Abstract
The “hygiene hypothesis” is a theory try to explain the dramatic increases in the prevalence of autoimmune and allergic diseases over the past two to three decades in developed countries. According to this theory, reduced exposure to parasites and microorganisms in childhood is the main cause for the increased incidences of both T helper 1 (Th1)-mediated autoimmunity and Th2-mediated allergy. In this study, we investigated the impact of Schistosoma japonicum infection on the allergic airway inflammation induced by repeated intracheal inoculations of house dust mites (HDM), which is a Th17 and neutrophils dominant murine asthma model, mimicking severe asthma. We found that S. japonicum infection downregulated airway hyperresponsiveness. The infiltrating cells, Th17 and Th2 effector cytokines in the bronchoalveolar lavage (BAL) fluids and lungs were significantly reduced in the infected mice. Our findings indicated that S. japonicum infection was able to effectively inhibit host’s allergic airway inflammation, which may be related to the upregulated Treg cells upon infection. To our knowledge, it is the first study to reveal the impact of S. japonicum infection on house dust mite induced severe asthma. More in depth investigation is need to elucidate the underlying mechanisms.
Introduction
In 1989, Strachan proposed the “hygiene hypothesis” to explain the dramatic increase in the prevalence of autoimmune and allergic diseases over the past two to three decades [1]. According to this hypothesis, a lack of early life exposure to microorganisms and parasites may cause an increased incidences of both T helper 1 (Th1)-mediated autoimmunity and Th2-mediated allergy [2–4]. Parasitic infections are becoming a major theme in the hygiene hypothesis [5]. Helminths, as long-lived parasites, possess their ability to manipulate host immunity to protect themselves from elimination and minimize severe pathology to the host [6]. Immunomodulation by parasitic helminths is a general and very conserved phenomenon [7].
Schistosomiasis is an immunopathogenic disorder. It is caused by blood flukes, of which there are three main species: Schistosoma japonicum, S. mansoni, and S. haematobium. The delayed-type hypersensitivity reactions against the schistosome eggs s trapped in the venules lead to the formation of circumoval granuloma in livers and intestines and subsequent fibrosis [8, 9]. Schistosomiasis is roughly divided into acute, chronic, and late phases upon infection progression. Th1 responses are elicited at early phase by the larval worms, succeeded by Th2 responses induced by the parasite eggs. Egg deposit in the tissues is a determining factor to drive Th2 response in S. mansoni and S. japonicum infections in mice [9, 10]. After infection progresses into chronic phase, usually 8–10 weeks (wks) post infection in mice, immunomodulation phenomenon occurs. The latter not only downregulates granuloma formation and other parasite-related immune responses, but also significantly affects host’s systemic immune responses. The underlying mechanisms are still elusive; among them, a wide range of excretory and secretory products from schistosomes may significantly modulate host’s immune responses [11, 12].
Helminth infection has a negative correlation with the severity of allergic airway inflammation. Several species of helminthes including S. japonicum have been shown to down-modulate airway hyperresponsiveness in human and murine models [13–15]. The interaction between helminthes infection and airway inflammation is affected by various factors, such as infection phases, parasite loads and species of helminthes [16]. Asthma is a chronic airway inflammation, in which Th2 cells are generally believed playing important roles in its pathogenesis. Allergic asthma accounts for about 80% of asthma cases, which is characterized by Th2 cytokines interleukin 4 (IL-4), IL-5 and IL-13 dependent increased immunoglobulin E (IgE) serum levels, lung eosinophilia, airway hyperresponsiveness and goblet cell metaplasia, respectively [17–19]. Recently, it is evident that other T cell subsets, such as regulatory T (Treg) [20, 21] and Th17 cells [22, 23] are actively involved in the airway hyperresponsiveness. Patients with severe asthma are often difficult to treat and resistant to the steroid therapy [22]. Th17 cells and neutrophils may play central roles in the pathogenesis of severe asthma [22]. It is generally believed that eosinophilic inflammation is mainly mediated by Th2 cells, whereas neutrophilic inflammation is a hallmark of Th17 responses [24]. Murine asthma models have been of invaluable importance in elucidating the pathogenesis of this disease [19]. In addition to classical ovalbumin-induced Th2 and eosinophils dominant allergic airway inflammation model [25–27], our laboratory recently established the house dust mite (HDM)-induced murine asthma model, in which Th17 and neutrophils are dominant responders, mimicking severe asthma [19, 28–31].
In this study, we investigated the impact of S. japonicum infection on HDM-induced allergic airway inflammation. We designed early and late HDM-immunization regimens to fit the time points before or on/after schistosome-induced immunomodulation initiated. We found that S. japonicum infection, at both early and later phases, was able to significantly inhibit the airway hyperresponsiveness. This inhibition may be related to upregulated Treg cells during S. japonicum infection.
Materials and methods
Ethics statement
The care and handling of the animals in this study were in strict accordance with the Guidelines for the Care and Use of Laboratory Animals at Jiangsu Institute of Parasitic Disease (JIPD). This study was prospectively approved by the Institutional Animal Care and Use Committee of JIPD (Permit Number: [2013] 016). All efforts were made to minimize animal suffering including the use of anesthesia (sodium pentobarbital, 50 mg/kg, intraperitoneally) for the administration of HDM. Mice were monitored daily by the staff of the animal facility. The human endpoint was used for mice that lost 25% or more of their initial body weight during the study. When the animals met the criteria, or at the experimental endpoint, they were euthanized under excess isoflurane anesthesia followed by cervical dislocation according to institutional guidelines.
