 278 (92) | 244 (86) | 126 (92) | ||||
| Yes | 33 (7) | 8 (6) | 56 (13) | 33 (6) | 33 (10) | 23 (8) | 39 (14) | 11 (8) | ||||
| Current season influenza vaccination | .088 | < .001 | .051 | .003 | ||||||||
| No | 428 (88) | 118 (93) | 377 (87) | 480 (94) | 304 (88) | 279 (93) | 238 (84) | 131 (94) | ||||
| Yes | 61 (12) | 9 (7) | 58 (13) | 32 (6) | 41 (12) | 22 (7) | 45 (16) | 8 (6) | ||||
| Onset-swabbing ≤ 4days | .129 | .103 | .980 | |||||||||
| No | 22 (5) | 2 (2) | 19 (4) | 22 (4) | .957 | 8 (2) | 14 (5) | 6 (2) | 3 (2) | |||
| Yes | 467 (95) | 125 (98) | 416 (96) | 490 (96) | 337 (98) | 287 (95) | 277 (98) | 136 (98) | ||||
aFor the influenza season 2010–2011, the previous vaccination was considered the administration of the pandemic vaccine
Influenza vaccine effectiveness results
The adjusted IVE against A(H1N1)pdm09 varied between 39% (95%CI: -13% to 67%) in 2013–2014 and 52% (95%CI: 20% to 78%) in 2015–2016 for all population. The pooled analysis revealed an IVE against A(H1N1)pdm09 of 50% (95% CI: 29% to 65%) and 42% (95%CI: 14% to 61%) for all population and target groups, respectively (Table 4).
Table 4. Influenza vaccine effectiveness in preventing laboratory-confirmed influenza by virus type/subtype in Spain, seasons 2010–2011 to 2015–2016.
| Type/Subtype | Season/Type of analysis | Cases vaccinated/Total(%) | Controls vaccinated/Total(%) | Crude VE % (95%CI) | Adjusted VEa (95%CI) |
|---|---|---|---|---|---|
|
A(H1N1)pdm09 |
2010–2011 | ||||
| All population | 22/507 (4) | 55/443 (12) | 68 (47; 81) | 49 (1; 73) | |
| Target groups | 19/118 (16) | 49/139 (35) | 65 (36; 81) | 36 (-32; 69) | |
| Onset-swabbing ≤ 4 days | 21/497 (4) | 49/425 (12) | 66 (43; 80) | 50 (1; 74) | |
| 2013–2014 | |||||
| All population | 22/303 (7) | 58/444 (13) | 48 (13; 69) | 39 (-13; 67) | |
| Target groups | 19/75 (25) | 54/142 (36) | 45 (-3; 70) | 40 (-21; 70) | |
| Onset-swabbing ≤ 4 days | 20/294 (7) | 58/434 (13) | 53 (19; 72) | 44 (-6; 70) | |
| 2015–2016 | |||||
| All population | 30/396 (8) | 42/265 (16) | 56 (28; 73) | 52 (20; 78) | |
| Target groups | 27/99 (27) | 36/90 (40) | 44 (-4; 69) | 48 (-9; 75) | |
| Onset-swabbing ≤ 4 days | 29/387 (8) | 41/260 (16) | 57 (28; 74) | 59 (20; 79) | |
| Pooled analysis | |||||
| All population | 74/1206 (6) | 155/1152 (14) | 58 (44; 68) | 50 (29; 65) | |
| Target groups | 65/292 (22) | 139/371 (38) | 52 (32; 66) | 42 (14; 61) | |
| Onset-swabbing ≤ 4 days | 70/1178 (6) | 148/1119 (13) | 59 (44; 69) | 51 (29; 66) | |
| A(H3N2) | 2011–2012 | ||||
| All population | 102/674 (15) | 59/430 (14) | -12 (-59; 21) | 29 (-11; 55) | |
| Target groups | 83/213 (39) | 44/108 (41) | 7 (-49; 42) | 39 (-14; 67) | |
| Onset-swabbing ≤ 4 days | 99/659 (15) | 54/412 (13) | -17 (-68; 18) | 28 (-14; 55) | |
| 2013–2014 | |||||
| All population | 49/322 (15) | 58/440 (13) | -18 (-78; 22) | -18 (-104; 31) | |
| Target groups | 40/105 (38) | 54/140 (39) | 2 (-65; 42) | -1 (-93; 47) | |
| Onset-swabbing ≤ 4 days | 45/316 (14) | 58/430 (14) | -7 (-62; 30) | -7 (-87; 39) | |
| 2014–2015 | |||||
| All population | 47/362 (13) | 41/358 (12) | -15 (-80; 26) | -15 (-101; 34) | |
| Target groups | 39/94 (42) | 35/91 (39) | -14 (-104; 37) | -5 (-106; 47) | |
