Abstract
Lipopolysaccharide (LPS) and Braun (murein) lipoprotein (Lpp) are major components of the outer membrane of gram-negative enteric bacteria that function as potent stimulators of inflammatory and immune responses. In a previous paper, we provided evidence that two functional copies of the lipoprotein gene (lppA and lppB) located on the chromosome of Salmonella enterica serovar Typhimurium contributed to bacterial virulence. In this study, we characterized lppA and lppB single-knockout (SKO) mutants and compared them with an lpp double-knockout (DKO) mutant using in vitro and in vivo models. Compared to the lpp DKO mutant, which was nonmotile, the motility of the lpp SKO mutants was significantly increased (73 to 77%), although the level of motility did not reach the level of wild-type (WT) S. enterica serovar Typhimurium. Likewise, the cytotoxicity was also significantly increased when T84 human intestinal epithelial cells and RAW264.7 murine macrophages were infected with the lpp SKO mutants compared to the cytotoxicity when cells were infected with the lpp DKO mutant. The level of interleukin-8 (IL-8) in polarized T84 cells infected with the lppB SKO mutant was significantly higher (two- to threefold higher), reaching the level in cells infected with WT S. enterica serovar Typhimurium, than the level in host cells infected with the lppA SKO mutant. The lpp DKO mutant induced minimal levels of IL-8. Similarly, sera from mice infected with the lppB SKO mutant contained 4.5- to 10-fold-higher levels of tumor necrosis factor-α and IL-6; the levels of these cytokines were 1.7- to 3.0-fold greater in the lppA SKO mutant-infected mice than in animals challenged with the lpp DKO mutant. The increased cytokine levels observed with the lppB SKO mutant in mice correlated with greater tissue damage in the livers and spleens of these mice than in the organs of animals infected with the lppA SKO and lpp DKO mutants. Moreover, the lppB SKO mutant-infected mice had increased susceptibility to death. Since the lpp DKO mutant retained intact LPS, we constructed an S. enterica serovar Typhimurium triple-knockout (TKO) mutant in which the lppA and lppB genes were deleted from an existing msbB mutant (msbB encodes an enzyme required for the acylation of lipid A). Compared to the lpp DKO and msbB SKO mutants, the lpp-msbB TKO mutant was unable to induce cytotoxicity and to produce cytokines and chemokines in vitro and in vivo. These studies provided the first evidence of the relative contributions of Lpp and lipid A acylation to Salmonella pathogenesis.
In gram-negative enteric bacteria, lipopolysaccharide (LPS) and Braun (murein) lipoprotein (Lpp) are the most abundant components of the outer membrane and are important in maintaining the integrity of the bacterial cell envelope (2, 16). The maturation of Lpp requires modification of a lipid moiety, which is catalyzed by glycerol transferase (Lgt), O-acyl transferase, signal peptidase II, and N-acyl transferase (Lnt) (19). LPS, on the other hand, is composed of a polysaccharide core, O antigen, and a lipid A domain. Lipid A contains fatty acids believed to contribute to the low-permeability barrier of the outer membrane of gram-negative bacteria (38). As is the case with Lpp, lipid modification of LPS by the addition of fatty acids is catalyzed by enzymes encoded by the genes msbB (multicopy suppressor of htrB), htrB (high temperature requirement), and pagP (PhoP-activated gene) that attach myristic, lauric, and palmitic acids, respectively, to lipid A (4, 18).
Both Lpp and LPS exhibit toxic and biological responses through their lipid domains by inducing high levels of cytokines, such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-1β (IL-1β), and IL-6, as well as by activating complement and other inflammatory mediators (16, 31, 35, 40, 49). In addition, Lpp synergizes with LPS to induce production of proinflammatory cytokines both in vitro and in vivo, leading to septic shock (56). While LPS activates cellular responses by binding to CD14 receptors and via Toll-like receptor 4 (15, 51, 53), Lpp binds to Toll-like receptor 2 to activate cells (1).
In a previous study, we demonstrated that there were two functional copies of the lpp gene (lppA and lppB, originally designated lpp1 and lpp2, respectively) on the chromosome of S. enterica serovar Typhimurium 14028 (46), which were adjacent and separated by 82 bp (46). Deletion of both copies of the lpp gene resulted in a Salmonella mutant that minimally invaded epithelial cells, was nonmotile, and was severely impaired in the ability to induce cytotoxicity in murine macrophages (RAW264.7) and T84 human intestinal epithelial cells, possibly through reduced production of inflammatory cytokines or chemokines (TNF-α and IL-8) (46). The lpp double-knockout (DKO) mutant was avirulent in mice following oral and intraperitoneal (i.p.) challenge, and mice immunized with this S. enterica serovar Typhimurium mutant were protected from death when they were challenged with a lethal dose of wild-type (WT) S. enterica serovar Typhimurium (46). We also generated lppA and lppB single-knockout (SKO) mutants of S. enterica serovar Typhimurium by marker exchange mutagenesis (46). The SKO strains were avirulent in mice when they were given by the i.p. route up to a dose of 1 × 105 CFU. However, their invasive potential for T84 cells was significantly higher than the invasive potential of the lpp DKO mutant, which was minimally invasive (46).
While the characteristics of the lpp DKO mutant are desirable for a vaccine candidate, the DKO strain still contains intact LPS, which could cause an undesirable inflammatory response in the host. Indeed, studies have shown that mutations in genes involved in lipid A biosynthesis reduce the ability of S. enterica serovar Typhimurium to cause a systemic infection. For example, Jones et al. (23) reported that a deletion in the htrB gene rendered Salmonella less cytotoxic and reduced systemic infections. In Escherichia coli and S. enterica serovar Typhimurium, the msbB gene product is involved in the terminal myristoylation of lipid A of LPS. A mutation in the msbB gene of S. enterica serovar Typhimurium (also known as waaN) impaired the ability of Salmonella to cause lethality in mice and to induce TNF-α, IL-1β, and inducible nitric oxide synthase production (25, 30). In a recent study, Watson et al. (55) demonstrated that a mutation in the S. enterica serovar Typhimurium msbB gene affected type III secretion system-dependent proteins and reduced the virulence of the organism. In this study, we constructed an lppA-lppB-msbB triple-knockout (TKO) mutant of S. enterica serovar Typhimurium. Furthermore, the lppA and lppB SKO and lpp-msbB TKO mutants of S. enterica serovar Typhimurium were functionally characterized by using in vitro and in vivo models. In addition, we investigated the relative contributions of lpp and msbB in S. enterica serovar Typhimurium virulence.
MATERIALS AND METHODS
Cell lines, bacteria, and plasmids.
The RAW264.7 murine macrophage and T84 human intestinal epithelial cell lines were obtained from the American Type Culture Collection (Manassas, Va.). The cells were grown and maintained as recently described (46). T84 polarized cells were established by seeding them in six-well transwell plates containing 0.4-μm-pore-size polycarbonate membranes (Corning, Corning, N.Y.) with 1.6 ml of prewarmed Dulbecco's modified Eagle's medium-F-12 medium (46) in the upper chamber and 2.6 ml of medium in the lower chamber according to the manufacturer's instructions. The cells were incubated for 5 to 7 days (with changes of medium every 3 days) to allow development of tight junctions and steady-state resistance as previously described (27). Polarized cells were infected apically with S. enterica serovar Typhimurium 14028 strains at a multiplicity of infection (MOI) of 10:1. Bacterial strains and plasmids used in this study are listed in Table 1. WT S. enterica serovar Typhimurium, lpp mutants of this organism (SKO and DKO), lpp complemented strains, and E. coli cultures were grown in either Luria-Bertani (LB) or salmonella-shigella (SS) agar plates. The S. enterica serovar Typhimurium YS1 strain (14028 background) with a mutation in the msbB gene (30) and the lpp-msbB TKO mutant were grown on MSB medium, which consisted of LB medium with no NaCl but supplemented with 2 mM MgSO4 and 2 mM CaCl2 along with appropriate antibiotics (36). The msbB SKO mutant was extensively characterized in several previous studies (25, 30, 36). The bacterial strains were grown at 37°C with or without shaking. A spontaneous, nalidixic acid-resistant (Nalr) YS1 strain was generated in our laboratory. The suicide vector pRE112 contained an R6K origin of replication (ori), a levansucrase gene (sacB) from Bacillus subtilis, and a chloramphenicol resistance (Cmr) gene (13). Low-copy-number plasmid pSM21, used for complementation of the msbB gene, was described previously (36).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| S. enterica serovar Typhimurium strains | ||
| 14028 | S. enterica serovar Typhimurium | 46 |
| STM-N | Nalidixic acid-resistant (Nalr) derivative of 14028 | 46 |
| Mutant lpp | Isogenic mutant in which two copies of the lipoprotein gene were deleted; Nalr Knr | 46 |
| Mutant msbB | 14028 in which the msbB gene was truncated; Tcr | 30 |
| lpp-msbB mutant | Mutant in which both copies of the lpp gene and the msbB gene were deleted; Nalr Tcr Knr | This study |
| Mutant lpp/pBR/pp | lpp mutant complemented with two copies of the lpp gene via the pBR322 vector; Nalr Tcr Knr | 46 |
| Mutant msbB/pSM21 | msbB mutant complemented with the msbB gene | 36 |
| Mutant lppA | Isogenic mutant in which only one copy of the lpp gene (lppA) was deleted; Nalr Knr | 46 |
| Mutant lppB | Isogenic mutant in which only one copy of the lpp gene (lppB) was deleted; Nalr Knr | 46 |
| E. coli strains | ||
| S17-1 | Streptomycin and trimethoprim resistance, λpir | Laboratory stock |
| SM10 | Knr, λpir | 13 |
| Plasmids | ||
| pBluelppK | Knr gene cassette was inserted at the XhoI site in plasmid pBluelpp that contained 5′ and 3′ flanking sequences to the lpp genes of S. enterica serovar Typhimurium | 46 |
| pRE112 | Suicide vector with R6K ori, sacB, and Cmr | 13 |
| pSM21msbB | pSM21 low-copy-number plasmid carrying the msbB gene | 36 |
Construction of S. enterica serovar Typhimurium lpp-msbB TKO mutants.