Mice and Parasitology
Female C57BL/6 mice (6–8 wks) were purchased from the College of Veterinary Medicine, Yangzhou University, China, and maintained under specific pathogen-free condition at JIPD. Schistosoma japonicum (Chinese mainland strain) were obtained from JIPD. Mice were percutaneously infected with 15 cercariae of S. japonicum (S.j.) 3 or 5 wks prior to the induction of allergic airway inflammation.
HDM-induced allergic airway inflammation
To induce allergic airway inflammation, infected and uninfected mice were inoculated intratracheally (i.t.) with house dust mite (HDM) (Dermatophagoides pteronyssinus) extract (Stallergenes Greer, Lenoir, NC), starting at 3 wks (early phase) or 5 wks (late phase) post infection. The immunization regimens included 4 times of sensitizations (15 μg of HDM in 40 μl PBS, i.t. per time), and 3 times of challenges, lasting for 24 days (Fig 1A and Fig 2A). Control groups of both infected and uninfected mice were inoculated i.t. with PBS alone. Mice were sacrificed 24 h after the last challenge. Their bronchoalveolar lavage (BAL) fluids were aspirated and centrifuged. Total cells in the pellet were counted by using a hemacytometer. Differential cell counts on >400 cells were performed on cytospins stained with Shandon Kwik-Diff Stain kit (Thermo Scientific, Rockford, IL). The BAL fluid from each mouse was concentrated to 0.5 ml by centrifugation with an Amicon Ultra-4 filter unit (Millipore, Billerica, MA) for determinations of cytokines by ELISA. For lung histology, the lower lobe of the right lung was fixed with 4% paraformaldehyde overnight, dehydrated, embedded in paraffin, cut into 4 μm sections, and processed for hematoxylin/eosin (H&E) staining. Lung tissue mRNA was analyzed by Real-time PCR. Serum levels of various HDM-specific antibodies were measured by ELISA with the use of biotinylated goat anti-mouse IgE (BD Bioscience, San Jose, CA), IgM, IgG1, IgG2a, and streptavidin-HRP (Southern Biotech, Birmingham, AL).
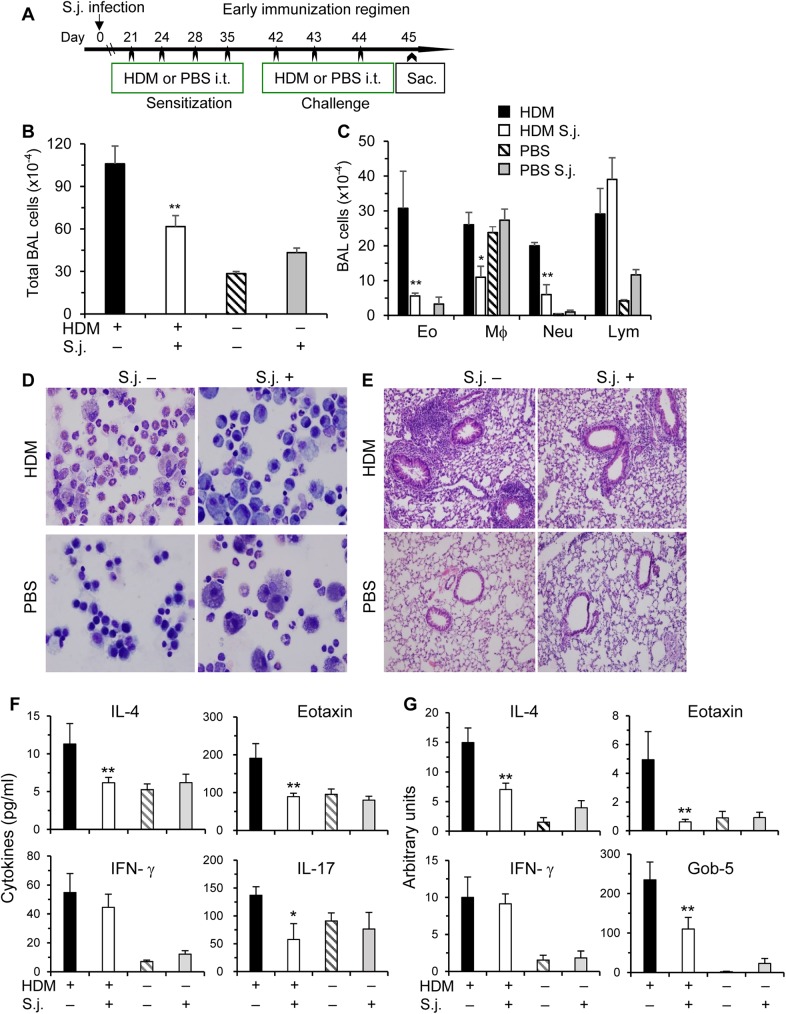
Fig 1. S. japonicum infection inhibits HDM-induced airway inflammation at early phase.
(A) To induce allergic airway inflammation, both infected and uninfected C57BL/6 mice were inoculated intratracheally (i.t.) with HDM. In the early immunization regimen, HDM inoculations started at 3 wks post the infection with S. japonicum (S.j.). Control groups were inoculated i.t. with PBS alone. Mice were sacrificed 24 h after the last challenge. (B) The total cells in BAL fluids were counted by using a hemacytometer. (C) Differential cell counts of >400 cells were performed on cytospins stained with Kwik-Diff. The numbers of eosinophils (Eo), macrophages (Mϕ), neutrophils (Neu), and lymphocytes (Lym) in BAL are shown. (D-E) Representative Kwik-Diff staining of BAL cells and H&E staining of lung tissue sections. (F) Cytokine levels in BAL fluids were determined by ELISA. (G) Total RNA from the right lower lobe of the lungs was extracted for real-time PCR analysis for IL-4, eotaxin, IFN-γ and Gob-5. Data are normalized to an 18S reference and expressed as arbitrary units. Results are expressed as mean+SE (B-C, F-G), representative of two independent experiments (n = 5–10 per group). Compared to uninfected and HDM immunized mice, *p<0.05; **p<0.01.