| Onset-swabbing ≤ 4 days | 47/359 (13) | 39/351 (11) | -21 (-90; 23) | -21 (-111; 31) | |
| Pooled analysis | |||||
| All population | 198/1358 (15) | 158/1228 (13) | -15 (-45; 8) | 4 (-28; 28) | |
| Target groups | 162/412 (39) | 133/339 (39) | 0 (-35; 25) | 10 (-27; 37) | |
| Onset-swabbing ≤ 4 days | 191/1334 (14) | 151/1193 (13) | -15 (-45; 8) | 6 (-26; 30) | |
| B | 2010–2011 | ||||
| All population | 9/127 (7) | 61/489 (13) | 46 (-11; 74) | 63 (1; 86) | |
| Target groups | 9/38 (24) | 55/159 (35) | 41 (-33; 74) | 55 (-36; 85) | |
| Onset-swabbing ≤ 4 days | 8/125 (6) | 54/467 (12) | 48 (-13; 76) | 66 (4; 88) | |
| 2012–2013 | |||||
| All population | 32/512 (6) | 58/435 (13) | 57 (32; 72) | 64 (37; 80) | |
| Target groups | 31/142 (22) | 47/129 (36) | 51 (17; 71) | 59 (22; 79) | |
| Onset-swabbing ≤ 4 days | 26/490 (5) | 54/416 (13) | 62 (39; 77) | 69 (44; 83) | |
| 2014–2015 | |||||
| All population | 22/301 (7) | 41/345 (12) | 41 (-1; 66) | 43 (-6; 69) | |
| Target groups | 17/72 (24) | 34/89 (38) | 50 (0; 75) | 47 (-13; 75) | |
| Onset-swabbing ≤ 4 days | 20/287 (7) | 39/337 (12) | 43 (-1; 67) | 45 (-5; 71) | |
| 2015–2016 | |||||
| All population | 8/139 (6) | 45/283 (16) | 68 (29; 85) | 55 (-17; 82) | |
| Target groups | 7/31 (23) | 38/96 (40) | 55 (-14; 82) | 29 (-132; 78) | |
| Onset-swabbing ≤ 4 days | 7/136 (5) | 44/277 (16) | 71 (34; 87) | 59 (-9; 85) | |
| Pooled analysis | |||||
| All population | 71/1079 (7) | 205/1552 (13) | 54 (39; 65) | 57 (40; 70) | |
| Target groups | 64/283 (23) | 174/473 (37) | 50 (30; 64) | 66 (34; 70) | |
| Onset-swabbing ≤ 4 days | 61/1038 (6) | 191/1497 (13) | 57 (42; 68) | 62 (45; 73) |
VE: Vaccine Effectiveness; CI: Confidence Intervals
aAdjusted VE by: age-groups (9–14; 15–44; 45–64; >64 years), sex, chronic conditions, sentinel network, week of swabbing and season (for the pooled analysis).
Against influenza A(H3N2), the adjusted IVE for all population was 29% (95%CI: -11% to 55%) in the 2011–2012 season; negative IVE point estimates were obtained in the 2013–2014 and 2014–2015. The pooled IVE point estimate against A(H3N2) was of 4% (95%CI: -28% to 28%) and 10% (95%CI: -27% to 37%) for the all population and target groups respectively (Table 4).
The adjusted IVE against influenza B, for all population, varied between 43% (95%CI: -6% to 69%) and 64% (95%CI: 37% to 80%) in 2014–2015 and 2012–2013 seasons, respectively. The pooled IVE against influenza B was 66% (95%CI: 34% to 70%) and 57% (95%CI: 40% to 70%), for the target groups and all population, respectively (Table 4).
When restricting the analysis to the patients swabbed 4 days or less after the onset of symptoms, we obtained similar results compared to all population analysis (Table 4).
Previous and current vaccination analysis
IVE against A(H1N1)pdm09 showed a null or low adjusted estimate for patients receiving only the previous vaccination, with the exception of the 2010–2011 season where patients had received the previous monovalent pandemic vaccine: 65% (95%CI: -13% to 89%) (Table 5).
Table 5. Effect of current and previous influenza vaccination in patients ≥9 years by virus type/subtype in Spain, seasons 2010–2011 to 2015–2016.