The strategy used for construction of the lpp-msbB TKO mutant was similar to that used for developing lpp SKO and lpp DKO mutants, as recently described (46). Briefly, 5′ and 3′ flanking DNA sequences to the lpp genes were amplified by PCR and cloned into the pBluescript vector to generate a pBluelppK plasmid. In this plasmid, the lpp genes were replaced with the kanamycin resistance (Knr) gene cassette. Subsequently, the pBluelppK recombinant plasmid was digested with the XbaI and KpnI restriction enzymes to isolate a fragment containing the Knr gene cassette flanked by the upstream and downstream flanking sequences to the lpp genes. This fragment was ligated into a pRE112 suicide vector digested with the XbaI and KpnI restriction enzymes and transformed by electroporation into an E. coli SM10 strain which contained λpir, which allowed replication of the suicide vector only in this strain (13). The pRE112lppK plasmid from the E. coli SM10 strain was then delivered to the Nalr S. enterica serovar Typhimurium YS1 strain (msbB deficient) by conjugation as previously described (46). The transconjugants were selected on MSB agar plates containing nalidixic acid (100 μg/ml), kanamycin (50 μg/ml), and 5% sucrose. These transconjugants represented double-crossover homologous recombination that resulted in replacement of the native lpp genes of S. enterica serovar Typhimurium with the Knr gene cassette. Such mutants should have been sensitive to chloramphenicol due to the loss of the suicide vector, pRE112. The identity of the TKO mutant was confirmed by Southern and Western blot analyses.
Southern blot analysis.
Chromosomal DNA from WT S. enterica serovar Typhimurium and the lpp-msbB TKO mutant was isolated by using a QIAamp DNA mini kit (QIAGEN Inc., Valencia, Calif.) and was subjected to Southern blot analysis as previously described (45). The Knr gene cassette, the lpp gene, and the suicide vector pRE112 labeled with [α-32P]dCTP (ICN, Irvine, Calif.) were used as probes. After hybridization, the blots were washed twice with 2× SSC-0.1% sodium dodecyl sulfate (SDS) for 20 min and then with 1× SSC-0.1% SDS at 68°C for 20 min and exposed to X-ray film (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate).
Western blot analysis.
Western blot analysis was performed to evaluate expression of the lpp gene in various S. enterica serovar Typhimurium mutants and the parental WT S. enterica serovar Typhimurium strain. An aliquot (20 to 25 μg) of total bacterial cell proteins was separated by SDS-15% polyacrylamide gel electrophoresis and subjected to Western blot analysis as recently described (46). The primary monoclonal antibodies used were developed to Yersinia enterocolitica Lpp. The secondary antibodies used were anti-mouse antibodies conjugated with horseradish peroxidase. Subsequently, an enhanced chemiluminescence substrate kit (Pierce, Rockford, Ill.) was used according to the manufacturer's instructions to detect signals on the X-ray film.
Complementation of the msbB SKO and lpp DKO mutants of S. enterica serovar Typhimurium.
The msbB gene mutant (YS1) was complemented by electroporation of a pSM21 low-copy-number plasmid (obtained from B. Low, Yale University, New Haven, Conn.) carrying the msbB gene. The complemented strain was used as a control in our study, along with the lpp DKO and msbB SKO mutants. The lpp DKO mutant was complemented by transformation of the pBR322 vector carrying both copies of the lpp gene (Table 1), as described previously (46). A mutant with the vector alone (pBR322 with no insert) served as a negative control.
Integrity of the outer membrane of the S. enterica serovar Typhimurium TKO mutant.
The outer membrane integrity of the msbB SKO, lpp DKO, and lpp-msbB TKO mutants was examined by membrane blebbing by using electron microscopy, sensitivity to detergents (Triton X-100 and SDS) and a hydrophobic antibiotic (rifampin), and the release of β-lactamase; the methods described recently were used (46).
In vitro virulence of S. enterica serovar Typhimurium mutants.
The in vitro virulence potentials of the lpp SKO and DKO, msbB SKO, and lpp-msbB TKO mutants were determined by examining the abilities of the mutants to invade, to induce cell death, and to survive inside the host cells, by examining motility, and by examining the induction of cytokines and chemokines by using RAW264.7 and T84 cells. The methods used for these assays were described recently (7, 46). The levels of cytokines (TNF-α and IL-6) and of a chemokine (IL-8) in the culture supernatants of the cells mentioned above were determined by using either an enzyme-linked immunosorbent assay (ELISA) (46) or a cytometric bead array (CBA) kit (BD Biosciences, San Diego, Calif.).
CBA.
The CBA assay is a multiplex assay, and in this assay a series of particles with discrete fluorescence intensities are combined with flow cytometry to simultaneously detect different cytokines. Both tissue culture supernatants and mouse sera from infected animals were used for measuring cytokines and chemokines with a CBA kit by following the manufacturer's instructions. These assays were performed in the Gastrointestinal Immunology Core of the Texas Digestive Disease Center, University of Texas Medical Branch, Galveston, Tex.
In vivo virulence of S. enterica serovar Typhimurium mutants.
Eight-week-old female Swiss Webster, C57BL/6, and BALB/c mice (Taconic Farms, Oxnard, Calif.) were used to determine the in vivo survival of and inflammatory cytokine and chemokine induction by S. enterica serovar Typhimurium mutants. The Swiss Webster mice were divided into seven groups and inoculated i.p. with the lpp DKO, msbB SKO, and lpp-msbB TKO mutants, complemented derivatives of the lpp DKO and msbB SKO mutants, and WT S. enterica serovar Typhimurium. One group of mice was inoculated with phosphate-buffered saline (PBS) and served as a control. Each group of 20 mice was inoculated with 2 × 103 CFU of an S. enterica serovar Typhimurium mutant or WT S. enterica serovar Typhimurium. Two days prior to inoculation, the mice that were to be infected with the complemented msbB SKO and lpp DKO strains were treated with tetracycline (5 mg/ml in drinking water) and chloramphenicol (0.5 mg/ml), respectively, to maintain the plasmid in these strains.
Five mice from each group were bled for sera and sacrificed at days 1, 3, 7, and 21 postinfection, and their livers and spleens were removed, weighed, and examined to determine bacterial survival. For studies of in vivo survival and replication, aliquots (0.1 g) of livers and spleens obtained on days 1, 3, 7, and 21 postinfection were homogenized in 1 ml of PBS, serially diluted, and plated on either MSB or LB agar plates with appropriate antibiotics or SS agar plates. The total bacterial loads in livers and spleens were determined from the resulting colony counts.
Liver and spleen sections were also fixed in 10% buffered formalin and submitted to the UTMB Histopathology Core Facility for processing and hematoxylin and eosin staining. Histopathological examinations of liver and spleen sections were performed by using four- and five-tier grading systems (Table 2). The levels of inflammatory cytokines and chemokines in the serum (TNF-α, IFN-γ, IL-6, and KC [equivalent of human IL-8]) were assayed.
TABLE 2.
Histopathological grading system for livers and spleens of mice infected with various S. enterica serovar Typhimurium
| Organ | Score | Description |
|---|---|---|
| Liver | 0 | Normal histology |
| + | Occasional portal, lobular, and perivascular histiocytic infiltrates; no evidence of necrosis | |
| ++ | Focal necrosis of hepatocytes with infiltration by macrophages and PMNs; histiocytic infiltrates observed focally and perivascularly | |
| +++ | Diffused histiocytic infiltrates in lobules and perivascular areas; focal necrosis of hepatocytes with PMNs and macrophages infiltrating necrotic areas | |
| ++++ | Diffused histiocytic infiltrates accompanied by areas of necrosis that are more extensive | |
| Spleen | 0 | Normal histology |
| + | Scattered macrophages infiltrating marginal zone; lymphoid follicles slightly increased in size, with preservation of their outlines; scattered immunoblasts and apoptotic lymphocytes observed in the lymphoid follicles | |
| ++ | Moderate architectural disruption of lymphoid follicles by macrophages infiltrating red pulp and marginal zone; moderate expansion of immunoblasts and apoptotic bodies | |
| +++ | Severe disruption of lymphoid follicles with marked infiltration of marginal zone and red pulp by sheets of macrophages; abundant immunoblasts and apoptotic bodies in follicles |
We also challenged BALB/c and C57BL/6 mice either orally (1 × 104 CFU) or by the i.p. route (2 × 103 CFU) with various S. enterica serovar Typhimurium strains. For orally infected mice, a 100-μl suspension of a bacterial culture was administered by using a mouse stomach tube (Sigma, St. Louis, Mo.). On days 1, 3, and 7 postinoculation, mice were bled and sera were obtained for measuring cytokine and chemokine levels. Also, livers and spleens were aseptically removed for determining bacterial loads and intracellular survival, as well as for assessing histopathology.
Statistical analysis.
Data were analyzed by using Student's t test, and P values of ≤0.05 were considered statistically significant. A minimum of three independent experiments were performed in triplicate unless otherwise indicated, and the data were expressed as arithmetic means ± standard deviations. For experiments for which no standard deviations are shown, typical experimental data obtained from three or four independent experiments are presented below.
RESULTS
In vitro characterization of lppA and lppB SKO mutants of S. enterica serovar Typhimurium.
Two copies of the lpp gene (lppA and lppB) that were located on the chromosome of S. enterica serovar Typhimurium were deleted to evaluate their role in bacterial virulence. The lpp DKO mutant was highly attenuated when it was tested in both in vitro and in vivo models (46). Subsequently, we generated S. enterica serovar Typhimurium lppA and lppB SKO mutants by marker exchange mutagenesis using a suicide vector and the lambda red system (46) to delineate the contribution of each of the lpp genes to bacterial virulence. Characterization of these lpp SKO mutants indicated that both lpp genes were functional and that they contributed equally when the mutants were tested for their invasive potential in T84 cells and for lethality in mice up to a bacterial dose of 1 × 105 CFU given by the i.p. route (46).
In this study, we also examined the contributions of LppA and LppB to Salmonella motility and the abilities of these proteins to induce cell death and to evoke cytokine and chemokine production by using RAW264.7 and T84 cells. The lpp DKO mutant was nonmotile. The motilities of the lpp SKO mutants (with either the lppA or lppB gene deleted) were significantly increased, as measured by the spreading of bacteria from the point of inoculation on LB or MSB medium plates containing 0.35% agar (46). The diameter of bacterial spreading for the LppA mutant was 37.3 ± 2.5 mm, and that for the LppB mutant was 39.3 ± 1.2 mm, indicating that the two lpp SKO mutants behaved similarly. The diameter of migration for WT S. enterica serovar Typhimurium from the point of inoculation was 52 ± 2.0 mm (data not shown). The reduced motilities of the lpp SKO mutants (23 to 27%) were still statistically significant compared to the mobility of WT S. enterica serovar Typhimurium.