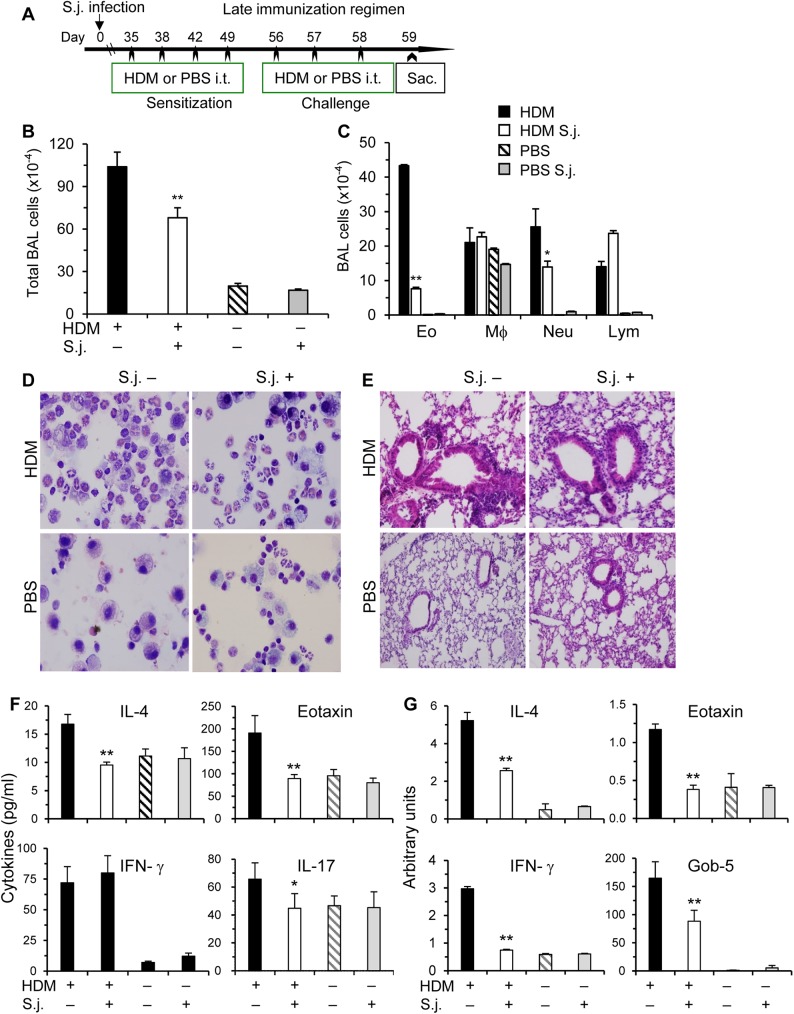
Fig 2. S. japonicum infection inhibits HDM-induced airway inflammation at late phase.
(A) In the late immunization regimen, HDM inoculations started at 5 wks post infection. The experiments were performed similarly as Fig 1. Total and differential cell counts of BAL cells (B-C), representative Kwik-Diff staining for BAL cytospins and H&E staining of lung tissue sections (D-E), cytokine levels in BAL fluids (F), and mRNA levels in the lungs (G) are shown. Results are representative of two independent experiments (n = 5–9 per group). Compared to uninfected and HDM immunized mice, *p<0.05; **p<0.01.
Cytokine assay
Cytokines in the culture supernatants and BAL fluids were measured by enzyme-linked immunosorbent assay (ELISA). IL-4, IL-10 and IFN-γ were measured with OptEIA kits (BD Bioscience); IL-13, IL-17 and eotaxin were measured with DuoSet ELISA kits (R&D Systems, Minneapolis, MN). ELISA plates were developed with TMB substrate (BD Bioscience), and read with a microplate reader. Cytokine mRNA levels were measured by real-time quantitative PCR.
In vivo cytokine capture assay (IVCCA)
The in vivo cytokine levels for IL-4, IL-10 and IFN-γ were detected by IVCCA [32, 33], following the manufacturer’s protocols (BD Bioscience). Mice were injected with biotinylated neutralizing monoclonal antibodies to capture the corresponding cytokines, and bled 2–4 h later. IVCCA facilitates measurement of cytokines in serum by increasing their in vivo half-lives. By this way, the sensitivity of in vivo cytokine assays increases for at least 30- to 1,000-fold [32]. IVCCA for IL-17 was developed in our laboratory with the use of biotinylated anti-mouse IL-17 (BD Bioscience), recombinant murine IL-17 and IL-17 ELISA kit (R&D Systems).
Flow cytometry
Cells were incubated with anti-CD16/32 (2.4G2) (BD Bioscience) to block FcγR II/III, and then stained with various conjugated antibodies as indicated. BD Cytofix/Cytoperm kit (BD Bioscience) was used for intracellular cytokine staining and Treg staining kit (e-Bioscience, San Diego, CA) was used for detection of Treg cells, following the manufacturer’s instructions Stained cells were analyzed by FACSVerse with FACS Suite (BD Bioscience) or FCS Express (De Novo Software, Los Angeles, CA) software.