| Type/ subtype | Season | Vaccine and circulating strains | Unvaccinated | Vaccinated previous season only |
Vaccinated current season only | Vaccinated both seasons | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Previous season vaccine strain | Current season vaccine strain |
Main Circulating strain |
Cases/ Controls |
Cases/ Controls |
VEa (95%CI) | Cases/ Controls |
VEa (95%CI) | Cases/ Controls | VEa (95%CI) | ||
| A(H1N1)pdm09 | 2010–2011 | A/California/7/2009 | A/California/7/2009 | A/California/7/2009b | 474/355 | 5/11 | 65 (-13; 89) | 18/35 | 45 (-11; 73) | 4/19 | 73 (1; 93) |
| 2013–2014 | A/California/7/2009 | A/California/7/2009 | A/StPetersburg/27/2011b | 276/376 | 5/10 | 20 (-147; 74) | 5/8 | 0 (-234; 70) | 17/50 | 46 (-5; 73) | |
| 2015–2016 | A/California/7/2009 | A/California/7/2009 | A/SouthAfrica/3626/2013b | 356/219 | 9/4 | -55 (-446; 66) | 4/10 | 74 (11; 92) | 26/32 | 49 (-5; 75) | |
| Pooled analysis | 1106/950 | 19/25 | 26 (-39; 61) | 27/53 | 53 (21; 72) | 47/101 | 50 (23; 68) | ||||
| A(H3N2) | 2011–2012 | A/Perth/16/2009 | A/Perth/16/2009 | 40%:A/England/259/2011c 36%:A/Victoria/361/2011c 22%: A/Iowa/19/2010c |
564/364 | 8/7 | 45 (-55; 89) | 17/10 | 14 (-107;65) | 83/49 | 34 (-6; 59) |
| 2013–2014 | A/Texas/50/2012 | A/Texas/50/2012d | A/Texas/50/2012b | 268/372 | 5/10 | 26 (-132; 77) | 7/8 | -1 (203;66) | 42/50 | -20 (-115;34) | |
| 2014–2015 | A/Texas/50/2012 | A/Texas/50/2012 | 35% A/Samara/73/2013b 50%A/HongKong/5738/2014c 15%A/Switzerland/9715293/2013c |
311/313 | 4/4 | -29 (-507; 72) | 6/11 | 45 (-59;80) | 41/30 | -45 (-171;21) | |
| Pooled analysis | 1143/1049 | 17/21 | 23 (-51; 61) | 30/29 | 17 (-43;52) | 166/129 | 3 (-33; 28) | ||||
| B | 2010–2011 | B/Brisbane/60/2008 (lin. Victoria) |
B/Brisbane/60/2008 (lin. Victoria) |
B/Brisbane/60/2008b (lin. Victoria) |
111/393 | 4/13 | -62 (-544; 69) | 2/14 | 51 (-182;91) | 7/46 | 62 (-13; 87) |
| 2012–2013 | B/Brisbane/60/2008 (lin. Victoria) |
B/Wisconsin/1/2010 (lin. Yamagata) |
B/Estonia/55669/2012b B/Wisconsin/1/2010b (lin. Yamagata) |
472/372 | 8/5 | -53 (-396; 53) | 6/7 | 44 (-84; 83) | 25/51 | 67 (40; 82) | |
| 2014–2015 | B/Massachusetts/02/2012 (lin. Yamagata) |
B/Massachusetts/02/2012 (lin. Yamagata) |
B/Phuket/3073/2013b (lin. Yamagata) |
275/314 | 4/4 | -22 (-416; 71) | 3/11 | 66 (-28; 91) | 19/31 | 30 (-40; 64) | |
| 2015–2016 | B/Massachusetts/02/2012 (lin. Yamagata) |
B/Phuket/3073/2013 (lin. Yamagata) |
B/Brisbane/60/2008c (lin. Victoria) |
125/236 | 4/4 | -151 (-1273; 54) | 1/10 | 69 (-160; 96) | 7/35 | 41 (-71; 79) | |
| Pooled analysis | 983/1300 | 20/25 | -54 (-200; 21) | 12/42 | 57 (12; 79) | 58/162 | 56 (36; 70) | ||||
VE: Vaccine Effectiveness; CI: Confidence Intervals; lin.: Lineage
a VE: adjusted VE by: age-groups (9–14; 15–44; 45–64; >64), sex, sentinel network and week of swabbing and season (pooled analysis).
b circulating strains antigenically matching the strain included in the current season vaccine
c circulating strains antigenically miss-matched with the strain included in the current season vaccine
d Similar to A/Victoria/361/2011
The IVE point estimates for patients receiving both current and previous vaccine were higher compared to those vaccinated only in the current season in the 2010–2011 and 2013–2014 seasons (pcomparison = .308 and .352, respectively), but lower in the 2015–2016 season (pcomparison = .321), although the differences were not significant. The pooled analysis revealed an IVE of 26% (95%CI: -39% to 61%) for those having received only the previous vaccination, and similar moderate values around 50% for those receiving either vaccines or only the current one (Table 5).
Against influenza A(H3N2), having received the previous season’s vaccine resulted in a moderate IVE of 45% (95%CI: -55% to 89%) in 2011–2012 season, and a lower or null value for the 2013–2014 and 2014–2015 seasons, respectively. The adjusted IVEs were 34% (95%CI: -6% to 59%) and 14% (95%CI: -107% to 65%) (pcomparison = .577) for those receiving both vaccines or only the current one, respectively, in the 2011–2012 season. However we found negative values for the season 2013–2014. For the 2014–2015 season, the IVE point estimate for both vaccines compared with only the current was lower: -45% (95%CI: -171% to 21%) vs 45% (95%CI: -59% to 80%) (pcomparison = .095). The pooled analysis revealed a low IVE of 3% (95%CI: -33% to 28%), for those receiving vaccinations in both seasons, and of 17% (95%CI: -43% to 52%) (pcomparison = .586) for those who received only the current vaccine (Table 5).
