Abstract
Rationale: Phenotypic distinctions between severe asthma (SA) and nonsevere asthma (NONSA) may be confounded by differential adherence or incorrect use of corticosteroids.
Objectives: To determine if there are persistent phenotypic distinctions between SA (as defined by 2014 American Thoracic Society/European Respiratory Society guidelines) and NONSA after intramuscular triamcinolone acetonide (TA), and to identify predictors of a corticosteroid response in these populations.
Methods: A total of 526 adults age 18 years and older (315 SA) and 188 children age 6 to less than 18 years (107 SA) in the NHLBI Severe Asthma Research Program III were characterized before and 3 weeks after TA. The primary outcome for corticosteroid response was defined as greater than or equal to 10-point improvement in percent predicted FEV1.
Measurements and Main Results: Adult asthma groups exhibited a small but significant mean FEV1% predicted improvement after TA (SA group mean difference, 3.3%; 95% confidence interval, 2.1–4.5%; P = 0.001), whereas children did not. Adult SA continued to manifest lower FEV1 and worse asthma control as compared with NONSA after TA. In children, after TA only prebronchodilator FEV1 distinguished SA from NONSA. A total of 20% of adults with SA and 21% of children with SA achieved greater than or equal to 10% improvement after TA. Baseline bronchodilator response and fractional exhaled nitric oxide had good sensitivity and specificity for predicting response in all groups except children with NONSA.
Conclusions: One in five patients with SA exhibit greater than or equal to 10% improvement in FEV1 with parenteral corticosteroid. Those likely to respond had greater bronchodilator responsiveness and fractional exhaled nitric oxide levels. In adults, differences in airflow obstruction and symptoms between SA and NONSA persist after parenteral corticosteroids, suggesting a component of corticosteroid nonresponsive pathobiology in adults with SA that may differ in children.
Clinical trial registered with www.clinicaltrials.gov (NCT 01606826).
Keywords: corticosteroid response phenotype, severe asthma, pediatric and adult asthma
At a Glance Commentary
Scientific Knowledge on the Subject
Whether phenotypic distinctions between severe and nonsevere asthma are related to the degree of corticosteroid use is unclear. Furthermore, in patients with severe asthma who respond to corticosteroids, the phenotypic or biomarker predictors of a response are not known. Whether the response or predictors vary by age has not been explored.
What This Study Adds to the Field
This study describes a distinction between adults and children with severe asthma in terms of their response to parenteral corticosteroids. Adults, but not children, with severe asthma remain phenotypically distinct from those with nonsevere asthma. Furthermore, it identified that, despite already being prescribed high-dose inhaled corticosteroids, approximately 20% of patients with severe asthma experience a clinically important improvement in airway caliber after triamcinolone acetonide. The baseline FEV1 response to bronchodilator and baseline fractional exhaled nitric oxide were good predictors of an FEV1 corticosteroid response. These findings suggest differences between children and adults in the pathobiologic underpinnings of severe asthma that require further investigation.
Defining features of severe asthma (SA) include poor control of symptoms, persistence of greater inflammatory marker expression, and/or airflow obstruction despite the use of high doses of corticosteroid therapy (1, 2). When individuals with SA are compared with those with non-SA (NONSA), they have lower FEV1, more symptoms, and poorer quality of life (2–6). Although these differences may be caused by fundamental pathobiologic differences in SA that lead to corticosteroid resistance, poor adherence or delivery of inhaled corticosteroid therapy may also contribute to these phenotypic distinctions, especially in those individuals in whom type 2 immune biomarkers remain elevated.
From 2001 to 2011 the National Institutes of Health/NHLBI supported the Severe Asthma Research Program (SARP) to study mechanisms differentiating severe from NONSA (5, 7). In 2012, NHLBI constituted SARP III, a panel of investigators from 11 U.S. pediatric and adult sites, including a data coordinating center, to further evaluate biologic processes that underlie asthma severity in adults and children that are associated with progression of severity. A unique feature of SARP III was an effort to eliminate the potential confounding of poor corticosteroid adherence, and variable deposition or absorption of inhaled therapies. All participants with SA and NONSA were characterized before and after administration of a single dose of intramuscular triamcinolone acetonide (TA) to answer several key questions. First, will clinical and biomarker distinctions between the SA and NONSA populations persist after parenteral corticosteroid administration? Second, do adults and children with SA differ in their response to parenteral corticosteroids? Third, are there phenotypic characteristics and/or biomarkers that predict an individual response to corticosteroid? Finally, are there defining characteristics associated with a corticosteroid response that provide insights into the pathobiology of SA?
We hypothesized that phenotypic/biomarker distinctions would persist between SA and NONSA after TA, but that children would have less of an airway response because of the lesser degree of baseline obstruction. To test these possibilities, we report characteristics of the SARP III cohort across age and severity at baseline characterization before and after TA.
Methods
A total of 714 adults and children age greater than or equal to 6 years old with asthma were recruited by 11 clinical centers for SARP III between 2011 and 2015 (see the online supplement for detailed inclusion/exclusion criteria). SARP III targeted a population of 60% SA and 40% NONSA (75% adults and 25% children). The institutional review board at each center approved the study. Subjects were classified as SA as defined by 2014 American Thoracic Society/European Respiratory Society guidelines (2). Briefly, this is defined as asthma that requires treatment with suggested Global Initiative for Asthma steps 4–5 for the previous year or systemic corticosteroids for greater than or equal to 50% of the previous year to prevent “uncontrolled” asthma or remains “uncontrolled” despite therapy (see online supplement for definitions of control).
After obtaining written informed consent/assent, subjects were characterized at baseline over two clinical research visits (see online supplement).
Questionnaires
Questionnaires assessed demographics, medication history, health care utilization, and asthma control (Asthma Control Questionnaire [ACQ]-6 permission obtained) (8).
Height and Weight and Body Mass Index
Height by stadiometry and weight were measured using National Health and Nutrition Examination Survey standards and body mass index (BMI) was calculated for adults and percentiles for age and sex in the children.
Measures of Atopy
Total IgE, and allergen-specific IgE by ImmunoCap were collected (see online supplement for details).