Real-time PCR
Total RNA was extracted from lung tissues with the RNeasy Mini Kit (Qiagen, Valencia, CA), and cDNA was prepared by using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative PCR was performed with the Platinum SYBR Green qPCR SuperMix-UDG w/RO or TaqMan Gene Expression Master Mix (Life Technologies, Carlsbad, CA) on a Light Cycler 480 II (Roche, Indianapolis, IN). The data were normalized to the 18S reference. Primers for IL-4, IL-5, IL-13, eotaxin, MUC-5AC, and Gob-5 were designed with OLIG 4.0 software as reported [26].
Statistical analysis
All experimental data were analyzed and compared for statistically significant differences using two-tailed Student’s t or Mann-Whitney U test, and a P value of < 0.05 was considered significant.
Results
S. japonicum infection inhibits HDM-induced airway inflammation
Helminth infection may significantly modulate host’s immune responses. We evaluated the impact of S. japonicum infection on the allergic airway inflammation induced by HDM. Mice were repeatedly inoculated HDM intracheally for about 4 wks, starting at 3 or 5 wks post infection respectively. The early (starting at 3 wks) or late (5 wks) HDM-immunization regimens were designed trying to fit the time points before or on/after schistosome-induced immunomodulation initiated. Airway inflammation was evaluated by quantitation of total cells in the bronchoalveolar lavage (BAL) fluids, the differential cell counts of BAL cells by Kwik-Diff staining, and infiltrating cells in lung tissues by H&E staining.
In the early immunization regimen (Fig 1A), total numbers of BAL cells in uninfected mice inoculated with HDM had over 3~4-fold increases as compared to PBS-inoculated control mice (Fig 1B). By differential counts with Kwik-Diff Staining, a mixed cell infiltration was observed, mainly including eosinophils, macrophages, neutrophils and lymphocytes (Fig 1C and 1D). However, S. japonicum infection led to a dramatic reduction in total numbers of BAL cells, particularly eosinophils and neutrophils, whereas, the proportions of macrophages were relatively increased. In the lungs, uninfected mice showed massive perivascular and peribronchial infiltration of inflammatory cells upon HDM-challenge as revealed by lung histological analysis. However, this inflammatory response was markedly attenuated in mice after S. japonicum infection (Fig 1E). We reasoned that this infection-induced inhibitory effect on allergic airway inflammation might be attributable to altered immune responses of various T helper (Th) subsets. In the following experiments, we determined effector cytokines of Th1, Th2 and Th17 cells. IL-4, a representative Th2 cytokine, was significantly reduced from infected mice in BAL fluids and lungs at protein (Fig 1F) and mRNA levels (Fig 1G), respectively. Similarly as IL-4, Th17 effector cytokine IL-17 was downregulated in infected mice in BAL fluids (Fig 1F). On the other hand, Th1 cytokine IFN-γ was comparable in both BAL fluids and lungs between the infected and uninfected mice (Fig 1F and 1G).
Hypersecretion of mucus plays an important role in the pathogenesis and severity of asthma [25]. Gob-5 gene has been suggested to promote the synthesis of mucin glycoproteins by upregulation of MUC-5AC gene expression [34]. Gob-5 was dramatically increased in HDM-inoculated uninfected mice (Fig 1G), whereas, over half of this increase was lost in the infected mice. Eotaxin, by acting on CCR3, selectively recruit eosinophils from the airway microvessels into the lung tissue, which plays a central role in the onset of allergic asthma [35]. This important chemokine was significantly reduced in the BAL fluid in S. japonicum infected mice (Fig 1F).
The findings from the late immunization regimen (Fig 2) basically followed the similar patterns as the early immunization regimen. Airway inflammation, Th2 and Th17 effector cytokines were consistently downregulated in infected mice in the early as well as late HDM-immunization regimens. These data suggest that S. japonicum infection significantly affects host’s allergic airway inflammation.
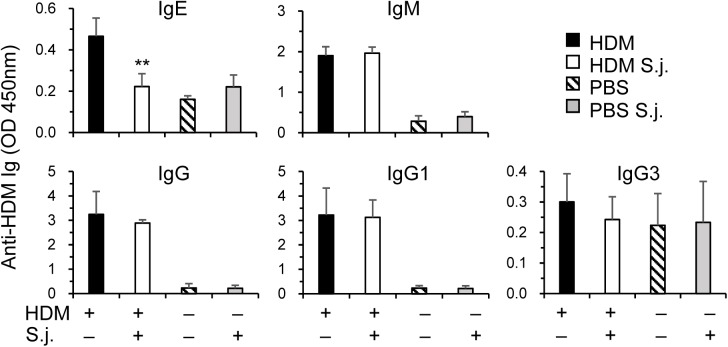
HDM-specific IgE downregulated upon S. japonicum infection
Apart from cellular responses in airway, humoral systemic responses to HDM are also important for asthma pathogenesis and development [25, 36]. IgE is an important mediator of allergic airway inflammation, which is related to Th2 cytokine IL-4 [36]. We found that serum levels of HDM-specific IgE were significantly reduced in S. japonicum infected mice. However, HDM-specific IgM, total IgG, and its subclasses IgG1, IgG3 did not differ significantly between infected and uninfected groups (Fig 3).
Fig 3. Serum OVA-specific IgE is reduced in S. japonicum infected mice.
In the same experiments as Fig 2, serum levels of OVA-specific IgE, IgM, IgG and IgG subclasses were assayed by ELISA (mean+SE). **p<0.01 compared to uninfected and HDM immunized mice.