Regarding influenza B, we found negative IVE point estimates for patients receiving only the previous season’s vaccine. Having received both the previous and current season’s vaccines resulted in an IVE similar to those receiving only the current one, with point estimates slightly higher in two seasons, 2010–2011 and 2012–2013, and slightly lower in the other two seasons studied, 2014–2015 and 2015–2016, with no significant differences. The pooled analysis revealed negative IVE point estimates for those receiving only the previous season vaccine and similar, moderate IVE of 56% and 57%, for those receiving both vaccines and the current season vaccine only (Table 5).
When estimating the IVE against influenza A(H3N2), A(H1N1)pdm09 and B and restricting the analysis to the population group targeted for vaccination, we obtained similar results (“S1 Table”).
Vaccine and circulating strains
The A(H1N1)pdm09 circulating strains for the three seasons included in the analysis matched the vaccine strain (Table 5).
In the three seasons with dominant or significant A(H3N2) circulation the previous season’s strain was similar to the current vaccine. The circulating strain matched the current season vaccine in 2013–2014 but only partially in 2014–2015, when 35% of the characterized strains belonged to A/Samara/73/2013 group, antigenically matching the A/Texas/50/2012 vaccine strain. 65% however, fell into two groups that had drifted from the A(H3N2) vaccine strain: A/HongKong/5738/2014 and A/Switzerland/9715293/2013. For the 2011–2012 season all circulating A(H3N2) strains were characterized as mismatched to the vaccine strain.
In all four seasons considered for IVE against influenza B, the previous and the current vaccine lineages were the same. Moreover they matched the current season’s circulating lineage. The only exception was the 2015–2016 season, when there was a mismatch between the current vaccine (Yamagata lineage) and the circulating strains (Victoria lineage) (Table 5).
Discussion
Clarifying the combined effect of previous and current influenza vaccination remains a complex challenge; we have tried to disentangle this effect by using data across six influenza seasons in Spain for the firs time. To better understand the role of the previous year’s vaccine, we discuss our results taking also into account the match between the circulating and vaccine influenza strains.
Overall, we have found better vaccine effectiveness against confirmed A(H1N1)pdm09 and B influenza than against A(H3N2), similar results were obtained both for all patients included in our study as well as for the target groups for vaccination (Table 1).This was suggested both by the pooled analysis and for each influenza season under consideration. These results appear to be in line with previous studies performed in Spain and elsewhere, and confirm the usual poor performance of influenza vaccines against A(H3N2) [22–25].
When taking into account the previous vaccination, we have found some residual protective effect of the pandemic vaccine against 2010–2011 A(H1N1)pdm09 infection, that could be related with an adjuvant vaccine administered universally in Spain during the pandemic. Also we have seen that having received both the pandemic and the current vaccine, protected against A(H1N1)pdm09 infection in 2010–2011, an effect also described in previous studies [14;26;27]. Within each studied season, we did not find any interference between the previous season’s vaccine and the current vaccine’s performance, findings also confirmed by the pooled analysis. Along the same lines, a recently published study suggested an optimal vaccine protection against A(H1N1)pdm09 as individuals having received the current season’s vaccine and 1–2 prior doses [28]. These results could be explained by the relative genetic stability of the A(H1N1)pdm09 influenza virus since the pandemic, leading to the inclusion of the same vaccine strain A/California/7/2009 and a continued good match with strains circulating within in each of the three studied seasons [14;18;29].
The low or null IVE estimates obtained against A(H3N2) do not always appear to be in line with the degree of matching between the vaccine and circulating strains. In 2011–2012 and 2014–2015 seasons, a mismatch was described, whereas in 2013–2014 the vaccine did match the circulating strain; however the overall IVE remained suboptimal in all three seasons (Table 5). We registered the highest protective effect against A(H3N2) (34%) in the 2011–2012 season, the first post-pandemic influenza season dominated by A(H3N2) which had circulating strains discordant with the vaccine strain. We also found some residual protective effect of the previous season’s vaccine against A(H3N2) infection in the 2011–2012 and 2013–2014 seasons, but not in the 2014–2015 season. The current season’s vaccines showed suboptimal but slightly better protection against A(H3N2) for the vaccination-targeted population than in the general population (S1 Table), findings which are in line with a previous study [30]. While previous research described a poor correlation between the IVE and the circulating-vaccine A(H3N2) strain match [31], others studies have shown a correlation of the IVE with the match between circulating and vaccine strains [32–34]. When taking into account the previous vaccination, our results suggest a negative interference between the previous season’s vaccine and the current one for the 2014–2015 season. This effect was observed and described in the same season in Canada [35;36] and is consistent with the antigenic distance theory proposed by Smith & al. [37]. Indeed in Spain in the 2014–2015 season the strains predominantly circulating were A/HongKong/5738/2014 and A/Switzerland/9715293/2013 strains, which had antigenically drifted from the A/Texas/50/2012 vaccine strain, the same vaccine strain being used in the 2013–2014 season. The negative influence of the previous season’s vaccine in 2014–2015 could be explained by the small antigenic distance between the current and the previous vaccines and high antigenic distance between the 2014–2015 vaccine and the circulating strains. However, similar conditions were present for the 2011–2012 season in Spain, with the circulating A(H3N2) strains mismatched with the vaccine[38] and unchanged A(H3N2) vaccine strains in the 2011–2012 and 2010–2011 seasons, but without a clear suggestion of a negative effect from the previous vaccination. A question remains as to whether the antigenic distances between the respective circulating strains and the current vaccine strains were comparable in the two situations, or if other factors are behind the final IVE estimates against AH3N2 in the 2011–2012 season. Finally, the pooled analysis of the vaccine protection against A(H3N2) suggests some kind of interference between the previous season’s vaccine and current vaccine performance.