Airflow, Airway Reactivity, and Fractional Exhaled Nitric Oxide
Pre- and postalbuterol spirometry (9) and maximum bronchodilation were performed in all subjects (see online supplement). Predicted values for spirometric variables were obtained using the 2012 Global Lung Initiative standard reference equations (10). Bronchodilator (BD) response was defined as absolute ∆ in FEV1% predicted between pre– and post–BD administration spirometry (11, 12). Methacholine challenge was performed using standardized methods (13). Fractional exhaled nitric oxide (FeNO) was measured per American Thoracic Society/European Respiratory Society guidelines (14).
Sputum/Blood Eosinophils
Blood eosinophils were quantified. Sputum induction was also performed using standard methods (15, 16).
Intramuscular Corticosteroid Administration
At the end of the second baseline characterization visit, subjects that satisfied inclusion/exclusion criteria received a single dose of intramuscular TA given intramuscularly deep in the gluteal region. Adults aged 18 years and older received 40 mg of TA; children ages 6–17 years received 1 mg/kg of TA (up to 40 mg maximum).
Characterization of Corticosteroid Response
Response to TA was evaluated at a third visit within 18 ± 3 days of the TA injection, which is a time frame used in prior TA studies (17, 18). Spirometry, maximum BD, blood eosinophil counts (subset), induced sputum eosinophil cell counts (ages ≥12), FeNO, and asthma control surveys were repeated after TA.
Corticosteroid Response
Corticosteroid response was defined as a dichotomous FEV1 outcome using a threshold of at least 10 percentage point absolute increase in pre-BD FEV1% predicted (FEV1 final minus FEV1 baseline) from before to after TA administration, based on other studies and the natural variation expected in spirometry (19–21).
Analytic Methods
Descriptive statistics are presented as means and SD for continuous variables and proportions or percentages for categorical variables. Generalized linear mixed effects models allowing for correlations between repeated measurements were used to compare outcomes pre and post corticosteroid administration, across severity and age groups. Models were fit under the normal distribution for FEV1, ACQ (minimally important difference = 0.5 and ACQ ≥1.5 defined as uncontrolled [22, 23]), or the log normal distribution for sputum (in adults only because of limited usable data in children), blood eosinophils, and FeNO. Because assessments were 2–3 weeks after TA and ACQ ascertained 2-week symptom recall (as opposed to 4-wk recall with Asthma Control Test/Childhood Asthma Control Test), ACQ6 was used as a marker of symptom control without lung function.
Corticosteroid response was also considered as a qualitative outcome defined as an increase of at least 10% in pre-BD FEV1% predicted after corticosteroid administration and predictors of response were examined using receiver operating characteristic curves. Sensitivity analyses were done to evaluate the choice of 10% as the threshold for defining response.
All analyses were conducted using SAS statistical software version 9.4 (SAS Inc., Cary, NC), without adjustment for multiple comparisons.
Results
Baseline Characteristics
The cohort (n = 714) was comprised of 526 adults (age ≥18 yr) and 188 children (age 6–17 yr) (Table 1). Thirteen percent (n = 41) of severe adults and 14.4% (n = 16) of severe children reported environmental tobacco smoke exposure without any apparent differences in reported exposure between groups (Table 1).
Table 1.
Summary Features of SARP III Participants
| Adult Nonsevere (n = 213) | Adult Severe (n = 313) | Pediatric Nonsevere (n = 77) | Pediatric Severe (n = 111) | |
|---|---|---|---|---|
| Age at baseline visit, yr | 44.5 ± 14.6* | 49.7 ± 12.8 | 11.6 ± 2.9 | 11.5 ± 2.8 |
| Age at diagnosis of asthma, yr | 19.1 ± 15.3 | 20.0 ± 16.5 | 3.2 ± 2.8 | 3.0 ± 2.7 |
| Age at onset of symptoms, yr | 16.3 ± 13.9 | 17.4 ± 16.0 | 2.3 ± 2.4 | 2.4 ± 2.6 |
| Years since diagnosis of asthma | 25.4 ± 15.6* | 29.7 ± 15.6 | 8.4 ± 3.8 | 8.4 ± 3.4 |
| Years since onset of asthma symptoms | 28.1 ± 15.9* | 32.3 ± 15.7 | 9.3 ± 3.4 | 9.1 ± 3.2 |
| Body mass index, kg/m2 (percentile for pediatric groups) | 31.0 ± 7.9* | 33.5 ± 8.5 | 73.7 ± 26.5 | 77.1 ± 26.8 |
| Pre-TA, pre-BD FEV1% predicted from visit 2 | 81.0 ± 16.4* | 66.7 ± 19.7 | 93.1 ± 14.4* | 86.3 ± 16.3 |
| Pre-TA, max FEV1% predicted | 92.2 ± 15.5* | 77.9 ± 19.7 | 105.6 ± 13.4 | 103.4 ± 17.5 |
| Pre-BD FEV1/FVC | 0.72 ± 0.09* | 0.66 ± 0.11 | 0.78 ± 0.08 | 0.75 ± 0.10 |
| Pre-BD FEV1/FVC% predicted | 87.9 ± 10.4* | 81.7 ± 13.9 | 88.4 ± 9.6† | 85.3 ± 11.1 |
| Maximum FEV1 albuterol response (absolute change in % predicted) | 10.5 ± 7.8† | 11.9 ± 7.9 | 12.4 ± 7.8† | 16.2 ± 11.0 |
| Methacholine PC20, median (IQR) | 1.6 (0.5–4.2) | 1.4 (0.4–4.1) | 1.4 (0.6–4.4) | 0.9 (0.4–4.9) |
| ACQ-6 | 1.1 ± 0.9* | 1.9 ± 1.1 | 0.9 ± 0.8* | 1.3 ± 0.9 |
| ACT/CACT(6–11) score | 19.2 ± 4.0* | 15.3 ± 4.8 | 19.8 ± 3.5* | 17.0 ± 4.4 |
| FeNO, ppb, median (IQR) | 24.0 (16.0–43.0)† | 21.0 (13.0–37.0) | 28.0 (12.0–49.0) | 23.0 (12.0–46.0) |