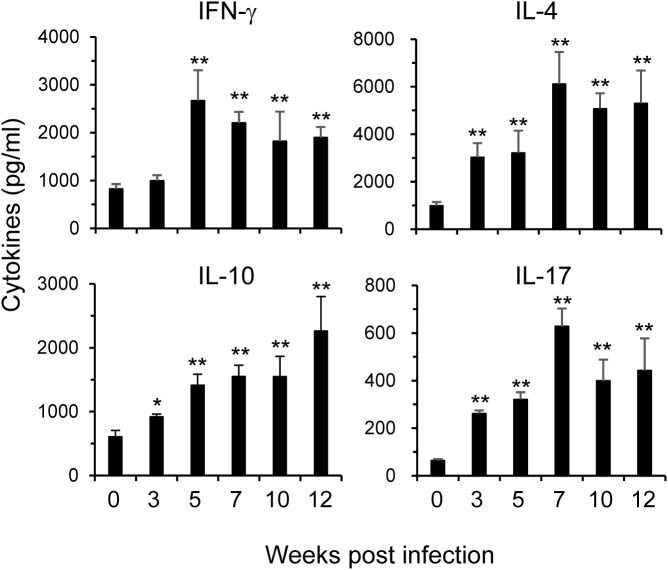
Dynamics of in vivo cytokine levels during S. japonicum infection
To begin to understand the underlying mechanisms of S. japonicum infection-induced inhibition to airway inflammation, we next studied the in vivo dynamics of various Th effector cytokines during the infection. Because most cytokines are utilized, catabolized, or excreted shortly after they are produced in vivo, it has been difficult to directly measure in vivo cytokine production [32, 33]. IVCCA was developed to determine the circulating levels of various cytokines. Once S. japonicum infected the host, a wide range of excretory and secretory products from schistosomes may downregulate host’s immune responses, including differentiation and function of various Th subsets. We monitored the in vivo dynamics of effector cytokines for Th1, Th2, Treg and Th17 upon infection progression. Serum levels of IFN-γ, IL-4, IL-10 and IL-17 were determined by IVCCA at 0, 3, 5, 7, 10 and 12 wks post infection (Fig 4).
Fig 4. Cytokine dynamics in vivo post infection.
C57BL/6 mice were infected with S. japonicum. The levels of IFN-γ, IL-4, IL-10, and IL-17 cytokines in the peripheral blood were assayed at indicated times by IVCCA. Results are representative of two independent experiments (n = 5–9 per group). Compared to uninfected mice, **p<0.01.
Serum level of IFN-γ started to increase at 3 wks, peaked at 5 wks post infection; then, significantly downregulated but remained at high levels compared to prior infection (0 wk). IL-4 reached its peak at 7 wks post infection; then gradually decreased but kept at high level till 12 wks after infection. On the other hand, serum level of IL-10 kept rising and maintained a plateau from 5 to 12 wks post infection. IL-17 in sera reached its peak at 7 wks after infection then gradually reduced but remained at elevated level 12 wks post infection. These data, for the first time, revealed the in vivo dynamics of cytokine profiles during S. japonicum infection.
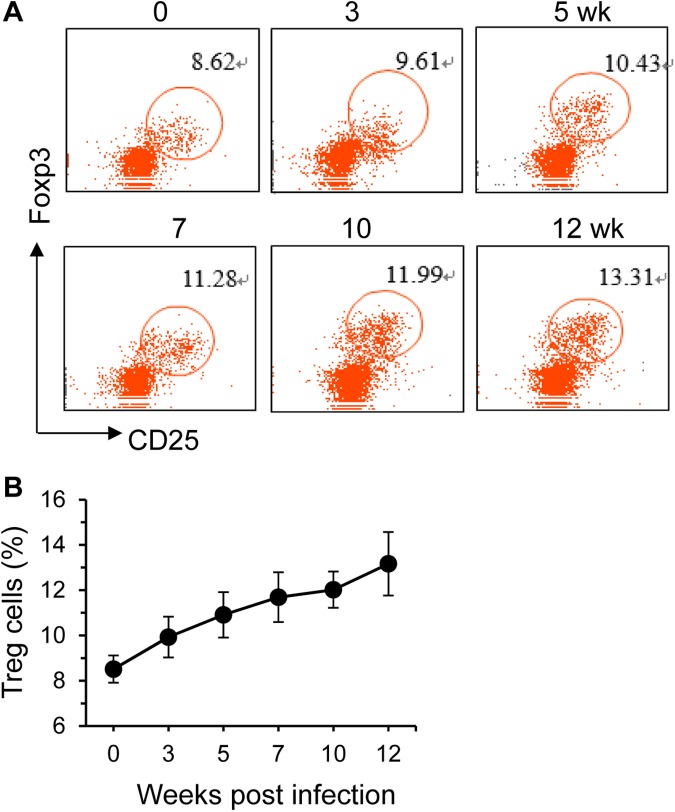
We also determined the dynamic changes of Treg cells in the spleens by FACS. These cells expanded upon S. japonicum infection (Fig 5). The main Treg effector cytokines are TGF-β1 and IL-10 [37]. The dynamics of Treg cells in the spleens were consistent with the in vivo IL-10 changes by IVCCA (Fig 4).
Fig 5. Treg cells expand after S. japonicum infection.
Treg cells in the spleens were determined by Flow cytometry gated on CD4+ cells. Percentages of Treg cells (CD4+CD25+Foxp3+) cells are shown in representative dot plots (A), and summarized in a graph (B). Results are representative of two independent experiments (n = 5–6 per group).