Regarding the protection against influenza B, the pooled analysis suggested no protective effect from the previous season’s vaccine, consistent with another similar study [39], and no negative interference between the previous season’s vaccine and current vaccine protection for all four studied seasons. By contrast, another study taking into account repeated vaccination over eight seasons in the United States found evidence of residual protection against influenza B [12]. For the 2010–2011 and 2012–2013 seasons, we have found no suggestion of negative interference between the previous and the current season’s vaccination; having received both vaccines resulted in moderate protective effect (around 65%) against confirmed Influenza B, significant for the 2012–2013 season and comparable to results described elsewhere [40]. For 2014–2015 and 2015–2016 seasons, a slightly decrease in IVE point estimates was registered for those receiving both vaccines compared to those having only the current, which was more pronounced for the 2014–2015 season. The small sample size and overlapping 95%CI however, do not allow strong conclusions to be drawn with respect to negative interference from the previous vaccination. In all but one influenza season, the circulating Influenza B strain matched the vaccine strain, partially explaining the moderate effectiveness of the vaccine. The 2015–2016 season was characterized by the discordance of circulating and vaccine lineages (Victoria and Yamagata, respectively), but the IVE was still moderate, suggesting possible cross-lineage protection, previously described in other settings [10;40].
In target groups for vaccination, the higher vaccine protection against A(H1N1)pdm09 and B was provided by vaccination in both previous and current seasons, whereas a possible interference between the previous and current vaccine was also observed against A(H3N2) (Table 5).
Our study has several limitations. The most important of which are the low vaccine coverage and small sample size, especially for the categories used when evaluating the previous vaccination effect, rendering imprecise estimations in some analysis, and reflected by wide confidence intervals. Moreover, for this analysis we could take into account the vaccination in the previous season, but we could not evaluate the repeated vaccination effect, since this information was not available. We discussed our results taking into account the match between circulating and vaccine strains, which was available for genetic characterization at national level but not in the cycEVA sentinel regions. Although differences in the distribution of circulating strains might exist, we consider these differences too small to significantly influence our results. We agree however with other researchers, however, that studies estimating the IVE against specific clade, with a randomized, and therefore unbiased selection of strains to be genetically characterized, would be of added value towards minimizing this limitation. Additionally, our study did not consider the effect that the use of several vaccine types might have on our results since it has varied by age group and Spanish sentinel network; however adjusting by these two variables might overcome this limitation.
In conclusion, our study results suggest an moderate protective effect against overall influenza A(H1N1)pdm09 and B, and a low vaccine effectiveness against A(H3N2). The pooled results of the studied seasons against predominant influenza type/subtype revealed no interference between the previous and current vaccine against A(H1N1)pdm09 and B and a possible negative interference against A(H3N2). Vaccine protection achieved against A(H1N1)pdm09 and B with the current vaccine or with both, the previous and current vaccine, is always superior to not being vaccinated. We have tried to explain these results by considering the previous vaccination effect and taking into account the match between circulating and vaccine strain. We have concluded that only the match between circulating and vaccine strains alone, cannot explain the obtained IVEs, and we underline the need of combined immunological and epidemiological studies, to better understand how these two elements are correlated.
Supporting information
VE: Vaccine effectiveness; CI: Confidence Intervals; lin: lineage.
a VE: adjusted VE by: age-groups (9–14; 15–44; 45–64; >64), sex, sentinel network and week of swabbing and season (pooled analysis).
b circulating strains antigenically matching the strain included in the current season vaccine.
c circulating strains antigenically miss-matched with the strain included in the current season vaccine. d Similar to A/Victoria/361/2011.