| Sputum eosinophils, %, median (IQR) | 0.7 (0.0–2.0) | 0.8 (0.2–4.3) | 1.1 (0.2–4.2) | 1.6 (0.3–10.2) |
| Blood eosinophils, cells/μl, median (IQR) | 189 (111–320)† | 228 (134–399) | 359 (208–575) | 324 (162–514) |
| IgE test result, IU/ml, median (IQR) | 141 (46–374) | 163 (45–384) | 490 (151–834) | 465 (164–1,207) |
| Number of positive specific IgEs (of 15 tests) | 4.8 ± 3.8* | 4.1 ± 4.1 | 6.9 ± 4.6 | 7.0 ± 4.6 |
| TA response: pre-BD FEV1% predicted | 2.3 ± 7.1 | 3.4 ± 10.1 | 1.3 ± 12.5 | 2.4 ± 13.6 |
| At least one positive specific IgE, n (%) | 173 (82) | 234 (75.2) | 67 (89.3) | 104 (94.5) |
| Race/ethnicity, n (%) | ||||
| Male sex | 71 (33.3) | 103 (32.9) | 50 (64.9) | 67 (60.4) |
| White race | 142 (66.7) | 194 (62) | 33 (42.9) | 39 (35.1) |
| African American race | 47 (22.1) | 85 (27.2) | 32 (41.6) | 49 (44.1) |
| Other race | 24 (11.3) | 34 (10.9) | 12 (15.6) | 23 (20.7) |
| Hispanic ethnicity | 9 (4.2) | 10 (3.2) | 11 (14.3) | 17 (15.3) |
| Medication use in the past 3 mo, n (%) | ||||
| Inhaled corticosteroids | 161 (75.6)* | 310 (99) | 65 (84.4)* | 111 (100) |
| Long-acting β-agonist | 108 (50.7)* | 300 (95.8) | 32 (41.6)* | 98 (88.3) |
| Long-acting anticholinergic | 3 (1.4)* | 48 (15.3) | 0 (0)† | 9 (8.1) |
| Leukotriene antagonists | 51 (23.9)* | 135 (43.1) | 29 (37.7)* | 84 (75.7) |
| Short-acting anticholinergic | 8 (3.8)* | 51 (16.3) | 1 (1.3)† | 11 (9.9) |
| Short-acting β-agonist | 183 (85.9)* | 294 (93.9) | 68 (88.3) | 105 (94.6) |
| Oral or systemic corticosteroids | 4 (1.9)* | 85 (27.2) | 1 (1.3)† | 11 (9.9) |
| >2 steroid courses in the past year | 28 (13.1)* | 164 (52.4) | 18 (23.4)* | 65 (58.6) |
| ≥3 mo with oral corticosteroid use in the past year | 0 (0)* | 70 (22.4) | 1 (1.3)† | 11 (9.9) |
| Biologics | 5 (2.3)* | 34 (10.9) | 1 (1.3) | 7 (6.3) |
| Eczema | 57 (26.8) | 96 (30.7) | 44 (57.1) | 64 (57.7) |
| ED visit for breathing problems in the past 12 mo | 24 (11.3)* | 101 (32.3) | 26 (33.8)* | 75 (67.6) |
| Hospitalized for asthma in the past year | 6 (2.8)* | 49 (15.7) | 9 (11.7)* | 41 (36.9) |
| Daily oral corticosteroids (current) | 1 (0.5)* | 70 (22.4) | 1 (1.3)* | 15 (13.5) |
| Environmental smoke exposure, n (%) | ||||
| Any | 25 (11.7) | 41 (13.1) | 10 (13.2) | 16 (14.4) |
| Home | 14 (6.6) | 24 (7.7) | 10 (13.2) | 12 (10.8) |
| Work | 6 (2.8) | 16 (5.1) | 0 (0) | 0 (0) |
| Other | 9 (4.2) | 9 (2.9) | 1 (1.3) | 5 (4.5) |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; ACT = Asthma Control Test; BD = bronchodilator; CACT = Childhood Asthma Control Test; ED = emergency department; FeNO = fractional exhaled nitric oxide; IQR = interquartile range; IU = international units; SARP = Severe Asthma Research Program; PC20 = provocative concentration (of methacholine in this case) that causes a 20% fall in FEV1; TA = triamcinolone acetonide.
Data are mean ± SD unless otherwise indicated.
P < 0.01; P values refer to comparison of severity by age group.
P < 0.05; P values refer to comparison of severity by age group.
Overall Change before and after TA among the Groups by Age and Severity
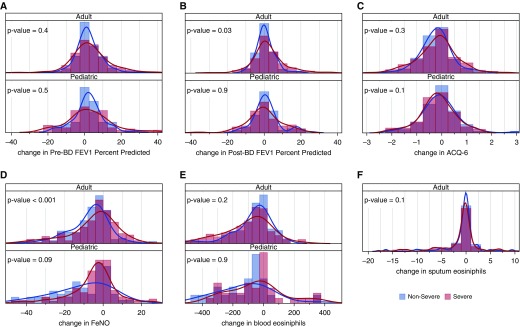
Pictorial distributions of intramuscular TA response in the population as quantified by absolute change in pre-BD FEV1% predicted, post-BD FEV1% predicted, blood and sputum eosinophils (sputum in adults only), and ACQ6 comparing adults and children, grouped by severity, are shown in Figure 1. Scatter plot distributions of pre- and post-TA response with the same variables are shown in Figure E1 in the online supplement. Overall, the severe patients had a wider distribution in lung function and ACQ6 (adults only) after TA (Figures 1A–1C), whereas in children the NONSA group had a wider FeNO distribution (Figure 1D). The scatter plot distributions before and after TA suggest that severe adult patients with higher baseline ACQ6, FeNO, and blood eosinophils responded less robustly than those with lower baseline levels (see Figures E1C–E1E), whereas lung function seemed to have a more direct correlation (see Figures E1A and E1B).
Figure 1.
(A–F) Distribution of intramuscular corticosteroid (triamcinolone) response in the population as quantified by absolute change in prebronchodilator FEV1% predicted (A), post-bronchodilator FEV1% predicted (B), Asthma Control Questionnaire-6 (C), fractional exhaled nitric oxide (D), and blood (E) and sputum (F; in adults only) eosinophils, comparing adults and children grouped by severity. Bars are histograms and lines are smooth density lines showing probabilities (area under the curve = 1.0). ACQ = Asthma Control Questionnaire; BD = bronchodilator; FeNO = fractional exhaled nitric oxide.
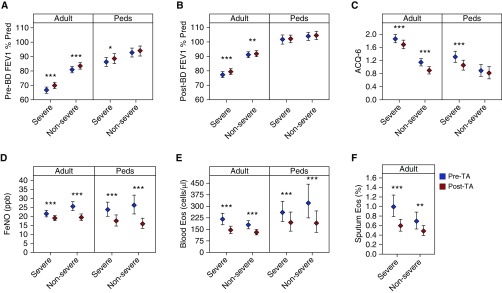
Baseline differences and changes in the characteristics and biomarkers measured before and after TA by severity and age groups are shown graphically in Figure 2 with actual values shown in Table 2. Ranges are reported as 95% confidence intervals unless otherwise indicated. Responses are described in the following sections separated by adults and children.