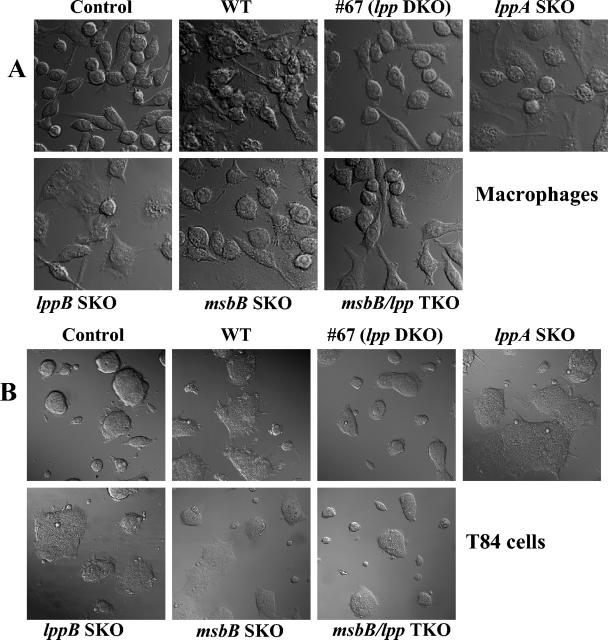
Laser scanning confocal microscopy was used to examine the death of RAW264.7 and T84 cells infected with the lppA and lppB SKO and lpp DKO mutants compared to the death of cells infected with WT S. enterica serovar Typhimurium (Fig. 1). Although the lpp DKO mutant (mutant #67) was severely impaired for inducing cell death (75% viability) in RAW264.7 macrophages (Fig. 1A) and T84 cells (Fig. 1B) compared to cells treated with WT S. enterica serovar Typhimurium (close to 100% cell death), the lpp SKO mutants (lppA and lppB mutants) produced more cell death (70%). Complementation of the lpp DKO mutant with both copies of the lpp gene resulted in the WT phenotype (data not shown). As discussed below, the cell death (around 25 to 30%) associated with lpp DKO mutant #67 was attributed to the presence of intact LPS.
FIG. 1.
Cell death induced by WT S. enterica serovar Typhimurium and S. enterica serovar Typhimurium mutants in RAW264.7 (A) and T84 (B) cells. Cells were infected with various S. enterica serovar Typhimurium mutants for 1 h at an MOI of 10:1. After incubation, the cells were washed with PBS and incubated in a gentamicin (100 μg/ml)-containing medium for 1 h. After washing, the cells were incubated in an antibiotic-free medium for 24 h. The cells were gently washed in PBS and examined with a laser scanning confocal microscope. Note the normal cell morphology of noninfected cells (control) and of cells infected with the lpp DKO mutant (#67) (75% viability) and the lpp-msbB TKO mutant (mostly normal) compared to the cell morphology of dead cells after infection with the WT and the lppA and lppB SKO mutants. The msbB SKO mutant-infected host cells showed around 30% destruction. At least 20 microscopic fields were examined.
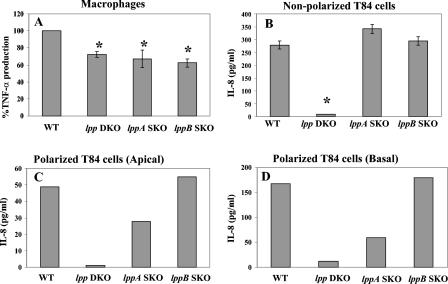
We infected RAW264.7 cells with heat-killed (65°C for 30 min) WT S. enterica serovar Typhimurium and the various mutant strains at an MOI of 0.1:1 as previously described (46). Addition of the heat-killed bacterial cells led to decreased cytokine production in macrophages. A 28 to 30% statistically significant decrease in TNF-α production (compared to the WT) was observed in macrophages infected with the lpp SKO and lpp DKO mutants (Fig. 2A). However, the differences in TNF-α production between the lpp SKO and lpp DKO mutants were not significant. In contrast, T84 cells (nonpolarized) infected with the lppA and lppB SKO mutants induced IL-8 levels similar to the level induced by WT S. enterica serovar Typhimurium. The lpp DKO mutant did not induce a detectable level of this chemokine (Fig. 2B), suggesting that Lpp plays a key role in IL-8 production in vitro.
FIG. 2.
Induction of TNF-α production in RAW264.7 murine macrophages (A) and induction of IL-8 production in T84 nonpolarized cells (B) and polarized T84 cells in the apical (C) and basal chambers (D) infected with WT S. enterica serovar Typhimurium and with lpp SKO and lpp DKO mutants as described in Materials and Methods. Cells were infected with various S. enterica serovar Typhimurium strains at an MOI of 10:1 and incubated for 1 h. After incubation, the cells were washed and incubated in gentamicin (100 μg/ml)-containing medium for 1 h, after which the cells were washed and incubated in an antibiotic-free medium for 12 h. IL-8 levels were determined by using a CBA and ELISA. The data in panels A and B are averages for four or five independent experiments performed in duplicate. The asterisks indicate statistically significant differences (P ≤ 0.05, as determined by Student's t test) between WT S. enterica serovar Typhimurium and the lpp mutants. The data in panels C and D are data from a representative experiment; a minimum of three independent experiments were performed.
To confirm these data, we infected (apically) polarized T84 cells with the WT S. enterica serovar Typhimurium and Lpp mutant strains and measured IL-8 production in the culture media in both the upper (apical) and lower (basal) chambers. As shown in Fig. 2C and D, the lpp DKO induced minimal levels of IL-8 production in the apical and basal chambers (1 and 12 pg/ml, respectively). Overall, more IL-8 was detected in the basal chamber (168 pg/ml) than in the apical chamber (49 pg/ml) in WT S. enterica serovar Typhimurium-infected T84 cells (Fig. 2C and D). A similar IL-8 profile was found in the apical (Fig. 2C) and basal (Fig. 2D) chambers for T84 cells infected with the lppA and lppB SKO mutants. Importantly, the amounts of IL-8 detected in the apical chamber with the lppA and lppB SKO mutants were 28 and 55 pg/ml, while the levels in the basal chamber were 59 and 180 pg/ml with these mutants, respectively (Fig. 2C and D). Furthermore, the levels of IL-8 detected in the apical and basal chambers with the lppB SKO mutant-infected T84 cells were similar to the levels found with WT S. enterica serovar Typhimurium. The levels of IL-8 were significantly lower (two to threefold lower) in both the apical and basal chambers with the lppA SKO mutant than with the lppB SKO mutant (Fig. 2C and D).
In vivo survival of lppA and lppB SKO and lpp DKO mutants of S. enterica serovar Typhimurium.
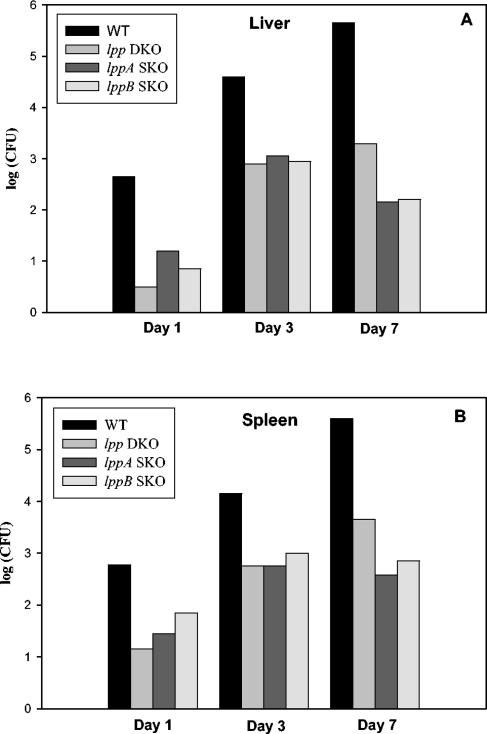
We examined the contributions of the lppA and lppB genes during systemic infection in mice. In these sets of experiments, we inoculated mice with S. enterica serovar Typhimurium mutants (lpp SKO and lpp DKO mutants) via the i.p. and oral routes. We used both outbred (Swiss Webster) and inbred (C57BL/6 and BALB/c) mice for all in vivo studies and obtained essentially similar results. As shown in Fig. 3, mice infected via the i.p. route with the lpp SKO and lpp DKO mutants were able to survive, and the mutants multiplied in the livers and spleens as they did following WT S. enterica serovar Typhimurium infection. The level of WT S. enterica serovar Typhimurium increased from 2.5 logs to 5.5 logs over a 7-day period in the livers and spleens.
FIG. 3.
In vivo survival and replication of lpp DKO and lppA and lppB SKO mutants of S. enterica serovar Typhimurium in livers (A) and spleens (B) of mice. Portions of liver and spleen harvested at days 1, 3, 7, and 21 postinoculation were homogenized in PBS, serially diluted, and plated on LB and SS agar plates, and the numbers of CFU per organ were determined. The animals were infected by the i.p. route with various S. enterica serovar Typhimurium strains by using 2 × 103 CFU in 100 μl. Three independent experiments were performed. Data from a representative experiment are shown.
For the lpp DKO mutant, the number of bacteria increased from 0.5 log to 3.0 logs in the livers and from 1.0 log to 3.5 logs in the spleens (Fig. 3). A similar pattern was obtained for the lpp SKO mutants, although the numbers of the lpp SKO mutants both in the liver and in the spleen on day 1 postinfection were little higher than the numbers of the lpp DKO mutant. Clearly, more WT bacteria (2.5 logs) than lpp SKO and DKO mutant bacteria (0.5 to 1.5 logs) invaded the liver and spleen, as indicated by the number of bacteria after 1 day of infection, indicating that there was attenuation in invasion of the S. enterica serovar Typhimurium mutants (Fig. 3). The bacterial populations were cleared from the livers and spleens of mice infected with the lpp mutants and WT S. enterica serovar Typhimurium in 3 weeks (data not shown).
The oral route of Salmonella infection is a natural infection route that leads to gastroenteritis. Therefore, C57BL/6 and BALB/c mice were also infected orally with 1 × 104 CFU of the lppA SKO, lppB SKO, and lppA-lppB DKO mutants and WT S. enterica serovar Typhimurium. It was found that mice infected with WT S. enterica serovar Typhimurium exhibited 60% mortality in 7 days, while 100% of the mice infected with either the lpp SKO or lpp DKO mutant survived for the 21-day test period. When the bacterial loads were determined in the livers and spleens of animals infected with various S. enterica serovar Typhimurium cultures (at days 1, 3, and 7), only WT S. enterica serovar Typhimurium was detected in these organs (data not shown).