(DOCX)
Acknowledgments
The authors within the cycEVA working group, during the seasons 2010–2011 to 2015–2016, are: Silvia Jimenez Jorge, Salvador de Mateo, Concepción Delgado Sanz, Jesus Angel Oliva Domínguez (National Centre of Epidemiology, CIBER de Epidemiología y Salud Pública (CIBERESP), Institute of Health Carlos III, Madrid); Camelia Savulescu (National Centre of Epidemiology, Institute of Health Carlos III, Madrid); Inmaculada Casas (National Centre of Microbiology, National Influenza reference Laboratory, WHO-National Influenza Centre, Institute of Health Carlos III, Madrid); Manuel García-Cenoz (Instituto de Salud Pública Navarra (IdiSNA), Pamplona); Jone Miren Altzíbar Arotzena, Rosa Sancho Martinez (CIBER de Epidemiología y Salud Pública (CIBERESP), Dirección de Salud Pública y Adicciones de Gipuzkoa, País Vasco); L. Etxebarriarteun-Aranzabal (Subdirección de Salud Pública y Adicciones de Álava, País Vasco); Inma Aspiritxaga Gamarra (Subdirección de Salud Pública y Adicciones de Bizkaia, País Vasco); Fernando González Carril (Servicio de Salud Pública. Departamento de Salud. Gobierno del País Vasco); Juana María Vanrell Berga, Jaume Giménez Durán (Servicio de Epidemiología. Dirección General de Salut Pública, Mallorca, Baleares); Carmen Quiñones Rubio, Eva Martínez Ochoa (Servicio de Epidemiología y Prevención Sanitaria, Dirección General de Salud Pública y Consumo de La Rioja, La Rioja); Tomás Vega Alonso, José Lozano, Carolina Rodríguez Gay, A. García (Dirección General de Salud Pública, Consejería de Sanidad de la Junta de Castilla y León, Valladolid); Daniel Castrillejo Pérez (Servicio de Epidemiología. Consejería de Bienestar Social y Sanidad de Melilla); Virtudes Gallardo García, Esteban Pérez Morilla (Servicio de Epidemiología, Consejería de Salud de la Junta de Andalucía); Ana Martínez i Mateo, Nuria Torner (CIBER de Epidemiología y Salud Pública (CIBERESP), Agencia de Salut Pública de Cataluña. Generalitat de Catalunya); Julián Mauro Ramos Aceitero, María del Carmen Serrano Martin (Dirección General de Salud Pública, Servicio Extremeño de Salud, Junta de Extremadura).
We also gratefully acknowledge the contribution of all the sentinel general practitioners and paediatricians, epidemiologists and virologists who have ever participated in the cycEVA study and in the Spanish Influenza Surveillance System.
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to restrictions imposed by National Epidemiological Surveillance Network, but are available from the corresponding author upon reasonable request. We follow a similar policy to other Public health Agencies, as European Centre for Disease Control, regarding access to the data within the National Epidemiological Surveillance Network (RENAVE). The RENAVE, managed and maintained by the National Centre of Epidemiology, has the mandate to collect, analyse and disseminate surveillance data on infectious diseases in Spain. There is not direct access to the RENAVE database, but requests from third parties not belonging to the RENAVE with research purposes are solved by the National Centre of Epidemiology. In this case, the person responsible for the database of this study is responsible for the National Influenza Surveillance Program in Spain, who is also the corresponding author of the manuscript: Amparo Larrauri. Data are available upon reasonable request to Amparo Larrauri, responsible for the National Influenza Surveillance Program in Spain.
Funding Statement
This study was funded by the Horizon 2020 program of the European Commission (I-MOVE-plus, EU 634446) and by the European Centre for Disease Prevention and Control (I-MOVE, ECDC tender OJ/16/07/2014-Proc/2014/024).
References
- 1.World Health Organization. Weekly epidemiological record. Vaccines against Influenza, WHO position paper—November 2012. 2012 Nov 23. Report No.: 47.
- 2.Spanish Ministry of Health SSaE. Recomendations for Influenza vaccination, season 2016–17 (available in Spanish). Spanish Ministry of Health, Social Services and Equality 2016 September 22Available from: URL: https://www.msssi.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/docs/Recomendaciones_vacunacion_gripe.pdf
- 3.Berlanda SF, Tsvetnitsky V, Donnelly JJ. Universal influenza vaccines: Shifting to better vaccines. Vaccine 2016. June 3;34(26):2926–33. doi: 10.1016/j.vaccine.2016.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan SG, Feng S, Cowling BJ. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines 2014. December;13(12):1571–91. doi: 10.1586/14760584.2014.966695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valenciano M, Kissling E, Ciancio BC, Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine 2010. October 28;28(46):7381–8. doi: 10.1016/j.vaccine.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 6.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013. March 13;31(17):2165–8. doi: 10.1016/j.vaccine.2013.02.053 [DOI] [PubMed] [Google Scholar]
- 7.Beyer WE, de Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch Intern Med 1999. January 25;159(2):182–8. [DOI] [PubMed] [Google Scholar]
- 8.Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997. July;15(10):1114–22. [DOI] [PubMed] [Google Scholar]