Discussion
Immunomodulation by parasitic infection has become a hot research topic recently. The ability of infectious agents to regulate their host’s immune system is becoming an interesting research attraction [6]. Ample evidences have now support the notion that both microbes and parasites can effectively modulate host’s immune responses to alleviate allergic and autoimmune disorders [3, 6]. Some helminthes seem to have anti-allergic or anti-inflammatory effects in humans. Experimental evidences have also shown the significant suppression for the development of airway hyperresponsiveness in mice infected with numerous helminths, including S. japonicum [15]. Infection by this parasite causes severe immunopathogenic injuries to the host; on the other hand, it may open a new avenue and idea to develop novel therapies to autoimmunity and allergy based on parasitic products and immunomodulation mechanisms according to the “hygiene hypothesis”.
Asthma is traditionally believed as Th2 cells and eosinophils-dominant chronic airway inflammation. Th2 cytokines IL-4 and IL-13 can activate epithelial and endothelial cells and fibroblasts to produce eotaxin, a chemotaxin important for recruiting eosinophil from the airway microvessels into the lung tissue [35]. Recently, compelling evidences have demonstrated that Th17 cells are a major player to the pathogenesis of allergic airway inflammation, especially to severe asthma [22, 23, 29, 38–40]. The latter is often induced by infection and resistant to steroid therapy in patients [41].
By intracheal inoculations of HDM, we developed a murine asthma model, in which Th17 cells and nertrophil are dominant responders, mimicking severe asthma [19, 22]. With this model, we investigated the impacts of S. japonicum infection on the allergic airway inflammation. Our previous study revealed that IL-4 reached a peak at 7 wks post infection, then declined steadily reaching the base line by week 20 post-infection from spleen cells of S. japonicum infected mice when stimulated with the soluble egg antigens [9]. The in vivo IL-4 level also peaked at 7 wks post infection, while, IFN-γ began to decline at this time point (Fig 4). It was found that egg deposition was the major factor driving Th2 responses, depressing Th1 cytokine expression as well as T-cell proliferation in S. japonicum-infected mice [9]. Therefore, we designed the early (starting at 3 wks) and late (5 wks) HDM-immunization regimens trying to fit the time points before or on/after schistosome-induced immunomodulation initiated in the current study. It was found that S. japonicum infection, at both early and later phases, was able to significantly inhibit the airway hyperresponsiveness. Th2 and Th17 effector cytokines in the BAL fluids and lungs were markedly downregulated. S. japonicum infection may inhibit eosinophil infiltration via the downregulation of eotaxin and IL-4 in the lung and BAL; whereas, IL-17 downregulation may contribute to the ameliorated neutrophil infiltration in airways. Not only cytokine secretions were inhibited, serum HDM-specific IgE was also inhibited by the infection, which may contribute to the reduced allergic airway inflammation too, as IgE plays an important role in the immunopathogenesis of asthma by activating mast cells and basophils through its binding to Fcε Receptors, etc [35]. On the other hand, HDM-specific IgG and IgM did not alter by the infection. Therefore, the reduced serum level of HDM-specific IgE was not caused by the generalized inhibition of B cells. Instead, it may be related to the impaired Th2 function, as IL-4 preferentially induces Ig isotype switching to IgE [26, 36].
The underlying mechanisms by which S. japonicum infection alleviates allergic airway inflammation are yet to be elucidated. We found that Treg cells in the spleens were continuously upregulated upon infection. TGF-β1 and IL-10 are the main Treg effector cytokines. In vivo IL-10 level was raised 5 wk post infection by IVCCA assay. These data are consistent with other reports that Treg cells participate in the regulation of schistosomiasis not only locally, but also systemically [42–44]. We found a systemic increase of IL-17 in the serum post S. japonicum infection (Fig 4); whereas, airway IL-17 in the BAL fluids was reduced (Fig 1F and Fig 2F). The mechanisms for this discrepancy remain elusive. The upregulation of systemic IL-17 might reflect the direct responses of Th17 cells to the parasite antigen stimulation; whereas, the inhibited BAL IL-17 secretion to HDM might be caused by activation of Treg cells in the airways. Further studies are needed to identify the components from schistosomes including their excretory and secretory products, which possess the ability to directly modify the responses of different Th subsets, such as Treg, Th17 cells.
In summary, our findings indicated that S. japonicum infection was able to effectively inhibit house dust mites-induced allergic airway inflammation. This inhibition may be related to the upregulated Treg cells upon infection. More in depth investigations are need to elucidate the underlying cellular and molecular mechanisms.
Data Availability
All relevant data are within the paper.