In vivo cytokine production by lppA and lppB SKO and lpp DKO mutants of S. enterica serovar Typhimurium.
During systemic Salmonella infection, cytokines and chemokines, such as TNF-α, IL-6, IFN-γ, and IL-8, play a critical role in regulating immune and inflammatory responses (12, 21). Therefore, production of these mediators was assayed on days 1, 3, and 7 postinoculation in the sera of C57BL/6 and BALB/c mice infected by the i.p. and oral routes with the lpp DKO and lppA and lppB SKO mutants and with WT S. enterica serovar Typhimurium.
In the sera of mice infected via the i.p. route with WT S. enterica serovar Typhimurium, an increase in the production of TNF-α was observed at day 3, and the level reached 945 pg/ml by day 7 postinoculation (Fig. 4A). There was a significant reduction (97.5%) in the level of TNF-α in the sera of mice infected with the lpp DKO mutant on day 7 postinfection compared to the level in mice infected with WT S. enterica serovar Typhimurium. Interestingly, the sera from mice infected with the lppB SKO mutant (with an intact lppA gene) reproducibly contained 4.5- to 5-fold-higher levels of TNF-α than the sera from mice infected with the lpp DKO mutant. Likewise, 1.7 to 2 times more TNF-α was detected in the sera of mice infected with the lppA SKO mutant (with an intact lppB gene) than in the sera of lpp DKO mutant-infected mice (Fig. 4A).
FIG. 4.
Induction of TNF-α (A), IL-6 (B), and KC (C) in mice infected with S. enterica serovar Typhimurium strains at a dose of 2 × 103 CFU by the i.p. route. Sera from mice infected with the lppA and lppB SKO and lpp DKO mutants were collected at days 1, 3, and 7 postinfection, and the levels of TNF-α, IL-6, KC, and IFN-γ were determined by ELISA and CBA as described in Materials and Methods. The data in panels A and B are data from a typical experiment; three independent experiments were performed. The data in panel C are arithmetic means ± standard deviations based on three independent experiments performed in duplicate. The asterisks indicate statistically significant differences (P ≤ 0.05, as determined by Student's t test) between the groups indicated by the horizontal lines.
Similarly, the sera of mice infected with WT S. enterica serovar Typhimurium contained a high level of IL-6 at day 3, which increased to 846 pg/ml at day 7 postinfection. Deletion of either both or single copies of the lpp gene dramatically reduced IL-6 production on day 7 postinfection (Fig. 4B). Once again, the sera from mice infected with the lppB SKO mutant reproducibly had 10- to 12-fold-higher IL-6 levels than the sera from animals infected with the lpp DKO mutant. The IL-6 levels were three- to fivefold higher in the sera of mice infected with the lppA SKO mutant than in the sera of lpp DKO mutant-infected mice (Fig. 4B). The levels of IFN-γ peaked on day 3 postinfection (1,597 pg/ml) and declined to 190 pg/ml on day 7 in mice infected with WT S. enterica serovar Typhimurium. As observed for the TNF-α and IL-6 levels, in lppB mutant-infected mice the IFN-γ levels were 1.5- to 2.5-fold higher than the levels in mice infected with the lppA SKO mutant on day 3 postinfection. The IFN-γ levels declined on day 7 after infection with lpp mutants, as noted with WT S. enterica serovar Typhimurium (data not shown). The IFN-γ levels in the lpp DKO mutant-infected mice were 83% lower than those in WT S. enterica serovar Typhimurium-infected mice on day 3 postinfection.
As shown in Fig. 4C, induction of KC (equivalent to IL-8) was highly reduced in mice infected with the lpp SKO and lpp DKO mutants compared to the induction in mice infected with WT S. enterica serovar Typhimurium. The KC level increased from 49 pg/ml on day 1 to 1,499 pg/ml on day 3 to 2,037 pg/ml on day 7 in mice infected with WT S. enterica serovar Typhimurium. No significant change in KC levels was noted in mouse sera at days 1 and 7 after infection with either the lpp SKO mutants or the lpp DKO mutant. Although the KC level appeared to be somewhat higher in the lppA SKO mutant-infected mice on day 7 postinfection (Fig. 4C), the difference was not statistically significant (P = 0.14) when the data were compared to the data for mice infected with the lpp DKO mutant. It was noted that the sera of animals infected with the lpp DKO mutant showed a spike in KC production on day 3 (310 pg/ml) and the level dropped to 48 pg/ml on day 7 postinfection, a value similar to the value seen on day 1 (Fig. 4C). The KC level detected in the sera of mice infected with the lppA SKO mutant did not change until day 3 (39 pg/ml on day 1 and 23 pg/ml on day 3); it increased to 89 pg/ml on day 7 postinfection. As observed with the lpp DKO mutant, sera from mice infected with the lppB SKO mutant showed an increase in KC levels from 45 pg/ml on day 1 to 101 pg/ml on day 3, and then the level decreased to 48 pg/ml on day 7 (Fig. 4C). On day 3 postinfection, the KC level induced by the lppB SKO mutant (101 pg/ml) was significantly higher (P = 0.02) than the level induced by the lppA SKO mutant (23 pg/ml). Likewise, the KC level induced by the lpp DKO mutant on day 3 postinfection (310 pg/ml) was significantly higher than the level in lppA SKO mutant-infected mice (23 pg/ml) (P = 0.004). The difference in the KC levels in sera from mice infected with the lpp DKO mutant (310 pg/ml) and the lppB SKO mutant (101 pg/ml) was also significant on day 3 postinfection (P = 0.006). Taken together, our findings indicated that the levels of the inflammatory mediators TNF-α, IL-6, and IFN-γ were consistently and reproducibly higher with the lppB SKO mutant than with the lppA SKO and lpp DKO mutants (except on day 3 for KC), suggesting that LppA contributes more to the induction of these cytokines.
The levels of cytokines (TNF-α, IL-6, and IFN-γ) and a chemokine (KC) in sera of the orally infected mice correlated with the systemic bacterial loads. Since (when given by the oral route) lpp SKO or DKO mutants were not detected in the spleen and liver, the levels of cytokines and chemokine detected in the sera of these mice were minimal compared to the levels in mice infected with WT S. enterica serovar Typhimurium. Mice infected with WT S. enterica serovar Typhimurium (1 × 104 CFU) generally exhibited a cytokine-chemokine response that was approximately 10-fold lower than the response observed when the bacteria were injected i.p. (data not shown).
Histopathological examination of tissue sections infected with lppA and lppB SKO and lpp DKO mutants of S. enterica serovar Typhimurium.
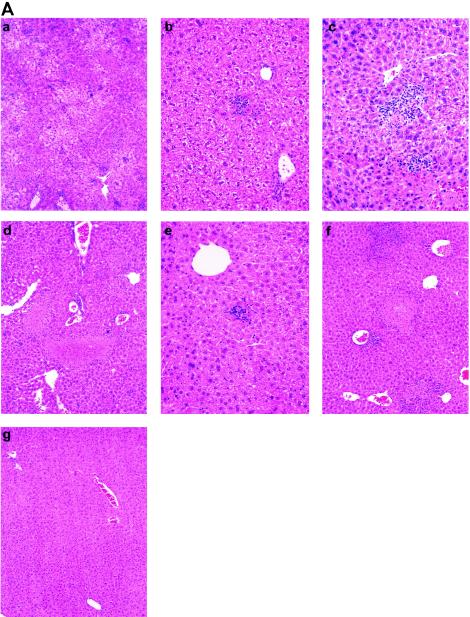
Our histopathological studies and analyses were performed with mice infected via the i.p. route with the lpp S. enterica serovar Typhimurium mutants and WT S. enterica serovar Typhimurium, and we used a five-tier grading system for the liver and a four-tier system for the spleen (Table 2). Postmortem gross examination of the livers and spleens of mice infected with WT S. enterica serovar Typhimurium showed hepatomegaly and splenomegaly, respectively. Both types of organs also had a diffuse pallor when they were compared to organs from normal controls. Figure 5 shows hematoxylin- and eosin-stained histological sections of livers (Fig. 5A) and spleens (Fig. 5B). Liver sections from mice infected with WT S. enterica serovar Typhimurium (score, ++++) had confluent areas of hepatocyte necrosis with extensive infiltration by macrophages and polymorphonuclear neutrophils (PMNs). The rest of the parenchyma had diffuse histiocytic infiltrates (HI) in and around the lobules and perivascular areas. In addition, moderate Kupffer cell hyperplasia was seen in liver sections from mice infected with WT S. enterica serovar Typhimurium, as was focal microvesicular fatty metamorphosis (Fig. 5A, panel a) when the tissues were compared to normal liver tissues (score, 0) from the uninfected mice (Fig. 5A, panel g).
FIG. 5.