- 9.Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, et al. Influenza Vaccine Effectiveness in the 2011–2012 Season: Protection Against Each Circulating Virus and the Effect of Prior Vaccination on Estimates. Clin Infect Dis 2013. November 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skowronski DM, Janjua NZ, Sabaiduc S, De SG, Winter AL, Gubbay JB, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–12 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014. January 19; doi: 10.1093/infdis/jiu048 [DOI] [PubMed] [Google Scholar]
- 11.Ohmit SE, Petrie JG, Malosh RE, Fry AM, Thompson MG, Monto AS. Influenza vaccine effectiveness in households with children during the 2012–2013 season: assessments of prior vaccination and serologic susceptibility. J Infect Dis 2015. May 15;211(10):1519–28. doi: 10.1093/infdis/jiu650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of Repeated Vaccination on Vaccine Effectiveness Against Influenza A(H3N2) and B During 8 Seasons. Clin Infect Dis 2014. September 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larrauri CA, Jimenez-Jorge S, Mateo OS, Pozo SF, Ledesma MJ, Casas F, I. Epidemiology of the 2009 influenza pandemic in Spain. The Spanish Influenza Surveillance System. Enferm Infecc Microbiol Clin 2012. October;30 Suppl 4:2–9. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Jorge S, Savulescu C, Pozo F, de Mateo S, Casas I, Ledesma J, et al. Effectiveness of the 2010–11 seasonal trivalent influenza vaccine in Spain: cycEVA study. Vaccine 2012. March 31;30:3595–602. doi: 10.1016/j.vaccine.2012.03.048 [DOI] [PubMed] [Google Scholar]
- 15.Jimenez-Jorge S, de Mateo S, Delgado-Sanz C, Pozo F., Casas I., Garcia Cenoz M., et al. Estimating Influenza vaccine effectiveness in Spain using sentinel surveillance data. Eurosurveillance 2015;20(28):pii = 21187 2015 Jul 16. [DOI] [PubMed] [Google Scholar]
- 16.European Centre for Disease Prevention and Control. Protocol for case-control studies to measure pandemic and seasonal influenza vaccine effectiveness in the European Union and European Economic Area Member States. TECHNICAL DOCUMENT 2009:1–27. Available from: URL: http://ecdc.europa.eu/en/publications/Publications/0907_TED_Influenza_AH1N1_Measuring_Influenza_Vaccine_Effectiveness_Protocol_Case_Control_Studies.pdf
- 17.European Comission. Commission Decision of 30 April 2009 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council, Luxembourg: Publications Office of the European Union. 1.5.2009. L 110/58. Available from. Publications Office of the European Union 2009 April 30Available from: URL: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:110:0058:0059:EN:PDF
- 18.Jimenez-Jorge S, Pozo F, de Mateo S, Delgado-Sanz C, Casas I, Garcia-Cenoz M, et al. Influenza vaccine effectiveness in Spain 2013/14: subtype-specific early estimates using the cycEVA study. Euro Surveill 2014;19(9). [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Jorge S, de Mateo S, Pozo F, Casas I, Garcia CM, Castilla J, et al. Early estimates of the effectiveness of the 2011/12 influenza vaccine in the population targeted for vaccination in Spain, 25 December 2011 to 19 February 2012. Euro Surveill 2012;17(12):1–6. [PubMed] [Google Scholar]
- 20.Jimenez-Jorge S, Pozo F, Larrauri A. Interim influenza vaccine effectiveness: A good proxy for final estimates in Spain in the seasons 2010–2014. Vaccine 2015. June 26;33(29):3276–80. doi: 10.1016/j.vaccine.2015.03.051 [DOI] [PubMed] [Google Scholar]
- 21.Savulescu C, Valenciano M, de Mateo S, Larrauri A, the cycEVA Study Team. Estimating the influenza vaccine effectiveness in elderly on a yearly basis using the Spanish influenza surveillance network—pilot case-control studies using different control groups, 2008–2009 season, Spain. Vaccine 2010. April 1;28(16):2903–7. doi: 10.1016/j.vaccine.2010.01.054 [DOI] [PubMed] [Google Scholar]
- 22.Savulescu C, Jimenez-Jorge S, Delgado-Sanz C, de Mateo S, Pozo F, Casas I, et al. Higher vaccine effectiveness in seasons with predominant circulation of seasonal influenza A(H1N1) than in A(H3N2) seasons: Test-negative case-control studies using surveillance data, Spain, 2003–2011. Vaccine 2014. July 31;32(35):4404–11 2014 Jun 23. doi: 10.1016/j.vaccine.2014.06.063 [DOI] [PubMed] [Google Scholar]
- 23.Gherasim A, Pozo F, de MS, Aspiritxaga G, I, Garcia-Cenoz M, Vega T, et al. Waning protection of influenza vaccine against mild laboratory confirmed influenza A(H3N2) and B in Spain, season 2014–15. Vaccine 2016. April 29;34(20):2371–7. doi: 10.1016/j.vaccine.2016.03.035 [DOI] [PubMed] [Google Scholar]
- 24.Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016. August;16(8):942–51. doi: 10.1016/S1473-3099(16)00129-8 [DOI] [PubMed] [Google Scholar]
- 25.Bragstad K, Emborg H, Kolsen FT, Voldstedlund M, Gubbels S, Andersen B, et al. Low vaccine effectiveness against influenza A(H3N2) virus among elderly people in Denmark in 2012/13—a rapid epidemiological and virological assessment. Euro Surveill 2013;18(6). [PubMed] [Google Scholar]
- 26.Pebody R, Hardelid P, Fleming D, McMenamin J, Andrews N, Robertson C, et al. Effectiveness of seasonal 2010/11 and pandemic influenza A(H1N1)2009 vaccines in preventing influenza infection in the United Kingdom: mid-season analysis 2010/11. Euro Surveill 2011;16(6):1–6. [PubMed] [Google Scholar]