Funding Statement
This work has been supported by grants from the National Natural Science Foundation of China (81373116 to J. Q.Y), the Research Capacity-strengthening Project from the Jiangsu Science and Technology Department, China (BM2015024 to J.Q.Y), and the National Institutes of Health, United States (GM 108661 and CA198358 to F.G.; CA193350 to Y.Z.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Strachan DP. Hay fever, hygiene, and household size. Bmj. 1989;299(6710):1259–60. Epub 1989/11/18. ; PubMed Central PMCID: PMCPmc1838109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiemsma LT, Turvey SE. Asthma and the microbiome: defining the critical window in early life. Allergy Asthma Clin Immunol. 2017;13:3 Epub 2017/01/13. doi: 10.1186/s13223-016-0173-6 ; PubMed Central PMCID: PMCPMC5217603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JQ, Zhou Y, Singh RR. Effects of Invariant NKT Cells on Parasite Infections and Hygiene Hypothesis. J Immunol Res. 2016;2016:2395645 Epub 2016/08/27. doi: 10.1155/2016/2395645 ; PubMed Central PMCID: PMC4987483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umetsu DT. Early exposure to germs and the Hygiene Hypothesis. Cell Res. 2012;22(8):1210–1. Epub 2012/04/25. doi: 10.1038/cr.2012.65 cr201265 [pii]. ; PubMed Central PMCID: PMC3411171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson MS, Maizels RM. Regulation of allergy and autoimmunity in helminth infection. Clin Rev Allergy Immunol. 2004;26(1):35–50. Epub 2004/02/03. doi: CRIAI:26:1:35 [pii] doi: 10.1385/CRIAI:26:1:35 . [DOI] [PubMed] [Google Scholar]
- 6.Maizels RM, McSorley HJ, Smyth DJ. Helminths in the hygiene hypothesis: sooner or later? Clin Exp Immunol. 2014;177(1):38–46. Epub 2014/04/23. doi: 10.1111/cei.12353 ; PubMed Central PMCID: PMC4089153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harnett W. Secretory products of helminth parasites as immunomodulators. Mol Biochem Parasitol. 2014;195(2):130–6. Epub 2014/04/08. doi: 10.1016/j.molbiopara.2014.03.007 S0166-6851(14)00038-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 8.Lukacs NW, Boros DL. Lymphokine regulation of granuloma formation in murine schistosomiasis mansoni. Clin Immunol Immunopathol. 1993;68(1):57–63. Epub 1993/07/01. doi: S0090122983710950 [pii]. . [DOI] [PubMed] [Google Scholar]
- 9.Yang JQ, Tasaka K, Chuang CK, Yoshikawa H, Nakajima Y. Dynamic analysis of T-lymphocyte function in relation to hepatopathologic changes and effect of interleukin-12 treatment in mice infected with Schistosoma japonicum. J Parasitol. 1999;85(2):257–62. . [PubMed] [Google Scholar]
- 10.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, Heiny S, et al. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146(4):1322–7. Epub 1991/02/15. . [PubMed] [Google Scholar]
- 11.Pineda MA, Al-Riyami L, Harnett W, Harnett MM. Lessons from helminth infections: ES-62 highlights new interventional approaches in rheumatoid arthritis. Clin Exp Immunol. 2014;177(1):13–23. Epub 2014/03/29. doi: 10.1111/cei.12252 ; PubMed Central PMCID: PMC4089150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11(6):375–88. Epub 2011/05/26. doi: 10.1038/nri2992 nri2992 [pii]. . [DOI] [PubMed] [Google Scholar]
- 13.Araujo MI, Hoppe B, Medeiros M, Alcantara L, Almeida MC, Schriefer A, et al. Impaired T helper 2 response to aeroallergen in helminth-infected patients with asthma. J Infect Dis. 2004;190(10):1797–803. Epub 2004/10/23. doi: 10.1086/425017 . [DOI] [PubMed] [Google Scholar]
- 14.Mangan NE, van Rooijen N, McKenzie AN, Fallon PG. Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J Immunol. 2006;176(1):138–47. Epub 2005/12/21. . [DOI] [PubMed] [Google Scholar]
- 15.Mo HM, Lei JH, Jiang ZW, Wang CZ, Cheng YL, Li YL, et al. Schistosoma japonicum infection modulates the development of allergen-induced airway inflammation in mice. Parasitol Res. 2008;103(5):1183–9. Epub 2008/07/26. doi: 10.1007/s00436-008-1114-1 . [DOI] [PubMed] [Google Scholar]
- 16.Carvalho EM, Bastos LS, Araujo MI. Worms and allergy. Parasite Immunol. 2006;28(10):525–34. Epub 2006/09/13. doi: 10.1111/j.1365-3024.2006.00894.x . [DOI] [PubMed] [Google Scholar]
- 17.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326(5):298–304. Epub 1992/01/30. doi: 10.1056/NEJM199201303260504 . [DOI] [PubMed] [Google Scholar]
- 18.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18(5):673–83. Epub 2012/05/09. doi: 10.1038/nm.2731 . [DOI] [PubMed] [Google Scholar]
- 19.Haspeslagh E, Debeuf N, Hammad H, Lambrecht BN. Murine Models of Allergic Asthma. Methods Mol Biol. 2017;1559:121–36. Epub 2017/01/08. doi: 10.1007/978-1-4939-6786-5_10 . [DOI] [PubMed] [Google Scholar]
- 20.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol. 2016;138(3):639–52. Epub 2016/09/07. doi: 10.1016/j.jaci.2016.06.003 S0091-6749(16)30499-7 [pii]. ; PubMed Central PMCID: PMC5023156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stelmaszczyk-Emmel A. Regulatory T cells in children with allergy and asthma: it is time to act. Respir Physiol Neurobiol. 2015;209:59–63. Epub 2014/12/03. doi: 10.1016/j.resp.2014.11.010 S1569-9048(14)00305-X [pii]. . [DOI] [PubMed] [Google Scholar]
- 22.Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190(10):1094–101. Epub 2014/08/28. doi: 10.1164/rccm.201405-0859PP . [DOI] [PubMed] [Google Scholar]
- 23.Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66(8):989–98. Epub 2011/03/08. doi: 10.1111/j.1398-9995.2011.02576.x . [DOI] [PubMed] [Google Scholar]