Histopathological examination of sections from livers (A) and spleens (B) of mice infected with Salmonella mutants. Five- and four-tier scoring systems (Table 2) were developed for the liver and the spleen, respectively, to evaluate the histopathological changes in the two organs. All sections were stained with hematoxylin and eosin. (A) Liver sections. (a) Mouse infected with WT S. enterica serovar Typhimurium (score, ++++). Note the confluent area of necrosis with PMN infiltration, diffuse HI, and Kupffer cell hyperplasia. Magnification, ×200. (b) Mouse infected with the lpp DKO mutant (score, +). Occasional HI in portal and lobular areas were observed. Magnification, ×200. (c) Mouse infected with the complemented lpp DKO mutant (score, ++). Lobular HI associated with focal hepatocyte necrosis and infiltration of PMNs were observed. Magnification, ×200. (d) Mouse infected with the msbB SKO mutant (score, +++). Note the focal area of hepatocyte necrosis with PMNs and macrophage infiltration and lobular and perivascular diffuse HI. Magnification, ×200. (e) Mouse infected with the lpp-msbB TKO mutant (score, +). Occasional portal, lobular, and perivascular HI were observed. Magnification, ×200. (f) Mouse infected with the complemented msbB SKO mutant (score, +++). Perivascular and lobular HI associated with focal necrosis of hepatic parenchyma and PMN infiltration at the edges of necrotic areas were observed. Magnification, ×200. (g) Noninfected animal. Note the normal histology and no inflammatory infiltrates and the absence of necrosis. Magnification, ×100. (B) Spleen sections. (a) Mouse infected with WT S. enterica serovar Typhimurium. Note the severe disruption of lymphoid follicles with marked infiltration of the marginal zone and red pulp by sheets of macrophages. Abundant immunoblasts and apoptotic bodies in follicles were observed. (b) Mouse infected with the lpp DKO mutant (score, +). Note the scattered macrophages infiltrating the marginal zone. Lymphoid follicles were slightly increased in size, and their outlines were preserved. Scattered immunoblasts and apoptotic lymphocytes were noted in lymphoid follicles. (c) Mouse infected with the complemented lpp DKO mutant (score, ++). Moderate infiltration of red pulp by macrophages was observed. Lymphocyte activation within splenic follicles was apparent. Magnification, ×100. (d) Mouse infected with the msbB SKO mutant (score, +++). Severe disruption of lymphoid follicles with marked infiltration of the marginal zone and red pulp by sheets of macrophages was observed. Abundant immunoblasts and apoptotic bodies in follicles were also observed. (e) Mouse infected with the lpp-msbB TKO mutant (score, +). Scattered macrophages infiltrating the marginal zone were apparent. Lymphoid follicles were slightly increased in size, and their outlines were preserved. Scattered immunoblasts and apoptotic lymphocytes were observed in lymphoid follicles. (f) Mouse infected with the complemented msbB SKO mutant (score, +++). Prominent infiltration of marginal zones and red pulp by macrophages and lymphocyte activation in splenic follicles were observed. Magnification, ×200. (g) Noninfected mouse. Note the normal splenic follicles with discrete marginal zones and normal-appearing red pulp. Magnification, ×100.
There was a dramatic reduction in the histopathology observed in the livers of mice infected with the lpp DKO mutant (score, +) (Fig. 5A, panel b). There was no evidence of hepatocyte necrosis, and only occasionally were small portal, lobular, and perivascular HI seen in the sections examined. Kupffer cell hyperplasia was mild. Complementation of the lpp DKO mutant resulted in an increase in the histopathological grade (score, ++), as indicated by the focal areas of necrosis in the parenchyma with infiltrates composed of macrophages and PMNs. Scattered HI were seen perivascularly and in the lobules. These infiltrates were larger than those seen in the lpp DKO mutant-infected mice. Moderate Kupffer cell hyperplasia was also seen in mouse livers infected with the lpp DKO complemented strain (Fig. 5A, panel c). While mice infected with the lppA SKO mutant exhibited pathological lesions similar to those in lpp DKO mutant-infected mice (score, +), the lppB SKO mutant caused more tissue damage (score, ++) than the lpp DKO mutant (score, +), which was similar to the tissue damage seen with the complemented DKO mutant (score, ++).
Similarly, spleen sections of mice infected with WT S. enterica serovar Typhimurium (score, +++) had a marked disruption of splenic follicles and severe infiltration of the marginal zone and red pulp by macrophages (Fig. 5B, panel a). Marked activation of lymphocytes was noted along with increased numbers of immunoblasts and apoptotic bodies. Spleen sections from the noninfected mice had normal splenic lymphoid follicles (score, 0), discrete marginal zones, and normal-appearing red pulp (Fig. 5B, panel g). On the other hand, spleens from the lpp DKO mutant-infected mice had well-preserved splenic architecture with well-formed splenic follicles (score, +) (Fig. 5B, panel b). Lymphocytic activation in splenic follicles and expansion of the marginal zone were mild. The red pulp showed minimal macrophage infiltration with scattered immunoblasts, and minimal apoptotic lymphocytes were noted in the lymphoid follicles.
In mice infected with the lpp DKO complemented strain, there was moderate architectural disruption of the lymphoid follicles by macrophage infiltrates in the red pulp and marginal zone (score, ++). Increased numbers of immunoblasts and apoptotic bodies and lymphocyte activation in the periarteriolar lymphoid sheets were also observed (Fig. 5B, panel c). The histopathological lesions in spleens from mice infected with the lppB SKO mutant (score, ++) were much more pronounced than those in spleens from mice infected with the lpp DKO mutant (score, 1+); however, in these spleens there were significantly fewer lesions than there were in the spleens from mice infected with WT S. enterica serovar Typhimurium (score, +++). The histology associated with the lppA SKO mutant was similar to that associated with the lpp DKO mutant (score, +).
Although the lppA and lppB SKO mutants seemed to have contributed equally to the overall Lpp phenotype in vitro, as determined by invasion, cytotoxicity, and motility assays, the in vivo studies indicated that LppA contributed more to the overall Lpp phenotype. Compared to the lppA SKO mutant (with an intact lppB gene), the lppB mutant (carrying an intact lppA gene) induced production of cytokines (TNF-α and IL-6) to a greater extent (Fig. 4A and B). Similarly, the lppB SKO mutant caused more tissue damage in the livers and spleens of mice than the lppA SKO mutant caused. We, therefore, performed a separate experiment, in which C57BL/6 mice were infected i.p. with a much higher bacterial dose (1 × 107 CFU). We noted that mice infected with the lppB SKO mutant died 3 days earlier than mice infected with the lppA SKO mutant. However, at this dose, mice in both groups died in 7 days due to an overwhelming number of bacteria in the circulation. Mice infected with WT S. enterica serovar Typhimurium died within 48 h at this dose.
Characterization of the lpp-msbB TKO mutant of S. enterica serovar Typhimurium.
We deleted both copies of the lpp gene from an msbB mutant (strain YS1), and the identity of the mutant was confirmed by Southern blot analysis by using the lpp gene, the Knr gene cassette, and the pRE112 plasmid vector as probes (data not shown). The BglII- and MluI-digested chromosomal DNA from the lpp-msbB TKO mutant reacted with the Knr gene probe and exhibited a band at 3.2-kb, while the WT S. enterica serovar Typhimurium and pRE112 suicide vector did not show any band with the Knr gene probe. Since both copies of the lpp gene were deleted from the msbB mutant, the lpp gene probe did not react with the digested chromosomal DNA of the TKO mutant, although a band of the correct size (2.4 kb) was detected in the digested chromosomal DNA of WT S. enterica serovar Typhimurium. Neither the digested chromosomal DNA from the mutants nor the digested chromosomal DNA from WT S. enterica serovar Typhimurium reacted with plasmid pRE112 when it was used as a probe, indicating the complete loss of the suicide vector from the S. enterica serovar Typhimurium lpp-msbB TKO mutant after homologous recombination. As a positive control, we used the pRE112 plasmid digested with the BamHI enzyme, which exhibited a band of the correct size (6.5 kb).
The absence of the Lpp protein in lpp TKO mutants was confirmed by Western blot analysis by using anti-Lpp monoclonal antibody (data not shown). A band at 6.3 kDa, representing Lpp, was visualized for WT S. enterica serovar Typhimurium, whereas the corresponding band for the lpp-msbB TKO mutant was missing, further indicating that there was successful deletion of the lpp genes. The msbB SKO mutant exhibited a 6.3-kDa band similar to that of WT S. enterica serovar Typhimurium (46), which served as a positive control.
Integrity of the bacterial cell envelope of the TKO mutant of S. enterica serovar Typhimurium.
We examined the integrity of the outer membrane of the lpp-msbB TKO mutant by evaluating outer membrane blebbing, the sensitivity to detergents, the permeability to rifampin, and the release of β-lactamase from bacterial cells. Transmission electron microscopy showed no blebbing of the bacterial outer membrane of the lpp TKO mutant. When the mutant was treated with Triton X-100 (up to a concentration of 5%), there was no difference between the sensitivity of the lpp-msbB TKO mutant and the sensitivity of WT S. enterica serovar Typhimurium (data not shown). While the lpp DKO and msbB SKO mutants were resistant to 1% SDS, a finding similar to the finding obtained with WT S. enterica serovar Typhimurium, the lpp-msbB TKO mutant showed sensitivity at this concentration of SDS (data not shown), indicating that there was some alteration in the membrane integrity of the TKO mutant.
Sensitivity to the antibiotic rifampin indicated that the lpp-msbB TKO mutant was sensitive at a concentration of 10 μg/ml, whereas the WT, lpp DKO mutant, and msbB SKO mutant were resistant at this concentration of rifampin (data not shown). More importantly, the β-lactamase assay indicated that WT S. enterica serovar Typhimurium and the lpp-msbB TKO mutant exhibited similar releases of β-lactamase into the medium (data not shown). These data suggested that the membrane integrity was affected, albeit minimally, in the lpp-msbB TKO mutant.
Virulence potential of the TKO S. enterica serovar Typhimurium mutant.
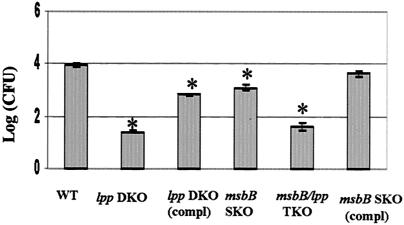
As we previously indicated (46), the lpp DKO mutant showed a 500- to 1,000-fold reduction in the invasion of T84 cells. The msbB SKO mutant was only 10-fold less invasive than WT S. enterica serovar Typhimurium and the complemented derivative of the msbB mutant (Fig. 6). The lpp-msbB TKO mutant showed a reduction in invasion of T84 cells similar to that of the lpp DKO mutant. The binding properties of WT S. enterica serovar Typhimurium and its various mutants to the host cells were very similar. The msbB SKO mutant of S. enterica serovar Typhimurium exhibited even greater motility than WT S. enterica serovar Typhimurium exhibited. The lpp-msbB TKO mutant, on the other hand, was nonmotile, like the lpp DKO S. enterica serovar Typhimurium mutant (data not shown).
FIG. 6.
Invasion of T84 cells by the lpp-msbB TKO and msbB SKO mutants of S. enterica serovar Typhimurium. The T84 cells were infected with the lpp-msbB TKO, lpp DKO, and msbB mutants. Likewise, T84 cells were infected with WT S. enterica serovar Typhimurium and the complemented derivatives of the lpp and msbB mutants at an MOI of 10:1 for 1 h. The monolayers were washed and incubated with medium containing gentamicin (100 μg/ml) for 1 h. After incubation, the cells were washed and incubated with fresh medium (without antibiotic) for another 1 h before they were lysed with 0.1% Triton X-100 (46) and different dilutions were plated on MSB agar plates. Three independent experiments were performed in triplicate, and an asterisk indicates statistical significance (P ≤ 0.05, as determined by Student's t test) compared to WT S. enterica serovar Typhimurium. The difference between the lpp DKO and lppDKO (compl) values was also significant. The arithmetic means ± standard deviations are shown. (compl) indicates a complemented strain.