- 27.Savulescu C, Jimenez-Jorge S, de Mateo S, Ledesma J, Pozo F, Casas I, et al. Effectiveness of the 2010/11 seasonal trivalent influenza vaccine in Spain: preliminary results of a case-control study. Euro Surveill 2011. March 17;16(11):1–6. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Baz I, Casado I, Navascues A, Diaz-Gonzalez J, Aguinaga A, Barrado L, et al. Effect of Repeated Vaccination With the Same Vaccine Component Against Influenza A(H1N1)pdm09. J Infect Dis 2017. February 7. [DOI] [PubMed] [Google Scholar]
- 29.Broberg E, Melidou A, Prosenc K, Bragstad K, Hungnes O. Predominance of influenza A(H1N1)pdm09 virus genetic subclade 6B.1 and influenza B/Victoria lineage viruses at the start of the 2015/16 influenza season in Europe. Euro Surveill 2016;21(13). [DOI] [PubMed] [Google Scholar]
- 30.Kissling E, Valenciano M, Larrauri A, Oroszi B, Cohen J, Nunes B, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Euro Surveill 2013;18(5). [DOI] [PubMed] [Google Scholar]
- 31.Kelly HA, Sullivan SG, Grant KA, Fielding JE. Moderate influenza vaccine effectiveness with variable effectiveness by match between circulating and vaccine strains in Australian adults aged 20–64 years, 2007–2011. Influenza Other Respi Viruses 2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis 199[2], 159–167. 15-1-2009. Ref Type: Journal (Full) doi: 10.1086/595861 [DOI] [PubMed] [Google Scholar]
- 33.Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng PY, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012. October;55(7):951–9. doi: 10.1093/cid/cis574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valenciano M, Kissling E, Reuss A, Jimenez-Jorge S, Horvath JK, Donnell JM, et al. The European I-MOVE Multicentre 2013–2014 Case-Control Study. Homogeneous moderate influenza vaccine effectiveness against A(H1N1)pdm09 and heterogenous results by country against A(H3N2). Vaccine 2015. June 4;33(24):2813–22. doi: 10.1016/j.vaccine.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 35.Skowronski D, Chambers C, Sabaiduc S, De Serres G, Dickinson J, Winter A, et al. Interim estimates of 2014/15 vaccine effectiveness against influenza A(H3N2) from Canada s Sentinel Physician Surveillance Network, January 2015. Euro Surveill 2015;20(4). [DOI] [PubMed] [Google Scholar]
- 36.Skowronski DM, Chambers C, De SG, Sabaiduc S, Winter AL, Dickinson JA, et al. Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010–11 to 2014–15. J Infect Dis 2017. February 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999. November 23;96(24):14001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgado-Sanz C., Jimenez-Jorge S., Lopez-Perea N., Pozo F., Gomez-Barroso D., Flores V., et al. Influenza surveillance in Spain. Season 2011–12 (week 40/2011-20/2012)—available in spanish. Carlos III Public Health Institute, National Centre of Epidemiology 2012. October 720:153–168. Available from: URL: http://revista.isciii.es/index.php/bes/article/view/756/855 [Google Scholar]
- 39.Martinez-Baz I, Navascues A, Pozo F, Chamorro J, Albeniz E, Casado I, et al. Influenza vaccine effectiveness in preventing inpatient and outpatient cases in a season dominated by vaccine-matched influenza B virus. Hum Vaccin Immunother 2015;11(7):1626–33. doi: 10.1080/21645515.2015.1038002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, et al. Influenza Vaccine Effectiveness in the United States During 2012–13: Variable Protection by Age and Virus Type. J Infect Dis 2014. November 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VE: Vaccine effectiveness; CI: Confidence Intervals; lin: lineage.
a VE: adjusted VE by: age-groups (9–14; 15–44; 45–64; >64), sex, sentinel network and week of swabbing and season (pooled analysis).
b circulating strains antigenically matching the strain included in the current season vaccine.
c circulating strains antigenically miss-matched with the strain included in the current season vaccine. d Similar to A/Victoria/361/2011.
(DOCX)
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to restrictions imposed by National Epidemiological Surveillance Network, but are available from the corresponding author upon reasonable request. We follow a similar policy to other Public health Agencies, as European Centre for Disease Control, regarding access to the data within the National Epidemiological Surveillance Network (RENAVE). The RENAVE, managed and maintained by the National Centre of Epidemiology, has the mandate to collect, analyse and disseminate surveillance data on infectious diseases in Spain. There is not direct access to the RENAVE database, but requests from third parties not belonging to the RENAVE with research purposes are solved by the National Centre of Epidemiology. In this case, the person responsible for the database of this study is responsible for the National Influenza Surveillance Program in Spain, who is also the corresponding author of the manuscript: Amparo Larrauri. Data are available upon reasonable request to Amparo Larrauri, responsible for the National Influenza Surveillance Program in Spain.