Figure 2.
(A–F) Intramuscular corticosteroid (triamcinolone) response in adult and pediatric participants by prebronchodilator FEV1% predicted (A), post-bronchodilator FEV1% predicted (B), Asthma Control Questionnaire-6 (C), fractional exhaled nitric oxide (D), and blood (E) and sputum (F; in adults only) eosinophils. Error bars represent 95% confidence intervals. ***P < 0.001; **P ≥ 0.001 to <0.01; *P ≥ 0.01 to <0.05. ACQ = Asthma Control Questionnaire; BD = bronchodilator; Eos = eosinophils; FeNO = fractional exhaled nitric oxide; Peds = pediatric; TA = triamcinolone acetonide.
Table 2.
Pre- and Post-Triamcinolone Response between Age and Severity Groups
| Before Triamcinolone | After Triamcinolone | Change after Triamcinolone | |
|---|---|---|---|
| Pre-BD FEV1% predicted | |||
| Adult | |||
| Severe (n = 313) | 66.7 (64.6 to 68.9) | 70.0 (67.9 to 72.2) | 3.3 (2.1 to 4.5) (P < 0.001) |
| Nonsevere (n = 212) | 81.0 (78.8 to 83.2) | 83.5 (81.2 to 85.8) | 2.5 (1.4 to 3.5) (P < 0.001) |
| Difference | −14.3 (−17.3 to −11.3) (P < 0.001) | −13.5 (−16.7 to −10.3) (P < 0.001) | 0.8 (−0.8 to 2.5) (P = 0.3) |
| Pediatric | |||
| Severe (n = 111) | 86.3 (83.3 to 89.4) | 88.6 (85.2 to 92.1) | 2.3 (−0.2 to 4.9) (P = 0.076) |
| Nonsevere (n = 77) | 93.1 (89.7 to 96.1) | 94.2 (94.2 to 97.5) | 1.1 (−1.9 to 3.9) (P = 0.5) |
| Difference | −6.8 (−10.9 to −2.2) (P = 0.003) | −5.5 (−10.3 to −0.8) (P = 0.025) | 1.2 (−2.5 to 5.2) (P = 0.5) |
| Post-BD FEV1% predicted | |||
| Adult | |||
| Severe (n = 311) | 77.9 (75.8 to 80.1) | 80.1 (78.0 to 82.2) | 2.2 (1.1 to 3.3) (P < 0.001) |
| Nonsevere (n = 213) | 92.3 (90.4 to 94.4) | 92.9 (90.8 to 95.2) | 0.7 (−0.1 to 1.4) (P = 0.084) |
| Difference | −14.3 (−17.4 to −11.3) (P < 0.001) | −12.8 (−15.9 to −9.8) (P < 0.001) | 1.5 (0.2 to 2.8) (P = 0.023) |
| Pediatric | |||
| Severe (n = 111) | 103.4 (100.0 to 106.6) | 103.8 (100.9 to 106.5) | 0.4 (−2.0 to 2.6) (P = 0.8) |
| Nonsevere (n = 77) | 105.6 (102.6 to 108.5) | 106.2 (103.2 to 109.3) | 0.6 (−1.5 to 2.6) (P = 0.6) |
| Difference | −2.2 (−6.7 to 2.2) (P = 0.3) | −2.5 (−6.5 to 1.5) (P = 0.2) | −0.3 (−3.4 to 3.0) (P = 0.9) |
| FeNO, ppb | |||
| Adult | |||
| Severe (n = 309) | 21.5 (19.7 to 23.3) | 19.0 (17.7 to 20.5) | −2.4 (−3.8 to −1.1) (P < 0.001) |
| Nonsevere (n = 213) | 25.6 (23.2 to 28.2) | 19.5 (17.7 to 21.4) | −6.1 (−7.8 to −4.4) (P < 0.001) |
| Difference | −4.1 (−7.3 to −1.1) (P = 0.010) | −0.4 (−2.7 to 1.8) (P = 0.7) | 3.6 (1.5 to 5.8) (P < 0.001) |
| Pediatric | |||
| Severe (n = 109) | 23.7 (20.0 to 27.9) | 17.5 (14.6 to 20.8) | −6.2 (−8.9 to −3.8) (P < 0.001) |
| Nonsevere (n = 75) | 26.2 (21.3 to 31.8) | 15.9 (13.3 to 19.0) | −10.3 (−14.4 to −6.6) (P < 0.001) |
| Difference | −2.5 (−9.2 to 4.2) (P = 0.5) | 1.6 (−2.5 to 5.7) (P = 0.5) | 4.1 (−0.4 to 8.8) (P = 0.086) |
| Sputum eosinophils, %* | |||
| Adult | |||
| Severe (n = 240) | 1.0 (0.8 to 1.2) | 0.6 (0.5 to 0.7) | −0.4 (−0.6 to −0.2) (P < 0.001) |
| Nonsevere (n = 166) | 0.7 (0.5 to 0.9) | 0.5 (0.4 to 0.6) | −0.2 (−0.4 to −0.1) (P = 0.015) |
| Difference | 0.3 (0.0 to 0.6) (P = 0.037) | 0.1 (−0.0 to 0.3) (P = 0.2) | −0.2 (−0.5 to 0.1) (P = 0.1) |
| Blood eosinophils, cells/μl* | |||
| Adult | |||
| Severe (n = 159) | 216 (180 to 255) | 146 (122 to 170) | −70 (−102 to −40) (P < 0.001) |
| Nonsevere (n = 119) | 179 (153 to 207) | 131 (114 to 149) | −48 (−71 to −26) (P < 0.001) |
| Difference | 37 (−7 to 81) (P = 0.1) | 14 (−15 to 45) (P = 0.4) | −22 (−61 to 15) (P = 0.2) |
| Pediatric | |||
| Severe (n = 43) | 261 (201 to 332) | 195 (138 to 262) | −66 (−110 to −26) (P = 0.002) |
| Nonsevere (n = 27) | 321 (224 to 444) | 190 (130 to 270) | −131 (-201 to -74) (P < 0.001) |
| Difference | −60 (−194 to 65) (P = 0.4) | 5 (−91 to 96) (P = 0.9) | 65 (−9 to 145) (P = 0.091) |
| ACQ-6 | |||
| Adult | |||
| Severe (n = 308) | 1.9 (1.7 to 2.0) | 1.7 (1.6 to 1.8) | −0.2 (−0.3 to −0.1) (P < 0.001) |
| Nonsevere (n = 213) | 1.1 (1.0 to 1.3) | 0.9 (0.8 to 1.0) | −0.2 (−0.3 to −0.2) (P < 0.001) |
| Difference | 0.7 (0.5 to 0.9) (P < 0.001) | 0.8 (0.6 to 1.0) (P < 0.001) | 0.1 (−0.1 to 0.2) (P = 0.3) |
| Pediatric | |||
| Severe (n = 111) | 1.3 (1.1 to 1.5) | 1.1 (0.9 to 1.2) | −0.3 (−0.4 to −0.1) (P < 0.001) |
| Nonsevere (n = 77) | 0.9 (0.7 to 1.1) | 0.8 (0.6 to 1.0) | −0.1 (−0.2 to 0.1) (P = 0.4) |
| Difference | 0.4 (0.2 to 0.7) (P = 0.001) | 0.2 (−0.0 to 0.5) (P = 0.059) | −0.2 (−0.4 to 0.0) (P = 0.1) |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; BD = bronchodilator; CI = confidence interval; FeNO = fractional exhaled nitric oxide.