The abilities of the lpp-msbB TKO and msbB SKO mutants to induce cell death in RAW264.7 and T84 cells were also examined. Host cells infected with the msbB SKO mutant exhibited cell death at a rate of approximately 30% compared to WT S. enterica serovar Typhimurium (Fig. 1). While the lpp DKO mutant showed 75% viability with macrophages and T84 cells, the findings with the lpp-msbB TKO mutant demonstrated that there was an almost complete absence of cell death (Fig. 1).
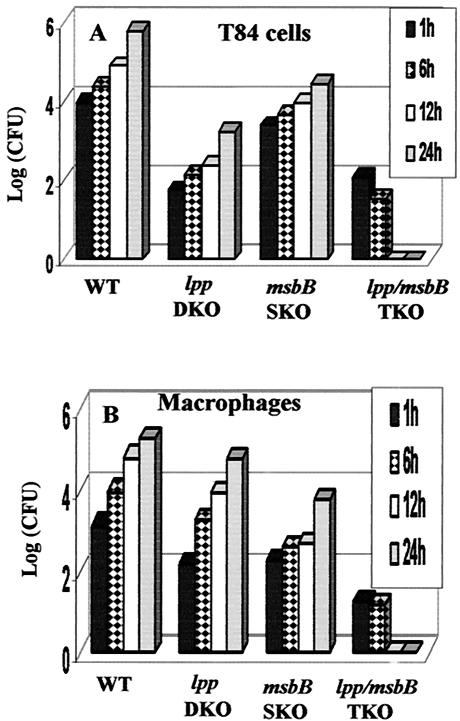
Survival inside the hostile environment of professional phagocytes, such as macrophages, is an important virulence mechanism of Salmonella (28). To examine whether the lpp and msbB genes have a role in this capacity, RAW264.7 macrophages and T84 epithelial cells were infected with the lpp-msbB TKO mutant, the msbB SKO mutant, and WT S. enterica serovar Typhimurium for 24 h. As shown in Fig. 7, WT S. enterica serovar Typhimurium and the lpp DKO and msbB SKO mutants were able to survive and replicate inside macrophages and T84 cells; however, the lpp-msbB TKO mutant was unable to survive in the intracellular environment of both cell types (Fig. 7). Similar intracellular survival data were obtained in vivo, in both liver and spleen homogenates (data not shown).
FIG. 7.
Intracellular replication of the lpp-msbB TKO mutant inside T84 cells (A) and RAW264.7 cells (B). Cells were infected with the various S. enterica serovar Typhimurium mutants and WT S. enterica serovar Typhimurium for 1 h. The cells were washed and incubated for 1 h with gentamicin (100 μg/ml)-containing medium. After incubation, the cells were washed and incubated in fresh medium containing a minimum concentration of gentamicin (5 μg/ml) for different times (1, 6, 12, and 24 h) (46). Finally, the cells were lysed with 0.1% Triton X-100 and plated on MSB agar plates to determine the number of CFU. Three independent experiments were performed; data from a representative experiment are shown.
In vitro cytokine production by the TKO mutant of S. enterica serovar Typhimurium.
To test whether deletion of the msbB and the lpp genes affected the overall induction of TNF-α and IL-8, we measured the levels of TNF-α and IL-8 after stimulation of RAW264.7 cells with heat-killed Salmonella mutants and infection of T84 cells with live bacteria. As shown in Fig. 8A, infection of RAW264.7 cells with the heat-killed lpp DKO mutant caused a 42% reduction in TNF-α production compared to the production in cells infected with WT S. enterica serovar Typhimurium, while the level of TNF-α production was decreased by 25% with the msbB SKO mutant. However, infection with the lpp-msbB TKO mutant resulted in a dramatic reduction (80%) in TNF-α production. Similarly, no IL-8 was detected in T84 cells infected with the lpp DKO and lpp-msbB TKO mutants (Fig. 8B). On the other hand, deletion of the msbB gene alone had no effect on in vitro IL-8 production (Fig. 8B).
FIG. 8.
Induction of TNF-α by various S. enterica serovar Typhimurium mutants in RAW264.7 cells (A) and IL-8 production in nonpolarized T84 cells (B). Macrophages were stimulated with the heat-killed bacteria at an MOI of 0.1:1 and incubated for 8 h. T84 cells were infected with live bacteria at an MOI of 10:1 and incubated for 1 h. After incubation, the cells were washed and incubated in gentamicin (100 μg/ml)-containing medium for 1 h, after which the cells were washed and incubated in an antibiotic-free medium for 12 h. TNF-α and IL-8 levels were determined by an ELISA. Three independent experiments were performed in duplicate, and an asterisk indicates a value that is statistically significantly different from the value for WT S. enterica serovar Typhimurium (P ≤ 0.05, as determined by Student's t test). The arithmetic means ± standard deviations are shown. The difference between the values for the lpp DKO- and lpp-msbB TKO-infected macrophages was also statistically significant.
In vivo characterization of the lpp-msbB TKO mutant of S. enterica serovar Typhimurium.
Quantitation of the bacterial loads in the livers and spleens of infected mice indicated that the lpp-msbB TKO mutant was unable to survive in an in vivo environment, although the lpp DKO and msbB SKO mutants survived and replicated under in vivo conditions (data not shown). Consequently, the TKO mutant was unable to induce any detectable levels of TNF-α, KC, or IFN-γ compared to the levels induced by WT S. enterica serovar Typhimurium and the lpp DKO and msbB SKO mutants in both BALB/c and Swiss Webster mice, even when the mice were inoculated by the i.p. route (Fig. 9). The levels of TNF-α, KC, and IFN-γ on day 7 postinfection were 850, 1,200, and 700 pg/ml, respectively, in the sera of WT S. enterica serovar Typhimurium-infected mice; the levels in the msbB SKO mutant-infected mice were 450, 175, and 225 pg/ml, respectively, which corresponded to reductions of 43, 85, and 68%, respectively, compared to findings for the WT S. enterica serovar Typhimurium-infected mice (Fig. 9).
FIG. 9.
Levels of TNF-α (A), KC (B), and IFN-γ (C) in mice infected with various S. enterica serovar Typhimurium mutants. Sera from mice infected with the WT and with lpp DKO, msbB SKO, and lpp-msbB TKO mutants were collected on days 1, 3, and 7 postinfection, and the levels of TNF-α, KC, and IFN-γ were determined by an ELISA and CBA as described in Materials and Methods. The animals were infected by the i.p. route with 2 × 103 CFU in 100 μl. In panels A and B, arithmetic means ± standard deviations from three independent experiments performed in duplicate are shown. In panel C, typical experimental data from the three independent experiments performed are shown. Asterisks denote statistically significant differences.
The lpp-msbB TKO mutant was only able to induce a minimal level of liver damage (score, +) (Fig. 5A, panel e), as indicated by histopathological examination, compared to the levels induced by WT S. enterica serovar Typhimurium (score, ++++) (Fig. 5A, panel a) and the msbB SKO mutant and its complemented derivative (score, +++) (Fig. 5A, panel d, and 5B, panel f). While the spleen sections of mice infected with the lpp-msbB TKO mutant exhibited mild histopathological changes (score, +) (Fig. 5B, panel e), the spleen sections of mice infected with the msbB SKO mutant and its complemented derivative showed greater histopathological changes (score, +++) (Fig. 5B, panels d and f), which were similar to the changes observed in the WT S. enterica serovar Typhimurium-infected mice (Fig. 5B, panel a).
DISCUSSION
In this study, we molecularly characterized the lppA and lppB SKO mutants using in vitro and in vivo models and compared them with the lpp DKO mutant generated in a previous study (46). We also constructed an lppA-lppB-msbB TKO mutant with the goal of developing a candidate vaccine against salmonellosis, which also could be used to deliver heterologous recombinant antigens.
Our motility and cytotoxicity data obtained with the lpp SKO mutants matched invasion data obtained in vitro, as the lpp SKO mutants were significantly more motile and cytotoxic than the lpp DKO mutant (Fig. 1). However, since the lppA and lppB SKO mutants exhibited comparable motilities and cytotoxicities, which were still significantly less than those of WT S. enterica serovar Typhimurium, these data indicated that both lpp genes could be required for fully restoring these bacterial phenotypes. We obtained essentially similar data when T84 cells were invaded with the WT and the various lpp mutants (46).
Compared to the TNF-α levels in macrophages infected with WT S. enterica serovar Typhimurium, the TNF-α levels in macrophages infected with the lpp SKO and lpp DKO mutants were reduced (Fig. 2A). These data also suggested that both lpp genes are required for the full virulence effect of Lpp in vitro. On the contrary, we demonstrated that while the lpp DKO mutant minimally produced IL-8, this chemokine was produced at a level similar to that seen in WT S. enterica serovar Typhimurium when nonpolarized T84 cells were infected with either the lppA or lppB SKO mutant (Fig. 2B). These data indicated that (i) Lpp is essential for induction of IL-8 and (ii) one copy of the lpp gene is sufficient to induce the production of this chemokine in vitro. It was shown previously that T84 cells did not respond to LPS in inducing IL-8 production (43). This study could explain the lack of detection of IL-8 in the lpp DKO mutant-infected T84 cells.
Since polarized cells more closely mimic the in vivo environment, we also examined IL-8 production in apically treated T84 cells with S. enterica serovar Typhimurium mutants. While the data obtained were similar to the data obtained with the nonpolarized cells, the following observations were noteworthy. First, higher IL-8 levels were observed in the basal chamber than in the apical chamber. Second, the lppB SKO mutant produced WT levels of IL-8. Third, the lppA mutant appeared to contribute less to IL-8 production (Fig. 2C and D). Nonetheless, it was clear that Lpp played a key role in IL-8 production in vitro.