- 24.Zhao S, Jiang Y, Yang X, Guo D, Wang Y, Wang J, et al. Lipopolysaccharides promote a shift from Th2-derived airway eosinophilic inflammation to Th17-derived neutrophilic inflammation in an ovalbumin-sensitized murine asthma model. J Asthma. 2016:1–9. Epub 2016/09/03. doi: 10.1080/02770903.2016.1223687 . [DOI] [PubMed] [Google Scholar]
- 25.Yang JQ, Leitges M, Duran A, Diaz-Meco MT, Moscat J. Loss of PKC lambda/iota impairs Th2 establishment and allergic airway inflammation in vivo. Proc Natl Acad Sci U S A. 2009;106(4):1099–104. Epub 2009/01/16. doi: 10.1073/pnas.0805907106 0805907106 [pii]. ; PubMed Central PMCID: PMC2633554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JQ, Liu H, Diaz-Meco MT, Moscat J. NBR1 is a new PB1 signalling adapter in Th2 differentiation and allergic airway inflammation in vivo. Embo J. 2010;29(19):3421–33. doi: 10.1038/emboj.2010.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JQ, Kalim KW, Li Y, Zhang S, Hinge A, Filippi MD, et al. RhoA orchestrates glycolysis for TH2 cell differentiation and allergic airway inflammation. J Allergy Clin Immunol. 2016;137(1):231–45 e4. Epub 2015/06/24. doi: 10.1016/j.jaci.2015.05.004 S0091-6749(15)00696-X [pii]. ; PubMed Central PMCID: PMC4684821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169(3):378–85. Epub 2003/11/05. doi: 10.1164/rccm.200308-1094OC . [DOI] [PubMed] [Google Scholar]
- 29.McGee HS, Stallworth AL, Agrawal T, Shao Z, Lorence L, Agrawal DK. Fms-like tyrosine kinase 3 ligand decreases T helper type 17 cells and suppressors of cytokine signaling proteins in the lung of house dust mite-sensitized and -challenged mice. Am J Respir Cell Mol Biol. 2010;43(5):520–9. Epub 2009/11/26. doi: 10.1165/rcmb.2009-0241OC 2009-0241OC [pii]. ; PubMed Central PMCID: PMC2970852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norimoto A, Hirose K, Iwata A, Tamachi T, Yokota M, Takahashi K, et al. Dectin-2 promotes house dust mite-induced T helper type 2 and type 17 cell differentiation and allergic airway inflammation in mice. Am J Respir Cell Mol Biol. 2014;51(2):201–9. Epub 2014/03/05. doi: 10.1165/rcmb.2013-0522OC . [DOI] [PubMed] [Google Scholar]
- 31.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132(5):1194–204 e2. Epub 2013/09/26. doi: 10.1016/j.jaci.2013.06.048 S0091-6749(13)01208-6 [pii]. ; PubMed Central PMCID: PMC3845500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkelman FD, Morris SC. Development of an assay to measure in vivo cytokine production in the mouse. Int Immunol. 1999;11(11):1811–8. Epub 1999/11/05. . [DOI] [PubMed] [Google Scholar]
- 33.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206(13):2947–57. doi: 10.1084/jem.20091268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busse PJ, Zhang TF, Srivastava K, Lin BP, Schofield B, Sealfon SC, et al. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116(6):1256–63. doi: 10.1016/j.jaci.2005.08.059 [DOI] [PubMed] [Google Scholar]
- 35.Conroy DM, Williams TJ. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res. 2001;2(3):150–6. Epub 2001/11/01. ; PubMed Central PMCID: PMCPMC2002069. doi: 10.1186/rr52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111(3):291–7. Epub 2003/02/06. doi: 10.1172/JCI17748 ; PubMed Central PMCID: PMC151878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palomares O, Martin-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, et al. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-beta. Genes Immun. 2014;15(8):511–20. Epub 2014/07/25. doi: 10.1038/gene.2014.45 gene201445 [pii]. . [DOI] [PubMed] [Google Scholar]
- 38.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180(8):720–30. doi: 10.1164/rccm.200904-0573OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong CK, Lun SW, Ko FW, Wong PT, Hu SQ, Chan IH, et al. Activation of peripheral Th17 lymphocytes in patients with asthma. Immunol Invest. 2009;38(7):652–64. Epub 2009/10/09. doi: 10.1080/08820130903062756 [pii]. . [DOI] [PubMed] [Google Scholar]
- 40.Gavino AC, Nahmod K, Bharadwaj U, Makedonas G, Tweardy DJ. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy. 2016;71(12):1684–92. Epub 2016/05/27. doi: 10.1111/all.12937 . [DOI] [PubMed] [Google Scholar]
- 41.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181(6):4089–97. Epub 2008/09/05. ; PubMed Central PMCID: PMCPMC3638757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen X, He L, Chi Y, Zhou S, Hoellwarth J, Zhang C, et al. Dynamics of Th17 cells and their role in Schistosoma japonicum infection in C57BL/6 mice. PLoS Negl Trop Dis. 2011;5(11):e1399 Epub 2011/11/22. doi: 10.1371/journal.pntd.0001399 PNTD-D-11-00351 [pii]. ; PubMed Central PMCID: PMC3216943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Zhao J, Yang Y, Zhang L, Yang X, Zhu X, et al. Schistosoma japonicum egg antigens stimulate CD4 CD25 T cells and modulate airway inflammation in a murine model of asthma. Immunology. 2007;120(1):8–18. Epub 2006/10/18. doi: IMM2472 [pii] doi: 10.1111/j.1365-2567.2006.02472.x ; PubMed Central PMCID: PMC1890919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osada Y, Kanazawa T. Parasitic helminths: new weapons against immunological disorders. J Biomed Biotechnol. 2010;2010:743758 Epub 2010/02/20. doi: 10.1155/2010/743758 ; PubMed Central PMCID: PMC2821776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.