Data are shown as mean (95% CI).
Sputum in adults only with usable data and blood eosinophils in a subset of participants were available.
Change in Markers after TA in Adults
Baseline distinctions before TA between SA and NONSA
Before TA, adults with SA were distinguished from NONSA by significantly lower pre-BD FEV1% predicted (difference, −14.3%; −17.3 to −11.3; P < 0.001), significantly lower post-BD FEV1% predicted, lower FeNO, higher sputum eosinophils, and higher ACQ6 (Table 2, column 1).
Post TA responses
Post-BD FEV1% predicted improved in adult SA after TA (2.2% predicted; 1.1–3.3; P < 0.001), but not in NONSA (0.7% predicted; −0.1 to 1.4; P = 0.08). Sputum eosinophil counts declined after TA in both SA and NONSA groups, and blood eosinophils declined by 30% in both SA and NONSA after TA. FeNO also decreased significantly after TA in SA and NONSA groups (SA, −2.4 ppb; −3.8 to −1.1; P < 0.001) (Figures 2A, 2B, 2D, and 2F; Table 2, column 3).
ACQ scores improved (declined) after TA in both groups (SA, −0.2; −0.3 to −0.1; P < 0.001) (NONSA, −0.2; −0.3 to −0.2; P < 0.001) (Figure 2C; Table 2, column 3). Although the average improvement did not exceed the threshold for the minimally important difference of 0.5, 32% of individuals achieved or exceeded that threshold change.
Differences between SA and NONSA Adults after TA
FeNO and sputum eosinophils in adults were significantly different between SA and NONSA at baseline but not after TA. When adults with SA and NONSA were compared after TA, pre- and post-BD FEV1% predicted remained significantly lower, and ACQ6 remained significantly higher in the adult SA group as compared with NONSA (Table 2, column 2; see Figure E2).
In children, blood eosinophils and FeNO declined significantly in SA and NONSA after TA. At baseline, children with SA were distinguished from NONSA only by lower pre-BD FEV1% predicted and higher ACQ6 (Table 2, column 1). There was no significant increase in pre-BD FEV1% predicted in children after TA. ACQ6 decreased (improved) significantly after TA only in children with SA (SA, −0.3; −0.4 to −0.1; P < 0.001) (Figures 2A–2E; Table 2, column 3).
Differences between SA and NONSA in Children after TA
ACQ6 was higher in children with SA as compared with NONSA at baseline, but this difference was eliminated after TA. Children with SA also had improvements in pre-BD FEV1% predicted after TA, although they were still significantly lower than NONSA. Values of post-BD FEV1% predicted, FeNO, and blood eosinophils were similar among children with SA and NONSA at baseline, and remained similar among the groups after TA (Table 2, column 2; see Figures E2A–E2E).
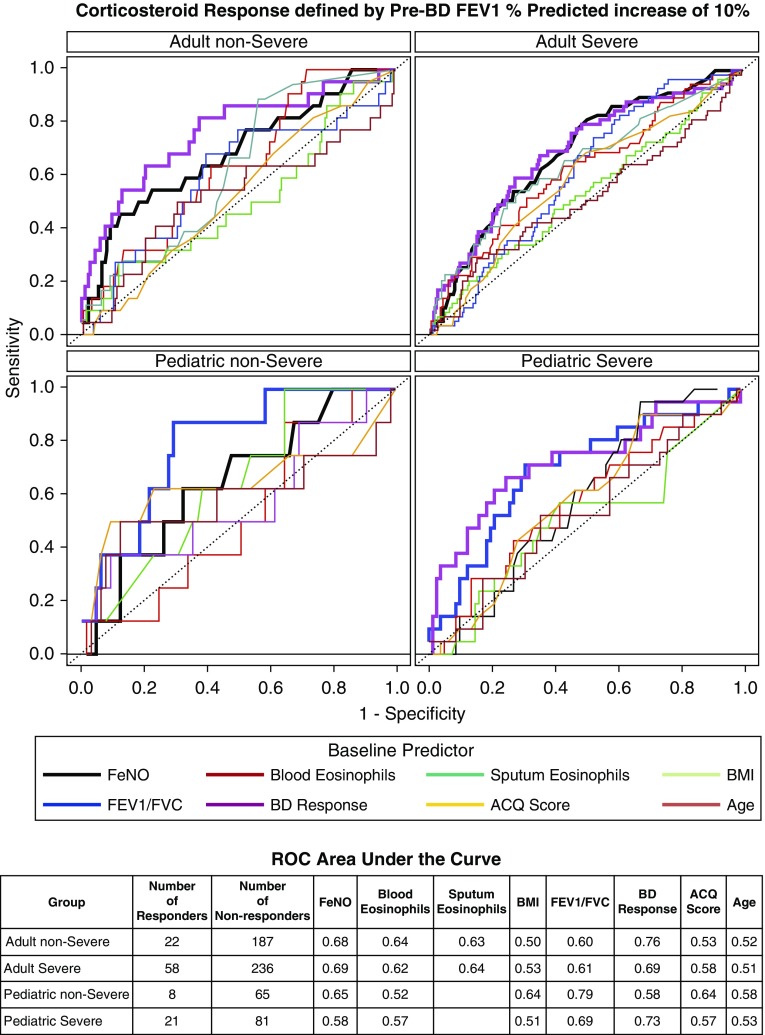
Predictors of Asthma Response to Corticosteroid
Twenty percent of the adult SA and 21% of the pediatric SA had an increase in FEV1 of greater than or equal to 10% predicted after parenteral administration of corticosteroids. We investigated if the baseline BD response, FeNO, blood eosinophils, sputum eosinophils (adult only), ACQ6, BMI, or age might identify individuals who would respond using receiver operating characteristic curve analyses (Figure 3).