A study of host-pathogen interactions in which an in vitro system is used could be a useful predictor of the behavior of pathogens in vivo (8, 22, 43). However, it is crucial that such in vitro studies are also conducted in an animal system to mimic human infections. A mouse model has been widely used to study Salmonella pathogenesis (11, 12, 26). It has been reported that during early systemic Salmonella infection, there is rapid clearance of the majority of the bacterial inoculum by macrophages and neutrophils (5, 6, 32, 44). This is followed by the exponential growth of residual bacteria within the liver and spleen, resulting in the production of inflammatory cytokines and chemokines, which together are thought to lead to the formation of pathological lesions and trafficking of various leukocyte subsets and macrophages to the subepithelial region (3, 10, 12, 20, 24, 33, 35, 40, 52, 56).
Our in vivo data indicated that the survival of both lpp SKO and DKO mutants was similar to the survival of WT strains in the liver and spleen of i.p. infected mice (Fig. 3). Likewise, lpp SKO mutants were more invasive than DKO mutants. These data matched our in vitro invasion data for these strains (46). The inability to detect lpp mutants in mouse organs after oral challenge could indicate either (i) that such mutants were unable to cross the intestinal mucosa or (ii) that such mutants were killed more efficiently in the lamina propria, which should be investigated in the future. Lpp mutants also showed a marked reduction in the induction of cytokines and chemokines in vivo, suggesting that Lpp plays a crucial role in producing these mediators (Fig. 4).
More interesting, however, were our findings that the lppB SKO mutant induced higher levels of TNF-α and IL-6 in mouse sera than lpp DKO and lppA SKO mutants induced (Fig. 4A and B). Similarly, the levels of these cytokines were higher with the lppA SKO mutant than with the lpp DKO mutant (Fig. 4A and B). These data suggested that (i) LppA might be more potent than LppB in cytokine induction, (ii) the lppA gene might be expressed at higher levels than the lppB gene in vivo, and (iii) LppA could be more accessible to receptor binding on host cells. In future studies we will determine the promoter activity of the lppA and lppB genes and the regulation of these two lpp genes in in vitro and in vivo environments.
Histopathological examination of the liver and spleen indicated that there was severe tissue damage in mice infected with WT S. enterica serovar Typhimurium, compared to the minimal damage in mice infected with the lpp DKO mutant strain (Fig. 5). Importantly, more extensive tissue damage with the lppB SKO mutant than with the lppA SKO mutant correlated with our finding that the lppB SKO mutant produced higher levels of TNF-α and IL-6 (Fig. 4). These results were further substantiated by the finding that mice infected with the lppB SKO mutant had a shorter mean time to death than animals infected with the lppA SKO or lpp DKO mutant.
The IFN-γ levels in sera from mice infected with the lppB SKO mutant were also higher than those in sera from mice infected with the lppA SKO mutant, although the levels were much reduced compared to the levels in sera from WT S. enterica serovar Typhimurium-infected mice, indicating that Lpp plays a significant role in IFN-γ production. IFN-γ plays a critical role in stimulation of Th1 immune responses and the clearance of intracellular pathogens, such as Salmonella (12, 17, 34, 37). High IFN-γ levels were previously reported during early Salmonella infection (42), as also noted in this study. Cytokine profiling for the presence of Th1 and Th2 cytokines in sera from WT S. enterica serovar Typhimurium-infected mice indicated increased IFN-γ levels and no detectable levels of IL-2 and IL-4, confirming the involvement of Th1 responses during salmonellosis (data not shown). Studies have indicated that TNF-α acts synergistically with IFN-γ to activate antimicrobial effects of macrophages (50). Since lpp mutants resulted in reduced TNF-α and IFN-γ levels, their intracellular survival would be enhanced. This could be an important characteristic of an attenuated vaccine candidate strain.
Although the lpp DKO mutant was avirulent in mice (46) and protected against a lethal challenge by WT S. enterica serovar Typhimurium, its intact LPS might induce host inflammatory reactions. Studies have shown that in an LPS-defective strain of Shigella flexneri with mutations in the rfcC, rfaL, or galU gene (coding for enzymes required for biosynthesis of the core polysaccharide region), there were reduced levels of adherence and invasion of intestinal epithelial cells, as well as a reduction in the migration of polymorphonuclear leukocytes across intestinal epithelial cells (41). It was also reported that mutation in the core polysaccharide region of LPS rendered S. enterica serovar Typhimurium and E. coli defective in motility and in the ability to penetrate mouse intestine (29, 39). In our studies, Salmonella strain SL3769 with a mutation in the rfaG gene (the rfa gene cluster is involved in the biosynthesis of the polysaccharide core unit) exhibited a 100-fold reduction in invasion of T84 cells compared to its parent strain (data not shown).
Mutations in the genes involved in lipid A biosynthesis (e.g., msbB and htrB) reduced the ability of S. enterica serovar Typhimurium to cause systemic infection by reducing host inflammatory responses and also by affecting secretion of type III secretion system effector proteins (23, 25, 30, 55). E. coli msbB mutants were also attenuated in the ability to stimulate E-selectin production by human endothelial cells (47). Therefore, to minimize the possible LPS-induced reaction in the host, we constructed and characterized an lpp-msbB TKO mutant.
Although acylation of lipid A was important for cytotoxicity and invasiveness of WT S. enterica serovar Typhimurium in T84 cells, Lpp's contribution was much more significant (Fig. 1 and 6). The msbB SKO S. enterica serovar Typhimurium mutant was shown to be more motile than WT S. enterica serovar Typhimurium (data not shown). The number of flagella could have been affected in this mutant, as previously reported for the lipid A mutant of S. enterica serovar Typhimurium defective in the htrB gene, which was shown to be hyperflagellated (48). However, the lpp-msbB TKO mutant was nonmotile, like the lpp DKO mutant, indicating that the lpp genes mainly contributed to bacterial motility. More importantly, the lpp-msbB TKO mutant failed to survive intracellularly in vitro, although the lpp DKO and msbB SKO mutants individually were not defective in intracellular survival (Fig. 7). We obtained similar data in vivo (data not shown), indicating that Lpp and acylation of lipid A together are contributing factors and are required for intracellular Salmonella survival.
Since Lpp and LPS are major components of the bacterial outer membrane required for structural integrity of the envelope, it is possible that deletion of the lpp and msbB genes compromised the bacterial cell envelope. Studies of Vaara and Nurminen (54) showed that an msbB mutant of E. coli had an intact permeability barrier against hydrophobic antibiotics, indicating that acylation of lipid A is not crucial for the bacterial outer membrane permeability function. Indeed, antimicrobial peptides are important components of the host innate immune system that act on the outer membranes of gram-negative bacteria, resulting in cell death (14). These antimicrobial peptides should be more effective in bacterial killing if the cell envelope is fragile. Our data indicated that the lpp-msbB TKO mutant was more sensitive to SDS and rifampin than WT S. enterica serovar Typhimurium. However, the mutant tolerated bile salts and released levels of β-lactamase similar to those seen with WT S. enterica serovar Typhimurium. Therefore, whether the membrane integrity of the lpp-msbB TKO mutant was compromised, resulting in its inability to survive intracellularly, must be explored further.
The lpp-msbB TKO mutant was severely impaired in inducing TNF-α in macrophages compared to the lpp DKO and msbB SKO mutants, thereby emphasizing the role of both Lpp and acylation of lipid A in TNF-α production (Fig. 8A). Additionally, it was clear that only Lpp contributed to the production of IL-8 in T84 cells (Fig. 8B).
The lpp-msbB TKO mutant was unable to induce any detectable levels of inflammatory cytokines in a mouse model (Fig. 9). This could have been due to (i) deletion of the lpp genes and an altered lipid A moiety of LPS and (ii) the inability of the lpp-msbB TKO mutant to survive intracellularly. It has been reported that msbB mutants of E. coli and Salmonella exhibited decreased cytokine (TNF-α) production in both in vitro and in vivo assays (4, 25, 30, 47). Likewise, inactivation of two copies of the msbB gene in S. flexneri inhibited TNF-α production and impaired the organism's ability to cause inflammatory destruction of the epithelial cells (9).
As shown in Fig. 9, the msbB SKO mutant induced expression of TNF-α, KC, and IFN-γ, albeit at much lower levels than the levels induced by WT S. enterica serovar Typhimurium, at 7 days postinfection. Compared to the msbB SKO mutant, the lpp DKO and lpp-msbB TKO mutants were highly attenuated in the induction of these inflammatory mediators. These data further indicated a greater role for Lpp in the induction of these cytokines and chemokines. We expected higher levels of KC in mice infected with the msbB SKO mutant. This was based on our finding that in T84 cells infected with the msbB SKO mutant, the IL-8 levels were similar to the levels in cells infected with WT S. enterica serovar Typhimurium (Fig. 8B). Whether differences in the production of KC and IL-8 represent a variation in the in vitro environment versus the in vivo environment needs to be explored further.
In conclusion, the data indicated that the lpp SKO and lpp DKO mutants were able to survive in the in vivo environment when mice were challenged via the i.p. route. Both lpp genes (lppA and lppB) were required for the full virulence of S. enterica serovar Typhimurium. However, LppA seemed to contribute more to producing proinflammatory cytokines that led to tissue damage in the animals. Moreover, deletion of the msbB gene from the lpp DKO mutant further attenuated S. enterica serovar Typhimurium virulence in both in vitro and in vivo models.
Acknowledgments
This work was supported by a grant from the Advanced Technology Program of the Texas Higher Education Coordinating Board to A.K.C. A.A.F. is a recipient of a McLaughlin Postdoctoral Fellowship, which supported a portion of this research.
We thank Mardelle Susman for editing the manuscript and B. Low (Yale University, New Haven, Conn.) for providing the msbB SKO mutant and the pSM21 plasmid for complementation and for critical reading of the manuscript. We also thank K. Sanderson, University of Calgary, Calgary, Canada, for providing Salmonella mutant SL3769.