Figure 3.
The receiver operating characteristic curve to depict sensitivity and specificity for a corticosteroid response (defined as absolute ∆ prebronchodilator FEV1% predicted of at least 10%) for each of the baseline predictors including Asthma Control Questionnaire-6, age, bronchodilator response, body mass index, blood eosinophils, and fractional exhaled nitric oxide. ACQ = Asthma Control Questionnaire; BD = bronchodilator; BMI = body mass index; FeNO = fractional exhaled nitric oxide; ROC = receiver operating characteristic.
BD response at baseline (defined as absolute ∆ in FEV1% predicted between pre– and post–BD administration spirometry [11, 12]) provided the best sensitivity/specificity in predicting a 10% FEV1 response to TA in all groups except pediatric NONSA (area under the curve [AUC], 0.76 NONSA adults; 0.69, SA adults; 0.58, NONSA children; 0.73, SA children). Baseline FeNO was also a good predictor of corticosteroid response, particularly in adult SA (AUC, 0.68) (see Table E2 for sensitivity/specificity and cutpoint values).
Notably, blood eosinophils performed similarly to sputum but both were inferior to FeNO in predicting response in adults. BMI, age, and ACQ6 were poor predictors of the FEV1 response in any of the severity/age groups (AUC ranged from 0.5 to 0.6) (Figure 3). Sensitivity analyses plotting the AUCs for each predictor at levels of change in pre-BD FEV1% continuously ranging from 5% to 15% suggested that 10% change in outcome gave the best inflection point for sensitivity and specificity for most of the predictors in the adult groups (see Figure E3).
Post Hoc Subgroup Analyses
Adults with higher FeNO at baseline and those with higher sputum eosinophils at baseline had a greater TA lung function response. Among adults with SA, corticosteroid response was three times more likely for those with FeNO greater than or equal to 20 ppb compared with those with FeNO less than 20 ppb and twice as likely for those with greater than or equal to 2% sputum eosinophils compared with those with less than 2% sputum eosinophils at baseline. There were no differences in TA response by race (see Table E1).
Discussion
SA is generally defined by poor control of symptoms and airflow obstruction even with the use of high doses of inhaled corticosteroid (1, 2). Although symptoms and airflow obstruction may persist because of unique pathobiologic mechanisms of asthma severity, poor adherence or suboptimal delivery of inhaled corticosteroid therapy may also underlie the poor control in patients with SA. In our well-characterized cohort of adult and children with asthma, we determined if phenotypic differences among SA and NONSA persist after uniformly administered systemic TA, and identified predictors of corticosteroid response. We found that children and adults differ in the pattern of their response to systemic corticosteroids. We identified that FeNO and sputum eosinophils in adults and ACQ6 in children were significantly different between SA and NONSA at baseline but not after intramuscular TA. Furthermore, post-BD FEV1% predicted improved in adult SA after TA, but not in NONSA. In children, blood eosinophils and FeNO declined significantly in SA and NONSA after TA. Overall, these findings indicate that SA is not simply related to poor use of or adherence to medications.
In adults, airflow obstruction differences and higher symptoms scores persisted between SA and NONSA even when all patients receive an “equalizing” dose of parenteral corticosteroids, suggesting that these features of SA in adults are not simply caused by differential exposure to corticosteroids. In contrast to adults, in children, corticosteroids reduced symptom differences between SA and NONSA and reduced, to a lesser extent, the difference in pre-BD FEV1. Furthermore, once BDs were administered, airway function in pediatric SA was not distinguishable from NONSA. The difference in persistence of the phenotype after corticosteroids and BD in adults and children is interesting and suggests that as patients move to adulthood their asthma may be a less reversible disease.
However, we found that about 20% of children or adults with SA (who are already on high-dose inhaled or oral corticosteroid) experienced a substantial lung function improvement after systemic corticosteroid administration. The ability to predict response of this subgroup of SA would be clinically meaningful. We demonstrate that using baseline BD response and/or FeNO one-third of patients already on intensive therapy and with substantial continued morbidity would have a substantial improvement in their lung function.
Our finding that type 2 inflammation, as indicated by FeNO and sputum eosinophils, was associated with a response to TA in adults with SA is consistent with the work of ten Brinke and coworkers (17) who reported a 12% (as a percent of baseline) improvement in FEV1 after systemic corticosteroids in patients with SA and greater than or equal to 2% sputum eosinophils. These findings were replicated in work by the BioAIR investigators with a 2-week oral corticosteroid intervention (24). These data suggest that persistent type 2 inflammatory processes contribute to ongoing airway obstruction in a proportion of patients with SA. Whether this persistence is related to pathobiologic underpinnings that make these patients more “resistant” to corticosteroids or whether these findings are indicators of poor adherence or incorrect medication use is unclear.
It is of interest that FeNO was as good, or better, for predicting a threshold response in FEV1 than blood or sputum eosinophils in adults with SA. We also found that a higher percentage of those with baseline FeNO greater than 20 ppb were corticosteroid responders as opposed to those with FeNO less than 20 ppb at baseline. This threshold for FeNO is similar to a cutpoint for patients with SA who respond to omalizumab (25) and lebrikizumab (26), therapies that target IgE and eosinophils, respectively, and lends further evidence that elevated FeNO may identify a subset of patients with SA who may benefit from additional systemic therapy targeting type 2 inflammation (27).
Very few studies have prospectively evaluated the response to parenteral corticosteroid therapy in patients with SA already on high-dose inhaled or oral corticosteroid therapy. Children with SA treated with oral or intramuscular corticosteroids found that environmental tobacco exposure was associated with a decreased likelihood of a “complete” response (1) and suggested multiple factors may be considered response in children (18). Adults also have differential inhaled corticosteroid response based on smoking status (28). Our cohort had approximately one in eight individuals exposed to environmental tobacco, but there were no apparent differences in response to triamcinolone among the exposed and nonexposed age and severity groups. A study by Kupczyk and coworkers (24) examined the effect of an oral corticosteroid course in patients with SA and NONSA. Similar to our study they found that nearly a fifth of the patients had a substantial improvement in FEV1. Interestingly, their population of adults with SA, which was less reversible at baseline than the population we studied, had markedly greater sputum (17.9 vs. 0.8%) and blood eosinophils (375 vs. 228). They did not find that the BD response predicted the oral corticosteroid response but rather found that sputum eosinophils were among their best predictors. These differences may reflect the major differences in the underlying populations studied.
Recently, a study of 85 children with SA who received parenteral triamcinolone (29) found approximately 25% had a substantial improvement in FEV1, similar to the proportion in our study. This study noted African American children (n = 16) were less likely to have an improvement in FeNO. We did not identify differences in FeNO response by race.
It is important to acknowledge that the study did not include a placebo intervention. The lack of a placebo does not affect our aim of examining the phenotypic differences among different asthma severity groups when all are treated with an “equalizing” dose of parenteral corticosteroids. Additionally, it does not have a significant effect on our aim to examine whether adults differ from children in their response to corticosteroids. Although it may be argued that it could affect our conclusions regarding phenotypic markers predicting and/or associated with a corticosteroid response, we have taken steps to minimize this possibility. In particular, we set our threshold for an FEV1 corticosteroid response to twice the FEV1% predicted response reported even in those with asthma who are highly reversible (30). Furthermore, lending credence that it is unlikely that the effect we are describing would occur from a placebo is the fact that studies in SA as opposed to milder asthma suggest that FEV1 is not particularly responsive to placebo (17).
The strength of our study is to the ability to discern whether lack of control is caused by severity of asthma, or by poor compliance. The administration of parenteral TA alleviates that latter concern, which makes the findings clinically and pathophysiologically informative. However, it is possible that those who improved the most with TA were the individuals with poor adherence at baseline, and/or with improved adherence over the evaluation period during the course of the study. In this context, measures of corticosteroid metabolites before intervention with TA would have been helpful to assess adherence to baseline inhaled or oral corticosteroid use. These measures are unavailable but may have provided some information on suppression of endogenous steroid pathways, primarily partial adherence or nonadherence. The strength of our study is the ability to discern whether lack of control is caused by severity of asthma, or by poor compliance. The administration of parenteral TA alleviates the latter concern, which makes the findings clinically and pathophysiologically informative. However, it is possible (and quite likely) that some of those who improved with TA were the individuals with poor adherence. In this context, measures of corticosteroid metabolites before intervention with TA would have been helpful to assess adherence to baseline inhaled or oral corticosteroid use. These measures are unavailable but may have provided some information on suppression of endogenous steroid pathways reflecting partial adherence or nonadherence.
Conclusions
Our study has several important findings. First, in adults with SA, “equalizing” corticosteroid treatment does not eliminate the distinction between SA and NONSA. In contrast, in children, parenteral corticosteroids lead to loss of most of the distinctions between SA and NONSA, aside from baseline degree of obstruction. The reasons for differences in response across age are unclear. Prolonged reduction in proresolving mediators in adults with SA (31, 32) and/or longer persistence of unopposed type 2 inflammation may result in remodeling and irreversible changes in adults. Recent work identifies separable effects of aging and asthma duration on asthma severity (33). Importantly, this study clearly defines corticosteroid-responsive and nonresponsive SA phenotypes, which will be useful to guide individualized therapy. Up to 20% of patients with SA have a substantial improvement in airway function after parenteral corticosteroids, which can be predicted by the baseline degree of reversibility of airway obstruction and levels of FeNO. High levels of FeNO, an indicator of type 2 inflammation, especially in adults, are associated with the corticosteroid responsive SA phenotype. Longitudinal lifespan studies are necessary to understand progression of SA and to guide approaches to alter the disease course in children and adults.
Acknowledgments
Acknowledgment
The authors thank the study participants, the Severe Asthma Research Program III clinical research coordinators, and the data coordinating center. Spirometers were provided for Severe Asthma Research Program by nSpire Health, Inc. (Longmont, CO).
Footnotes
This study was conducted with the support of grants that were awarded by the NHLBI to the Severe Asthma Research Program Principal Investigators (PIs), Clinical Centers, and Data Coordinating Center as follows: Wake Forest University (E.R.B. and D.A.M.) and Emory University (U10 HL109164, A.M.F. subaward PI); Washington University (U10 HL109257, M.C.); University of California San Francisco (U10 HL109146, J.V.F.); Case Western Reserve University (U10 HL109250, B.M.G.); Cleveland Clinic (U10 HL109250, S.C.E. Co-PI, Virginia-Cleveland Consortium); University of Virginia (U10 HL109250, W.G.T. Co-PI, Virginia-Cleveland Consortium); Brigham and Women’s Hospital, Harvard Medical School (E.I. and B.D.L.) and Boston Children’s Hospital, Harvard Medical School (U10 HL109172, W.P. subaward PI); University of Wisconsin (U10 HL109168, N.N.J.); University of Pittsburgh (U10 HL109152, S.E.W.); and Penn State University (U10 HL109086, Data Coordinating Center, D.T.M.). In addition, this program is supported through the following National Institutes of Health National Center for Advancing Translational Sciences awards: UL1 TR001420 to Wake Forest University, UL1 TR000427 to the University of Wisconsin, UL1 TR001102 to Harvard University, UL1 TR000454 to Emory University, K24 AI 106822, and K23 AI106945.
Author Contributions: Conception and design, W.P., D.T.M., F.H., L.B.B., W.C.M., E.R.B., D.A.M., M.C., A.M.F., B.M.G., N.N.J., B.D.L., S.P.P., W.G.T., S.E.W., S.C.E., and E.I. Data collection, W.G.T., J.V.F., S.E.W., A.M.F., W.C.M., A.T.H., E.R.B., D.A.M., S.P.P., M.C., L.B.B., N.P.L., R.L.S., B.M.G., S.C.E., E.I., B.D.L., W.P., J.M.G., F.H., M.F., and N.N.J. Data analysis, W.P., D.T.M., R.L.S., F.H., P.G.W., N.P.L., L.B.B., N.R.B., W.C.M., S.C.E., and E.I. Manuscript writing committee, W.P., D.T.M., R.L.S., F.H., P.G.W., N.P.L., L.B.B., N.R.B., W.C.M., S.C.E., and E.I. Manuscript review and editing, W.P., D.T.M., W.G.T., J.V.F., S.E.W., A.M.F., W.C.M., A.T.H., E.R.B., D.A.M., S.P.P., M.C., L.B.B., N.P.L., R.L.S., B.M.G., B.D.L., J.M.G., F.H., M.F., N.N.J., S.C.E., and E.I.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
This is a corrected version of this article; it was posted online on March 30, 2018.