Editor: J. T. Barbieri
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 2.Braun, V. and K. Hantke. 1974. Biochemistry of bacteria cell envelopes. Annu. Rev. Biochem. 43:89-121. [DOI] [PubMed] [Google Scholar]
- 3.Cater, P. B., and F. M. Collins. 1974. The route of enteric infection in normal mice. J. Exp. Med. 139:1189-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clementz, T., Z. Zhou, and C. R. Raetz. 1997. Function of the E. coli msbB gene, a multicopy suppressor of hlrB knockouts, in the acylation of lipid A. J. Biol. Chem. 272:10353-10360. [DOI] [PubMed] [Google Scholar]
- 5.Conlan, J. W. 1996. Neutrophils prevent extracellular colonization of the liver microvasculature by Salmonella typhimurium. Infect. Immun. 64:1043-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conlan, J. W. 1997. Critical roles of neutrophils in host defense against experimental infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect. Immun. 65:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dailey, F. E., and R. M. Macnab. 2002. Effects of lipoprotein biogenesis mutations on flagellar assembly in Salmonella. J. Bacteriol. 184:771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darwin, K. H., and V. L. Miller. 1999. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 12:405-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Hauteville, H., S. Khan, D. J. Maskell, A. Kussak, A. Weintraub, J. Mathison, R. H. Ulevitch, N. Wuscher, C. Parsot, and P. J. Sansonetti. 2002. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J. Immunol. 168:5240-5251. [DOI] [PubMed] [Google Scholar]
- 10.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckmann, L., J. Fierer, and M. F. Kagnoff. 1996. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with Salmonella Dublin. J. Immunol. 156:2894-2900. [PubMed] [Google Scholar]
- 12.Eckmann, L., and M. F. Kagnoff. 2001. Cytokines in host defense against Salmonella. Microb. Infect. 3:1191-1200. [DOI] [PubMed] [Google Scholar]
- 13.Edwards, R. A., L. H. Keller, and D. M. Schifferli. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbriae gene expression. Gene 207:149-157. [DOI] [PubMed] [Google Scholar]
- 14.Ernst, R. K., T. Guina, and S. I. Miller. 1999. How intracellular bacteria survive: surface modifications that promote resistance to host innate immune responses. J. Infect. Dis. 179:5326-5330. [DOI] [PubMed] [Google Scholar]
- 15.Fenton, M. J., and D. T. Golenbock. 1998. LPS-binding proteins and receptors. J. Leukoc. Biol. 64:25-32. [DOI] [PubMed] [Google Scholar]
- 16.Glauser, M. P., G. Zanetti, J. D. Baumgartner, and J. Cohen. 1991. Septic shock: pathogenesis. Lancet 338:732-739. [DOI] [PubMed] [Google Scholar]
- 17.Gulig, P. A. T. J. Doyle, M. J. Clare-Salzler, R. I. Maiese, and H. Marsui. 1997. Systemic infection of mice by wild-type but not spv− Salmonella typhimurium is enhanced by neutralization of gamma interferon and tumor necrosis alpha. Infect. Immun. 65:5191-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveu, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, S., and H. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 20.Henderson, B., S. Poole, and M. Wilson. 1996. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol. Rev. 60:316-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu, H. S. 1989. Pathogenesis and immunity in murine salmonellosis. Microbiol. Rev. 53:390-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurley, B. P., and B. A. McCormick. 2003. Translating tissue culture results into animal models: the case of Salmonella typhimurium. Trends Microbiol. 11:562-569. [DOI] [PubMed] [Google Scholar]
- 23.Jones, B. D., W. A. Nichols, B. W. Gibson, M. G. Sunshine, and M. A. Apicella. 1997. Study of the role of the htrB gene in Salmonella typhimurium virulence. Infect. Immun. 65:4778-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung, H. C., L. Ekmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan, S. A., P. Everest, S. Servos, N. Foxwell, U. Zahringer, H. Brade, E. Rietchel, G. Dougan, I. Charles, and D. J. Muskell. 1998. A lethal role for lipid A in Salmonella infections. Mol. Microbiol. 29:571-579. [DOI] [PubMed] [Google Scholar]
- 26.Klimpel, G. R., M. Asuncion, J. Haithcoat, and D. W. Niesel. 1995. Cholera toxin and Salmonella typhimurium induce different cytokine profiles in the gastrointestinal tract. Infect. Immun. 63:1134-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, C. A., M. Silva, A. M. Siber, A. J. Kelly, E. Galyov, and B. A. McCormick. 2000. A selected Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl. Acad. Sci. USA 97:12283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung, K. Y., and B. B. Finlay. 1991. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 88:11470-11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Light, T. R., K. A. Krogfelt, P. S. Cohen, L. K. Poulsen, J. Urbance, and S. Molin. 1996. Role of lipopolysaccharide in colonization of the mouse intestine by Salmonella typhimurium studied by in situ hybridization. Infect. Immun. 64:3811-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low, K. B., M. Ittensohn, T. Le, J. Platt, S. Sodi, M. Amoss, O. Ash, E. Carmichael, A. Chakraborty, J. Fischer, S. Lin, X. Luo, S. I. Miller, L. Zheng, I. King, J. Pawelek, and D. Bermuudes. 1999. Lipid A mutant Salmonella with suppressed virulence and TNF-α induction retain tumor-targeting in vivo. Nat. Biotechnol. 17:37-41. [DOI] [PubMed] [Google Scholar]
- 31.Luderitz, O., C. Galanos, V. Lehman, M. Nurminen, E. T. Rietschel, G. Rosenfelder, M. Simon, and O. Westphal. 1973. Lipid A: chemical structure and biological activity, J. Infect. Dis. 128:17-29. [DOI] [PubMed] [Google Scholar]
- 32.Mastroeni, P., J. A. Harrison, and C. E. Hormaeche. 1994. Natural resistance and acquired immunity to Salmonella. Fundam. Clin. Immunol. 2:83-88. [Google Scholar]
- 33.McCormick, B. A., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. I. Madara. 1993. Salmonella attachment to human intestinal epithelial monolayers: transcellular signaling to subepithelial neutrophils. J. Cell Biol. 123:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuno, Y., H. Takada, A. Nomura, C. H. Jin, H. Hattori, K. Ihara, T. Aoki, K. Egughi, and T. Hara. 2003. Th1 and Th1-inducing cytokines in Salmonella infection. Clin. Exp. Immunol. 131:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison, D. C., and J. L. Ryan. 1987. Endotoxins and disease mechanisms. Annu. Rev. Med. 38:417-432. [DOI] [PubMed] [Google Scholar]
- 36.Murray, S. R., D. Bermudes, K. S. De Felipe, and K. B. Low. 2001. Extragenic suppressors of growth defects in msbB Salmonella. J. Bacteriol. 183:5554-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nauciel, C., and F. Espinase-Maes. 1992. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 60:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neidhardt, F. C., J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.). 1987. Salmonella and E. coli: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 39.Nevola, J. J., D. C. Laux, and P. S. Cohen. 1987. In vivo colonization of the mouse large intestine and in vitro penetration of intestinal mucus by an avirulent smooth strain of Salmonella typhimurium and its lipopolysaccharide-deficient mutant. Infect. Immun. 55:2884-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parrillo, J. E. 1993. Pathogenic mechanisms of septic shock. N. Engl. J. Med. 328:1471-1477. [DOI] [PubMed] [Google Scholar]
- 41.Rajakumar, K., B. H. Jost, C. Sasakawa, N. Okada, M. Yoshikawa, and B. Adler. 1994. Nucleotide sequence of the rhamnose biosynthetic operon of Shigella flexneri 2a and role of lipopolysaccharide in virulence. J. Bacteriol. 176:2362-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramarathinam, L., R. A. Shaban, D. W. Niesel, and G. R. Klimpel. 1991. Interferon gamma production by gut-associated lymphoid tissues and spleen following oral Salmonella typhimurium challenge. Microb. Pathog. 11:347-356. [DOI] [PubMed] [Google Scholar]
- 43.Schuerer-Maly, C. C., L. Eckmann, M. F. Kagnoff, M. T. Falco, and F. E. Maly. 1994. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology 81:85-91. [PMC free article] [PubMed] [Google Scholar]
- 44.Sebastiani, G., V. Blais, V. Sancho, S. N. Vogel, M. M. Stevenson, P. Gros, J. Lapointe, S. Rivest, and D. Malo. 2002. Host immune response to Salmonella enterica serovar Typhimurium infection in mice derived from wild strains. Infect Immun. 70:1997-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sha, J., M. Lu, and A. K. Chopra. 2001. Regulation of cytotoxic gene in Aeromonas hydrophila: characterization of an iron uptake regulator. Infect. Immun. 69:6370-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sha, J. A., A. Fadl, G. R. Klimpel, D. W. Niesel, V. L. Popov, and A. K. Chopra. 2004. The two murein lipoproteins of Salmonella enterica serovar Typhimurium contribute to the virulence of the organism. Infect. Immun. 72:3987-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somerville, J. E., L. Cassiano, B. Bainbride, M. D. Cunningham, and R. P. Darveau. 1996. A novel E. coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J. Clin. Investig. 97:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sunshine, M. G., B. W. Gibson, J. J. Engstrom, W. A. Nicols, B. D. Jones, and M. A. Apicella. 1997. Mutation of htrB gene in a virulent Salmonella typhimurium strain by intergenic transduction: strain construction and phenotypic characterization. J. Bacteriol. 179:5521-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenner, A. J., R. J. Ziccardi, and N. R. Cooper. 1984. Antibody-independent C1 activation by E. coli. J. Immunol. 133:886-891. [PubMed] [Google Scholar]
- 50.Tite, J. P., G. Dougan, and S. N. Chatfield. 1991. The involvement of tumor necrosis factor in immunity to Salmonella infection. J. Immunol. 147:3161-3164. [PubMed] [Google Scholar]
- 51.Tobias, P. S., R. I. Tapping, and J. A. Gegner. 1999. Endotoxin interaction with lipopolysaccharide-responsive cells. Clin. Infect. Dis. 28:476-481. [DOI] [PubMed] [Google Scholar]
- 52.Tracey, K. J., and A. Cerami. 1993. Tumor necrosis factor: an update of its biology. Crit. Care Med. 21:S415-S422. [PubMed] [Google Scholar]
- 53.Ulevitch, R. J., and P. S. Tobias. 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13:437-457. [DOI] [PubMed] [Google Scholar]
- 54.Vaara, M., and M. Nurminen. 1999. Outer membrane permeability barrier in E. coli mutants that are defective in the late acyltransferase of lipid A biosynthesis. Antimicrobial Agents Chemother. 43:1459-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson, P. R., A. Benmore, S. A. Khan, P. W. Jones, D. J. Maskell, and T. S. Wallis. 2000. Mutation of waaN reduces Salmonella enterica serovar Typhimurium-induced enteritis and net secretion of type III secretion system 1-dependent proteins. Infect. Immun. 68:3768-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, H., J. W. Peterson, D. W. Niesel, and G. R. Klimpel. 1997. Bacterial lipoprotein and LPS act synergistically to induce lethal shock and proinflammatory cytokine production. J. Immunol. 159:4868-4878. [PubMed] [Google Scholar]