Originally Published in Press as DOI: 10.1164/rccm.201607-1453OC on December 14, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bossley CJ, Saglani S, Kavanagh C, Payne DN, Wilson N, Tsartsali L, Rosenthal M, Balfour-Lynn IM, Nicholson AG, Bush A. Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma. 2009;34:1052–1059. doi: 10.1183/09031936.00186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 3.European Network for Understanding Mechanisms of Severe Asthma. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. 2003;22:470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 4.Chan MT, Leung DY, Szefler SJ, Spahn JD. Difficult-to-control asthma: clinical characteristics of steroid-insensitive asthma. 1998;101:594–601. doi: 10.1016/S0091-6749(98)70165-4. [DOI] [PubMed] [Google Scholar]
- 5.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. National Heart, Lung, Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, et al. NHLBI Severe Asthma Research Program (SARP) Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. 2012;185:356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. 2010;36:1410–1416. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 9.Brusasco V, Crapo R, Viegi G. ATS/ERS task force: standardisation of lung function testing. 2005;26:153–161. [Google Scholar]
- 10.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand PL, Quanjer PH, Postma DS, Kerstjens HA, Koëter GH, Dekhuijzen PN, Sluiter HJ The Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group. Interpretation of bronchodilator response in patients with obstructive airways disease. 1992;47:429–436. doi: 10.1136/thx.47.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward H, Cooper BG, Miller MR. Improved criterion for assessing lung function reversibility. 2015;148:877–886. doi: 10.1378/chest.14-2413. [DOI] [PubMed] [Google Scholar]
- 13.Popa V. ATS guidelines for methacholine and exercise challenge testing. 2001;163:292–293. doi: 10.1164/ajrccm.163.1.16310b. [DOI] [PubMed] [Google Scholar]
- 14.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djukanović R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. 2002;37:1s–2s. doi: 10.1183/09031936.02.00000102. [DOI] [PubMed] [Google Scholar]
- 16.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. 2010;125:1028–1036. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. “Refractory” eosinophilic airway inflammation in severe asthma: effect of parenteral corticosteroids. 2004;170:601–605. doi: 10.1164/rccm.200404-440OC. [DOI] [PubMed] [Google Scholar]
- 18.Bossley CJ, Fleming L, Ullmann N, Gupta A, Adams A, Nagakumar P, Bush A, Saglani S. Assessment of corticosteroid response in pediatric patients with severe asthma by using a multidomain approach. 2016;138:413–420. doi: 10.1016/j.jaci.2015.12.1347. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 20.Pellegrino R, Brusasco V. Point: Is an increase in FEV1 and/or FVC ≥ 12% of control and ≥ 200 mL the best way to assess positive bronchodilator response? Yes. 2014;146:536–537. doi: 10.1378/chest.14-0810. [DOI] [PubMed] [Google Scholar]
- 21.Goleva E, Hauk PJ, Boguniewicz J, Martin RJ, Leung DY. Airway remodeling and lack of bronchodilator response in steroid-resistant asthma. 2007;120:1065–1072. doi: 10.1016/j.jaci.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meltzer EO, Busse WW, Wenzel SE, Belozeroff V, Weng HH, Feng J, Chon Y, Chiou CF, Globe D, Lin SL. Use of the Asthma Control Questionnaire to predict future risk of asthma exacerbation. 2011;127:167–172. doi: 10.1016/j.jaci.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 23.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 24.Kupczyk M, Haque S, Middelveld RJ, Dahlén B, Dahlén SE, Investigators B BIOAIR Investigators. Phenotypic predictors of response to oral glucocorticosteroids in severe asthma. 2013;107:1521–1530. doi: 10.1016/j.rmed.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, Lal P, Arron JR, Harris JM, Busse W. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. 2013;187:804–811. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 26.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 27.Brightling CE, Nordenmark LH, Jain M, Piper E, She D, Braddock M, Colice G, Tornling G. Effect of anti-IL-13 treatment on airway dimensions in severe asthma. 2016;194:118–120. doi: 10.1164/rccm.201511-2224LE. [DOI] [PubMed] [Google Scholar]
- 28.Roche N, Postma DS, Colice G, Burden A, Guilbert TW, Israel E, Martin RJ, van Aalderen WM, Grigg J, Hillyer EV, et al. Differential effects of inhaled corticosteroids in smokers/ex-smokers and nonsmokers with asthma. 2015;191:960–964. doi: 10.1164/rccm.201411-2116LE. [DOI] [PubMed] [Google Scholar]
- 29.Koo S, Gupta A, Fainardi V, Bossley C, Bush A, Saglani S, Fleming L. Ethnic variation in response to intramuscular triamcinolone in children with severe therapy resistant asthma. 2016;149:98–105. doi: 10.1378/chest.14-3241. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler ME, Kelley JM, Boyd IO, Dutile S, Marigowda G, Kirsch I, Israel E, Kaptchuk TJ. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. 2011;365:119–126. doi: 10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu SH, Yin PL, Zhang YM, Tao HX. Reversed changes of lipoxin A4 and leukotrienes in children with asthma in different severity degree. 2010;45:333–340. doi: 10.1002/ppul.21186. [DOI] [PubMed] [Google Scholar]
- 32.Kazani S, Planaguma A, Ono E, Bonini M, Zahid M, Marigowda G, Wechsler ME, Levy BD, Israel E. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. 2013;132:547–553. doi: 10.1016/j.jaci.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zein JG, Dweik RA, Comhair SA, Bleecker ER, Moore WC, Peters SP, Busse WW, Jarjour NN, Calhoun WJ, Castro M, et al. Severe Asthma Research Program. Asthma is more severe in older adults. 2015;10:e0133490. doi: 10.1371/journal.pone.0133490. [DOI] [PMC free article] [PubMed] [Google Scholar]